Uncommon Diagnoses that Cannot Be Missed: An Update
Authors
R. Lane Coffee, Jr., PhD, MS
Assistant Professor of Medicine, Department of Medicine, University of Central Florida College of Medicine, Orlando
Stephen John Cico, MD, MEd
ACGME Designated Institutional Official (DIO), UCF-HCA Florida Healthcare GME Consortium, Associate Dean for Graduate Medical Education, Professor of Emergency Medicine and Pediatrics, Department of Clinical Sciences, University of Central Florida College of Medicine, Orlando
Derya Caglar, MD
Associate Professor of Pediatrics, Program Director for Pediatric Emergency Medicine, Division of Emergency Medicine, Department of Pediatrics, University of Washington School of Medicine, Seattle Children’s Hospital, Seattle
Peer Reviewer
Steven M. Winograd, MD, FACEP
Attending Emergency Physician, Trinity Health Care, Samaritan, Troy, NY
Executive Summary
- Clinical features of necrotizing fasciitis include severe pain that may seem out of proportion to exam findings. The hallmark symptoms include reports of pain and tenderness beyond areas of erythema and induration seen on examination, consistent with bacterial spread along the fascia. Patients may have fevers, but tachycardia out of proportion to fever is a common finding. On exam, areas that are extremely tender to palpation may feel edematous because of third spacing of fluid in deeper tissues. There may be crepitus caused by bacterial gas production, but this is a late finding and often is not present.
- The amount of epidermal detachment that is present defines the following illnesses, with erythema multiforme having no epidermal detachment, Stevens-Johnson syndrome having < 10% of the body surface with epidermal detachment, and toxic epidermal necrolysis having > 30% of the body surface with epidermal detachment.
- Eczema herpeticum (EH), which is caused by an infection of eczematous skin with herpes simplex virus (HSV), is a potentially serious infection that leads to disseminated vesicles with skin erosion, viremia, fever, and lymphadenopathy. Examination may reveal erythematous skin with punched-out erosions, excoriations, and/or vesicles with or without crusting. If lesions are close to the eyes, HSV can spread to the eyes and lead to corneal scarring if not recognized quickly.
- Purpura is the presenting symptom in about three-fourths of patients with immunoglobulin A vasculitis (IgAV), preceding other symptoms by an average of four days. It often begins as a painless, erythematous, macular rash that evolves over the course of days to weeks into the typical palpable purpura and ecchymoses. It usually is symmetric in distribution and occurs primarily in dependent areas (i.e., lower extremities and buttocks in toddlers and older children). Arthralgias and arthritis are seen in more than 80% of patients and usually are transient and migratory, typically affecting the lower extremity large joints (knees, ankles). Significant swelling and tenderness may occur, limiting joint movement, and may precede the purpura by a few days. Abdominal pain may be mild or significant throughout the illness. Guaiac-positive stools are common, but significant bleeds are rare. Intussusception should be considered in all children with IgAV who report significant pain, since it is the most common gastrointestinal complication. Renal disease is more common in older children who present with IgAV.
- Lemierre’s syndrome is a rare infection caused by Fusobacterium necrophorum or mixed anaerobic flora and is associated with internal jugular venous thrombophlebitis and septic emboli.
- Retropharyngeal abscess (RPA) is an infection of the retropharyngeal nodes and is seen in children 2 to 4 years of age. After age 5 years, the retropharyngeal lymph nodes involute, making this infection rare in older children and adults. Children present with fever, pain and difficulty swallowing, drooling, stiff neck, or torticollis. Blood cultures are rarely positive in children with RPAs. Lateral neck radiographs will show a widening of the retropharyngeal space. At the level of C3, the prevertebral soft tissues should measure less than two-thirds of the width of the vertebral body. If the soft tissues measure > 7 mm at C2 or 14 mm at C6, if air-fluid levels are seen in the tissues, or if there is loss of the normal cervical lordosis due to muscle spasm, RPA should be highly considered.
- Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, potentially life-threatening hypersensitivity reaction that involves skin changes, hematologic abnormalities (eosinophilia, atypical lymphocytosis), lymphadenopathy, and internal organ involvement (dysfunction of the liver, kidney, or lungs).
- Clinicians should consider incomplete Kawasaki disease (IKD) when a child has an unexplained fever for seven days or longer, or a fever for five days or longer with two or three diagnostic criteria. IKD also should be considered in children who have three or four clinical or lab findings but have had a fever for fewer than five days.
There are a variety of uncommon pediatric conditions that, if not detected, may result in devastating consequences. The authors review and update the current standard of care for a variety of conditions, including necrotizing fasciitis, DRESS syndrome, Kawasaki disease, MIS-C, Lemierre's, and RPA.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
In the emergency department (ED), pediatric patients can present with a wide range of concerns, spanning from harmless upper respiratory infections to life-threatening necrotizing fasciitis. It is common for urgent conditions to imitate ordinary and non-threatening childhood ailments. Therefore, emergency physicians need to promptly and accurately identify and manage these rare yet critical illnesses. Likewise, emergency physicians should recognize those illnesses that may appear severe but only require supportive care. This review will concentrate on evaluating, diagnosing, and managing infrequent but crucial diagnoses in pediatric patients.
Skin and Soft Tissue Diseases
Necrotizing Fasciitis
Skin and soft tissue infections (SSTIs) are common in children, accounting for an increasing amount of healthcare utilization; studies report that SSTIs are diagnosed in approximately 2% of pediatric ED visits.1,2 With so many children presenting with skin infections, it is important to consider necrotizing fasciitis, a much more serious and fulminant skin infection with high morbidity and mortality in children who present for evaluation.
Necrotizing fasciitis is characterized by extensive soft tissue necrosis of the superficial, fascial, and deep dermal layers of the skin that can lead to thrombosis, necrosis, systemic toxicity, and death.3, 4 However, early in the course, these infections can appear deceptively benign. Infections typically begin with direct invasion of the soft tissues from external trauma, which could be as small as an insect bite. Once bacteria are in the subcutaneous tissues, they invade deeper tissues and release toxins, leading to capillary thrombosis, tissue ischemia, liquefaction necrosis, and systemic toxicity. This ischemic environment leads to rapid bacteria growth that spreads infection along fascial lines, and patients can deteriorate within a matter of hours.
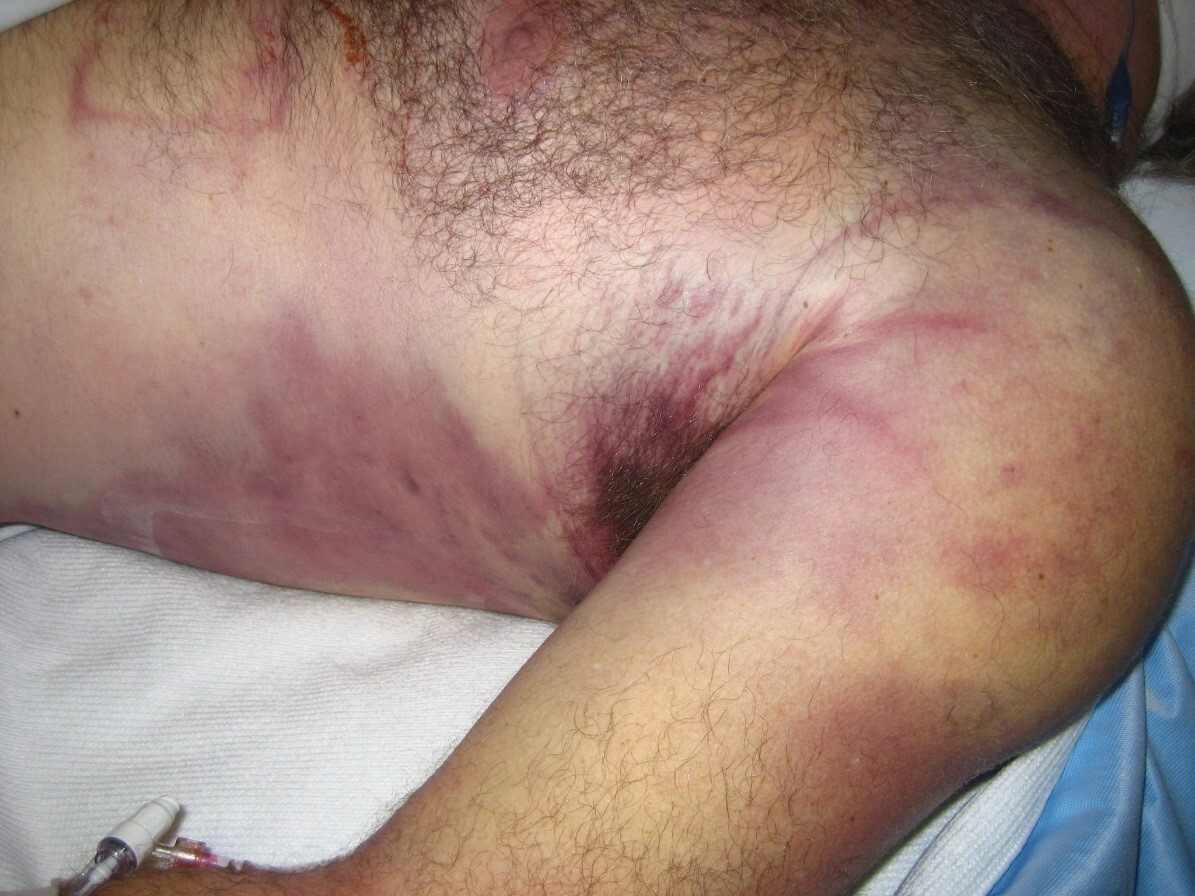
Clinical features of necrotizing fasciitis include severe pain that may seem out of proportion to exam findings. The hallmark symptoms include reports of pain and tenderness beyond areas of erythema and induration seen on examination, consistent with bacterial spread along the fascia. Patients may or may not have fevers, but tachycardia out of proportion to fever is a common finding. On exam, areas that are extremely tender to palpation may feel edematous because of third spacing of fluid in deeper tissues. There may be crepitus due to bacterial gas production, but this is a late finding and often is not present. Late in the disease process, patients may develop blisters and frank tissue necrosis. Erythema may spread as far as 1 inch/hour, with rapid deterioration of the patient’s mental status.
Diagnosis is based on clinical assessment and rapid progression of symptoms.5 (See Figure 1.) Lab findings to support the diagnosis of necrotizing fasciitis include elevated C-reactive proteins and elevated white blood cell count with bandemia, anemia, hyponatremia, hyperglycemia, or thrombocytopenia. Because many lab indicators will miss the diagnosis, physicians should maintain a high level of suspicion for necrotizing fasciitis. Patients should be treated with broad-spectrum antibiotics and intravenous (IV) hydration, pending transfer to the operating room.6,7 Emergent surgical involvement with aggressive debridement of the area is required, since delay leads to increased morbidity and mortality.
Figure 1. Evolution of Necrotizing Fasciitis Over 12 Hours |
 |
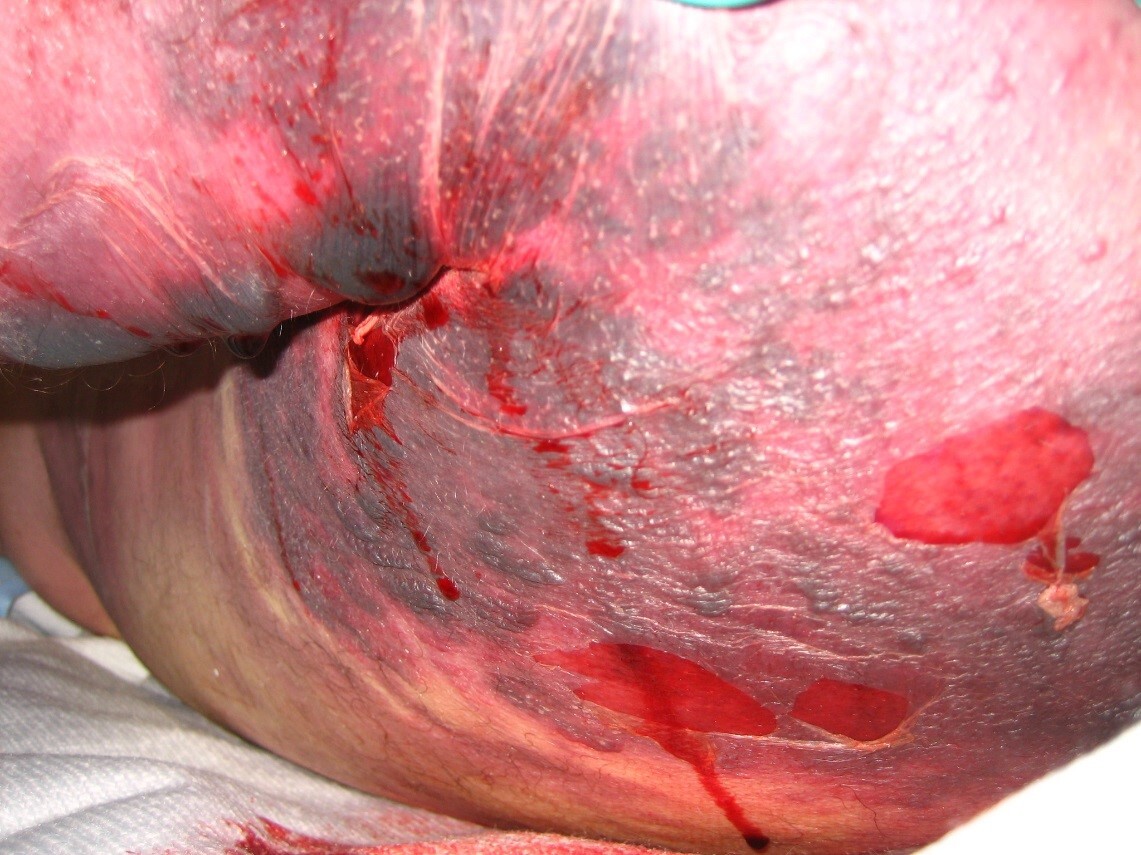 |
Images courtesy of Richard Kwun, MD. |
Erythema Multiforme and Stevens-Johnson Syndrome
Erythema multiforme (EM) is an acute inflammatory disease of the skin that may begin as a localized papular rash that can progress to much more severe, multisystemic illnesses with widespread bullous lesions and erosions of the mucosal surfaces, known as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).8-10 The illnesses are defined by the amount of epidermal detachment that is present, with EM having no epidermal detachment, SJS having < 10% of the body surface with epidermal detachment, and TEN having > 30% of the body surface with epidermal detachment. SJS and TEN can occur in patients of any age. They are more common in women than in men, with a female to male ratio of about 2:1. Estimates of incidence for SJS and TEN range from two to seven cases per 1 million people per year. Common precipitating factors include infection (Mycoplasma and herpes simplex virus [HSV]), drugs (antibiotics and anticonvulsants), and malignancy (more common in adults than children).11 Although the exact causes are unknown, SJS and TEN are considered to be hypersensitivity reactions with immune complex deposition leading to skin changes.
Clinical symptoms include fever, myalgias, and rash. Patients may report a diffuse, generalized burning or itching sensation before skin lesions develop. Maculopapular and targetoid lesions are most common, but the rashes may be polymorphic. The rash typically evolves into the classic “target lesions” over the next 24-48 hours and involves the palms and soles of the feet.8 Patients may develop areas of central duskiness or purpura. If the disease progresses to SJS or TEN, the patient will exhibit mucosal changes with ulcerations and bleeding and sloughing of the skin. Ocular involvement is common and is seen in almost 70% of patients with SJS and TEN, manifesting as a purulent conjunctivitis, often continuing to corneal ulceration.12 Patients who develop TEN have an increased risk of sepsis and frequently develop pneumonia or multiple organ dysfunction.
Systemic steroids are used commonly to localize the disease and provide symptomatic relief, but they have no effects on duration or outcomes. Patients with SJS and TEN need to be admitted for IV hydration, pain control, and close monitoring. Patients with extensive lesions or TEN often need treatment in a burn unit. The overall mortality rate among patients with SJS and TEN ranges from about 10% for SJS to up to 50% for TEN.
Eczema Herpeticum
Eczema, or atopic dermatitis, is very common in the pediatric population, with a prevalence of 10% to 20% in industrialized countries.13 Patients typically experience dry skin and severe pruritus that is associated with skin hyperreactivity to various stimuli, including exposure to food and environmental allergens, irritants, and infection. Erythema, lichenification, and excoriation frequently occur and lead to a breakdown in the skin barrier, leaving the patient susceptible to a variety of infections.
Eczema herpeticum (EH), which is caused by an infection of eczematous skin with HSV, is a potentially serious infection that leads to disseminated vesicles with skin erosion, viremia, fever, and lymphadenopathy.14 EH can result in the more serious complications of corneal scarring and meningitis. Examination may reveal erythematous skin with punched-out erosions, excoriations, and/or vesicles with or without crusting. The involved skin may be pruritic and painful, and lesions may be widespread.
If lesions are close to the eyes, HSV can spread to the eyes and lead to corneal scarring if not recognized quickly.15 In disseminated disease, patients may be febrile with altered mental status and may exhibit signs of meningitis. Coinfection with bacteria, particularly Staphylococcus aureus, also is common and can lead to cellulitis, abscesses, and systemic diseases.
Diagnosis primarily is clinical but can be confirmed with viral testing of skin lesions. The Tzanck smear, viral cultures, skin biopsy, or detection of viral DNA by polymerase chain reaction may be helpful in questionable cases. Patients with lesions near the eyes should have an ophthalmic evaluation to look for keratoconjunctivitis and corneal ulceration. In patients with toxic appearance, abnormal vital signs, or concern for disseminated disease, the physician should maintain suspicion for meningitis and send cerebrospinal fluid studies for HSV testing.
Depending on the severity, either oral or IV acyclovir can be used. Oral acyclovir should be considered only in mild cases in which the patient has very localized disease without systemic concerns or eye involvement. Patients with more diffuse lesions or disseminated disease should be hospitalized and receive IV acyclovir.16 Intravenously administered acyclovir can precipitate as crystals in the kidneys, resulting in renal impairment, so the patient’s medication should be given with adequate hydration. Since bacterial coinfection is common, the patient also should receive antibiotics with adequate Staphylococcus and Streptococcus coverage. The patient should receive adequate medication for pain control.
Immunoglobin A Vasculitis
Immunoglobulin A (IgA) vasculitis (IgAV), formerly known as Henoch-Schönlein purpura (HSP), is the most common systemic vasculitis of childhood that occurs primarily between 2 and 15 years of age.17, 18 It presents with a combination of symptoms, including purpura, arthritis or arthralgias, and abdominal pain in association with renal disease. While the presence of purpura is ubiquitous for the diagnosis of IgAV, other signs and symptoms are present more variably. Symptoms are due to the deposition of IgA containing immune complexes in the walls of small vessels (arterioles, capillaries, and venules).19
The majority of IgAV cases are preceded by an upper respiratory tract infection, suggesting a potential infectious trigger. Streptococcus, Staphylococcus, and parainfluenza are implicated most commonly, but virtually all respiratory pathogens have been linked to IgAV.19 Anecdotal reports also describe cases after vaccination, with multiple vaccines implicated, including the mumps, measles, and rubella (MMR) and the pandemic influenza A (H1N1) vaccine, although no studies support avoiding vaccinations to decrease risk of disease.20 An association between drugs and IgAV also has been reported, but the role of these medications in the pathogenesis is uncertain since most were being used at the onset of the disease to treat a concurrent infection.
Clinical findings may develop over the course of days to weeks and may vary in their order of presentation. Purpura and arthralgia of the lower extremity joints usually are early symptoms, but this is not always the case.
In the absence of the purpuric rash, the diagnosis may not be obvious. Patients who initially present with significant joint or abdominal pain before skin manifestations occur may be thought to have an infectious or surgical process.
Purpura is the presenting symptom in about three-fourths of patients, preceding other symptoms by an average of four days.17 It often begins as a painless, erythematous, macular rash that evolves over the course of days to weeks into the typical palpable purpura and ecchymoses. It usually is symmetric in distribution and occurs primarily in dependent areas (i.e., lower extremities and buttocks in toddlers and older children). Arthralgias and arthritis are seen in more than 80% of patients and usually are transient and migratory, typically affecting the lower extremity large joints (knees, ankles). Significant swelling and tenderness may occur, limiting joint movement, and may precede the purpura by a few days.
Abdominal pain may be mild or significant throughout the illness. Guaiac-positive stools are common, but significant bleeds are rare. Intussusception should be considered in all children with IgAV who report significant pain, since it is the most common gastrointestinal complication. Renal disease is more common in older children who present with IgAV.17 This may present most commonly as hematuria, but proteinuria, elevated creatinine, and hypertension can be seen in a minority of patients.
The diagnosis of IgAV primarily is based on physical findings.21 Bloodwork usually is not necessary. Urine studies are indicated to look for renal disease, and positive results warrant ongoing follow-up and evaluation until resolution, which may take months. Patients presenting with significant abdominal pain should have an ultrasound evaluation for intussusception.
Although patients may appear quite uncomfortable, with impressive and extensive purpuric lesions and swollen joints, most do not require significant workup and generally will make a full recovery with supportive care. Nonsteroidal anti-inflammatory drugs may be used for treatment. Some data suggest glucocorticosteroids may shorten the duration of abdominal symptoms, but they are not routinely recommended.22
Ear, Nose, and Throat and Gastrointestinal Diseases
Lemierre’s Syndrome
Acute pharyngitis is commonly associated with tonsillitis resulting from inflammation of the throat and tonsils. It accounts for 1% to 2% of all visits to outpatient clinics and EDs, resulting in 7.3 million annual visits for children.23 Rarely, throat pain can indicate a deeper and more dangerous infection. Lemierre’s syndrome is a rare infection caused by Fusobacterium necrophorum or mixed anaerobic flora and is associated with internal jugular venous thrombophlebitis and septic emboli.24
Infection spreads from the oropharynx to the parapharyngeal or lateral pharyngeal space, extending into the carotid sheath and vessels. Patients often are previously healthy adolescents and young adults. Clinically, patients often appear ill and present with high fevers, rigors, and complaints of a severe sore throat and neck pain. They often are tachycardic, tachypneic, and may be hypoxic on room air. On exam, the oropharynx may show significant erythema, ulceration, a pseudomembrane, or posterior pharyngeal swelling, and patients may have limited range of motion at the neck because of significant pain.
Septic emboli to the lungs are a common finding in a majority of cases.25 Chest radiography may demonstrate infiltrates, which can cavitate to form lung abscesses and empyema. Patients may develop septic arthritis and/or osteomyelitis. Complications include a mortality rate as high as 10%, especially when antibiotic treatment is delayed.23 Patients should be admitted for close monitoring for sepsis and respiratory distress and should receive broad-spectrum parenteral antibiotics. Anticoagulation is a controversial issue. Based on the current evidence, it is not clear whether anticoagulation therapy is beneficial. There were no apparent differences in mortality or clinical course in those who received anticoagulants and those who did not.23,25 Unfortunately, no randomized studies are available.25
Retropharyngeal Abscess
Retropharyngeal abscess (RPA) is an infection of the retropharyngeal nodes and is seen in children 2 to 4 years of age. After age 5 years, the retropharyngeal lymph nodes involute, making this infection rare in older children and adults.24,26 Children present with fever, pain and difficulty swallowing, drooling, stiff neck, or torticollis.27 This presentation can be similar to meningitis; however, these children tend to hold their necks in flexion and have trouble with neck extension, rather than holding their necks extended and having difficulty with flexion, as seen with meningitis. Trismus and voice change also may be present. Infections typically are polymicrobial, including Streptococcus pyogenes, S. aureus (including methicillin-resistant S. aureus [MRSA]), F. necrophorum, and respiratory anaerobic bacteria.24
The initial evaluation should include an attempt to visualize the posterior pharynx, which may show a mass or bulging enlargement of the anterior cervical lymph nodes. Complete blood count may show a leukocytosis with left shift; blood cultures are rarely positive in children with RPAs. Lateral neck radiographs will show a widening of the retropharyngeal space. At the level of C3, the prevertebral soft tissues should measure less than two-thirds of the width of the vertebral body. If the soft tissues measure > 7 mm at C2 or 14 mm at C6, if air-fluid levels are seen in the tissues, or if there is loss of the normal cervical lordosis due to muscle spasm, RPA should be highly considered.24 (See Figure 2.) Computed tomography of the neck can make the diagnosis definitively, aid with visualization of the vessels of the neck, determine any extension of the abscess, and help plan for surgical intervention and drainage.27 (See Figure 2.) Clindamycin is the current drug of choice for initial treatment of RPAs, given the increased incidence of MRSA.26 Children with respiratory compromise may require operative drainage by otolaryngology.
Figure 2. Retropharyngeal Abscess |
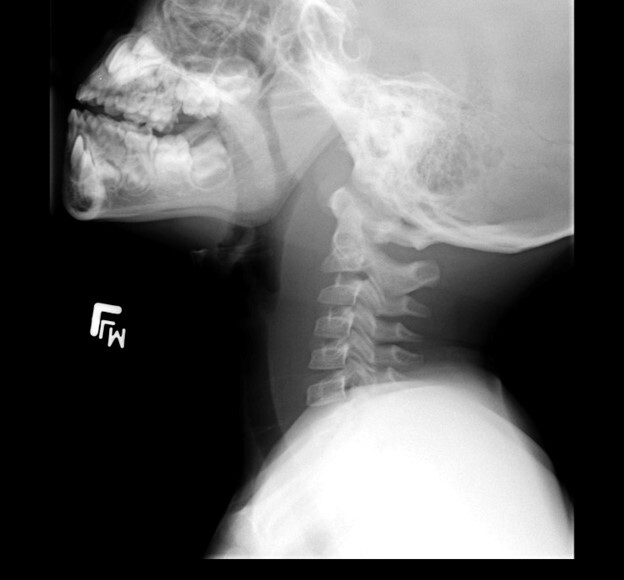
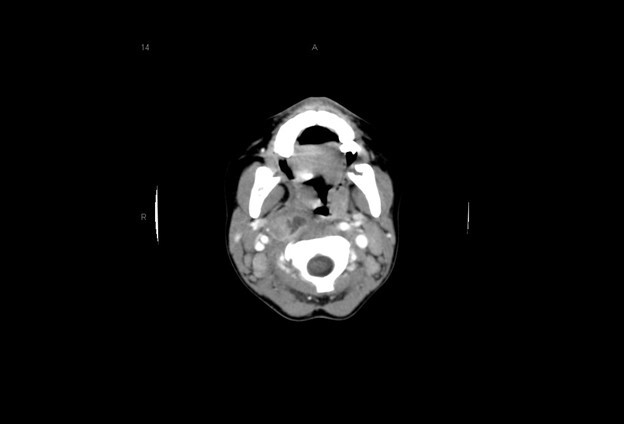 |
The first image is a lateral neck radiograph showing widening in the prevertebral soft tissues, raising concern for a retropharyngeal abscess. The second image is a computed tomography of the same retropharyngeal abscess. Images courtesy of Stephen John Cico, MD, MEd. |
Intussusception
Intussusception is the most common cause of intestinal obstruction in children 6 months to 6 years of age.28,29 It is twice as common in males as in females, and it rarely is associated with a pathologic lead point.28, 29 It occurs when a portion of the intestines telescopes into another portion of the intestines. Small intestine intussusception is seen with gastroenteritis and rarely is pathologic. Classic intussusception is ileocecal (also called ileocolic) and frequently requires intervention for reduction. The incidence of intussusception peaks in spring and fall, suggesting viral infections are an associated factor, with rotavirus, adenovirus, and herpesvirus 6 all having associations with intussusception.28 Lymphoid hyperplasia may serve as the lead point in these cases of intussusception. Meckel’s diverticulum can serve as a lead point in the pediatric population, and this is the most common pathologic cause of intussusception.30 Other conditions, such as intestinal polyps, lymphoma, and IgAV, all increase a child’s risk of intussusception.17
The classic presentation triad of intussusception — abdominal pain that is intermittent in nature, currant jelly stool, and a palpable abdominal mass or “sausage sign” — is seen in only 7.5% to 40% of children, particularly since currant jelly stools are a late finding, indicating mucosal sloughing due to ischemia.29,31 The waves of abdominal pain tend to be episodic, involving screaming and crying, and often involve flexing of the limbs, particularly the legs toward the chest. The child may appear to be straining but then return to baseline. Episodes occur 10 to 20 minutes apart.28
However, children may have nonspecific signs and symptoms upon presentation, which can lead clinicians away from intussusception and cause them to miss this diagnosis on initial presentation. Infants may have a more obscure presentation, including vomiting, painless intussusception, lethargy, and altered mental status.32
Evaluation may show a right upper quadrant mass or “sausage sign.” However, the absence of this on examination does not eliminate the need for further evaluation for intussusception, nor does the absence of pain at the time of presentation. Radiographs may show a paucity of bowel gas on the right, loss of the liver edge, and may even reveal the intussusception itself. (See Figure 3.) This is identified as a donut-like shape or “target sign” in the right upper quadrant, or as an intraluminal mass within the transverse colon, which may be identified by the “crescent sign” or “meniscus sign” of air within the lumen of the colon at the leading edge of the intussusceptum.28 The abdominal radiographs are abnormal in only 45% of cases of intussusception.30
Figure 3. Intussusception |
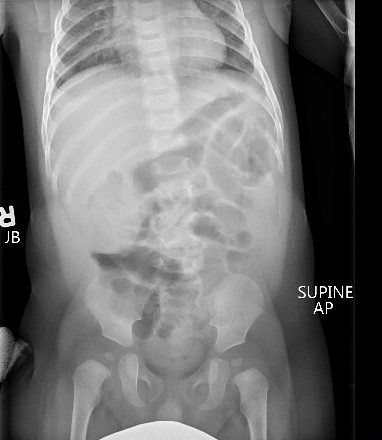
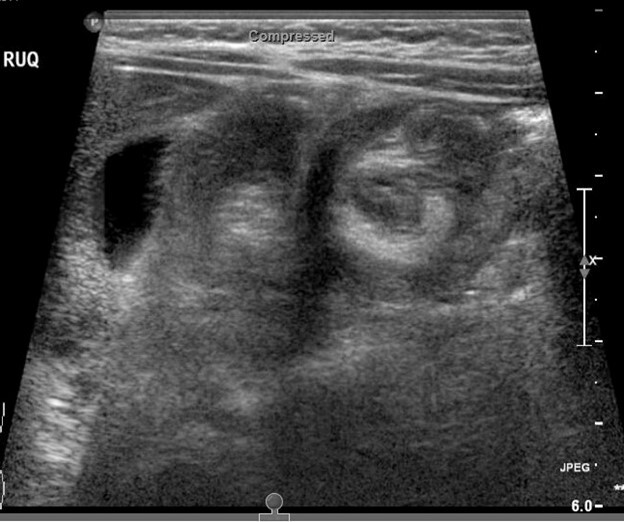 |
The top image is a radiograph with intussusception visible along the edge of the liver. The bottom image is an ultrasound of intussusception. Images courtesy of Derya Caglar, MD. |
Ultrasound has become the primary and preferred method for diagnosing intussusception in the pediatric population. It has high sensitivity (98% to 100%) and specificity (88% to 100%) in the diagnosis of intussusception.33,34 (See Figure 3.) Air contrast enema reduction of intussusception has become standard, although it occasionally can be unsuccessful (less than 20% of primary attempts are unsuccessful at reduction) or cause perforation of the intestines (less than 1% incidence), necessitating surgical intervention.35 Unsuccessful first attempts at reduction can be repeated, with delays reported between 30 minutes to 24 hours between attempts. Success rates after delayed repeat attempts at reduction enemas are 50% to 82% successful.36 A meta-analysis comparing barium enema to air enema showed a higher success rate, lower perforation rate, decreased cost, and decreased fluoroscopy time for air enema.35
After successful reduction of intussusception, recurrence is seen in less than 10% of patients.37 Most children with uncomplicated intussusception reduction (two or fewer attempts at enema reduction, low maximum insufflation pressure of < 120 mmHg) can be safely discharged from the ED after a four-hour asymptomatic observation period.38
Systemic Illnesses
DRESS Syndrome
Allergic reactions often occur in children. It is important that the physician be aware of more serious types of reactions. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a rare, potentially life-threatening hypersensitivity reaction that involves skin changes, hematologic abnormalities (eosinophilia, atypical lymphocytosis), lymphadenopathy, and internal organ involvement (dysfunction of the liver, kidney, or lungs).39
DRESS typically develops two to eight weeks after the drug exposure and may involve frequent relapses despite the discontinuation of the inciting drug in 80% of cases.40 Antiepileptic agents (e.g., carbamazepine, lamotrigine, phenytoin, phenobarbital) and allopurinol frequently are the most reported causes, although antibiotics (sulfonamides, dapsone, minocycline, and vancomycin) also have been implicated. The rash typically starts as a morbilliform eruption on the face and trunk that spreads and becomes confluent to involve > 50% of the body surface area. Patients may have significant associated edema and/or purpura that evolves into blisters, pustules, and severe dermatitis. They often will develop mucosal inflammation and edema that do not evolve to erosions.
Systemic symptoms include fevers, malaise, lymphadenopathy, abdominal pain, nausea, and/or vomiting. Diffuse lymphadenopathy is reported in 30% to 60% of cases.41 Hepatomegaly and jaundice may be present, but most liver involvement is asymptomatic. The kidneys also may be affected and present as an acute interstitial nephritis with elevated creatinine levels and/or proteinuria. These changes generally are mild and transient, but severe dysfunction can occur and should be monitored. Patients also may report a cough or chest pain and may have hypoxia or tachypnea on exam caused by pneumonitis. Patients may have myocarditis, gastrointestinal bleeds, encephalitis, myositis, or uveitis with prolonged or severe reactions that can lead to organ failure and death if unrecognized.
Treatment involves hospitalization with the immediate removal of the suspected drug and initiation of supportive care via IV hydration, correction of electrolyte abnormalities, and nutritional support. In cases of severe liver dysfunction, the patient may require transplantation.
Kawasaki Disease, MIS-C, and COVID-19
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an acute systemic vasculitis seen in the pediatric population. There is a genetic predisposition to the disease, with the highest incidence found in children of Asian descent (about 250 cases per 100,000 children younger than 5 years of age).42,43 However, it is seen in children of all ethnic groups, again with the highest incidence in children younger than 5 years of age. KD affects males more than females by a ratio of 3:2, but the exact cause currently is unknown.43,44 It is believed that the combination of a genetic predisposition with an infective agent predisposes a patient to develop KD.44
Although the development of coronary artery aneurysms is the most serious complication, the generalized vasculitis can manifest as various signs and symptoms throughout the patient. No single sign or symptom is specific to KD. Diagnosis is based on the American Academy of Pediatrics/American Heart Association diagnostic criteria listed in Table 1. As with physical exam findings, there is no one laboratory test that is diagnostic for KD; however, supportive laboratory findings are listed in Table 2. Other findings of KD are listed in Table 3.
Table 1. Diagnostic Criteria for Kawasaki Disease |
|
Fever for at least five days, usually high and unremitting despite antipyretics |
|
Four of the following five criteria with no alternative explanation: |
|
Cervical lymphadenopathy |
|
Conjunctivitis |
|
Polymorphous rash |
|
Extremity changes |
|
Oral mucous membrane changes |
|
Table 2. Laboratory Findings Supportive of Kawasaki Disease |
|
Complete blood count |
|
Inflammatory markers |
|
Liver function tests |
|
Urinalysis |
|
Cerebrospinal fluid (CSF) |
|
Basic metabolic panel |
|
Other |
|
Table 3. Other Findings of Kawasaki Disease |
|
Cardiac |
Congestive heart failure; myocarditis; pericarditis; coronary artery abnormalities; valvular insufficiency; aneurysms of medium-sized, noncoronary arteries; Raynaud’s; peripheral gangrene |
Musculoskeletal |
Arthralgias, arthritis |
Gastrointestinal |
Vomiting, diarrhea, abdominal pain, hepatic dysfunction, hydropic gall bladder |
Central Nervous System |
Extreme irritability, aseptic meningitis, sensorineural hearing loss |
Genitourinary |
Urethritis, meatitis |
Hematology |
Infants: thrombocytopenia, disseminated intravascular coagulation |
Other |
Anterior uveitis (mild), desquamating rash in the groin region, erythema, induration at Bacillus Callmette-Guerin site |
Echocardiography should be used to assess for the presence of coronary artery aneurysms, but treatment should not be delayed nor reliant on echocardiography. Echocardiogram findings consistent with KD include coronary dilatation, found in up to one-third of patients at the time of diagnosis. Coronary aneurysms typically are not present before day 10 of illness. The absence of coronary aneurysms does not preclude their development, and follow-up echocardiography is recommended at two weeks and between six and eight weeks, with continued follow-up for 10 to 20 years for most individuals.45 More frequent echocardiography is recommended for medium- and high-risk patients.45
Initial treatment for KD includes a combination of high-dose aspirin and IV immunoglobulin (IVIG). The use of IVIG is associated with faster resolution of fever and lower development of coronary abnormalities.44 Initiation of high-dose aspirin and IVIG within the first 10 days of symptoms provides maximum protection from long-term sequelae. After 14 days, low-dose aspirin therapy is continued for six to eight weeks if there are no cardiac abnormalities found on follow-up echocardiography. IV steroids typically are reserved only for patients with refractory KD (those who have remained febrile despite two treatments with IVIG).44
There is a subset of children who do not fulfill the diagnostic criteria for KD but in whom the clinician may have a high suspicion for the disease. About 10% of children who develop coronary artery aneurysms do not fulfill the criteria for KD. These children are considered to have atypical or incomplete Kawasaki disease (IKD) and are less likely to have cervical lymphadenopathy than children diagnosed with KD.45 Rash, peripheral extremity changes, and mucous membrane changes also are less likely in children with IKD. Clinicians should consider IKD when a child has an unexplained fever for seven days or longer, or a fever for five days or longer with two or three diagnostic criteria. IKD also should be considered in children who have three or four clinical or lab findings but have had a fever for fewer than five days.45,46 An algorithm for the workup of KD and IKD is found at https://bit.ly/45oRpfx.
COVID-19, caused by the SARS-CoV-2 virus, has been a major global health concern, affecting millions of patients worldwide since its emergence in late 2019. While initially thought to primarily affect adults, it soon became clear that children also could contract the virus. According to the Centers for Disease Control and Prevention (CDC), as of March 2023, there have been more than 5.5 million cases of COVID-19 in children in the United States alone.47 However, the majority of children who become infected with SARS-CoV-2 experience only mild symptoms or are asymptomatic.48 The clinical signs and symptoms of COVID-19 in children are similar to those seen in adults and can include fever, cough, sore throat, shortness of breath, fatigue, headache, body aches, and loss of taste or smell.49 Nevertheless, a small percentage of children who contract COVID-19 may develop a rare but severe complication known as multisystem inflammatory syndrome in children (MIS-C).50
MIS-C is a condition that typically develops in children and adolescents several weeks after they have been infected with SARS-CoV-2.50 It is characterized by inflammation in multiple organ systems, including the heart, lungs, kidneys, brain, and gastrointestinal tract. Symptoms of MIS-C can include fever, abdominal pain, vomiting, diarrhea, rash, and fatigue. In severe cases, children may require hospitalization, and some may even require admission to the intensive care unit.
Although the exact cause of MIS-C is still unknown, it is believed to be an immune system reaction that occurs after a child has been infected with SARS-CoV-2.50 Early recognition and treatment of MIS-C are crucial to prevent long-term complications, such as heart damage. Diagnosis of COVID-19 in pediatric patients is based on clinical symptoms, travel history, and testing for SARS-CoV-2 through polymerase chain reaction or antigen testing. Diagnosis of MIS-C is based on clinical signs and symptoms, a positive SARS-CoV-2 test or exposure, and evidence of inflammation in multiple organ systems.
Treatment of COVID-19 in pediatric patients primarily is supportive, including hydration, fever control, and oxygen therapy if necessary.49 In severe cases, hospitalization may be required. Treatment of MIS-C typically involves hospitalization and treatment with IVIG, steroids, and other supportive measures. While most pediatric patients with COVID-19 experience mild symptoms or are asymptomatic, healthcare providers must remain vigilant in monitoring for MIS-C, a rare but serious complication that can develop several weeks after infection with SARS-CoV-2. Prompt recognition and treatment of MIS-C are crucial to prevent long-term complications, and healthcare providers should have a high level of suspicion for this condition in children who present with persistent fever and signs of inflammation in multiple organ systems.
Trauma
Hair Tourniquet
Hair tourniquets are rare but should be considered in pediatric patients, particularly infants being evaluated for fussiness, irritability, or crying. It is characterized by a hair or fiber that becomes tightly wrapped around an appendage, such as a toe, finger, uvula, tongue, or a genital structure, causing strangulation and compromised blood flow.51,52 (See Figure 4.) Tissue ischemia or amputation is rare with prompt recognition. However, diagnosis may be difficult, and careful inspection of the affected area with magnification is recommended.53
Figure 4. Hair Tourniquet on Toes |
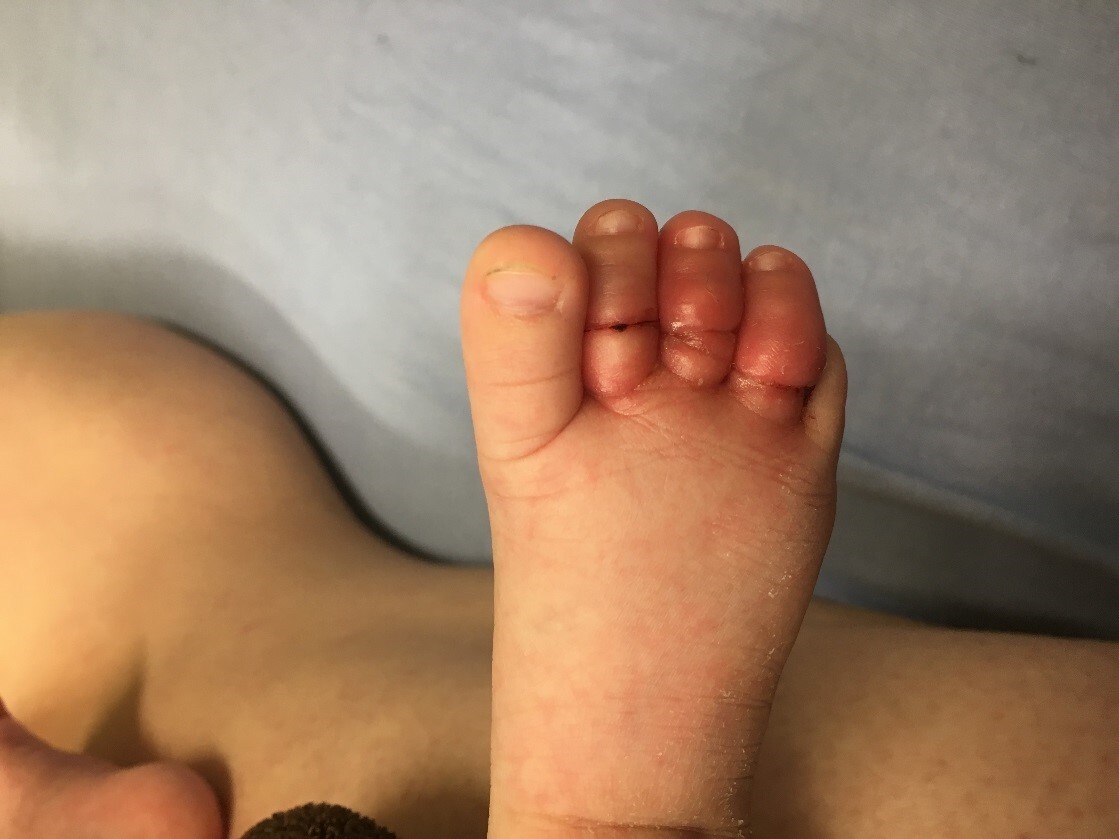 |
Image courtesy of Kaileen Jafari, MD. |
Management includes unwrapping, cutting, or dissolving the strangulating fiber. Chemical depilatory agents are very effective in the treatment of hair tourniquets when not contraindicated because of the location near mucous membranes.51,53 Infectious complications, like cellulitis, are rare after hair tourniquet release, so the physician should re-evaluate for persistent, unreleased tourniquets in any patient who presents with persistent erythema, swelling, or tenderness.
Child Abuse
During training, most physicians hear the mantra that child abuse is always in the differential diagnosis. This is especially true because of the varied, nonspecific presentation of nonaccidental trauma, as well as the inability of the treating physician to obtain a complete and accurate history from the patient and often from the caregiver. To complicate matters, the parent or guardian presenting with the child to the ED, urgent care, or primary care physician’s office may have no knowledge of the true nature of the child’s current symptoms or illness. However, he or she may know and be withholding the information, or even may be lying about the symptoms and timeline, complicating the picture for medical professionals. In 2021, the latest year for which data are available, 3.987 million reports were made to child protective services (CPS) across the country, 16% of which were victims of physical abuse (the remainder were neglect concerns).54 Of the total, 15% were classified as substantiated or indicated, meaning the victim rate of child abuse and neglect was 8.1 per 1,000 children in the United States.54
Because of the complexities surrounding child abuse, obtaining and documenting a complete history of the current presentation, as well as an accurate developmental history, can help the healthcare team assess the plausibility of presumed accidents and injuries.55 Risk factors for child abuse are complex, but mainly fall into three categories: those related to the child, those related to the parent, and those related to the environment. Factors associated with the child that increase the risk of child abuse include children with emotional and behavioral problems, children with chronic illnesses or disabilities, children who were born prematurely, and children who were unplanned or are unwanted.
Parental factors associated with an increased risk of child abuse include low self-esteem, poor impulse control, alcohol and substance abuse, young parental age, the parent’s history of abuse as a child, depression, mental illness, poor understanding of developmental stages of children or unrealistic expectations of the child, and a negative perception of a child’s behavior. Environmental factors related to child abuse include social isolation, poverty, unemployment, low educational achievement, single parenthood, a nonbiologically related male living in the home, and a history of intimate partner violence.54
Although accidental injuries are more common than abuse injuries in pediatric patients, identifying those children with injuries that are nonaccidental is an opportunity that often is missed by physicians, but one that can be lifesaving.56-58 Up to three-quarters of all cases of child abuse can be missed by ED providers.59 Previous isolated injuries or sentinel injuries (injuries recognized by physicians or parents but not attributed to abuse at that time) are identified in up to 25% of children with nonaccidental trauma, and in up to one-third of those with abusive head trauma.56,60 The most commonly identified injuries are skin bruises, intraoral injuries or frenula tears, and fractures.61-63
Bruising is the most common feature of physical abuse and should raise particular concern to physicians, especially in children who are developmentally unable to sustain bruising injuries. Because bruising is common, it is easily overlooked by physicians and other healthcare providers. Yet bruising can be the only visual manifestation of underlying injuries in victims of child abuse. The TEN-4 FACES Bruising Clinical Decision Rule can guide the evaluation of bruises in the clinical setting. Children at high risk include those younger than 4 months of age with any bruises, or any child younger than 4 years of age presenting with bruising in the TEN region (torso, ears, or neck, where the torso includes the chest, abdomen, back, buttocks, genitourinary, and hip area). If there is no confirmed accident in a public setting that accounts for the bruising in the TEN region or in a child younger than 4 months of age, the sensitivity is 97% and the specificity is 84% for identifying child abuse.57 Special areas to pay attention to when evaluating children include the torso, ears, and neck (TEN) in children younger than 4 years of age, and the frenulum, angle of the jaw, cheek, eyelid, and subconjunctiva (FACES).57 Bite marks and patterned bruising also should raise concern for the possibility of abuse. This bruising clinical decision rule is commonly referred to by the pneumonic TEN-4 FACESp Bruising Rule. This clinical decision rule is 95.6% sensitive and 87.1% specific for distinguishing abusive trauma from nonabusive trauma.63
Head injuries are the most common cause of death in abuse victims, and abusive head trauma is the most common cause of head injury deaths in infants and young children.64 Fractures, thoracic injuries (including rib fractures), and intra-abdominal injuries all are seen in child abuse.56 Evaluation of child abuse should be based on the age of the child and the injuries identified or suspected. Siblings and other young children with whom the suspected perpetrator has contact also should be evaluated. CPS should be contacted to facilitate these evaluations. As mandated reporters, healthcare professionals must report suspicion of child abuse and should never rely on others or assume others will report. The transfer of care to another physician or facility does not excuse the physician from reporting the suspicion of child abuse to the appropriate authorities.56
Conclusion
Children present to the ED with a variety of issues ranging from benign to life-threatening. Serious illnesses may have initially benign presentations and require a high degree of suspicion to recognize quickly and accurately. The ability to recognize these potentially devastating diseases early facilitates care and ensures optimal outcomes for children.
References
- Mistry RD, Hogan PG, Parrish KL, et al. Skin and soft tissue infection treatment and prevention practices by pediatric emergency medicine providers. Pediatr Emerg Care 2022;38:e1348-e1354.
- Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Summaries of infectious diseases. Red Book 2021-2024 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics;2021:186.
- Zundel S, Lemaréchal A, Kaiser P, Szavay P. Diagnosis and treatment of pediatric necrotizing fasciitis: A systematic review of the literature. Eur J Pediatr Surg 2017;27:127-137.
- Breyre A, Frazee BW. Skin and soft tissue infections in the emergency department. Emerg Med Clin North Am 2018;36:723-750.
- Marks A, Patel D, Sundaram T, et al. Ultrasound for the diagnosis of necrotizing fasciitis: A systematic review of the literature. Am J Emerg Med 2023;65:31-35.
- Parrish KL, Salwan NK, Thompson RM, et al. Skin and soft tissue infection treatment and prevention practices by pediatric infectious diseases providers. J Pediatric Infect Dis Soc 2020;9:760-765.
- Bouza E, Burillo A, Muñoz P. How to manage skin and soft-tissue infections in the emergency department. Curr Opin Infect Dis 2023;36:81-88.
- Stoopler ET, Houston AM, Chmieliauskaite M, et al. Erythema multiforme. J Emerg Med 2015;49:e197-198.
- Lerch M, Mainetti C, Beretta-Piccoli BT, Harr T. Current perspectives on erythema multiforme. Clin Rev Allergy Immunol 2018;54:177-184.
- Lerch M, Mainetti C, Beretta-Piccoli BT, Harr T. Current perspectives on Stevens-Johnson Syndrome and toxic epidermal necrolysis. Clin Rev Allergy Immunol 2018;54:147-176.
- Langley A, Anooshiravani N, Kwan S. Erythema multiforme in children and Mycoplasma pneumoniae aetiology. J Cutan Med Surg 2016;20:453-457.
- Sotozono C, Ueta M, Nakatani E, et al. Predictive dactors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol 2015;160:228-237.e2.
- Langan SM, Mulick AR, Rutter CE, et al. Trends in eczema prevalence in children and adolescents: A Global Asthma Network Phase I Study. Clin Exp Allergy 2023;53:337-352.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med 2017;377:e9.
- Naoum S, Bencherifa F. [Atopic dermatitis complicated by eczema herpeticum in a child]. J Fr Ophtalmol 2016;39:e247-e248.
- Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: Clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am 2015;35:161-183.
- Song Y, Huang X, Yu G, et al. Pathogenesis of IgA vasculitis: An up-to-date review. Front Immunol 2021;12:771619.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): Current state of knowledge. Curr Opin Rheumatol 2013;25:171-178.
- Xu L, Li Y, Wu X. IgA vasculitis update: Epidemiology, pathogenesis, and biomarkers. Front Immunol 2022;13:921864.
- Watanabe T. Henoch-Schönlein purpura following influenza vaccinations during the pandemic of influenza A (H1N1). Pediatr Nephrol 2011;26:795-798.
- Ting TV. Diagnosis and management of cutaneous vasculitis in children. Pediatr Clin North Am 2014;61:321-346.
- Dudley J, Smith G, Llewelyn-Edwards A, et al. Randomised, double-blind, placebo-controlled trial to determine whether steroids reduce the incidence and severity of nephropathy in Henoch-Schonlein purpura (HSP). Arch Dis Child 2013;98:756-763.
- Patel PN, Levi JR, Cohen MB. Lemierre’s syndrome in the pediatric population: Trends in disease presentation and management in literature. Int J Pediatr Otorhinolaryngol 2020;136:110213.
- Centor RM, Atkinson TP, Ratliff AE, et al. The clinical presentation of Fusobacterium-positive and streptococcal-positive pharyngitis in a university health clinic: A cross-sectional study. Ann Intern Med 2015;162:241-247.
- Johannesen KM, Bodtger U. Lemierre’s syndrome: Current perspectives on diagnosis and management. Infect Drug Resist 2016;9:221-227.
- Coffee Jr. R, Cico S, Caglar D. An update on soft tissue neck infection in children. Pediatr Emerg Med Rep 2023;28:37-47.
- Esposito S, De Guido C, Pappalardo M, et al. Retropharyngeal, parapharyngeal, and peritonsillar abscesses. Children (Basel) 2022;9:618.
- Waseem M, Rosenberg HK. Intussusception. Pediatr Emerg Care 2008;24:793-800.
- Kelley-Quon LI, Arthur LG, Williams RF, et al. Management of intussusception in children: A systematic review. J Pediatr Surg 2021;56:587-596.
- Tang XB, ang X, Ma Y, Bai YZ. Radiological and clinical characteristics of intussuscepted, inverting, and inverted Meckel’s diverticulum: A case series. Eur J Radiol 2022;157:110611.
- Gluckman S, Karpelowsky J, Webster AC, McGee RG. Management for intussusception in children. Cochrane Database Syst Rev 2017;6:CD006476.
- Khasawneh R, El-Heis M, Al-Omari M, et al. The radiological characteristics of childhood intussusception including unusual features and rare pathological lead points. Heliyon 2021;7:e07231.
- Edwards EA, Pigg N, Courtier J, et al. Intussusception: Past, present, and future. Pediatr Radiol 2017;47:1101-1108.
- Rahmani E, Amani-Beni R, Hekmatnia Y, et al. Diagnostic accuracy of ultrasonography for detection of intussusception in children: A systematic review and meta-analysis. Arch Acad Emerg Med 2023;11:e24.
- Sadigh G, Zou KH, Razabi SA, et al. Meta-analysis of air vs. liquid enema for intussusception reduction in children. AJR Am J Roentgenol 2015;205:W542-W549.
- Applegate KE. Intussusception in children: Evidence-based diagnosis and treatment. Pediatr Radiol 2009;39 (Suppl 2):S140-S143.
- Chien M, Willyerd FA, Mandeville K, et al. Management of the child after enema-reduced intussusception: Hospital or home? J Emerg Med 2013;44:53-57.
- Raval MV, Minneci PC, Deans KJ, et al. Improving quality and efficiency for intussusception management after successful enema reduction. Pediatrics 2015;136:e1345-e1352.
- Darlenski RJ, Kazandjieva J, Tsankov N. Systemic drug reactions with skin involvement: Stevens-Johnson syndrome, toxic epidermal necrolysis, and DRESS. Clin Dermatol 2015;33:538-541.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): An original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013;169:1071-1080.
- Mori F, Carlo C, Silvia C, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) in children. Acta Biomed 2019;90(Suppl 3):66-79.
- Harnden A, Tulloh R, Burgner D. Kawasaki disease. BMJ 2014;349:g5336.
- Ramphul K, Mejias SG. Kawasaki disease: A comprehensive review. Arch Med Sci Atheroscler Dis 2018;3:e41-e45.
- McCrindle BW, Rowley AH, Newburger JW, et al; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017;135:e927-e999.
- Hedrich CM, Schnabel A, Hospach T. Kawasaki disease. Front Pediatr 2018;6:198.
- Son MBF, Newburger JW. Kawasaki disease. Pediatr Rev 2018;39:78-90.
- Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Reviewed Jan. 3, 2023. https://www.cdc.gov/mis/mis-c.html
- Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088-1095.
- Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to U.S. and Canadian pediatric intensive care units. JAMA Pediatr 2020;174:868-873.
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334-346.
- Bean JF, Hebal F, Hunter CJ. A single center retrospective review of hair tourniquet syndrome and a proposed treatment algorithm. J Pediatr Surg 2015;50:1583-1585.
- Adjei NN, Lynn AY, Grimshaw A, et al. Systematic literature review of pediatric male and female genital hair thread tourniquet syndrome. Pediatr Emerg Care 2022;38:e799-e804.
- O’Gorman A, Ratnapalan S. Hair tourniquet management. Pediatr Emerg Care 2011;27:203-204.
- Children’s Bureau. Child maltreatment. U.S. Department of Health and Human Services. Reviewed Feb. 9, 2023. https://www.acf.hhs.gov/cb/data-research/child-maltreatment
- Zamalin D, Hamlin I, Shults J, et al. Predictors of making a referral to child protective services prior to expert consultation. Acad Pediatr 2023 May 11;S1876-2859(23)00150-X. doi: 10.1016/j.acap.2023.05.002. [Online ahead of print].
- Christian CW; Committee on Child Abuse and Neglect; American Academy of Pediatrics. The evaluation of suspected child physical abuse. Pediatrics 2015;135:e1337-e1354.
- Pierce MC, Kaczor K, Aldridge S, et al. Bruising characteristics discriminating physical child abuse from accidental trauma. Pediatrics 2010;125:67-74.
- Diyaolu M, Ye C, Huang Z, et al. Disparities in detection of suspected child abuse. J Pediatr Surg 2023;58:337-343.
- Kunen S, Hume P, Perret JN, et al., Underdiagnosis of child abuse in emergency departments. Acad Emerg Med 2003;10:546-a.
- Dias MS, Cappos KM, Rottmund CM, et al. Preventing abusive head trauma: Can educating parents reduce the incidence? Pediatr Radiol 2021;51:1093-1096.
- Barr RG, Barr M, Rajabali F, et al. Eight-year outcome of implementation of abusive head trauma prevention. Child Abuse Negl 2018;84:106-114.
- Dubowitz H, Feigelman S, Lane W, Kim J. Pediatric primary care to help prevent child maltreatment: The Safe Environment for Every Kid (SEEK) Model. Pediatrics 2009;123:858-864.
- Pierce MC, Kaczor K, Lorenz DJ, et al. Validation of a clinical decision rule to predict abuse in young children based on bruising characteristics. JAMA Netw Open 2021;4:e215832.
- Gill JR, Goldfeder LB, Armbrustmacher V, et al. Fatal head injury in children younger than 2 years in New York City and an overview of the shaken baby syndrome. Arch Pathol Lab Med 2009;133:619-627.
There are a variety of uncommon pediatric conditions that, if not detected, may result in devastating consequences. The authors review and update the current standard of care for a variety of conditions, including necrotizing fasciitis, DRESS syndrome, Kawasaki disease, MIS-C, Lemierre's, and RPA.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
