AUTHORS
Ethan Gerdts, MD
Department of Emergency Medicine, University of North Carolina, Chapel Hill
Daniel Migliaccio, MD, FDP, FAAEM
Clinical Assistant Professor, Director of Emergency Ultrasound, Ultrasound Fellowship Director, University of North Carolina, Chapel Hill
PEER REVIEWER
Steven M. Winograd, MD, FACEP
Attending Emergency Physician, Trinity Health Care, Samaritan, Troy, NY
Executive Summary
- Certain patient populations are at increased risk for respiratory syncytial virus (RSV) infection compared to others. There is a clear correlation between increased risk of RSV infection and morbidity in pediatric patients who were born premature, who have underlying lung disease, who have congenital heart disease, who are immunocompromised, or who are oncology patients. There also appears to be an increased risk of death in these high-risk groups if they are hospitalized with RSV infection.
- RSV infections, specifically those that result in symptomatic bronchiolitis, are primarily in children younger than the age of 2 years. If a child presents outside this normal age range, clinicians should consider alternative diagnosis, since RSV bronchiolitis does not normally cause severe symptomatic infection above this age.
- The usual course of this illness starts with symptoms of an upper respiratory tract infection, including rhinorrhea, cough, and conjunctivitis congestion, with or without a sore throat. Around day 3, symptoms progress as the lower airways start to be affected by the destruction of respiratory epithelium. Persistent cough and increased work of breathing are the hallmark findings in lower respiratory illness, often accompanied by fever. Increased work of breathing can manifest differently depending on the age of the patient, but it can include tachypnea, nasal flaring, intercostal or supraclavicular retractions, abdominal breathing, or grunting. Crackles and wheezing are both frequently heard on auscultation of the patient’s lungs.
- Apnea is a unique clinical feature of RSV bronchiolitis and can be the sole presenting symptom of infant patients with disease. Between 5% and 20% of infants younger than 6 months of age will have apnea associated with RSV infection. The mechanism behind apnea remains unclear.
- The American Academy of Pediatrics and many other worldwide pediatric organizations, including the Canadian, United Kingdom, and Spanish equivalents, do not recommend routine RSV testing for the diagnosis of RSV bronchiolitis. In their clinical practice guidelines, these groups agree that the diagnosis of bronchiolitis is a clinical diagnosis and does not necessarily require testing.
- Imaging in the diagnosis of RSV bronchiolitis also is not routinely recommended by clinical practice guidelines. There has been no demonstrated benefit, and it does not alter clinical outcomes. Chest radiographs may even lead to an increase in inappropriate antibiotic administration. Imaging may be useful only if the child’s clinical course or severity may suggest a superimposed bacterial infection requiring antibiotic administration.
- Administering saline spray into the nares prior to suctioning also can improve the amount of secretion aspirated. Suctioning when the patient has the highest amount of respiratory distress, before feeds, and before sleep will give the patient the most amount of symptomatic relief.
- In preparation for discharge, one of the most important management points of mild RSV bronchiolitis is return precautions and patient counseling. RSV bronchiolitis is a labile and dynamic illness, and a parent or caregiver needs to know what physical exam findings may indicate decompensation and need for escalation of care.
- Supportive care remains the mainstay of treatment, but in cases of severe bronchiolitis, the patient most likely will require ventilatory support. High flow nasal cannula or continuous positive airway pressure are oft-used noninvasive ventilatory support options. They can deliver increased flow rates and increase the fraction of inspired oxygen in the hypoxic patient.
Respiratory syncytial virus is a common virus encountered in the ED, with myriad presentations and complications that clinicians must be able to identify and manage. The authors provide state-of-the-art diagnostic and management strategies for the acute care clinician.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Respiratory syncytial virus (RSV) is a virus that causes primarily seasonal respiratory illness, like myriad others. However, RSV differs from other pathogens because of its abnormally high rate of morbidity and mortality in children and extremely high prevalence in children younger than 1 year of age. Additionally, it has unique clinical characteristics and requires different treatment than other similar viral infections. For these reasons, clinicians must be intimately aware of this potentially deadly virus and its management — mismanagement can have deadly consequences.
Epidemiology
RSV is a single-stranded ribonucleic acid (RNA) virus of the Pneumovirus subfamily of the Paromyxovirus family, similar to human metapneumovirus (HMPV).1,2 There are A and B subtypes of RSV, and it is commonly accepted that the ‘A’ subtype confers more severe disease.2-5 It primarily causes a lower respiratory illness, meaning it affects the bronchi, bronchioles, and alveoli, compared to upper respiratory tract infections, which affect the pharynx, larynx, sinuses, or large airways (trachea and mainstem bronchi).1,6 Upper respiratory tract infections are more common and typically are caused by rhinovirus, enterovirus, and adenovirus, among others.3 Upper respiratory tract infections primarily have self-limited inflammation and irritation that typically do not require antimicrobials to treat.6,7 Common diagnoses of the upper respiratory tract include sinusitis, laryngitis, pharyngitis, epiglottitis, and tracheitis.7
Lower respiratory tract infections are caused by both viruses and bacteria. The most common viruses include RSV, influenza, and HMPV viruses, whereas bacterial causes range from the typical (Staphylococcus aureus, Streptococcus pneumoniae, and Streptococcus pyogenes) to the atypical (Chlamydia trachomatis/pneumoniae and Mycoplasma species).6,8,9 These infections are defined by inflammation, irritation, and eventual propagation of bacteria/viruses/fungi into the lung parenchyma. They may require antimicrobials to treat.1,6,10 These infections result in bronchitis, bronchiolitis, and pneumonia.7 The anatomical breakdown of an upper vs. lower respiratory tract infection is detailed in Figure 1.
Figure 1. Anatomical Structures in the Respiratory System |
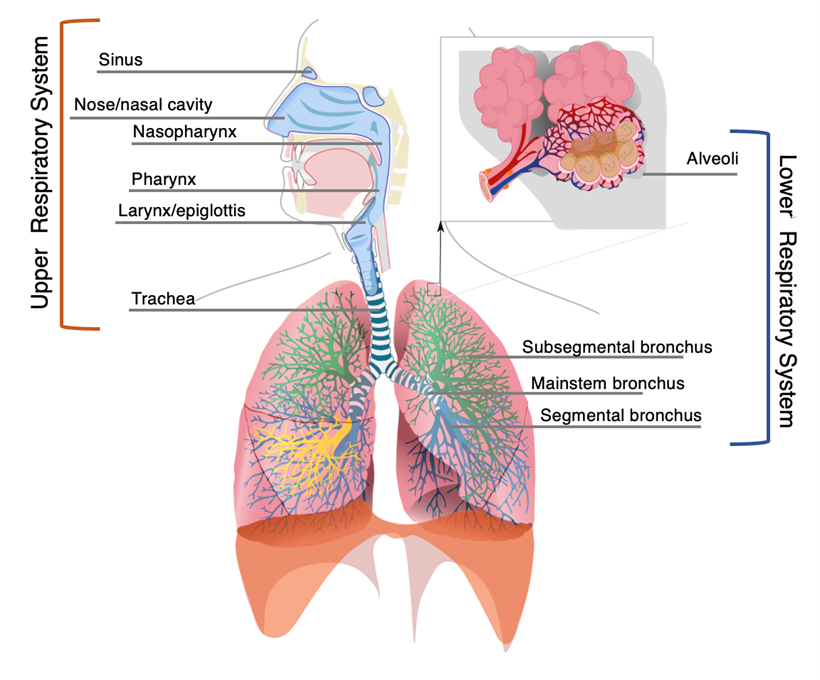 |
Adapted from: Respiratory system. Published Dec. 13, 2007. https://commons.wikimedia.org/wiki/Category:Respiratory_system#/media/File:Respiratory_system_complete_en.svg |
RSV accounts for nearly 80,000 hospitalizations and more than 2 million outpatient visits per year in the United States, which alone sets it apart from other viral illnesses in children.3,4,11 Many studies have demonstrated that RSV remains the most common cause of lower respiratory illness in children younger than 1 year old, accounts for nearly 20% of all outpatient visits for acute respiratory infection, and is isolated in about 31% of cases of all pediatric patients admitted for severe pneumonia.3-5,11,12 Some studies indicate that RSV accounts for about 7% of all pediatric deaths between 1 month and 1 year of age.13
RSV exhibits a unique seasonal pattern compared to other viruses that has been well-described thanks to the monitoring and reporting efforts of the Centers for Disease Control and Prevention (CDC).4 The commonly accepted RSV season has been described as mid-October through mid-May.4,14-16 This season not only indicates a higher prevalence of disease, but also increased severity in patients testing positive for RSV.15 Worldwide, RSV tends to peak in months that are colder or with more precipitation relative to the year-round climate, since this causes people to stay indoors, where transmission is easier and causes greater accumulations of infective droplets compared to the drier months.16-18 Notably, in equatorial countries and territories, RSV lacks a specific season but rather spreads endemically year-round.16,19
Interestingly, during the recent COVID-19 pandemic, the typical RSV season ended sooner than expected in the United States, and the following expected RSV season leading into 2021 was notably devoid of nearly all cases.20-22 A plethora of nonpharmacological interventions (NPIs) were implemented to help decrease the spread of COVID-19, including mask-wearing, social distancing, school and daycare closures, and frequent hand hygiene.19,20 These methods to decrease the spread of COVID-19 inadvertently decreased the spread of other viral illnesses as well, including RSV.18 These data make sense, given that COVID-19, influenza, RSV, and most other respiratory viruses are spread through droplets found in saliva and nasal secretions, so attempts to decrease the spread of these droplets affect multiple viral strains. Data from 2020 and 2021 reveal a marked decrease in incidence, prevalence, morbidity, and mortality from RSV, but subsequently led to an off-season spike in RSV cases at an atypical time.18,20-25 Between 90% and 99% of cases were eliminated in many areas worldwide by NPIs, but since these NPIs have eased, RSV outbreaks have returned to their normal patterns.20,22,25 The atypical epidemic pattern spanning from 2019 to 2022 is detailed in Figure 2, adapted from Hamid et al, who compiled the data from the CDC’s Morbidity and Mortality Weekly Report, where they track case numbers and patterns for multiple pathogens, including RSV.22 These data suggest that NPIs are highly effective in reducing the spread of infective droplets of multiple respiratory viruses, RSV notwithstanding, but these data are better described outside of this review.20-24
Figure 2. Yearly RSV-Positive PCR Tests in the United States, 2017-2023 |
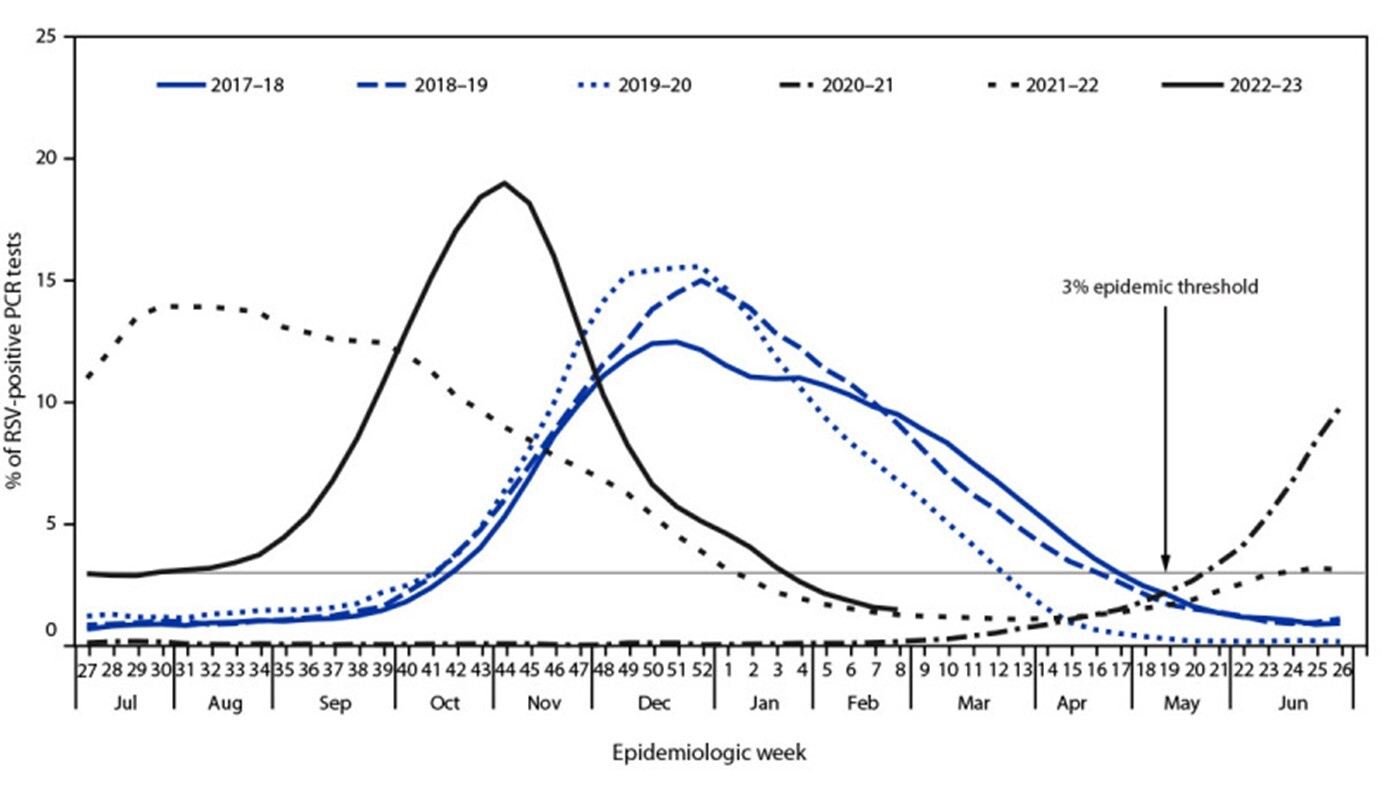 |
RSV: respiratory syncytial virus; PCR: polymerase chain reaction Reprinted from Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus — United States, 2017-2023. MMWR Morb Mortal Wkly Rep 2023;72:355-361. |
Certain patient populations also are at increased risk for RSV infection compared to others. There is a clear correlation between increased risk of RSV infection and morbidity in pediatric patients who were born premature, who have underlying lung disease, who have congenital heart disease, who are immunocompromised, or who are oncology patients.4,26,27 There also appears to be an increased risk of death in these high-risk groups if they are hospitalized with RSV infection.28 Age itself also is considered an independent risk factor, mainly if the patient is younger than 6 months old.3,4,27,29 There is a bimodal distribution in how morbid RSV is based on the patient’s age. The scope of this review covers pediatric patients, but it should be noted that patients older than 5 years of age and adults younger than 50 years of age get infected at similar rates. That means the overall incidence is similar, but the manifestations of disease are more severe in the very young and the elderly patient populations.3,4,30,31 Data and information reviewing the effects and presentations of RSV in the elderly are outside the scope of this review.
Pathophysiology
RSV is spread by infected respiratory droplets that inoculate the new host through the nasal or ocular mucus membranes.32 Generally, any droplets in the oropharynx are not considered adequate to lead to infection of the new host.32 Viral particles can survive on surfaces for many hours, making cleansing surfaces and hands essential to help decrease spread.8 After the viral particles enter the mucosa, the virus incubates and replicates for four to five days on average, then proceeds to the lung tissue through aspiration of secretions or ciliated respiratory epithelium.4,33,34 The direct invasion of these viral particles elicits an innate immune response mediated by various toll-like receptors (TLRs) and chemokines described elsewhere, which then cause destruction of epithelium in the lower lung.33-37
This destruction of respiratory epithelium, regardless of whether it is induced by the inflammatory response or the apoptosis induced by viral replication, is what causes the hallmark features of RSV.4,36-38 Decreased permeability and inflammation of the lower airways decreases the diameter of usable airway space used for ventilation, highlighted in the image at https://bit.ly/3oTxx4m. This increase in resistance also causes collapse in alveoli, which results in decreased usable lung tissue for ventilation.
Simultaneously, some alveoli become over-distended (air-trapping), which limits gas exchange. The decreased ability for ventilation triggers tachypnea as the patient tries to compensate, breathing faster to achieve the same amount of gas exchange with only the usable alveoli. Because the smaller airways have a decreased luminal radius, bronchiolitis frequently causes wheezing, which also is seen in obstructive lung disease pathology like asthma.37,39,40 For this reason, some providers will use bronchodilators to help with bronchiolitis, albeit with mixed results, which will be discussed more in-depth later in this review.37,39 Bronchiolitis is caused by a variety of viruses, including RSV, but it also can be seen in exposure to certain chemicals, chronic aspiration, hypersensitivity reaction to allergens, adverse effect of medication, and as a result of lung transplant. These other etiologies of bronchiolitis are seen more often in adults.40,41 This review focuses on RSV, but it should be noted that a clinical diagnosis of bronchiolitis may not be solely indicative of RSV infection, and other etiologies must be considered as well.40,41
Clinical Features
RSV infections, specifically those that result in symptomatic bronchiolitis, are primarily in children younger than the age of 2 years.39,42 If a child presents outside this normal age range, clinicians should consider alternative diagnosis, since RSV bronchiolitis does not normally cause severe symptomatic infection above this age.39,42 The usual course of this illness starts with symptoms of an upper respiratory tract infection, including rhinorrhea, cough, and conjunctivitis congestion, with or without a sore throat.4,39 Around day 3, symptoms progress as the lower airways start to be affected by the destruction of respiratory epithelium, detailed previously. Persistent cough and increased work of breathing are the hallmark findings in lower respiratory illness, often accompanied by fever.4,39,42,43 Increased work of breathing can manifest differently depending on the age of the patient, but it can include tachypnea, nasal flaring, intercostal or supraclavicular retractions, abdominal breathing, or grunting.4,39,42,43 Crackles and wheezing are both frequently heard on auscultation of the patient’s lungs.4,38
Apnea is a unique clinical feature of RSV bronchiolitis and can be the sole presenting symptom of infant patients with disease.42-44 Between 5% and 20% of infants younger than 6 months of age will have apnea associated with RSV infection.44,45 The mechanism behind apnea remains unclear, but the presence of apnea is well-described. Similar populations of patients are predisposed to apneic events during RSV infection and are correlated with higher morbidity and mortality from disease.44-46 For this reason, RSV has been implicated as a potential explanation for sudden infant death syndrome, since the apnea exhibited by these young patients can be severe enough to cause life-threatening hypoxia.4,44-47
Another hallmark clinical feature of bronchiolitis is the minute-to-minute variability in a patient’s examination.39 This can cause discrepancies between healthcare providers, leading to different treatment plans. The variability in exams often comes after suctioning, waxing/waning levels of agitation, or coughing fits because the patient can clear part of the mucus from their lower airway, freeing that functional lung unit to improve aeration and/or decrease adventitious breath sounds otherwise heard on auscultation.39 Frequent reassessment during a patient’s emergency department (ED) stay, strict return precautions, and appropriate caregiver teaching of signs and symptoms to look out for if a patient is discharged are of paramount importance to reduce any negative patient outcome that may result from inter-provider variability.
Diagnostic Studies
Interestingly, the American Academy of Pediatrics (AAP) and many other worldwide pediatric organizations, including the Canadian, United Kingdom, and Spanish equivalents, do not recommend routine RSV testing for the diagnosis of RSV bronchiolitis.39,42,48-50 In their clinical practice guidelines, these groups agree that the diagnosis of bronchiolitis is a clinical diagnosis and does not necessarily require testing.
Other governing bodies, like the Italian and Scottish groups, recommend testing for the purposes of inpatient cohorting.39,51,52 The patient’s history of present illness alone should be sufficient for clinicians to diagnose this disease process. Many clinicians will pursue testing if it will change the patient’s management — for example, if a positive RSV test will prevent the unnecessary administration of antibiotics or bronchodilators.4 It has become more routine during the years of the COVID pandemic to obtain testing for numerous viruses during acute illness for cohorting and provider/patient safety, with some studies indicating a five-fold increase in the number of tests performed.53 Additionally, although RSV testing is not required, most laboratories across the nation voluntarily participate in the CDC’s reporting system, initially established in the 1980s, which has been used to analyze seasonality, trends, and outcomes of children and adults across the nation.54
The role of imaging in the diagnosis of RSV bronchiolitis also is not routinely recommended by the aforementioned clinical practice guidelines.42,48-52 There has been no demonstrated benefit, and it does not alter clinical outcomes.55 Chest radiographs may even lead to an increase in inappropriate antibiotic administration.55,56 Imaging may be useful only if the child’s clinical course or severity may suggest a superimposed bacterial infection requiring antibiotic administration.57 This diagnosis is quite rare and is not easily excluded in the very ill patient, but it may be considered if the child becomes febrile after a prolonged initial illness course.57,58 Certain clinical findings, such as focal adventitious breath sounds or a new cardiac murmur, also may warrant investigation with a chest radiograph.42,57
Continuous pulse oximetry also should not be used routinely in the ED setting for the monitoring of this condition.39,42,48-52 Of course, any patient in extremis or who requires supplemental oxygen should be monitored per unit protocol and provider discretion, but, as discussed, frequent changes in the clinical features of the patient can yield vastly different pulse oximetry readings on a minute-to-minute basis and certainly can alter the course of the patient’s ED stay. This may prompt unnecessary admission depending on the cutoffs used in the ED.39,59 There are good data to support that regular pulse oximetry checks above 90% do not warrant admission and demonstrate no difference in adverse clinical outcomes.39,60 Additionally, there has been a decade-long trend indicating higher rates of admission for bronchiolitis, which may be explained by increased usage of continuous pulse oximetry.8,61,62
Routine blood work, which may include complete blood counts, blood cultures, erythrocyte sedimentation rate (ESR), urinalysis with culture, or C-reactive protein (CRP), are not recommended.39,42,48-52 Generally, clinicians assess laboratory values to help determine a disposition. For example, an elevated white blood cell count might indicate the presence of concomitant bacterial infection like lobar pneumonia. They may obtain blood cultures as a routine part of the febrile child workup for an institution, or they main obtain inflammatory markers because they may believe that elevated inflammatory markers correlate with the severity of disease. However, none of the mentioned tests have been clinically validated.
White blood cell counts are poorly correlated with serious bacterial infection or superinfection.39,63 CRP and ESR are elevated in a wide variety of conditions and are nonspecific regardless of value. Concomitant bacteremia has an estimated rate of less than 0.1% occurrence when a patient has RSV bronchiolitis, making the assessment of blood cultures more likely to false-positive from contamination than to represent a true positive.39,64,65
Multiple studies have looked at the utility of routine urinalyses in patients admitted with RSV bronchiolitis and concluded that routine testing is not necessary given the low incidence.66 There have been different recommendations made, and there have been a non-negligible incidence of positive urine cultures in patients admitted with RSV, but these studies were primarily done in 0- to 90-day-old infants.66-68 The incidence of concurrent urinary tract infection is between 1% and 4% in patients admitted with RSV bronchiolitis, but a lack of homogeneity in defining a urinary tract infection limits the comparability between studies.69 Regardless of the incidence, testing for concomitant serious bacterial infection with the aforementioned laboratory analysis should follow the AAP’s febrile infant guidelines, especially in patients younger than 60 days of age.70 Patients in that age range should, by these guidelines, have testing of the urine, blood work including cultures, a chest X-ray, and consideration for a lumbar puncture. There may be utility in these evaluations in other special populations or by clinician gestalt, but these are not routinely recommended to be obtained in all patients simply because of RSV infection. The use of continuous pulse oximetry, obtaining laboratory workup, and RSV viral testing, along with common treatments used in RSV bronchiolitis, are summarized in Table 1.
Table 1. Common Diagnostic Tests and Treatments for RSV Bronchiolitis8,39,42,48-52,59-62 | |||
| Test/Treatment | Recommended by AAP? | When to Consider | |
Testing/diagnosis | Chest X-ray | Not routinely | Persistent focal crackles, temperature > 39.0°C |
RSV viral testing | Not routinely | Cohorting patients during an endemic outbreak; any inpatient admission | |
Continuous pulse oximetry | No (spot checks recommended in all patients) | ICU admission or impending respiratory failure | |
CBC/blood cultures | No | Strong suspicion for concomitant bacterial superinfection or in patients younger than 60 days of age | |
Treatment | Bronchodilators (beta-agonist) | No | Strong history of asthma or previously documented wheezing-associated respiratory illness responsive to bronchodilators |
Steroids | No | ||
Nebulized hypertonic saline | No | Long-stay ICU patients | |
RSV: respiratory syncytial virus; AAP: American Academy of Pediatrics; ICU: intensive care unit; CBC: complete blood count | |||
Differential Diagnosis
Summarizing the previous section on presenting symptoms, RSV typically has a few days of cough, coryza, congestion, and possibly conjunctivitis, followed by the hallmark increased work of breathing after destruction/irritation of respiratory epithelium. Cough, coryza, and congestion with or without fever can be explained by virtually any upper or lower respiratory tract infection, including RSV, human metapneumovirus, rhinovirus, enterovirus, coronavirus, adenovirus, influenza, and parainfluenza.8 Lower respiratory disease, such as pneumonia, is thought to cause fever more commonly and should be considered as well. Although pneumonia can be caused by viruses, classic teaching lends consideration to bacterial causes as well. Clinicians also should consider rarer causes of these symptoms, such as foreign body aspiration, in the right clinical context and age group.
Depending on the age of the child, toxidromes also can present with tachypnea and/or fever. The absence of congestion, cough, or this typical viral prodrome should prompt consideration of cardiac causes (congenital cardiac abnormalities) or airway structural abnormalities (tracheoesophageal fistula, vascular ring, or tracheomalacia/bronchomalacia), especially in the neonatal period.39,57 Naturally, any respiratory infection also can cause an asthma exacerbation, which clinicians should consider if indicated by the patient’s history.
Management and Disposition
RSV bronchiolitis does not have a cure. However, discussing the management of bronchiolitis is most helpful when subdivided into three categories, which are summarized in Table 2. The well-appearing, nontoxic patient with only mild respiratory distress can be classified as mild bronchiolitis. This type of patient likely is in the outpatient or ED setting and only requires minimal intervention. There are some considerations for treatment in these patients, including antipyretics and nasal suctioning.
Table 2. Characteristics and Proposed Management of Patients with RSV Bronchiolitis8,39,59,74,75 | ||||||
| Mild Bronchiolitis | Moderate Bronchiolitis | Severe Bronchiolitis | ||||
Characteristics | Treatment | Characteristics | Treatment | Characteristics | Treatment | |
|
|
|
|
|
| |
Any patient being discharged home needs strict and detailed return precautions because of the labile nature of the RSV disease process. | Infants younger than 60 days old should be worked up and treated according to separate AAP guidelines. | |||||
RSV: respiratory syncytial virus; IV: intravenous; CPAP: continuous positive airway pressure; AAP: American Academy of Pediatrics | ||||||
Suctioning in the ED involves attaching a variety of attachments to suction tubing. Turning on the suction elicits negative pressure at the tip, which can be inserted into the anterior nares. There are various methods to suctioning based on the type of attachment, insertion depth (nasal suctioning, nasotracheal, and nasopharyngeal delineating the depth within the name), and whether the patient has any respiratory assist devices, such as a tracheostomy. Suctioning at home involves using a bulb syringe and positioning it in the nares, inserting it as far as it will go. Depressing the bulb creates a small vacuum that, when released, causes a low-strength suction of the anterior nares. Retracting the bulb while suctioning helps ensure clearing the nares of nasal secretions. This should be performed with the child in the sniffing position (slight neck extension and chin anterior).
Administering saline spray into the nares prior to suctioning also can improve the amount of secretion aspirated. Suctioning when the patient has the highest amount of respiratory distress, before feeds, and before sleep will give the patient the most amount of symptomatic relief.
Antipyretics should be administered to the febrile patient, particularly if the temperature is above 40.0°C, since controlling a patient’s fever also can improve a patient’s overall comfort level and work of breathing.71,72 Nasal suctioning does not have sufficient evidence to recommend it widely, but it generally is accepted as an effective and noninvasive way to clear upper airway secretions and improve a child’s work of breathing. In preparation for discharge, one of the most important management points of mild RSV bronchiolitis is return precautions and patient counseling. As discussed previously, RSV bronchiolitis is a labile and dynamic illness, and a parent or caregiver needs to know what physical exam findings may indicate decompensation and need for escalation of care.
Severe bronchiolitis also is straightforward to identify. The patient will be in clear respiratory distress with tachypnea often greater than 60 or 70 respirations per minute with increased accessory muscle use (subcostal, intercostal, supraclavicular retractions).8,57 They may be hypoxic or altered secondary to the impending respiratory failure.8,57
Supportive care remains the mainstay of treatment, but in cases of severe bronchiolitis, the patient most likely will require ventilatory support.8,73 High flow nasal cannula (HFNC) or continuous positive airway pressure (CPAP) are oft-used noninvasive ventilatory support options. They can deliver increased flow rates and increase the fraction of inspired oxygen in the hypoxic patient. If a patient does not improve on HFNC, they may require invasive ventilatory support with endotracheal intubation. Peri-intubation management is outside the scope of this review, but providers should take note of the minute ventilation of the patient and may want to match the patient’s minute ventilation with initial ventilatory settings to help prevent respiratory and cardiovascular collapse.
Moderate bronchiolitis may take some characteristics from both severe and mild bronchiolitis. The patient may have intermittent retractions or may be transiently hypoxic but may improve with coughing or suctioning, they may experience tachypnea when feeding, or they may be irritable.57 Providers should follow antipyretic use and nasal suctioning as with mild bronchiolitis, but they also may consider more controversial treatments. Bronchodilators, epinephrine, systemic glucocorticoids, antiviral treatment like ribavirin, or nebulized hypertonic saline are common treatments, but none have been shown to improve outcomes.8,39,57,74,75 These treatments will be discussed later in this review, but it is important to note that none should be used as a rule — they may be tried in select cases based on a provider’s discretion.
Treatment and disposition of patients with RSV bronchiolitis largely are provider- and institution-dependent. Some providers may try bronchodilators, especially if the child has wheezing. The use of bronchodilators in RSV bronchiolitis has been studied widely and is not recommended by the AAP or other governing bodies in other similar countries.39,42,48-52 They do not improve oxygenation and do not reduce overall length of stay.8,57,74 A recent Cochrane review (published by Gadomski et al) of multiple randomized controlled trials did not show any difference in rate of admission, time to resolution of illness, or duration of hospitalization in the bronchodilator group compared to placebo.74 This provides strong evidence against using bronchodilators, but, given their safety profile, some providers may consider trialing their use in cases of severe bronchiolitis with impending respiratory failure in an attempt to stave off intubation or in select patients with a long-documented history of asthma, reactive airway disease, or a previous positive response to bronchodilators.8
Glucocorticoids (including prednisolone and dexamethasone), when pooled in a meta-analysis, were not shown to reduce the length of stay or duration of mechanical ventilation across multiple studies.76 However, there are small studies that show small benefits of glucocorticoid use in length of stay and possibly prevention of admission in moderate cases of RSV bronchiolitis.77,78
Nebulized hypertonic saline is not recommended in the ED because of limitations on the duration of therapy inherent to the treatment location.39,42,48 There have been studies on nebulized hypertonic saline in patients who are admitted to the intensive care unit (ICU) for RSV bronchiolitis that showed a hint of reduction in length of stay, but meta-analyses have failed to show benefit. Therefore, nebulized hypertonic saline is not routinely recommended by any of the previously mentioned pediatric societies, with the exception of Spain’s 2010 recommendations.39,42,48-52
Antivirals like ribavirin also have been studied with conflicting results. Smaller studies, when pooled, show a trend toward decreased length of stay and decreased ventilator days, but others highlight the barriers of availability, cost of administration, particularly with inhaled ribavirin, and stewardship considerations of antiviral administration for such a common disease.8,57,76,79-81 Ribavirin also is a teratogen, precluding its use in pregnant females and males whose partner is pregnant. There also are data suggesting that it can cause bronchoconstriction, cough, or dyspnea as adverse effects in some patients.82 Regardless, it is a Food and Drug Administration (FDA)-approved treatment for RSV bronchiolitis in immunocompromised patients.82 Other treatments, including surfactant, heliox, leukotriene inhibitors, epinephrine, vitamin A, and immune globulin, have failed to show a significant benefit for widespread use.8,76
Palivizumab and RSV-IGIV (Respigam)
These two treatments are RSV-specific and do not apply to other causes of bronchiolitis. RSV immunoglobulin (RSV-IGIV, trade name Respigam) was developed in the late 1980s to reduce RSV infections, given the seasonality witnessed by clinicians and researchers. In-vivo studies revealed that it prevented RSV replication after inoculation without any clinical features of the disease.83 Clinical trials showed decreased hospitalization and decreased lengths of stay, and it was approved by the FDA for use in immunocompromised patients.83-85 Later studies questioned the efficacy of RSV-IGIV and showed conflicting results and that there was a high cost associated with immunoglobulin administration.1,83 Eventually, RSV-IGIV was removed from the treatment/prevention arsenal after palivizumab was developed because palivizumab was much more effective with the same outcome measures.83
Palivizumab is a monoclonal antibody licensed in 1998 after studies showed an approximately 50% decrease in hospitalization rate after regular treatments with the monoclonal antibody.4,86,87 There also was a decrease in hospital length of stay, days requiring oxygen, and a decreased rate of admission to the ICU, which was confirmed by a recent meta-analysis by the Cochrane Review group.86-88 It is recommended by the AAP to be given only to certain at-risk populations, including those with hemodynamically significant congenital heart and lung disease (formerly called bronchopulmonary dysplasia) and patients who were born prematurely (defined as less than 32 weeks’ gestation), since these groups have demonstrated more than a three-fold increase in mortality from an acute RSV infection.4,76,86-89
However, there is some discussion over the cost-effectiveness of palivizumab. Some studies quote a cost of $12,000 per hospitalization averted and $33,000 per life-year saved with a number needed to treat to avoid one hospitalization of 7.4.90,91 Interestingly, groups from other countries have performed similar analyses and reached different conclusions, including expanding the role of palivizumab use to a gestational age of 35 weeks or younger.90,92 In examination of these studies, the conclusion of “cost-effectiveness” differs by the actual dollar amount assigned to what is cost effective per life-year saved. The cost of palivizumab was expected to decrease with the expiration of the patent in 2015, but no similar products have been made commercially available at the time of this review. The ethics of assigning a dollar amount to a year of life saved for a child is certainly worth discussing on a larger platform, particularly that this differs based on the cost of healthcare in various countries.
Complications
Generally, RSV infection is self-limited, requires no antibiotic treatment, and is managed with supportive care. The morbidity and mortality of this disease have been discussed. However, there are many studies that correlate early childhood RSV infection with an increased risk of obstructive lung disease both in the pediatric population and as an adult.93-95 RSV infection was found to sensitize the innate immune system for the development of allergy and asthma by about twofold compared to controls.94,95 However, given that, epidemiologically, the vast majority of children are infected with RSV at some point before the age of 2 years, this clearly is not a linear correlation, or there would be a similar number of children with asthma. Although asthma is not uncommon, its incidence is less than the incidence of pediatric RSV infection. In immunocompromised patients, RSV infection, especially if recurrent, can lead to tracheobronchitis and long-term pulmonary dysfunction.4,96,97
Future Directions/Vaccine Development
The structure and identity of the virus have been known for more than 60 years, and it exhibits low antigenic variation, both of which portend a good candidate for vaccine development.98 Given the high morbidity and mortality associated with this disease and the total healthcare expenditures across the nation regarding management and testing, a vaccine to prevent RSV infection seems prudent. Groups have been working on an effective and safe vaccine for about 60 years with mixed results.
The first vaccines to be developed in the 1960s were administered to uninfected children, but when they became infected, they had worse outcomes (including deaths) compared to nonvaccinated children.99,100 This unexpected and unfortunate result halted efforts of vaccine development and highlighted the need for safety, given the proposed patient population. Today, multiple types of vaccines against RSV are in development, including live-attenuated, protein-based, mRNA, and other nucleic acid vaccines all are making headway, including a bivalent protein-based vaccine that is supposed to confer immunity to RSV and human metapneumovirus.98,99,101 Meanwhile, the FDA approved an RSV vaccine for older adults in May, marking a momentous step forward for prevention in that patient population.102
Interestingly, since the most at risk populations for severe RSV infection include children younger than 6 months of age, one of the novel vaccine approaches attempts to achieve temporary immunity through maternal vaccination, since immunoglobulin G antibodies are passed through breast milk.99 In all, 144 current or recently completed clinical trials are registered worldwide for RSV vaccine development, giving hope for protection from this illness in the near future.91
Summary
RSV infection, usually manifesting as bronchiolitis, is the most important cause of lower respiratory tract infection in the pediatric population. Understanding its clinical course, lack of demonstrable benefit of many commonly used treatments, and identifying at-risk populations for severe disease are essential for any pediatric emergency medicine provider. Treatments have mixed results, but there are some strategies for treatment and prevention that are available for the highest risk populations. Through robust clinical trials across the world, there is a high likelihood of decreasing the healthcare burden of this disease in the near future.
References
- Domachowske JB, Rosenberg HF. Respiratory syncytial virus infection: Immune response, immunopathogenesis, and treatment. Clin Microbiol Rev 1999;12:298-309.
- Welliver RC. Respiratory syncytial virus and other respiratory viruses. Pediatr Infect Dis J 2003;22(2 Suppl):S6-S10; discussion S10-S12.
- Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588-598.
- Barr FE, Graham BS. Respiratory syncytial virus infection: Clinical features and diagnosis. UpToDate. Updated May 22, 2023. https://www.uptodate.com/contents/respiratory-syncytial-virus-infection-clinical-features-and-diagnosis
- Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019;8:284-286.
- Thomas M, Bomar PA. Upper respiratory tract infection. StatPearls [Internet]. Updated June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK532961/
- Dasaraju PV, Liu C. Infections of the respiratory system. In: Baron S, ed. Medical Microbiology. 4th ed. University of Texas Medical Branch at Galveston; 1996. https://www.ncbi.nlm.nih.gov/books/NBK8142/
- Piedra PA, Stark AR. Bronchiolitis in infants and children: Treatment, outcome, and prevention. UpToDate. Updated July 19, 2022. https://medilib.ir/uptodate/show/6020
- Byington CL, Bradley JS. Pediatric community-acquired pneumonia. In: Cherry JD, Harrison GJ, Kaplan SL, et al, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 7th ed. Elsevier-Saunders;2014:283.
- Mahowald M, Shahan B, Forbes D. Respiratory conditions: Lower respiratory tract infections. FP Essent 2019;486:19-25.
- Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I, et al. Respiratory syncytial virus seasonality: A global overview. J Infect Dis 2018;217:1356-1364.
- Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019;394:757-779. [Erratum in: Lancet 2019;394:736].
- Shi T, McAllister DA, O’Brien KL, et al; RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017;390:946-958.
- Rose EB, Wheatley A, Langley G, et al. Respiratory syncytial virus seasonality - United States, 2014-2017. MMWR Morb Mortal Wkly Rep 2018;67:71-76.
- Staat MA, Henrickson K, Elhefni H, et al. Prevalence of respiratory syncytial virus-associated lower respiratory infection and apnea in infants presenting to the emergency department. Pediatr Infect Dis J 2013;32:911-914.
- Yu J, Liu C, Xiao Y, et al. Respiratory syncytial virus seasonality, Beijing, China, 2007-2015. Emerg Infect Dis 2019;25:1127-1135.
- Haynes AK, Manangan AP, Iwane MK, et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis 2013;208(Suppl 3):S246-S254.
- Di Mattia G, Nenna R, Mancino E, et al. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol 2021;56:3106-3109.
- Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: The ups and downs of a seasonal virus. Pediatr Infect Dis J 2003;22(2 Suppl):S21-S32.
- Sherman AC, Babiker A, Sieben AJ, et al. The effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mitigation strategies on seasonal respiratory viruses: A tale of 2 large metropolitan centers in the United States. Clin Infect Dis 2021;72:e154-e157.
- Yeoh DK, Foley DA, Minney-Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis 2021;72:2199-2202.
- Hamid S, Winn A, Parikh R, et al. Seasonality of respiratory syncytial virus — United States, 2017-2023. MMWR Morb Mortal Wkly Rep 2023;72:355-361.
- Kuitunen I, Artama M, Mäkelä L, et al. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J 2020;39:e423-e427.
- Eden JS, Sikazwe C, Xie R, et al; Australian RSV study group. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun 2022;13:2884.
- Binns E, Koenraads M, Hristeva L, et al. Influenza and respiratory syncytial virus during the COVID-19 pandemic: Time for a new paradigm? Pediatr Pulmonol 2022;57:38-42.
- Paes B, Fauroux B, Figueras-Aloy J, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with chronic lung disease. Infect Dis Ther 2016;5:453-471.
- Gijtenbeek RG, Kerstjens JM, Reijneveld SA, et al. RSV infection among children born moderately preterm in a community-based cohort. Eur J Pediatr 2015;174:435-442.
- Welliver RC Sr, Checchia PA, Bauman JH, et al. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin 2010;26:2175-2181.
- Meissner HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatr Infect Dis J 2003;22(2 Suppl):S40-S44; discussion S44-S45.
- Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev 2000;13:371-384.
- Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis 2013;57:1069-1077.
- Hall CB, Douglas RG Jr, Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect Immun 1981;33:779-783.
- Johnson JE, Gonzales RA, Olson SJ, et al. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007;20:108-119.
- Villenave R, Shields MD, Power UF. Respiratory syncytial virus interaction with human airway epithelium. Trends Microbiol 2013;21:238-244.
- Savón C, Goyenechea A, Valdés O, et al. Respiratory syncytial virus group A and B genotypes and disease severity among Cuban children. Arch Med Res 2006;37:543-547.
- Noah TL, Becker S. Chemokines in nasal secretions of normal adults experimentally infected with respiratory syncytial virus. Clin Immunol 2000;97:43-49.
- Ali S, Plint AC, Klassen TP. Bronchiolitis. In: Wilmott RW, Kendig EL, Boat TF, et al, eds. Kendig and Chernick's Disorders of the Respiratory Tract in Children. 8th ed. Elsevier-Saunders;2012:443-452.
- Hoffman SJ, Laham FR, Polack FP. Mechanisms of illness during respiratory syncytial virus infection: The lungs, the virus and the immune response. Microbes Infect 2004;6:767-772.
- Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. Lancet 2017;389:211-224.
- Ryu JH, Azadeh N, Samhouri B, Yi E. Recent advances in the understanding of bronchiolitis in adults. F1000Res 2020;9:F1000 Faculty Rev-568.
- Papiris SA, Malagari K, Manali ED, et al. Bronchiolitis: Adopting a unifying definition and a comprehensive etiological classification. Expert Rev Respir Med 2013;7:289-306.
- Bronchiolitis in children: Diagnosis and management. NICE. Updated Aug. 9, 2021. https://www.nice.org.uk/Guidance/NG9
- Anas N, Boettrich C, Hall CB, Brooks JG. The association of apnea and respiratory syncytial virus infection in infants. J Pediatr 1982;101:65-68.
- Bruhn FW, Mokrohisky ST, McIntosh K. Apnea associated with respiratory syncytial virus infection in young infants. J Pediatr 1977;90:382-386.
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543-546.
- Arms JL, Ortega H, Reid S. Chronological and clinical characteristics of apnea associated with respiratory syncytial virus infection: A retrospective case series. Clin Pediatr (Phila) 2008;47:953-938.
- Lindgren C, Jing L, Graham B, et al. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatr Res 1992;31(4 Pt 1):381-385.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014;134:e1474-e1502. [Erratum in: Pediatrics 2015;136:782].
- Friedman JN, Rieder MJ, Walton JM; Canadian Paediatric Society, Acute Care Committee, Drug Therapy and Hazardous Substances Committee. Bronchiolitis: Recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr Child Health 2014;19:485-498.
- Working Group of the Clinical Practice Guideline on Acute Bronchiolitis. Quality plan for the Spanish National Healthcare System of the Spanish Ministry for Health and Social Policy; Catalan Agency for Health Technology Assessment, 2010. Clinical Practice Guidelines in the Spanish National Healthcare System: CAHTA no. 2007/05.
- Scottish Intercollegiate Guidelines Network (SIGN). Bronchiolitis in Children: A National Clinical Guideline. Edinburgh; 2006.
- Baraldi E, Lanari M, Manzoni P, et al. Inter-society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr 2014;40:65.
- Stamm P, Sagoschen I, Weise K, et al. Influenza and RSV incidence during COVID-19 pandemic-an observational study from in-hospital point-of-care testing. Med Microbiol Immunol 2021;210:277-282.
- Centers for Disease Control and Prevention. Respiratory syncytial virus infection (RSV). Updated Oct. 28, 2022. https://www.cdc.gov/rsv/research/index.html
- Bordley WC, Viswanathan M, King VJ, et al. Diagnosis and testing in bronchiolitis: A systematic review. Arch Pediatr Adolesc Med 2004;158:119-126.
- Piedra PA, Stark AR. Bronchiolitis in infants and children: Clinical features and diagnosis. UpToDate. Updated Feb. 17, 2022. https://medilib.ir/uptodate/show/6018
- Fitzgerald DA, Kilham HA. Bronchiolitis: Assessment and evidence-based management. Med J Aust 2004;180:399-404.
- Friis B, Andersen P, Brenoe E, et al. Antibiotic treatment of pneumonia and bronchiolitis. A prospective randomised study. Arch Dis Child 1984;59:1038-1045.
- Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: A randomized clinical trial. JAMA 2014;312:712-718.
- Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): A double-blind, randomised, equivalence trial. Lancet 2015;386:1041-1048.
- Mallory MD, Shay DK, Garrett J, Bordley WC. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics 2003;111:e45-e51.
- Schuh S, Freedman S, Coates A, et al. Effect of oximetry on hospitalization in bronchiolitis: A randomized clinical trial. JAMA 2014;312:712-718.
- Purcell K, Fergie J. Lack of usefulness of an abnormal white blood cell count for predicting a concurrent serious bacterial infection in infants and young children hospitalized with respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J 2007;26:311-315.
- Librizzi J, McCulloh R, Koehn K, Alverson B. Appropriateness of testing for serious bacterial infection in children hospitalized with bronchiolitis. Hosp Pediatr 2014;4:33-38.
- Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis a systematic review. Arch Pediatr Adolesc Med 2011;165:951-956.
- Hendaus MA. Why are children with bronchiolitis at risk of urinary tract infections? Risk Manag Healthc Policy 2019;12:251-254.
- Titus MO, Wright SW. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics 2003;112:282-284.
- Hendaus MA, Alhammadi AH, Khalifa MS, et al. Risk of urinary tract infection in infants and children with acute bronchiolitis. Paediatr Child Health 2015;20:e25-e29.
- Purcell K, Fergie J. Concurrent serious bacterial infections in 2,396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med 2002;156:322-324.
- Pantell RH, Roberts KB, Adams WG, et al; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8 to 60 days old. Pediatrics 2021;148:e2021052228. [Erratum in: Pediatrics 2021;148:e2021054063].
- El-Radhi A, Barry W, Patel S. Association of fever and severe clinical course in bronchiolitis. Arch Dis Child 1999;81:231-234.
- El-Radhi ASM. Fever management: Evidence vs current practice. World J Clin Pediatr 2012;1:29-33.
- Sinha IP, McBride AKS, Smith R, Fernandes RM. CPAP and high-flow nasal cannula oxygen in bronchiolitis. Chest 2015;148:810-823.
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev 2014;2014:CD001266.
- Quinonez RA, Garber MD, Schroeder AR, et al. Choosing wisely in pediatric hospital medicine: Five opportunities for improved healthcare value. J Hosp Med 2013;8:479-485.
- Davison C, Ventre KM, Luchetti M, Randolph AG. Efficacy of interventions for bronchiolitis in critically ill infants: A systematic review and meta-analysis. Pediatr Crit Care Med 2004;5:482-489.
- Schuh S, Coates AL, Binnie R, et al. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. J Pediatr 2002;140:27-32.
- van Woensel JBM, van Aalderen WMC, de Weerd W, et al. Dexamethasone for treatment of patients mechanically ventilated for lower respiratory tract infection caused by respiratory syncytial virus. Thorax 2003;58:383-387.
- Randolph AG, Wang EE. Ribavirin for respiratory syncytial virus infection of the lower respiratory tract. Cochrane Database Syst Rev 2000;2:CD000181.
- Law BJ, Wang EE, MacDonald N, et al. Does ribavirin impact on the hospital course of children with respiratory syncytial virus (RSV) infection? An analysis using the pediatric investigators collaborative network on infections in Canada (PICNIC) RSV database. Pediatrics 1997;99:E7.
- Pelaez A, Lyon GM, Force SD, et al. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J Heart Lung Transplant 2009;28:67-71.
- Barr FE, Graham BS. Respiratory syncytial virus: Treatment. UpToDate. Updated April 22, 2022. https://www.uptodate.com/contents/respiratory-syncytial-virus-infection-treatment
- Wu H, Pfarr DS, Losonsky GA, Kiener PA. Immunoprophylaxis of RSV infection: Advancing from RSV-IGIV to palivizumab and motavizumab. Curr Top Microbiol Immunol 2008;317:103-123.
- Groothuis JR, Simoes EA, Levin MJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 1993;329:1524-1530.
- [No authors listed]. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics 1997;99:93-99.
- Meissner HC, Long SS; American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003;112(6 Pt 1):1447-1452.
- [No authors listed]. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102(3 Pt 1):531-537.
- Andabaka T, Nickerson JW, Rojas-Reyes MX, et al. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 2013;4:CD006602.
- Scott LJ, Lamb HM. Palivizumab. Drugs 1999;58:305-311; discussion 312-313.
- Lanctôt KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: A Canadian-based analysis. Curr Med Res Opin 2008;24:3223-3237.
- Wang D, Cummins C, Bayliss S, et al. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: A systematic review and economic evaluation. Health Technol Assess 2008;12:iii, ix-x, 1-86.
- Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: A UK analysis. Pharmacoeconomics 2007;25:55-71.
- Berry CE, Billheimer D, Jenkins IC, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med 2016;194:607-612.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000;161:1501-1507.
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137-141.
- Hertz MI, Englund JA, Snover D, et al. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: A clinical approach and review of the literature. Medicine (Baltimore) 1989;68:269-281.
- Whimbey E, Champlin RE, Englund JA, et al. Combination therapy with aerosolized ribavirin and intravenous immunoglobulin for respiratory syncytial virus disease in adult bone marrow transplant recipients. Bone Marrow Transplant 1995;16:393-399.
- Graham BS. Vaccine development for respiratory syncytial virus. Curr Opin Virol 2017;23:107-112.
- Higgins D, Trujillo C, Keech C. Advances in RSV vaccine research and development — A global agenda. Vaccine 2016;34:2870-2875.
- Kapikian AZ, Mitchell RH, Chanock RM, et al. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol 1969;89:405-421.
- PATH. RSV Clinical Trial Tracker. Published January 2023. https://www.path.org/resources/rsv-and-mab-trial-tracker/
- Food and Drug Administration. FDA approves first respiratory syncytial virus (RSV) vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine
