Traumatic Hemorrhagic Shock
September 15, 2023
AUTHORS
Erin Falk, MD, Emergency Medicine Resident, NewYork-Presbyterian, New York, NY
Anne R. Katz, MD, Assistant Program Director, NewYork-Presbyterian Emergency Medicine Residency Program, Assistant Professor of Clinical Emergency Medicine, Weill Cornell Medicine, New York, NY
PEER REVIEWER
Dennis Hanlon, MD, FAAEM, Medical Director, Department of Emergency Medicine, Allegheny General Hospital, Pittsburgh, PA
EXECUTIVE SUMMARY
- Resuscitation efforts for trauma-associated hypotension should have a goal of 80 mmHg to 90 mmHg systolic, since levels above that may lead to rebleeding and other complications. Exceptions are the presence of spinal or head injury.
- Two scores — the Shock Index and the ABC score — may predict patients who could need massive transfusion.
- Viscoelastic testing provides information on the state of a patient’s coagulation and helps to direct transfusion with blood components, such as platelets and fresh frozen plasma.
- Tranexamic acid may decrease mortality from severe trauma but needs to be given within three hours of the acute trauma.
Introduction
Traumatic injury affects individuals across all ages, races, and socioeconomic backgrounds. Traumatic hemorrhage, a direct consequence of traumatic injury, is a major cause of morbidity and mortality, accounting for 40% of traumatic deaths worldwide and is the leading cause of death in the young.1 This issue will review the management of traumatic hemorrhage in the emergency department (ED), highlighting prehospital care, recognition of hemorrhagic shock, initial resuscitative measures, massive hemorrhage protocol, reversal agents, and technological advancements in medical and mechanical support for traumatic hemorrhage.
Definition
Hemorrhagic shock is defined as an acute loss of circulating blood volume leading to inadequate oxygen delivery to tissues.2 It is the most common cause of shock in the trauma patient. A common four-tier classification system proposed by Advanced Trauma Life Support (ATLS) can be used to estimate the percentage of blood loss and guide initial therapy based on presenting signs and symptoms.3 (See Table 1.)
Caution should be used when interpreting heart rate alone as a predictor of mortality and estimation for degree of blood loss. Post-traumatic heart rate patterns in the setting of hemorrhagic shock typically present as biphasic or triphasic rather than a linear increase as suggested by ATLS. For example, the heart rate may appear to stabilize between class II and III because of vagal activity caused by paradoxical activation of the Bezold-Jarisch reflex, a cardioinhibitory reflex causing hypotension, peripheral vasodilation, and bradycardia, at around 30% blood loss. The lack of severe tachycardia expected during class III may provide false reassurance to the emergency medical team. In addition, bradycardia also can develop in class IV, shortly before cardiac arrest.4
Table 1. Classification of Hemorrhage Based on Estimated Blood Loss |
||||
Class I |
Class II |
Class III |
Class IV |
|
Blood loss (mL) |
Up to 750 |
750-1,500 |
1,500-2,000 |
> 2,000 |
Blood loss (% blood volume) |
Up to 15 |
15-30 |
30-40 |
40 |
Pulse rate |
< 100 |
100-129 |
120-140 |
> 140 |
Blood pressure |
Normal |
Normal |
Decreased |
Decreased |
Differential Diagnosis
The most common reason for shock in the trauma patient is hemorrhagic shock.5 While this represents the overwhelming etiology of hypotension in this patient population, it is important to keep a broad differential for shock in trauma. Alternative etiologies of shock in trauma include obstructive shock, cardiogenic shock, and distributive shock. (See Table 2.)
Table 2. Differential Diagnosis for Trauma-Related Shock |
||
Category |
Subtype |
Comments |
Hypovolemic |
|
Most common cause |
Obstructive |
|
|
Cardiogenic |
|
*MI is unlikely the presenting cause of cardiogenic shock unless it precipitated the trauma or is caused by stress-induced cardiomyopathy |
Distributive |
|
Maintain high index of suspicion in patients with bradycardia |
Obstructive Shock
Obstructive shock can be defined as physical obstruction to the normal path of blood flow, leading to tissue hypoperfusion. The most common causes of obstructive shock in trauma are tension pneumothorax/hemothorax and cardiac tamponade. Cardiac tamponade is an uncommon etiology of shock in traumatic injuries, but it can be alleviated with needle aspiration followed by a resuscitative thoracotomy and accounts for the majority of cases in reported thoracotomy survivors.6
Cardiogenic Shock
Cardiogenic shock is a rare cause of shock in trauma unless the patient experienced a blunt cardiac injury (BCI), the trauma was precipitated by a heart attack, or the trauma itself caused a stress-induced cardiomyopathy. BCI is a spectrum of cardiac injuries that include cardiac contusions, arrhythmias, valvular and septal injuries, and myocardial rupture. The initial work-up may include an electrocardiogram (ECG), cardiac biomarkers, and a bedside echocardiogram. Unfortunately, there is no gold standard for the diagnosis or management of suspected BCI, and therapeutic interventions are case-specific.7
Neurogenic Shock
Neurogenic shock is a spinal cord injury that disrupts the descending sympathetic tracts above the level of T6, resulting in unopposed parasympathetic activity, peripheral vasodilation, and autonomic instability. Clinical signs and symptoms of neurogenic shock can manifest as hypotension, bradycardia, and temperature dysregulation. Once other causes of shock are ruled out, the major treatment goal for neurogenic shock is avoidance of hypotension and maintenance of adequate spinal cord perfusion with a mean arterial pressure (MAP) target of 85 mmHg to 90 mmHg.8
Pathophysiology
Commonly known as the “lethal triad,” the development of hypothermia, coagulopathy, and acidosis in the injured patient describes the physiological insult that causes disseminated intravascular coagulopathy and directs resuscitation efforts. More recently, “the lethal diamond” has been introduced to include calcium, since all of these mechanisms potentiate one another and exacerbate traumatic hemorrhage and shock.9 (See Figure 1.)
Figure 1. The Lethal Diamond |
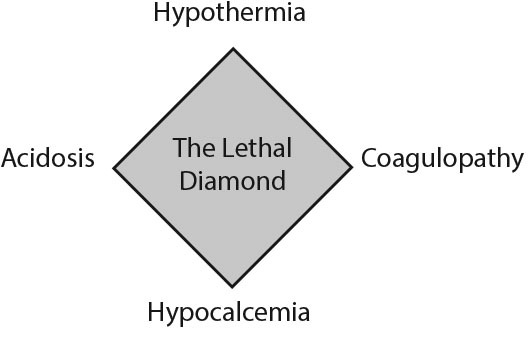 |
Hypothermia in the trauma setting is caused by multiple factors, such as direct environmental exposure, cold crystalloid infusions, hypotension, and impaired ability to thermoregulate in patients who present with traumatic brain injury (TBI), burns, or alcohol intoxication.
Acidosis can result from anemia in the setting of acute blood loss, peripheral vasoconstriction, and decreased cardiac output. This can lead to impaired oxygen delivery to the tissues, causing a shift to anaerobic metabolism and production of lactic acid. Additional causes of acidosis include excessive use of normal saline leading to metabolic acidosis and traumatic injuries resulting in respiratory acidosis from hypoventilation.
The coagulation cascade is a temperature- and pH-dependent system. Acidosis and hypothermia impair the body’s ability to synthesize clots, leading to coagulopathy. Furthermore, iatrogenic dilutional coagulopathy can develop if the resuscitative fluid and/or blood products do not contain the same clotting factors that were lost.10
Finally, calcium is a vital co-factor in the clotting cascade. Transfusions of large amounts of packed red blood cells (PRBCs) contain significant amounts of citrate preservative, which can bind to a patient’s endogenous calcium, rendering it inactive.10,11 Low ionized calcium levels should be replaced to avoid precipitating coagulopathy.
Prehospital Care
The management of traumatic hemorrhage begins in the prehospital stage with aggressive prevention of the lethal diamond and identification of life-threatening bleeding. According to the Prehospital Trauma Life Support (PHTLS), the initial assessment of the patient should focus on control of major bleeding, airway, breathing, circulation, disability, and exposure (ABCDE).12
Hemorrhage Control
Control of major bleeding is the first and most vital step in the prehospital setting. For a compressible site, direct and firm pressure should be applied to the wound. Axillary and groin wounds may be difficult to compress and can be packed with non-hemostatic materials and, if available, hemostatic dressings that contain oxidized cellulose, fibrin glue, synthetic adhesives, zeolite, or chitosan. In the case of the exsanguinating extremity, prehospital tourniquet use in the civilian population has been associated with decreased hemorrhagic shock upon arrival to the trauma center without increasing limb complications.13 For suspected unstable pelvic fractures, a pelvic binder may be placed in the presence of hypotension. The evidence and benefits of a prehospital pelvic binder remain unclear and have not been shown to improve clinical outcomes such as early vital signs, number of blood transfusions, or mortality during admission.14
ABCDE
Once obvious signs of bleeding have been addressed, prehospital resuscitation should continue with the ABCs (airway, breathing, and circulation). Oxygen supplementation should be provided if needed. Optimizing circulation in the prehospital setting with crystalloid infusions remains controversial. There is weak evidence suggesting that standard fluid resuscitation does not improve mortality when compared to restricting or withholding intravenous (IV) fluids in the context of the severely injured trauma patient.15 C-collars should be placed for suspected spinal cord injuries and all wet clothing should be removed.
ED Evaluation
Upon the patient’s arrival to the ED, the ED team should place two large bore IVs and obtain a complete set of vital signs. Assessment should begin with the primary survey with a focus on circulation and exposure in the patient presenting with suspected hemorrhagic shock.
Primary Survey
The evidence for the sequence ABCDE is minimal, and data that support this algorithm are based on the development of shared mental models and emphasis on life-threatening injuries. In recent years, this sequence has come under scrutiny, with an emphasis on a more nuanced approach to critically ill patients with traumatic injury.16-18
In traumatic injury, there are two immediately life-threatening and actionable injury mechanisms: massive external hemorrhage and critical airway compromise.
For the majority of patients who do not have hypoxia or airway compromise leading to circulatory collapse, a “circulation first” approach to resuscitation is supported by the literature. Data published on prehospital intubation and positive pressure ventilation in hypovolemic trauma patients have shown higher morbidity and mortality in the intervention arm.19 Early intubation without circulatory support, even in cases of patients who clearly have an imminent need for intubation, results in higher mortality.16,18
The vasodilatory response to rapid sequence intubation (RSI) can be profound, worsened by medication side effects, and more pronounced in hemodynamically fragile patients. Additionally, the application of positive pressure ventilation after intubation increases intrathoracic pressure and decreases preload. Ultimately, this combination of medication-induced blunting of adrenergic response and decreased venous return through positive pressure ventilation can result in decreased cardiac output and circulatory collapse. For this reason, experts have advocated for a more circulation-forward approach, considering blood products and hemodynamic support prior to intubation.16-19 Additionally, if there is concern for obstructive shock, procedures like thoracostomy or chest tubes should be considered before the application of positive pressure, which may cause development of tension physiology. (The medical literature emphasizes high-quality cardiopulmonary resuscitation [CPR] and compression-only CPR over early airway stabilization, resulting in improved patient outcomes.)20,21
Diagnosing Internal Hemorrhage
In the hemodynamically stable patient, the computed tomography (CT) scan is the gold standard for diagnosis of intrathoracic and intra-abdominal bleeding. A chest X-ray, pelvic X-ray, and the extended focused assessment with sonography for trauma (E-FAST) are considered adjuncts to the primary survey and can assist with timely diagnosis of internal hemorrhage, especially in the hemodynamically unstable patient. The E-FAST is a particularly useful tool for ruling in pericardial effusions (sensitivity 91% and specificity 94%) and detecting intra-abdominal free fluid (sensitivity 74% and specificity 98%).22
Trauma Scoring Systems to Predict Bleeding
Numerous prediction scores risk stratify trauma patients and can help predict occult shock, need for major hemorrhage protocol (MHP), and mortality. The shock index (SI) and the ABC score are two commonly used prediction tools that are used in the ED.23
The Shock Index (SI = HR/SBP)
The SI is calculated as the ratio of the heart rate (HR) to the systolic blood pressure (SBP) and has been found to be superior to either vital sign in isolation for predicting occult hemorrhage.23 Studies have observed a correlation between elevated prehospital SI and mortality, patient-centered outcomes, and resource utilization. An SI ≥ 0.9 portends a higher likelihood of disposition to the intensive care unit (ICU), operating room, or death, and an index > 1 is associated with a four-fold increase in mortality.23,24 An elevated SI also correlates with a higher likelihood of requiring > 10 units of PRBCs in the first 24 hours and the need for activating MHP. In a pooled analysis of 11 studies, an SI > 1 had a 93% specificity and 40% sensitivity across all serious injury indicators, suggesting the absence of an elevated SI does not rule out serious injury.25 It is important to note that there currently are no large prospective studies evaluating the SI score, and it should not be used in isolation to determine the need for MHP. However, an elevated SI should prompt the ED team to have a higher index of suspicion for occult hemorrhage and potential need for blood transfusion.
ABC Score for Massive Transfusion
The assessment of blood consumption (ABC) score for massive transfusion is a validated scoring system developed to identify patients who may require MHP early in their resuscitation course.26,27 (See Table 3.) The score consists of four items: penetrating mechanism, systolic blood pressure < 90 mmHg in the ED, heart rate > 120 bpm in the ED, and positive FAST. A score > 2 is predictive of MHP activation.
Table 3. The ABC Score |
||
Yes |
No |
|
Systolic BP < 90 mmHg |
1 point |
0 points |
HR > 120 bpm |
1 point |
0 points |
Positive FAST |
1 point |
0 points |
Penetrating trauma |
1 point |
0 points |
* Score > 2: Patient may require MHP ABC: assessment of blood consumption; BP: blood pressure; HR: heart rate; FAST: focused assessment with sonography for trauma; MHP: major hemorrhage protocol |
||
A retrospective cohort study comparing the SI to the ABC score recommended that both can be used to predict the need for MHP, with the SI slightly more sensitive and less difficult to perform than the ABC score. Both had similar specificity.28
Management of Hemorrhagic Shock
Blood Products
Patients with severe trauma and major bleeding often require massive transfusions of blood to replace what has been lost secondary to injury. Whole blood contains all components, while blood products are specific components of blood that have been extracted for individual use. Blood products include red blood cells (RBCs), fresh frozen plasma (FFP), and platelets. FFP can be further purified into prothrombin complex concentrate (PCC), which contains concentrated clotting factors, and cryoprecipitate, which is used to replace fibrinogen.
Whole blood offers a readily available, balanced transfusion of lifesaving RBCs, plasma, and clotting factors. It is theorized to reduce the rates of citrated blood product transfusion, which may cause life-threatening hypocalcemia.29,30 Disadvantages to whole blood transfusions are increased rates of transfusion reactions and limited shelf life.31,32 The current literature supporting the use of whole blood transfusions in traumatic injuries is varied. A recent regional trauma center pilot program used whole blood for their air and ground-based emergency medical services (EMS) units. Although it had a very small data set, they reported a marked decrease in mortality in patients who received whole blood compared to standard component therapy or crystalloid.31 Whole blood likely is non-inferior to component transfusions and may be particularly useful in prehospital or military settings where transport to higher levels of care may be prolonged.29,31
Component transfusion is more common in the hospital setting than whole blood transfusion. A landmark study, the PROPPR randomized clinical trial, studied the optimal ratio of blood products (plasma: platelets: RBCs), comparing 1:1:1 to 1:1:2.33 Although there were no significant differences in mortality at 24 hours or 30 days, more patients in the 1:1:1 group achieved hemostasis and fewer deaths occurred due to exsanguination by 24 hours.33 Notably, this group did have higher rates of thromboembolic events and a higher incidence of transfusion-related acute lung injury. Despite these mixed results, the PROPPR study established the current standard of care of 1:1:1 transfusions in traumatic hemorrhagic shock.
Recent research has considered early transfusion of plasma in the patient in severe hemorrhagic shock. The PAMPer trial in 2018 studied the impact of prehospital plasma administration on mortality in patients with traumatic injuries and concluded that 30-day mortality was significantly lower in the group administered plasma as the first transfused product.34 However, another recent trial found no mortality benefit with early plasma in situations where transport to a trauma center is readily available.35 Plasma is stored frozen, and it takes 30-45 minutes to thaw. Early resuscitation may favor RBCs as plasma thaws, resulting in an unbalanced early transfusion.36 Plasma-first transfusion may be useful in certain patient populations.
Permissive Hypotension
Optimal resuscitative blood pressure for trauma patients has evolved over the years. Early research idealized large volume fluid resuscitations, neglecting side effects of significant volume expansion and higher MAP goals, namely edema, hemodilution, and risk of rebleeding.37 In traumatic and hemorrhagic shock, newer research has suggested that permissive hypotension is associated with improved mortality.37,38 Lower MAP goals are theorized to cause fewer rebleeding injuries and less disruption of formed clots by limiting tissue edema and microvascular stress.38,39
Additionally, lower MAP goals often cause reduction in the overall amount of transfused blood products, which may be associated with decreased downstream risk of acute respiratory distress syndrome, acute kidney injury, and acute compartment syndrome.37,40 Multiple meta-analyses and retrospective randomized controlled trials (RCTs) have shown a mortality benefit associated with permissive hypotension.37,41-44 These studies also have shown trends toward improvements in secondary outcomes, such as intensive care unit (ICU) length of stay, nosocomial infections, and trauma-induced coagulopathy.
The MAP or systolic pressure goal in these studies has varied greatly. A 2018 meta-analysis of randomized controlled trials on permissive hypotension demonstrated significant variability in blood pressure goals.44 Although a clinical consensus on a target systolic or MAP goal is not well established, the 2023 European guidelines suggested targeting a systolic blood pressure goal of 80 mmHg to 90 mmHg.11 Permissive hypotension should not be applied to patients with TBI and spinal cord injuries who require higher MAPs to maintain brain and spinal cord perfusion.
Viscoelastic Testing
Trauma-induced coagulopathy (TIC) has been observed for centuries, with the first published evidence of traumatic coagulopathy documented in the 1960s.45,46 In the 1980s, a case series of patients with severe vascular intra-abdominal injuries showed that nearly 90% of the early post-injury deaths in this cohort was bleeding-related, but half of those occurred after mechanical control of bleeding sites.46,47 This implication that a complex coagulopathy underlies bleeding in traumatic injuries motivated the development of research and innovation for the next half-century.
TIC has no consensus laboratory definition, and, thus, the incidence of this condition in trauma patients is difficult to discern. A recently published review article on coagulopathy in traumatic injuries found an overall incidence of TIC in about 25% in severely injured patients.47 Studies in civilian and military populations found a higher frequency of TIC when TBI, shock, metabolic acidosis, and penetrating injury are present.46,48-50 Additionally, the current literature suggests the presence of TIC is an independent risk factor for mortality.46,49-55
Early literature on TIC used conventional laboratory markers of coagulation partial thromboplastin time (PTT)/activated partial thromboplastin time (aPTT), international normalized ratio (INR), fibrinogen, and platelet levels to describe ongoing coagulopathy.56-59 However, the pathophysiologic mechanisms that underlie TIC are characterized by unique hemostatic abnormalities, such as inadequate thrombin generation, impaired platelet function, fibrinogen depletion, and dysregulated fibrinolysis.46 These mechanisms are complex, change quickly over time with acid-base status, hypothermia, electrolyte derangements, and ongoing resuscitation and, therefore, are inadequately described by conventional laboratory markers of coagulation.46,55 Rotational thromboelastometry (ROTEM) and thromboelastography (TEG) are diagnostic tools that can be used to assess the coagulation status of patients in real time.
ROTEM and TEG are both methods used for viscoelastic testing (VET), which is a type of hemostasis testing that evaluates the clotting cascade of whole blood. Compared to conventional assessments of coagulopathy, VET measures the dynamic changes in the kinetics of a clot as it forms, stabilizes, and dissolves.60 ROTEM and TEG are similar but not identical methods of VET, which analyze and plot coagulation variables on a graph for interpretation.55 The results of this testing are readily available within 15 minutes.
The data produced by TEG and ROTEM help to direct targeted rather than fixed transfusion blood products; however, the impact on patient-centered outcomes has been variable.55 A recent meta-analysis analyzed bleeding patients in both elective and traumatic settings and concluded that VET lowered blood product use and mortality.61 Two RCTs have been published comparing conventional coagulation testing to VET. In 2016, the Goal-Directed Hemostatic Resuscitation of Trauma-Induced Coagulopathy Trial showed a mortality benefit at 28 days post-injury, as well as less platelets and plasma transfused in patients receiving TEG-guided resuscitation.62 However, a more recent RCT in 2021 demonstrated no difference between VET-directed and conventional laboratory-directed transfusions with regard to mortality or patient-
centered outcomes.63 Additional retrospective trials have not demonstrated improvements in mortality and had an inconsistent impact on blood product usage, either leading to fewer transfusions in the VET intervention arms or no impact on number of transfusions.64-68
The use of VET is an emerging science that may help guide resuscitations and improve patient outcomes, but the impact on patient-centered outcomes and number of transfusions is unclear, and currently is conditionally recommended by the Eastern Association for the Surgery of Trauma (EAST) trauma guidelines.69
Reversal Agents
An increasing amount of the U.S. population takes anticoagulation medication.70 In severe traumatic hemorrhage and life-threatening bleeding, anticoagulation should be reversed.70 Table 4 illustrates common anticoagulants and their reversal agents. In general, if it has been less than eight hours since a patient’s last dose of anticoagulant medication, they should be considered fully anticoagulated.70,71
Table 4. Anticoagulants and Reversal Agents |
|||
Drug |
Mechanism |
Reversal Agent |
Comments |
Warfarin |
Vitamin K antagonism |
PCC, vitamin K71 |
PCC preferred to FFP71 |
DOAC/DTI |
Inhibition of factor X (DOAC) Inhibition of thrombin (DTI) |
PCC FFP Drug-specific reversal agents70-72 |
Drug-specific reversal agents: Andexanet alfa for apixaban/rivaroxaban Idarucizumab for dabigatran These agents are more expensive and are not more effective. PCC is recommended over use of these more directed agents at this time.71,72 |
LMWH |
Activation of antithrombin III |
Protamine73,74 |
|
DOAC: direct oral anticoagulant; DTI: direct thrombin inhibitor; PCC: prothrombin complex concentrate; LMWH: low molecular weight heparin; FFP: fresh frozen plasma |
|||
Pressors
The use of pressors in traumatic hemorrhage is controversial. Vasoconstriction is thought to result in decreased organ perfusion and increased rates of multi-organ dysfunction.75 However, the pathophysiologic mechanism of shock in trauma is complex, with some animal models suggesting that late stages of traumatic injury may be characterized by vasodilation.11,75 The European guidelines on major bleeding and coagulopathy following trauma conditionally recommend that vasopressors may be used to supplement adequate intravascular resuscitation.11 Rigorous studies on the use of vasopressors in traumatic hemorrhage are limited. A 2011 double-blinded RCT studied the efficacy and safety of adding bolus and drip dose vasopressin to standard resuscitations, finding no difference in mortality or adverse events with lower amounts of blood products transfused.76 However, more recent data found increasing mortality and negative patient-centered outcomes with the use of vasopressors in this patient population.77-79
Current evidence does not support the use of vasopressors in hemorrhagic shock. In the face of life-threatening hypotension while resuscitation is ongoing or efforts are made to secure an airway, the transient use of vasopressors may have some limited utility.11 If vasopressors are used, permissive hypotension with systolic blood pressure of 80 mmHg to 90 mmHg still should be targeted.
Steroids
It has been postulated that in the setting of significant injury, the body activates the systemic inflammatory response syndrome (SIRS).80 As steroids have been evaluated in other SIRS conditions such as septic shock, it was theorized that steroids may have a role in traumatic hemorrhagic shock. However, multiple studies have shown no mortality benefit with steroid use in patients with hemorrhagic shock.80-83 Furthermore, the CRASH-1 trial in 2004 found increased mortality with steroid administration and concomitant serious TBI.84 Overall, with no clear benefit and some evidence of harm, empiric steroid administration is not recommended at this time.
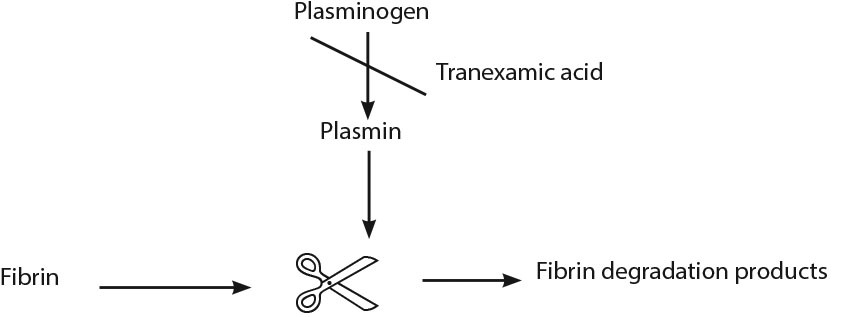
Advanced Medical: Tranexamic Acid
Tranexamic acid (TXA) prevents clot breakdown by preventing plasmin from degrading fibrin. (See Figure 2.) TXA, first synthesized in 1962, became a popular treatment option for hemorrhagic shock after the landmark study, the CRASH-2 trial, was published in 2010. This study was a large, placebo randomized controlled trial, enrolling more than 20,000 patients, and concluded that TXA reduced all-cause mortality without increasing vascular occlusive complications.
Figure 2. Mechanism of Tranexamic Acid |
 |
The recommended dose of TXA is a 1 g IV bolus over 10 minutes followed by 1 g over eight hours and should be given within three hours of injury onset. Indications for TXA include signs of severe hemorrhage (SBP < 90 mmHg or heart rate > 110 bpm, or both) or patients deemed at risk for significant hemorrhage.85-86 Limitations of the CRASH-2 trial include concerns of external validity to advanced trauma centers in developing countries. Additionally, despite a reduction in mortality, TXA did not reduce the need for blood transfusions.
In 2012, the MATTERs study, a retrospective observational study, evaluated combat-wounded soldiers treated in an Afghanistan surgical center, receiving at least 1 U PRBC. This study found a mortality benefit in the TXA group; however, transfusion requirements and risk of deep vein thrombosis/pulmonary embolism (DVT/PE) were higher in the TXA group. The increased rate of thromboembolism in the TXA group may be due to survivorship phenomenon and the possibility of increased thrombotic events as a result of high injury burden.87
A recent meta-analysis of TXA administration in both the civilian and military trauma environments revealed no statistically significant difference between the two groups. Subgroup analysis did reveal a statistically significant lower mortality rate in the civilian population who received TXA. Interestingly, the military population demonstrated higher mortality rates in the TXA arm, although this was not statistically significant. The higher mortality rate in the combat environment may be attributed to more severe injuries at baseline as suggested by the higher injury severity score on admission.88
Ultimately, TXA is a cost-effective treatment option that is easy to administer and may lower mortality in trauma patients with shock or at risk for hemorrhage. Risk of thromboembolism remains possible. Discussion with the trauma department should assist with TXA administration guidelines, since practice patterns vary among different institutions.89-91
Advanced Mechanical: Resuscitative Thoracotomy
Resuscitative thoracotomy (RT) is a maximally invasive procedure for treatment of traumatic cardiac arrest that involves surgically accessing the mediastinum to control ongoing hemorrhage and devastating cardiac, pulmonary, or vessel injury.92
There are two initial technical approaches to resuscitative thoracotomy: left anterolateral thoracotomy (LAT) and modified clamshell thoracotomy (MCT). While both approaches are acceptable, the MCT approach is the preferred approach among many. It allows for better exposure of the mediastinum, both lungs and hilum, as well as large venous and arterial structures.93,94 It also takes about the same amount of time as the LAT, which offers a more limited view.94 In a small prospective RCT, a comparison of the two techniques on cadavers showed significantly higher success rates with the MCT compared to the LAT, and the MCT technique was associated with less iatrogenic injury.94 Both approaches are acceptable, although the MCT approach may provide improved visualization without increasing time or complications.
Indication
The most commonly accepted indications for RT are patients with penetrating thoracic injury with profound, refractory shock or witnessed loss of pulses in the ED with signs of life (SOL). RT also can be considered in patients who are suspected to be exsanguinating from abdominal vascular injuries to obtain control of proximal blood flow through cross-clamping of the aorta as a means to bridge patients to definitive surgical repair. Guidelines from the EAST and Western Trauma Association (WEST) are summarized.95,96 (See Table 5.) RT should not be performed in cases where there is suspected TBI or other injuries deemed unsurvivable.92-99
Table 5. EAST and WEST Resuscitative Thoracotomy Guidelines |
|||
Eastern Association for the Surgery of Trauma (EAST) Trauma Guidelines |
|||
Recommendation |
Mechanism |
Location |
Signs of Life |
Strong |
Penetrating |
Thoracic |
With signs of life |
Conditional |
Penetrating |
Thoracic |
Without signs of life |
Extra-thoracic |
With signs of life |
||
Extra-thoracic |
Without signs of life |
||
Blunt |
- |
With signs of life |
|
Do Not Recommend |
Blunt |
- |
Without signs of life |
Western Trauma Association (WEST) Trauma Guidelines |
|||
Recommendation |
Mechanism |
Location |
CPR Time |
Strong |
Blunt |
- |
< 10 |
Penetrating |
Torso |
< 15 |
|
Penetrating |
Neck or extremity |
< 5 |
|
WEST guidelines also recommend resuscitative thoracotomy in patients with profound refractory shock (signs of life or systolic blood pressure CPR: cardiopulmonary resuscitation |
|||
Although the data are limited by patient and provider heterogeneity, thoracotomy should be performed as soon as possible after the need is identified, with times > 10 minutes from patient arrival to RT consistently associated with increased mortality.97
The survivability and mortality of RT is not well understood. A retrospective study of a nationwide database of more than 2,000 patients who underwent ED thoracotomy within 60 minutes of arrival noted overall survival of 19.9%. Notably, this cohort overwhelmingly presented with signs of life (87.5%). This study found a survival rate of 26.0% in penetrating trauma compared to 7.6% in blunt trauma. No patients with blunt trauma without SOL survived.98 Another large study found around a 14% survival in penetrating trauma and a 3% survival in blunt trauma, and severe neurological sequelae survivors were frequent.99
Advanced Mechanical: REBOA
In addition to the resuscitative techniques described earlier, advanced mechanical support in traumatic injury has gained traction in recent years. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is an endovascular technique that allows for mechanical control of hemorrhage.100
REBOA involves insertion of a balloon catheter into the aorta to temporarily occlude blood flow distally. Arterial access is obtained by the femoral artery via a percutaneous sheath, and the balloon is inflated in the aorta. There are three zones of occlusion that may be considered. Zone 1 placement occludes the descending thoracic aorta just below the level of the left subclavian artery. (See Figure 3.) Zone 2 involves inflation of the balloon in the abdominal aorta between the celiac and renal arteries, and zone 3 involves placement into the infra-renal aorta above the bifurcation of the iliacs.100-102
Figure 3. Zones for REBOA Placement |
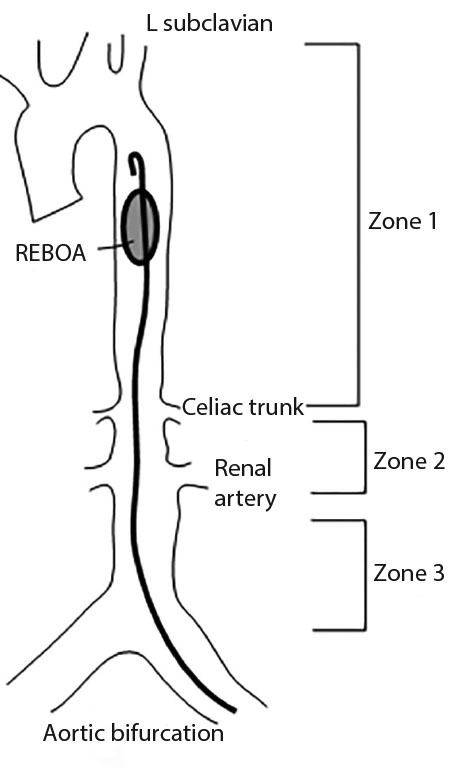 |
REBOA: resuscitative endovascular balloon occlusion of the aorta |
Indication
REBOA is used for severe, refractory hemorrhagic shock. Like RT, it is indicated for patients with non-compressible sources of internal bleeding who require ongoing resuscitation. It may be particularly useful in patients with pelvic or lower extremity hemorrhage.100-102 It also may be indicated in traumatic cardiac arrest as an attempt to increase afterload and allow for adequate myocardial perfusion.101
The use of REBOA has increased significantly in recent years with studies reporting some promising outcomes; however, the efficacy and impact on patient survival still is being debated. A 2020 systematic review with meta-analysis of 11 studies compared REBOA to RT with aortic cross-clamping and showed a survival benefit for the use of REBOA.102 Data persistently have shown improvement in mortality and secondary outcomes for patients using REBOA when compared to RT.103 However, this benefit may be explained by a bias in patient selection: RT is a maximally invasive procedure for patients who are dead or nearly dead, whereas REBOA may be considered more frequently in patients with severe and refractory shock who are not yet pulseless.102,103 Additionally, in comparisons between REBOA and no-REBOA, there was no mortality benefit of the procedure.102 In fact, a case control study published in 2019 found significantly higher rates of acute kidney injury, lower-limb amputation, and mortality in patients who underwent REBOA as a life-saving measure compared to patients who did not.104
Research on REBOA use in traumatic hemorrhage is ongoing. Unfortunately, no RCTs exist that compare REBOA to continued noninvasive resuscitation or to RT and open aortic cross-clamping. While the data overall have shown mixed results, it is likely that REBOA is a viable alternative to RT in crashing or pulseless trauma patients.
Conclusion
The development of novel medical technologies and treatment strategies can help emergency medicine physicians have a significant impact on patient outcomes in these complex cases. The use of permissive hypotension, balanced blood product transfusion, hemostatic agents, and advances in mechanical control of hemorrhage are subjects of current research and may help guide resuscitation in these critically ill patients. New advances in point-of-care testing, such as ultrasound and VET, can quickly assess a patient’s response to interventions and help physicians manage ongoing hemorrhage.
REFERENCES
- Faria I, Thivalapill N, Makin J, et al. Bleeding, hemorrhagic shock, and the global blood supply. Crit Care Clin 2022;38:775-793.
- Pitotti C, David J. An evidence-based approach to nonoperative management of traumatic hemorrhagic shock in the emergency department. Emerg Med Pract 2020;22:1-24.
- Henry S. Advanced Trauma Life Support. 10th ed. American College of Surgeons, 2018:49-50.
- Jávor P, Hanák L, Hegyi P, et al. Predictive value of tachycardia for mortality in trauma-related haemorrhagic shock: A systematic review and meta-regression. BMJ Open 2022;12:e059271.
- Cocchi MN, Kimlin E, Walsh M, Donnino MW. Identification and resuscitation of the trauma patient in shock. Emerg Med Clin North Am 2007;25:623.
- Paulich S, Lockey D. Resuscitative thoracotomy. BJA Educ 2020;20:242-248.
- Nair L, Winkle B, Senanayake E. Managing blunt cardiac injury. J Cardiothorac Surg 2023;18:71.
- Dave S, Dahlstrom JJ. Neurogenic shock. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated Feb. 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK459361/
- Wray JP, Bridwell RE, Schauer SG, et al. The diamond of death: Hypocalcemia in trauma and resuscitation. Am J Emerg Med 2021;41:104-109.
- Gerecht R. The lethal triad. Hypothermia, acidosis & coagulopathy create a deadly cycle for trauma patients. JEMS 2014;39:56-60.
- Rossaint R, Afshari A, Bouillon B, et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit Care 2023;27:80.
- Teuben M, Löhr N, Jensen KO, et al. Improved pre-hospital care efficiency due to the implementation of pre-hospital trauma life support (PHTLS) algorithms. Eur J Trauma Emerg Surg 2020;46:1321-1325.
- Schroll R, Smith A, Alabaster K, et al. AAST multicenter prospective analysis of prehospital tourniquet use for extremity trauma. J Trauma Acute Care Surg 2022;92:997-1004.
- Bangura A, Burke CE, Enobun B, et al. Are pelvic binders an effective prehospital intervention? Prehosp Emerg Care 2023;27:24-30.
- Hébert S, Kohtakangas E, Campbell A, Ohle R. The efficacy of prehospital IV fluid management in severely injured adult trauma patients: A systematic review and meta-analysis. CJEM 2023;25:200-208.
- Ferrada P, Callcut RA, Skarupa DJ, et al. Circulation first — the time has come to question the sequencing of care in the ABCs of trauma; an American Association for the Surgery of Trauma multicenter trial. World J Emerg Surg 2018;13:8.
- Gondek S, Schroeder ME, Sarani B. Assessment and resuscitation in trauma management. Surg Clin North Am 2017;97:985-998.
- Nolan B, Hillier M. Unlearning the ABCs: A call to reprioritize prehospital intubation for trauma patients. CJEM 2021;23:271-273.
- Petrosoniak A, Hicks C. Resuscitation resequenced: A rational approach to patients with trauma in shock. Emerg Med Clin North Am 2018;36:41-60.
- Xu Y, Wu QQ. Standard cardiopulmonary resuscitation versus chest compressions only after out-of-hospital cardiac arrest: A protocol for systematic review and meta-analysis. Inplasy protocol 202130109. doi: 10.37766/inplasy2021.3.0109.
- Riva G, Ringh M, Jonsson M, et al. Survival in out-of-hospital cardiac arrest after standard cardiopulmonary resuscitation or chest compressions only before arrival of emergency medical services: Nationwide study during three guideline periods. Circulation 2019;139:2600-2609.
- Netherton S, Milenkovic V, Taylor M, Davis PJ. Diagnostic accuracy of eFAST in the trauma patient: A systematic review and meta-analysis. CJEM 2019;21:727-738.
- Gianola S, Castellini G, Biffi A, et al. Accuracy of risk tools to predict critical bleeding in major trauma: A systematic review with meta-analysis. J Trauma Acute Care Surg 2022;92:1086-1096.
- Vang M, Østberg M, Steinmetz J, Rasmussen LS. Shock index as a predictor for mortality in trauma patients: A systematic review and meta-analysis. Eur J Trauma Emerg Surg 2022;48:2559-2566.
- Totten AM, Cheney TP, O’Neil ME, et al. Physiologic predictors of severe injury: Systematic review. AHRQ Comparative Effectiveness Review No. 205. Agency for Healthcare Research and Quality; April 2018. doi: https://doi.org/10.23970/AHRQEPCCER205
- Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: Simple as ABC (assessment of blood consumption)? J Trauma 2009;66:346-352.
- Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma 2010;69(Suppl 1):S33-S39.
- Schroll R, Swift D, Tatum D, et al. Accuracy of shock index versus ABC score to predict need for massive transfusion in trauma patients. Injury 2018;49:15-19.
- Kronstedt S, Lee J, Millner D, et al. The role of whole blood transfusions in civilian trauma: A review of literature in military and civilian trauma. Cureus 2022;14:e24263.
- Cruciani M, Franchini M, Mengoli C, et al. The use of whole blood in traumatic bleeding: A systematic review. Intern Emerg Med 2021;16:209-220.
- Zhu CS, Pokorny DM, Eastridge BJ, et al. Give the trauma patient what they bleed, when and where they need it: Establishing a comprehensive regional system of resuscitation based on patient need utilizing cold-stored, low-titer O+ whole blood. Transfusion 2019;59:1429-1438.
- Assen S, Cardenas J, George M, et al. Hemostatic potential of cold-stored non-leukoreduced whole blood over time: An assessment of platelet function and thrombin generation for optimal shelf life. J Trauma Acute Care Surg 2020;89:429-434.
- Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015;313:471-482.
- Sperry JL, Guyette FX, Brown JB, et al; PAMPer Study Group. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med 2018;379:315-326.
- Moore HB, Moore EE, Chapman MP, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: A randomised trial. Lancet 2018;392:283-291.
- Pusateri AE, Moore EE, Moore HB, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: A post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg 2020;155:e195085.
- Woodward L, Alsabri M. Permissive hypotension vs. conventional resuscitation in patients with trauma or hemorrhagic shock: A review. Cureus 2021;13:e16487.
- Ho KH, Tarng YW, Chou YP, Lin HL. Permissive hypotensive resuscitation in patients with traumatic hemorrhagic shock. Scand J Trauma Resusc Emerg Med 2019;27:14.
- Cohen M, Monaghan SF. Hemorrhagic shock and fluid dynamics. Physiol Rep 2021;9:e14813.
- Chipman AM, Jenne C, Wu F, Kozar RA. Contemporary resuscitation of hemorrhagic shock: What will the future hold? Am J Surg 2020;220:580-588.
- Lu X, Ying L, Wang H, et al. Efficacy comparison of restrictive versus massive fluid resuscitation in patients with traumatic hemorrhagic shock. Am J Transl Res 2022;14:7504-7511.
- Safiejko K, Smereka J, Filipiak KJ, et al. Effectiveness and safety of hypotension fluid resuscitation in traumatic haemorrhagic shock: A systematic review and meta-analysis of randomised controlled trials. Cardiol J 2020;29:463-471.
- Guyette FX, Sperry JL, Peitzman AB, et al. Prehospital blood product and crystalloid resuscitation in the severely injured patient: A secondary analysis of the prehospital air medical plasma trial. Ann Surg 2021;273:358-364.
- Tran A, Yates J, Lau A, et al. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. J Trauma Acute Care Surg 2018;84:802-808.
- Innes D, Sevitt S. Coagulation and fibrinolysis in injured patients. J Clin Pathol 1964;17:1-13.
- Moore EE, Moore HB, Kornblith LZ, et al. Trauma-induced coagulopathy. Nat Rev Dis Primers 2021;7:30. Erratum in: Nat Rev Dis Primers 2022;8:25.
- Kashuk JL, Moore EE, Millikan JS, Moore JB. Major abdominal vascular trauma — a unified approach. J Trauma 1982;22:672-679.
- Samuels JM, Moore EE, Silliman CC, et al. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg 2019;86:686-693.
- Nakae R, Murai Y, Wada T, et al. Hyperfibrinolysis and fibrinolysis shutdown in patients with traumatic brain injury. Sci Rep 2022;12:19107.
- Zhang L, Lin M, Tang X, Tang Y. Correlation between coagulation fibrinolysis function and outcomes during hospitalization in patients with severe traumatic hemorrhagic shock. Emerg Med Int 2022;2022:3775868.
- Reed CR, Williamson H, Vatsaas C, et al. Higher mortality in pediatric and adult trauma patients with traumatic coagulopathy, using age-adjusted diagnostic criteria. Surgery 2019;165:1108-1115.
- Ishii K, Kinoshita T, Kiridume K, et al. Impact of initial coagulation and fibrinolytic markers on mortality in patients with severe blunt trauma: A multicentre retrospective observational study. Scand J Trauma Resusc Emerg Med 2019;27:25.
- Sun C, Xi F, Li J, et al. Longitudinal D-dimer trajectories and the risk of mortality in abdominal trauma patients: A group-based trajectory modeling analysis. J Clin Med 2023;12:1091.
- Fröhlich M, Mutschler M, Caspers M, et al; TraumaRegister DGU. Trauma-induced coagulopathy upon emergency room arrival: Still a significant problem despite increased awareness and management? Eur J Trauma Emerg Surg 2019;45:115-124.
- Brill JB, Brenner M, Duchesne J, et al. The role of TEG and ROTEM in damage control resuscitation. Shock 2021;56:52-61.
- Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr Opin Crit Care 2007;13:680-685.
- Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma 2003;54:1127–1130.
- Hoyt DB, Dutton RP, Hauser CJ, et al. Management of coagulopathy in the patients with multiple injuries: Results from an international survey of clinical practice. J Trauma 2008;65:755-764.
- Stahel PF, Moore EE, Schreier SL, et al. Transfusion strategies in postinjury coagulopathy. Curr Opin Anaesthesiol 2009;22:289-298.
- Sayce AC, Neal MD, Leeper CM. Viscoelastic monitoring in trauma resuscitation. Transfusion 2020;60 (Suppl 6):S33-S51.
- WikkelsØ A, Wetterslev J, MØller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: A systematic review with meta-analysis and trial sequential analysis. Anaesthesia 2017;72:519-531.
- Gonzalez E, Moore EE, Moore HB, et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: A pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg 2016;263:1051-1059.
- Baksaas-Aasen K, Gall LS, Stensballe J, et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med 2021;47:49-59.
- Nardi G, Agostini V, Rondinelli B, et al. Trauma-induced coagulopathy: Impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care 2015;19:83.
- Prat NJ, Meyer AD, Ingalls NK, et al. Rotational thromboelastometry significantly optimizes transfusion practices for damage control resuscitation in combat casualties. J Trauma Acute Care Surg 2017;83:373-380.
- Netherton S, Milenkovic V, Taylor M, Davis PJ. Diagnostic accuracy of eFAST in the trauma patient: A systematic review and meta-analysis. CJEM 2019;21:727-738.
- Schaden E, Kimberger O, Kraincuk P, et al. Perioperative treatment algorithm for bleeding burn patients reduces allogeneic blood product requirements. Br J Anaesth 2012;109:376-381.
- Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: Thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care 2011;15:R83.
- Unruh M, Reyes J, Helmer SD, Haan JM. An evaluation of blood product utilization rates with massive transfusion protocol: Before and after thromboelastography (TEG) use in trauma. Am J Surg 2019;218:1175-1180.
- Josef AP, Garcia NM. Systemic anticoagulation and reversal. Surg Clin North Am 2022;102:53-63.
- Hofer S, Schlimp CJ, Casu S, Grouzi E. Management of coagulopathy in bleeding patients. J Clin Med 2022;11:1.
- Parsels KA, Seabury RW, Zyck S, et al. Andexanet alfa effectiveness and safety versus four-factor prothrombin complex concentrate (4F-PCC) in intracranial hemorrhage while on apixaban or rivaroxaban: A single-center, retrospective, matched cohort analysis. Am J Emerg Med 2022;55:16-19.
- Lauer BR, Nelson RA, Adamski JH, et al. Protamine sulfate for the reversal of enoxaparin associated hemorrhage beyond 12 h. Am J Emerg Med 2019;37.1:174-e5.
- de Olano J, Howland MA, Su MK. Massive intentional enoxaparin overdose managed with minimal protamine: A single case report. Am J Health-System Pharm 2023;80:e98-e103.
- Richards JE, Harris T, Dünser MW, et al. Vasopressors in trauma: A never event? Anesth Analg 2021;133:68-79.
- Cohn SM, McCarthy J, Stewart RM, et al. Impact of low-dose vasopressin on trauma outcome: Prospective randomized study. World J Surg 2011;35:430-439.
- Sperry JL, Minei JP, Frankel HL, et al. Early use of vasopressors after injury: Caution before constriction. J Trauma 2008;64:9-14.
- Van Haren RM, Thorson CM, Valle EJ, et al. Vasopressor use during emergency trauma surgery. Am Surg 2014;80:472-478.
- Barmparas G, Dhillon NK, Smith EJ, et al. Patterns of vasopressor utilization during the resuscitation of massively transfused trauma patients. Injury 2018;49:8-14.
- Hogarty JP, Jones ME, Jassal K, et al. Early steroid administration for traumatic haemorrhagic shock: A systematic review. Emerg Med Australas 2023;35:6-13.
- Kim G, Young J. Clinical characteristics of trauma patients requiring hydrocortisone treatment for refractory hypotension. Am Surg 2017;83:821-824.
- Roquilly A, Mahe PJ, Seguin P, et al. Hydrocortisone therapy for patients with multiple trauma: The randomized controlled HYPOLYTE study. JAMA 2011;305:1201-1209.
- Rygård SL, Butler E, Granholm A, et al. Low-dose corticosteroids for adult patients with septic shock: A systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2018;44:1003-1016.
- Wasserberg J; CRASH Trial Collaborative Group. The MRC CRASH trial — a large, simple randomized trial of steroids in head injury. Acta Neurochir Suppl 2004;89:109-112.
- Williams-Johnson JA, McDonald AH, Strachan GG, Williams EW. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH-2): A randomized, placebo-controlled trial. West Indian Med J 2010;59:612-624.
- CRASH-2 collaborators; Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377:1096-101, 1101.e1-2.
- Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch Surg 2012;147:113-119.
- Al-Jeabory M, Szarpak L, Attila K, et al. Efficacy and safety of tranexamic acid in emergency trauma: A systematic review and meta-analysis. J Clin Med 2021;10:1030.
- Wang K, Santiago R. Tranexamic acid — A narrative review for the emergency medicine clinician. Am J Emerg Med 2022;56:33-44. Erratum in: Am J Emerg Med 2022;May 23. PMID: 35364476.
- Guerriero C, Cairns J, Perel P, et al; CRASH 2 trial collaborators. Cost-effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH-2 trial. PLoS One 2011;6:e18987.
- Jawa RS, Singer A, Mccormack JE, et al. Tranexamic acid use in United States trauma centers: A national survey. Am Surg 2016;82:439-447.
- Aseni P, Rizzetto F, Grande AM, et al. Emergency department resuscitative thoracotomy: Indications, surgical procedure and outcome. A narrative review. Am J Surg 2021;221:1082-1092.
- Newberry R, Brown D, Mitchell T, et al. Prospective randomized trial of standard left anterolateral thoracotomy versus modified bilateral clamshell thoracotomy performed by emergency physicians. Ann Emerg Med 2021;77:317-326.
- DuBose JJ, Morrison J, Moore LJ, et al. Does clamshell thoracotomy better facilitate thoracic life-saving procedures without increased complication compared with an anterolateral approach to resuscitative thoracotomy? Results from the American Association for the Surgery of Trauma Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery Registry. J Am Coll Surg 2020;231:713-719.
- Seamon MJ, Haut ER, Van Arendonk K. An evidence-based approach to patient selection for emergency department thoracotomy: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;79:159-173.
- Burlew CC, Moore EE, Moore FA, et al. Western Trauma Association critical decisions in trauma: Resuscitative thoracotomy. J Trauma Acute Care Surg 2012;73:1359-1363.
- Gore AV, Burlew CC, Moore EE. Resuscitative thoracotomy. In: Wilson KL, Rogers SO Jr, eds. Difficult Decisions in Trauma Surgery: An Evidence-Based Approach. Springer;2022:241-249.
- Panossian VS, Nederpelt CJ, El Hechi MW, et al. Emergency resuscitative thoracotomy: A nationwide analysis of outcomes and predictors of futility. J Surg Res 2020;255:486-494.
- Joseph B, Khan M, Jehan F, et al. Improving survival after an emergency resuscitative thoracotomy: A 5-year review of the trauma quality improvement program. Trauma Surg Acute Care Open 2018;3:1-6.
- Granieri S, Frassini S, Cimbanassi S, et al. Impact of resuscitative endovascular balloon occlusion of the aorta (REBOA) in traumatic abdominal and pelvic exsanguination: A systematic review and meta-analysis. Eur J Trauma Emerg Surg 2022;48:3561-3574.
- Aoki M, Abe T. Traumatic cardiac arrest: Scoping review of utilization of resuscitative endovascular balloon occlusion of the aorta. Front Med (Lausanne) 2022;9:888225.
- Castellini G, Gianola S, Biffi A, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in patients with major trauma and uncontrolled hemorrhagic shock: A systematic review with meta-analysis. World J Emerg Surg 2021;16:41.
- Khalid S, Khatri M, Siddiqui MS, Ahmed J. Resuscitative endovascular balloon occlusion of aorta versus aortic cross-clamping by thoracotomy for noncompressible torso hemorrhage: A meta-analysis. J Surg Res 2022;270:252-260.
- Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg 2019;154:500-508.
