Influenza-Like Illness in the Time of the COVID-19 Pandemic
October 26th, 2022
AUTHORS
Cherrelle Smith, MD,Clinical Assistant Professor of Emergency Medicine, Stanford University School of Medicine, Palo Alto, CA
Mia L. Karamatsu, MD,Clinical Assistant Professor of Emergency Medicine, Stanford University School of Medicine, Palo Alto, CA
Guillermo A. De Angulo, MD, MSCR, Clinical Assistant Professor of Emergency Medicine, Stanford University School of Medicine, Palo Alto, CA
N. Ewen Wang, MD,Professor of Emergency Medicine, Stanford University School of Medicine, Stanford, CA
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Clinical Assistant Professor of Emergency Medicine, Mt. Sinai Medical School and Bon Secours Hospital, NY
Executive Summary
• Of coronavirus cases in the United States, only 10.6% of cases were in children ages 0 to 17 years, with a mortality rate of < 0.1%.
• The influenza A and B viruses are negative-sense, single-stranded, segmented ribonucleic acid (RNA) viruses of the Orthomyxoviridae family.
• The coronavirus family is a type of enveloped, positive-sense, single-stranded RNA viruses with multiple subgroups. COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus), which is part of the β subgroup.
• Although COVID-19 has caused more than 1 million deaths worldwide, as of October 2020, influenza deaths have claimed the lives of many more in the last decade. This is especially true of children. Thus, it is important to keep influenza in the forefront while caring for children with influenza-like illnesses during this pandemic.
• In pediatric patients, a review of 11 international coronavirus case series, including 333 children, stated that 35% of patients were asymptomatic. The most common presenting symptoms were cough, fever, and pharyngitis. Tachypnea, nasal congestion, rhinorrhea, wheezing, diarrhea, vomiting, fatigue, and headache occurred less often.
• Gastrointestinal symptoms, as well as chest pain, are more common in COVID-19 than influenza. These may be factors to aid in distinguishing between these two respiratory presentations. Anosmia (loss of smell) and ageusia (loss of taste) also can be demarcating findings when differentiating between influenza and COVID-19.
• The American Academy of Pediatrics’ influenza 2019-2020 guidelines recommend rapid polymerase chain reaction (PCR) testing should be performed in children younger than 5 years of age, and especially in those younger than 2 years of age; children with respiratory illnesses, including cystic fibrosis or asthma; and children in high-risk groups.
• There are rapid antigen and molecular assay PCR tests for both influenza and SARS-CoV-2. Rapid tests can provide results in 15 to 30 minutes, whereas molecular assay tests (e.g., reverse transcription PCR [RT-PCR]) generally have a turnaround time of at least one hour. The rapid antigen tests are helpful when they are positive, but they are less sensitive than RT-PCR tests. Therefore, rapid antigen tests should be interpreted with caution because they may represent false negatives.
• In alignment with recommendations published by the Pediatric Infectious Diseases Society and Infectious Diseases Society of America, a chest radiograph is not necessary unless the patient has hypoxemia, significant respiratory distress, or requires hospitalization.
• An international consensus statement on chest imaging for patients with confirmed or suspected COVID-19 was published by pediatric radiologists in April 2020. Although pediatric data is limited, they determined that imaging is not indicated in children who are well appearing, > 3 months of age, are immunocompetent, and do not require hospitalization. Pediatric patients hospitalized with severe illness benefited the most from imaging. They were found to have “bilateral patchy consolidation with associated ground glass opacity in a peripheral and lower lung zone predominant pattern.
• Oseltamivir (Tamiflu) is the antiviral drug of choice for a child with an influenza illness. Previously healthy children with mild disease may benefit from oseltamivir if taken within the first 48 hours of illness. It can reduce hospitalization rates and secondary bacterial infections, such as pneumonia. It also can shorten the course of illness by up to 1.5 days.
• In pediatric patients, there appears to be a risk for MIS-C, which resembles Kawasaki disease or toxic shock syndrome. This syndrome is defined per the CDC as a patient younger than 21 years of age with measured or tactile fevers of at least 24 hours’ duration and laboratory-confirmed SARS-CoV-2 infection via PCR testing, antibody testing, or epidemiologic link to a person with a known infection with no alternative diagnosis. Most patients present with laboratory findings of previous SARS-CoV-2 infection and evidence of inflammation with multisystem involvement.
The sudden appearance of COVID-19 has created an additional challenge to the evaluation of children with "flu-like" symptoms. This article compares and contrasts influenza and coronavirus and provides a critical update on a timely topic.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Early fall through March, respiratory season, traditionally is a busy time of year in a pediatric emergency department (ED). While most children will have an uncomplicated, and undiagnosed, viral syndrome, diagnosis of influenza and identification of children at risk of severe disease always have been paramount.
The present human coronavirus (COVID-19) pandemic adds increased complexity to this setting. Although only a minority of children with COVID-19 develop severe disease, it is important to diagnose it for public health reasons. Likewise, the need for special measures to avoid COVID-19 disease transmission, and to improve ED throughput efficiency and efficacy, requires thoughtful protocols and workflows for respiratory virus testing.
This article first will discuss the epidemiology and pathophysiology of influenza and COVID-19, the differential diagnosis, and ED testing algorithms. Next, it will speak specifically of the clinical course of influenza, ED management, disposition, and vaccination strategies. Lastly, it will cover COVID-19 disease in children as known at the writing of this report.
Epidemiology
Influenza
While the full analysis of the Centers for Disease Control and Prevention (CDC) influenza surveillance data for the 2019-2020 year has not been released, preliminary data analysis estimates that, during this season, 39-56 million people were infected, causing 18-26 million medical visits, 410,000-740,000 hospitalizations, and roughly 24,000-62,000 deaths.1 In patients ages 0 to 4 years, the incidence of influenza A was 55%, while patients from ages 5 to 24 years had a higher incidence of influenza B infection. There were 188 deaths in children ages 0 to 17 years, with 41.4% having an underlying high-risk medical condition and 48.5% having a confirmed bacterial co-infection.1 While these numbers are significant, the 2019-2020 season affected smaller numbers of children and was shorter in duration than expected when compared to prior influenza seasons.2 It has been suggested that this was because of the effects of COVID-19 transmission-reducing behaviors like improved hand hygiene and self-isolation.2 This is further supported by an observed decrease of positive influenza rates in pediatric patients — from 22% to 0.3% — after local school closures and stay-at-home orders were initiated.3
Coronavirus Disease (COVID-19/SARS-CoV-2)
Regarding the coronavirus, as of Jan. 5, 2021, there were about 84 million cases worldwide and more than 1.8 million deaths. In the United States, there were 20.5 million cases and 350,664 deaths. Of those cases in the United States, only 10.6% of cases were in children ages 0 to 17 years, with a mortality rate of < 0.1%.4
Pathophysiology of Influenza
The influenza A and B viruses are negative-sense, single-stranded, segmented ribonucleic acid (RNA) viruses of the Orthomyxoviridae family. The viruses are categorized into subtypes based on the type of hemagglutinin and neuraminidase protein on their envelope. The hemagglutinin glycoprotein induces endocytosis and viral RNA release by binding to sialic acid residues on the epithelial cell surface. The neuraminidase protein cleaves the bond between the viral progeny and host cell, thus allowing viral progeny to spread.5 The influenza viruses, particularly influenza A viruses, have the ability to undergo changes in the antigenic characteristic of the hemagglutinin and neuraminidase glycoproteins via antigenic drift or shifts. Drift occurs when there are mutations or transcription errors that create a new variant. An antigenic shift is when there is a major change in the glycoproteins that occurs through re-assortment. In general, antigenic shifts lead to new epidemics/pandemics.
Influenza transmission occurs primarily through droplets and direct contact. Indirect contact and airborne transmission occur infrequently.6 The incubation period for influenza is virus-dependent, although, in general, it is thought to be one to four days.7,8 The average duration of viral shedding is 4.8 days, starting one to two days prior to symptom onset, and it can last up to 10 days. Viral shedding normally stops within seven days.6,9
Pathophysiology of COVID
The coronavirus family is a type of enveloped, positive-sense, single-stranded RNA viruses with multiple subgroups. COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 virus), which is part of the β subgroup.10 SARS-CoV-2 enters cells by binding to the spike (S) glycoprotein within the angiotensin converting enzyme 2 (ACE 2) receptor, then activating endocytosis.11 This receptor is expressed in numerous types of tissues, including pulmonary, intestinal, cardiac, and renal tissues, which may explain some of the clinical manifestations. At the time of this writing, the literature suggests that COVID-19 is transmitted through direct, indirect, or close contact with infected persons. The main route of transmission is thought to be through droplets that are released when a person breathes, speaks, coughs, sings, or sneezes.12 These droplets can travel about 1 m in distance and can infect someone by getting into the mouth, nose, or eyes. Certain medical procedures, such as nebulization therapies, noninvasive ventilation, intubation/extubation, and cardiopulmonary resuscitation, can cause the virus to be aerosolized for up to three hours and can cause airborne transmission.13 Viable viral particles have been shown to survive for prolonged periods of time on different surfaces, making fomites another major source of direct and indirect transmission.14,15 Fecal viral shedding has been demonstrated in SARS-CoV-2-infected patients, although fecal-oral transmission has not been proven as of yet.10,16
Once infection occurs, the incubation period is thought to be two to 14 days, although some studies have shown incubation periods of up to 25 days, with a mean of seven days.15,17 While the duration of viral shedding is still not completely understood, some studies have demonstrated that children can carry a high level of virus in their upper airways, particularly in the early stages of infection, while having mild or no symptoms.18
Also, at the time of this writing, it is suggested that COVID-19 disease has two phases. During the first phase, the virus directly affects the function of the lungs, and respiratory symptoms predominate. Approximately two weeks after the first phase, an inflammatory stage occurs. While the patient no longer has active virus, the patient’s immunomodulatory system is dysregulated and causes a post-infectious syndrome named multisystem inflammatory syndrome in children (MIS-C), which will be covered in the “Complications” section. Although COVID-19 has caused more than 1 million deaths worldwide, as of October 2020, influenza deaths have claimed the lives of many more in the last decade.19 This is especially true of children. Thus, it is important to keep influenza in the forefront while caring for children with influenza-like illnesses during this pandemic.
Differentiating Influenza from COVID-19 in Children
Influenza and COVID-19 are respiratory viruses that can cause very similar clinical presentations varying from asymptomatic to severe disease. The diagnosis of influenza is important for vulnerable and high-risk populations, who can receive oseltamivir prophylaxis or treatment, which is especially effective if diagnosed early in the disease course. In children who are admitted, further laboratory diagnosis of different classes of respiratory pathogens (respiratory syncytial virus [RSV] and influenza) is important for cohorting purposes. During the pandemic, diagnosis of COVID-19 is likewise important for quarantining, cohorting, and for healthcare practitioner safety.
Influenza Clinical Disease and Course
The classic symptoms of influenza are cough, sore throat, rhinitis, fevers, headaches, myalgia, and malaise.20 Although younger children may be asymptomatic, they can present with more gastrointestinal complaints, or have localizing signs like conjunctival erythema, cervical adenopathy, or parotitis. In one study of 353 children who were treated in outpatient settings for influenza infection, the initial presentations showed a rate of 95% with fever, 77% with a cough, 78% with rhinitis, 26% with headaches, and 7% with myalgia.21
Regarding COVID-19, in a review of 55,924 patients in China that included children and adults, the most common presenting symptoms were fever, cough, and sputum production.22 Patients also presented with dyspnea, sore throat, nasal congestion, myalgias, headaches, nausea, vomiting, and diarrhea.15 In pediatric patients, a review of 11 international case series, including 333 children, stated that 35% of patients were asymptomatic. The most common presenting symptoms were cough, fever, and pharyngitis. Tachypnea, nasal congestion, rhinorrhea, wheezing, diarrhea, vomiting, fatigue, and headache occurred less often.17 This was further reinforced by another study of 341 pediatric patients from mainland China that demonstrated fever and cough as the most prevalent symptoms, with 5.9% of those patients being asymptomatic and 99% classified as having mild/moderate disease.23 Pediatric patients admitted for COVID-19 had statistically significantly higher reports of fever, diarrhea, vomiting, headache, myalgia, and chest pain when compared to patients admitted for influenza A or B infection.3
As discussed, many presenting symptoms of influenza and COVID-19 are not only similar to each other, but nonspecific. Acute onset of high fever is a common presentation of influenza. Gastrointestinal symptoms, as well as chest pain, are more common in COVID-19 than influenza.3 These may be factors to aid in distinguishing between these two respiratory presentations. Anosmia (loss of smell) and ageusia (loss of taste) also can be demarcating findings when differentiating between influenza and COVID-19. There are several case-based studies in the current literature associating olfactory dysfunction with SARS-CoV-2, but that is difficult to assess because these symptoms are subjective and can be reported on a spectrum from hyposmia to dysgeusia.24,25 Even with the variety of reported symptoms, olfactory nerve dysfunction is still the most positive predictor of COVID-19 testing positivity.26 However, diagnostic testing is the definitive way to differentiate influenza from COVID-19.
Influenza Complications and Vulnerable Populations
While all children are susceptible to the influenza virus and are at potential risk for serious influenza-related complications, certain populations are at higher risk of developing more severe illness. Risk factors include patients who are immunosuppressed, < 5 years of age (especially < 2 years of age), obese, have chronic conditions (pulmonary, cardiac, metabolic, endocrine, neurologic and neurodevelopmental, hematologic, renal, and hepatic diseases or disorders), pregnant or recently postpartum, on long-term aspirin or salicylate therapy, or are Native American or Alaskan Native American. (See Table 1.)
Recent studies report a decreased risk of COVID-19 infection in children, especially those younger than 10-14 years of age.27 Children with COVID-19 also have fewer complications. However, the risk of infection in adolescents is closer to that of adults.27 According to the National Institutes of Health (NIH), children who are severely immunocompromised or have cardiopulmonary disease may be at higher risk of severe illness.28 Based on adult data, the NIH also reports that children with obesity, hypertension, or diabetes may be at risk of severe COVID-19 disease.28
Practical Approach to a Child with Influenza-Like Illness in the ED
Given that both illnesses are clinically similar in pediatric patients, several considerations should be made to optimize workflow and to promote safe, high-quality care to special populations.
All patients, symptomatic or asymptomatic, should be masked. If possible, waiting areas should allow for patients to be six feet apart. Clinicians should wear personal protective equipment (PPE), per hospital policy. Usually, this would consist of a mask and gloves when caring for any child. When caring for children under investigation, this typically would require eye protection, disposable gowns, gloves, and an appropriately fitted N-95 face mask. Hospitals should provide training in donning and doffing procedures.
A patient under investigation should be placed in a private room, ideally with negative pressure ventilation, if available.4 Safe practice measures must be followed to prevent the spread of influenza (and COVID-19). There is no recent literature to support the efficacy of high-efficiency particulate air (HEPA) filters against SARS-CoV-229; however, historically, they have been studied to reduce airborne viral particulates. They can be used per hospital protocols, especially in areas where spaces are shared.
Respiratory Virus Testing Types and ED Testing Algorithms
All febrile children should be tested for SARS-CoV-2 using the hospital’s usual testing platform. COVID-19 status is necessary to guide cohorting and quarantining practices, both in the community upon discharge or in the hospital. (Asymptomatic children should be tested according to the area prevalence and hospital policy.) Children with flu symptoms and who are either within the 48-hour treatment window or in a high-risk category for increased influenza complications should be tested for influenza.
The American Academy of Pediatrics’ influenza 2019-2020 guidelines recommend rapid polymerase chain reaction (PCR) testing should be performed in children younger than 5 years of age, and especially in those younger than 2 years of age; children with respiratory illnesses, including cystic fibrosis or asthma; and children in high-risk groups.30 (See Table 1.) Testing will not only identify children who have the influenza virus, but it also can reduce unnecessary antibiotic use. It will guide management for influenza chemoprophylaxis of household members who are at high risk of developing severe illness. Those children with possible admission should be tested for influenza, as well as RSV and other respiratory viruses for cohorting purposes. It is important to understand the types of tests available, as well as their turnaround times. There are rapid antigen and molecular assay PCR tests for both influenza and SARS-CoV-2. Rapid tests can provide results in 15 to 30 minutes, whereas molecular assay tests (e.g., reverse transcription PCR [RT-PCR]) generally have a turnaround time of at least one hour. The rapid antigen tests are helpful when they are positive, but they are less sensitive than RT-PCR tests. Therefore, rapid antigen tests should be interpreted with caution because they may represent false negatives. An RT-PCR should be sent on any patient who requires laboratory confirmation of influenza or SARS-CoV-2 infection. Figure 1 details an algorithm to consider when diagnosing pediatric patients with influenza-like illnesses.
Table 1. Risk Factors for More Severe Illness with Influenza in Children |
|
Adapted from Centers for Disease Control and Prevention. Flu & young children. Updated Oct. 15, 2020. https://www.cdc.gov/flu/highrisk/children.htm |
Figure 1. Influenza-Like Illness Diagnostic Algorithm |
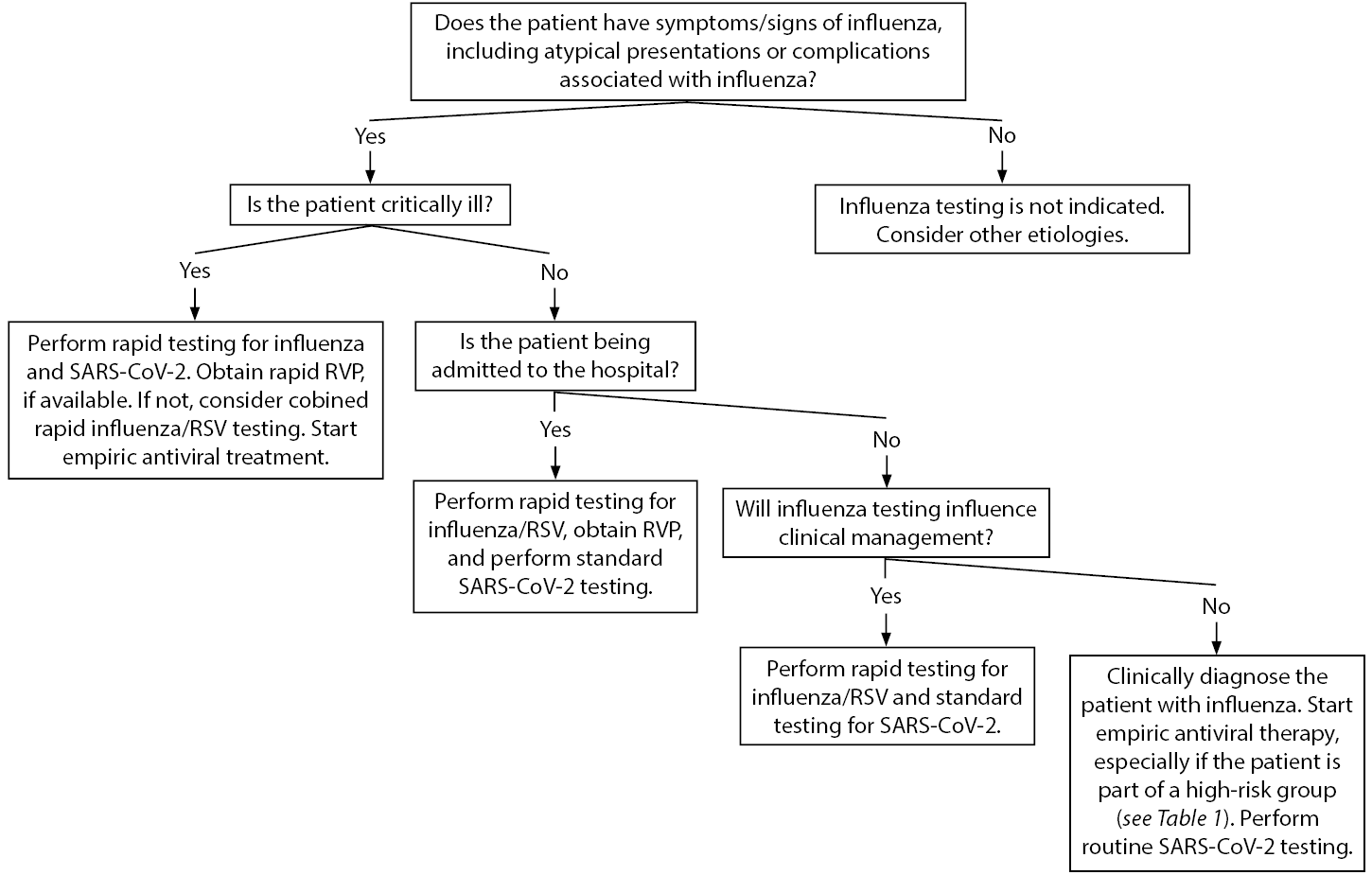 |
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; RVP: respiratory viral panel; RSV: respiratory syncytial virus Adapted from Centers for Disease Control and Prevention. Guide for considering influenza testing when influenza viruses are circulating in the community. Updated Sept. 1, 2020. https://www.cdc.gov/flu/profes... |
Influenza Management and Disposition
If a child presents with an emergent condition, the usual resuscitation, with attention to airway, breathing, and circulation, should be performed.
Symptomatic Care
Symptomatic and supportive care are the mainstays of treatment for both influenza and COVID-19. They include antipyretics, analgesia, fluid hydration, and, possibly, antiemetics. Although there was initial concern with the use of ibuprofen and worsening symptoms of COVID-19, data do not support this in children.31 Children may present with wheezing or shortness of breath, which poses a challenge when treating a patient with suspected COVID-19. If the patient is clinically stable, albuterol can be given via a metered-dose inhaler with a spacer. Those with moderate or severe illness who require aerosol-generating procedures, such as nebulized medications, positive pressure ventilation (high-flow nasal cannula), continuous positive airway pressure, bilevel positive airway pressure , or intubation, should ideally be placed in a negative pressure room or a room with a HEPA filter.
Adjunct Laboratory Testing
Children with moderate-severe illness may have a bacterial or viral co-infection. Consider a bacterial superinfection in patients who are ill-appearing or present with a more progressive course. These patients require a comprehensive workup, including a complete blood count (CBC) with a manual differential, C-reactive protein (CRP) testing, procalcitonin (PCT) testing, a comprehensive metabolic panel (CMP), and blood culture. Check blood gas for patients with moderate-severe respiratory distress. Results from these tests can help identify those who may have a bacterial infection or confirm those who have severe illness and require intensive care unit admission.
For infants 3 months of age or younger, it is imperative to rule out a serious bacterial infection (e.g., pneumonia, urinary tract infection) or invasive bacterial infection (e.g., bacteremia, meningitis). For febrile neonates (aged 0-28 days), complete the standard sepsis workup, which includes blood, urine, and cerebrospinal fluid, and may include chest radiographs. If there is suspected cardiac involvement, such as myocarditis or MIS-C, add a B-type natriuretic peptide (BNP) and troponin.
The other laboratory studies for MIS-C include a CBC, CMP, CRP, erythrocyte sedimentation rate (ESR), lactate dehydrogenase, partial thromboplastin time, international normalized ratio, activated partial thromboplastin time, and a D-dimer.
A respiratory pathogen panel can be useful in further identifying the etiology of the patient’s illness. This is important for patients who are admitted and may need to cohort. A typical respiratory pathogen panel includes RSV, influenza (A and B), parainfluenza (serotypes 1-4), rhinovirus/enterovirus, adenovirus, human metapneumovirus, and human coronavirus. Some panels also include mycoplasma pneumoniae and chlamydia pneumoniae. If a child is infected with a respiratory virus, it does not exclude them from having a co-infection with another respiratory pathogen.16,32
Imaging
Imaging should be done under the usual “As Low as Reasonably Achievable” principles.
Chest Radiograph
In alignment with recommendations published by the Pediatric Infectious Diseases Society and Infectious Diseases Society of America, a chest radiograph is not necessary unless the patient has hypoxemia, significant respiratory distress, or requires hospitalization.33
Children with influenza often do not have findings on a chest radiograph (CXR) that will change their medical management. In patients with moderate-severe disease who require hospitalization, a CXR can help guide the management of their pneumonia. Typical CXR findings for pneumonia due to influenza are bilateral patchy peribronchial and interstitial opacities. The most common abnormalities for a bacterial pneumonia are lobar infiltrates or focal consolidation. The CXR also will identify complications of bacterial pneumonia, such as parapneumonic effusions, necrotizing pneumonia, or pneumothorax.33
An international consensus statement on chest imaging for patients with confirmed or suspected COVID-19 was published by pediatric radiologists in April 2020.34 Although pediatric data is limited, they determined that imaging is not indicated in children who are well appearing, > 3 months of age, are immunocompetent, and do not require hospitalization. Pediatric patients hospitalized with severe illness benefited the most from imaging. They were found to have “bilateral patchy consolidation with associated ground glass opacity in a peripheral and lower lung zone predominant pattern.”34
CT Chest
A chest computed tomography (CT) scan is generally reserved for hospitalized patients with specific, severe clinical indications when the CT findings would potentially change the course of management. Pediatric patients with COVID-19 can have ground glass opacities early in their disease course, but typically have a lower rate of positive findings when compared to adult patients.34 However, ground glass opacities are a nonspecific finding. The same findings can be seen with influenza and other respiratory viral illnesses.35 The American College of Radiology recommends against using CT as a first line screening test to diagnose COVID-19.
Electrocardiogram
An electrocardiogram (ECG) is indicated for patients with concern for an arrhythmia or myocardial injury resulting in an arrhythmia or conduction abnormalities. With rare exceptions, an ECG is almost always abnormal in a patient with myocarditis. Although the findings are nonspecific, patients with myocarditis may have low-voltage QRS complexes, abnormal ST-T waves, or ST elevations. Patients with MIS-C also may have cardiac dysfunction, and obtaining an ECG may be considered.36
Treatment
Antiviral treatment is recommended for all children with suspected or confirmed influenza who are at high risk for complications, are hospitalized, or have severe or progressive disease. The antiviral medications approved for children are neuraminidase inhibitors or an endonuclease inhibitor. All of these medications, ideally, should be started within the first 48 hours of illness.
Oseltamivir (Tamiflu) is the antiviral drug of choice for a child with an influenza illness. It is a neuraminidase inhibitor that is Food and Drug Administration (FDA)-approved for children 14 days of age or older. It is given orally as a liquid suspension or in capsules. The dose is dependent on age and weight (see Table 2). Previously healthy children with mild disease may benefit from oseltamivir if taken within the first 48 hours of illness. It can reduce hospitalization rates and secondary bacterial infections, such as pneumonia. It also can shorten the course of illness by up to 1.5 days. Patients with severe or progressive disease requiring hospitalization should be started on oseltamivir regardless of time of onset, since even late dosing has been shown to provide some benefit.37 The most common side effect is nausea and vomiting. There is no known resistance to oseltamivir. It is used for chemoprophylaxis of influenza in people 1 year of age and older who are at high risk of developing severe illness. (See Table 1.)
Table 2. Recommended Dosage and Duration of Oral Oseltamivir for Treatment or Chemoprophylaxis for Influenza | ||
Age Group | Treatment Dosing | Chemoprophylaxis Dosing |
Adults | 75 mg twice daily | 75 mg once daily |
Pregnancy (any trimester) | 75 mg twice daily | 75 mg once daily |
Children (1 year or older) ≤15 kg | 30 mg twice daily | 30 mg once daily |
Children >15 kg to 23 kg | 45 mg twice daily | 45 mg once daily |
Children > 23 kg to 40 kg | 60 mg twice daily | 60 mg once daily |
Children > 40 kg | 75 mg twice daily | 75 mg once daily |
Infants 9 to 11 months | 3 mg/kg to 3.5 mg/kg per dose twice daily* | 3 mg/kg to 3.5 mg/kg per dose once daily* |
Term infants 0 to 8 months | 3 mg/kg per dose twice daily | 3 mg/kg per dose once daily if aged ≥ 3 months; because of a lack of safety and efficacy data, chemoprophylaxis is not recommended for infants < 3 months of age unless the situation is judged critical |
*For children 9 to 11 months of age, the American Academy of Pediatrics recommends 3.5 mg/kg per dose twice daily; the Centers for Disease Control and Prevention and the U.S. Food and Drug Administration-approved dosing is 3 mg/kg per dose twice daily. Source: Centers for Disease Control and Prevention. Influenza antiviral medications: Summary for clinicians. Updated Nov. 30, 2020. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm | ||
Two other neuraminidase inhibitors (zanamivir [Relenza] and peravimir [Rapivab]) and one endonuclease inhibitor (baloxavir [Xofluza]) are FDA-approved for children. Zanamivir is approved for patients 7 years of age or older. It is an inhaled powder and is the most potent of all neuraminidase inhibitors. It is not recommended for patients with asthma or other chronic lung diseases because it may cause bronchospasm. It also can be prescribed for chemoprophylaxis. Peravimir is approved for patients 2 years of age or older and is given intravenously. Its main side effect is diarrhea. Baloxavir is approved for those 12 years of age or older. It is given as a single oral dose, with a long half-life of 91 hours. It is not recommended for pregnant women, breastfeeding mothers, patients with complex or progressive illness, or hospitalized patients. The main side effect is diarrhea.38
Antibiotics are given to patients when a bacterial infection is suspected. They are also indicated in patients with severe illness or clinical deterioration. While the most common complication of influenza is acute otitis media, bacterial pneumonia is another common complication, with Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus being the most common pathogens.39 If a patient presents with sepsis or shock, treat with a broad-spectrum antibiotic.
At this time, remdesivir (an intravenous nucleotide prodrug of an adenosine analog), is the only drug the FDA has approved for the treatment of patients hospitalized with COVID-19. In October 2020, the FDA approved remdesivir for children older than 12 years of age and weighing more than 40 kg who are hospitalized with COVID-19. Under an FDA Emergency Use Authorization (EUA), it can be given to children younger than 12 years of age, weighing 3.5 kg to < 40 kg for the same indications.
In November 2020, the FDA approved EUAs for several SARS-CoV-2 monoclonal antibody therapies for use in children. Bamlanivimab and casirivimab plus imdevimab were given FDA EUAs for individuals older than 12 years of age and weighing > 40 kg. Baricitinib, when given with remdesivir, is available through an FDA EUA for use in children older than 2 years of age. For more information, visit the FDA website at http://bit.ly/38qVepJ.
Complications
Since both influenza and SARS-CoV-2 viruses have similar clinical manifestations, they also have similar clinical complications, although our understanding of COVID-19 is still evolving. Influenza has been associated with sinusitis, pneumonia, myositis, myocarditis, pericarditis, and respiratory failure in pediatric patients. Pneumonia in patients not at high risk tends to be mild and usually occurs in those younger than 2 years of age. Bacterial co-infection of the pneumonia or bacteremia can be very severe and, at times, fatal. The rate of bacterial co-infection ranged from 2% in patients seen in the ED to 26% in hospitalized patients to 50% in patients within the intensive care unit.40 The most common pathogens were Streptococcus pneumoniae and Staphylococcus aureus.39
While the complications related to the COVID-19 disease process in pediatric patients are being elucidated, pneumonia, acute respiratory distress syndrome, sepsis, and septic shock have been reported, albeit rarely.13 In one study, 6% of the patients were asymptomatic, while 93% had either mild to moderate disease.23 The moderate group included patients with fever, respiratory symptoms, and radiographic evidence of pneumonia. Only 0.6% of patients had a severe disease process, and 0.3% had a critical case presentation.
In pediatric patients, there appears to be a risk for MIS-C, which resembles Kawasaki disease (KD) or toxic shock syndrome. This syndrome is defined per the CDC as a patient younger than 21 years of age with measured or tactile fevers of at least 24 hours’ duration and laboratory-confirmed SARS-CoV-2 infection via PCR testing, antibody testing, or epidemiologic link to a person with a known infection with no alternative diagnosis.18 Most patients present with laboratory findings of previous SARS-CoV-2 infection and evidence of inflammation with multisystem involvement.
Most patients have at least four-system involvement, with the most common being gastrointestinal, cardiovascular, hematologic, mucocutaneous, and respiratory.36,41 Associated symptoms have been vomiting, abdominal pain, diarrhea, conjunctivitis, rashes, headaches, and encephalopathy. Cardiovascular involvement includes hypotension and development of coronary artery aneurysms. Most of these patents require admission to an intensive care unit, since 20% require mechanical ventilation, 50% to 74% developed hypotension requiring vasoactive support, and 4% to 16% are placed on extracorporeal membrane oxygenation.36,41
Laboratory results usually showed elevation in ESR, CRP, and PCT; neutrophilia, lymphopenia; elevated D-dimer and ferritin; anemia; thrombocytopenia; and a suggestion of myocardial damage through elevation of troponin or pro-BNP.
In a review of 186 patients diagnosed with MIS-C, 78% had a fever lasting five or more days, 55% had conjunctivitis, 42% had mucosal changes, 37% had peripheral extremity changes, 59% had rashes, and 10% had cervical lymphadenopathy greater than 1.5 cm in diameter, showing an overlap with KD — although most of those patients would not meet criteria for complete or incomplete KD.41 Although knowledge regarding MIS-C is still evolving, it has been shown to be a life-threatening complication.
Disposition
Children with influenza illness present with symptoms ranging from mild to severe. Previously healthy children who have mild symptoms, are drinking well, and have no signs of distress usually can be managed at home. These children typically recover in a few days (up to two weeks) without complications.
Similarly, children with COVID-19 who are well appearing and have mild illness can be safely discharged home. Discharge criteria include normal vital signs, normal work of breathing, and the ability to maintain adequate oxygenation and hydration. All high-risk patients require close follow-up. All patients diagnosed with COVID-19 must self-isolate for at least 10 days from the onset of symptoms, have no fever for at least 24 hours without using antipyretics, and symptoms must be improving, with the exception of lack of taste or smell.4
Children with moderate-severe influenza usually require hospitalization. There are no standard admission guidelines or criteria for children with influenza illness. Young children (0 to 4 years of age) tend to have the highest hospitalization rate for influenza, 95.1 children per 100,000 in the overall population.30 The hospitalization rate for influenza during the 2019-2020 season for ages 0-18 years was 68.2 children per 100,000 in the overall population.30
Tufts Medical Center published admission guidelines (available online at https://bit.ly/3hmf48q) distinguishing patients who should be admitted to the pediatric ward vs. the pediatric intensive care unit. Vanderbilt University Medical Center published a “Clinical Guidance for the Care & Treatment of COVID-19 Pediatric Patients.”42 Their admission criteria reflect standard admission criteria for patients with viral respiratory infections. “Pediatric patients with confirmed COVID-19, or suspected COVID-19 awaiting test results, who have respiratory distress (e.g., tachypnea, shortness of breath), hemodynamic instability, per os refusal, inadequate oral intake, or who cannot be monitored safely at home should be hospitalized.”42
Vaccination/Public Health
A seasonal influenza vaccine is available starting in September every year. The Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics (AAP) both recommend all children 6 months of age or older (who do not have contraindications) receive the vaccine.30 Vaccination has been proven to lower the risk of hospitalizations, influenza-related complications, and death. With the SARS-CoV-2 virus still circulating in the community, the AAP recommends that all children be vaccinated by the end of October. In the setting of the COVID-19 pandemic, influenza vaccination can reduce hospitalizations and the burden on the healthcare infrastructure.
All influenza vaccines approved for children are quadrivalent. The inactivated influenza vaccine (IIV) is approved for children 6 months of age and older and is given intramuscularly. A live attenuated influenza vaccine (LAIV), given intranasally, also is available for children 2 years of age and older.
The use of LAIV is limited to healthy children who do not have underlying conditions, such as respiratory disease or an immunocompromised status. Children 6 months through 8 years of age who are receiving the influenza vaccine for the first time, did not receive two influenza vaccines prior to July 1, 2020, or have an unknown vaccine status require a two-dose series at least four weeks apart.
As long as it is not contraindicated, the two doses can be both the IIV, LAIV, or a combination of the two. It is recommended that all children with underlying medical conditions receive an influenza vaccine. The only contraindication is a severe allergy to the vaccine. For those with an egg allergy, ovalbumin-free vaccines are available for children 4 years of age and older. (See Table 3.)
Table 3. Comparing Live Attenuated Influenza Vaccine (LAIV) with Inactivated Influenza Vaccine (IIV) | ||
LAIV | IIV | |
Route of administration | Intranasal | Intramuscular |
Approved ages | 2-49 years | ≥ 6 months |
Interval between the two doses administered to children between the ages of 6 months to 8 years | ≥ 4 weeks | ≥ 4 weeks |
OK to give simultaneously with other vaccines? | Yes | Yes |
OK to give in those with medical risk factors for influenza-related complications?* | No | Yes |
Can be given to children with asthma or in children ages 2-4 years with wheezing | No | Yes |
Can be given to those in close contact with immunosuppressed persons requiring | No | Yes |
*See Table 1 for high-risk populations. Other contraindications include persons with active communication between the cerebrospinal fluid (CSF) and the oropharynx, nasopharynx, nose, ear, or any other cranial CSF leak; persons with cochlear implants; receipt of influenza antiviral medication within the previous 48 hours for oseltamivir and zanamivir, five days for peramivir, and 17 days for baloxavir. Precautions: moderate or severe acute illness with or without fever; asthma in persons aged ≥ 5 years; Guillain-Barré syndrome within six weeks following a previous dose of influenza vaccine. Adapted from Grohskopf LA, Alyanak E, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2020–21 influenza season. MMWR Morb Mortal Wkly Rep 2020;69:1-24. | ||
According to the CDC, the annual influenza vaccine effectiveness typically is 40% to 60%. In the 2019-2020 season, more than half of the pediatric deaths due to influenza-associated illness were in unvaccinated children. In the past, up to 80% of the pediatric deaths due to influenza were in patients who did not receive the seasonal influenza vaccine.30
While the timing and severity of the 2020-2021 influenza season is difficult to predict, vaccine manufacturers are expected to produce 194-198 million doses of influenza vaccines, which is approximately 20 million more doses than last year. Vaccinations during the 2018-2019 season prevented 4.4 million illnesses, 2.3 million medical visits, 58,000 hospitalizations, and 3,500 deaths.43 The study also showed that vaccinations that year prevented 43% of projected hospitalizations in children aged 6 months to 4 years due to influenza A infection. This further confirms the importance of influenza vaccinations.
In December 2020, the FDA approved EUAs for two COVID-19 vaccines, the Pfizer-BioNTech and Moderna mRNA-based vaccines. The Pfizer-BioNTech vaccine was approved for individuals 16 years of age and older. The Moderna vaccine was approved for individuals 18 years of age and older. A vaccine for children under 12 years of age has not been developed at time of this publication.
ACIP recommends that “health care personnel and long-term care facility residents be offered COVID-19 vaccination first (Phase 1a). In Phase 1b, COVID-19 vaccine should be offered to persons aged > 75 years and non-health care frontline essential workers, and in Phase 1c, to persons aged 65-74 years, persons aged 16-64 years with high-risk medical conditions, and essential workers not included in Phase 1b.”44 A vaccine for children younger than 12 years of age has not been developed at the time of this publication.
Conclusion
In these uncertain times, where there is still much to learn from the pandemic of the novel coronavirus, it is imperative to provide appropriate care to pediatric patients who present with influenza and other common respiratory diseases. Emergency practitioners will serve as the front-line care providers for this population, and this season has the potential to be challenging.
Will the volume of patients with respiratory disease be decreased by school closures and social distancing? Or will there be a surge of patients due to decreased influenza vaccination rates? Will there be a surge in patients requiring testing due to limited testing capabilities in the community? These, along with many other unanswered questions, can be addressed prophylactically with preparation.
This report advises developing systems and protocols for triage, testing, and cohorting symptomatic individuals when in shared waiting areas, providing care in full PPE, avoiding of aerosolizing procedures, and, if necessary, performing procedures in negative pressure rooms. Vaccination against influenza should be encouraged. The goals should be to continue to keep children safe while recognizing the complications associated with both of these deadly viruses — and providing quality care while limiting exposure to others within pediatric emergency departments.
References
- Centers for Disease Control and Prevention. Influenza Hospitalization Surveillance Network (FluSurv-NET). Updated Oct. 1, 2020. https://www.cdc.gov/flu/weekly...
- Zipfel CM, Bansal S. Assessing the interactions between COVID-19 and influenza in the United States. medRxiv 2020; doi:10.1101/2020.03.30.20047993 [Preprint].
- Song X, Delaney M, Shah RK, et al. Comparison of clinical features of COVID-19 vs. seasonal influenza A and B in U.S. children. JAMA Netw Open 2020;3:e2020495
- Centers for Disease Control and Prevention. COVID-19 Response Team. Coronavirus disease 2019 in children — United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422-426.
- Wagner R, Matrosovich M, Klenk H-D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol 2002;12:159-166.
- Brankston G, Gitterman L, Hirji Z, et al. Transmission of influenza A in human beings. Lancet Infect Dis 2007;7:257-265.
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis 2005;5:718-725.
- Coburn BJ, Wagner BG, Blower S. Modeling influenza epidemics and pandemics: Insights into the future of swine flu (H1N1). BMC Med 2009;7: doi: 10.1186/1741-7015-7-30.
- Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis 2010;201:1509-1516.
- Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020;12:372.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-280.e8.
- Chan JFW, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020;395:514-523.
- De Luca CD, Esposito E, Cristiani L, et al. Covid-19 in children: A brief overview after three months experience. Paediatr Respir Rev 2020;35:9-14.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564-1567.
- Kakodkar P, Kaka N, Baig MN. A comprehensive literature review on the clinical presentation, and management of the pandemic coronavirus disease 2019 (COVID-19). Cureus 2020;12:e7560.
- Wu D, Wu T, Liu Q, Yang Z. The SARS-CoV-2 outbreak: What we know. Int J Infect Dis 2020;94:44-48.
- Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: A review of epidemiologic and clinical features. Pediatr Infect Dis J 2020;39:469-477.
- Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Clinical presentation, infectivity, and immune responses. J Pediatr 2020;227:45-52.e5.
- Johns Hopkins University and Medicine. Coronavirus resource center. Updated Dec. 15, 2020. https://coronavirus.jhu.edu/ma...
- Paules C, Subbarao K. Influenza. Lancet 2017;390:697-708.
- Silvennoinen H, Peltola V, Lehtinen P, et al. Clinical presentation of influenza in unselected children treated as outpatients. Pediatr Infect Dis 2009;28:372-375.
- World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19).
https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf - Guo C-X, He L, Yin J-Y, et al. Epidemiological and clinical features of pediatric COVID-19. BMC Med 2020;18:250.
- Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: A review based on up-to-date knowledge. Am J Otolaryngol 2020;41:102581.
- Erdede O, Sarı E, Külcü NU, et al. An overview of smell and taste problems in paediatric COVID-19 patients. Acta Paediatr 2020;109:2184-2186.
- Rocke J, Hopkins C, Philpott C, Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin Otolaryngol 2020;45:914-922.
- Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: A systematic review and meta-analysis. JAMA Pediatr 2020:e204573. doi: 10.1001/jamapediatrics.2020.4573.
- National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated Dec. 14, 2020. https://www.covid19treatmentgu...
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2020-2021. Pediatrics 2020;146:e2020024588.
- Christopherson D, Yao WC, Lu M, et al. High-efficiency particulate air filters in the era of COVID-19: Function and efficacy. Otolaryngol Head Neck Surg 2020;163:1153-1155.
- Martins-Filho PR, do Nascimento-Júnior EM, Santos VS. No current evidence supporting risk of using ibuprofen in patients with COVID-19. Int J Clin Pract 2020;74:e13576.
- Kim D, Quinn J, Pinsky B, et al. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020;323:2085-2086.
- Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011;53:e25-e76.
- Foust AM, Winant AJ, Chu WC, et al. Pediatric SARS, H1N1, MERS, EVALI and now coronavirus disease (COVID-19) pneumonia: What radiologists need to know. AJR Am J Roentgenol2020;215:736-744.
- Yoshinobu T, Abe K, Shimizu H, et al. CT findings in pediatric novel influenza A (H1N1)-associated pneumonia. Iran J Pediatr 2012;22:213-217.
- Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-system inflammatory syndrome in children (MIS-C) following SARS-CoV-2 infection: Review of clinical presentation, hypothetical pathogenesis, and proposed management. Children (Basel) 2020;7:69.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 2019;68:e1-e47.
- Centers for Disease Control and Prevention. Influenza antiviral medications: Summary for clinicians. Updated Nov. 30, 2020. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm
- Mani CS, Murray DL. Acute pneumonia and its complications. Principles and Practice of Pediatric Infectious Disease 2008:245-257.
- Klein EY, Monteforte B, Gupta A, et al. The frequency of influenza and bacterial coinfection: A systematic review and meta-analysis. Influenza Other Respir Viruses 2016;10:394-403.
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334-346.
- Vanderbilt University Medical Center Divisions of Pediatric Infectious Disease, Hospital Medicine, Outreach Medicine, Pediatric ED, PICU, Pulmonary Medicine, and MCJCHV Nursing Leadership. Clinical guidance for the care & treatment of COVID-19 pediatric patients: Initial evaluation, diagnosis, and management. Published March 23, 2020. https://www.vumc.org/coronavir...
- Chung JR, Rolfes MA, Flannery B, et al. Effects of influenza vaccination in the United States during the 2018-2019 influenza season. Clin Infect Dis 2020;72:e368-e376.
- Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 vaccine — United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69:1657-1660.
