Spontaneous Subarachnoid Hemorrhage
January 1, 2023
Related Articles
-
Echocardiographic Estimation of Left Atrial Pressure in Atrial Fibrillation Patients
-
Philadelphia Jury Awards $6.8M After Hospital Fails to Find Stomach Perforation
-
Pennsylvania Court Affirms $8 Million Verdict for Failure To Repair Uterine Artery
-
Older Physicians May Need Attention to Ensure Patient Safety
-
Documentation Huddles Improve Quality and Safety
AUTHORS
Caleb P. Canders, MD, Department of Emergency Medicine, UCLA Ronald Reagan Medical Center, Los Angeles, CA
Michelle P. Choi, MD, Department of Emergency Medicine, UCLA Ronald Reagan Medical Center, Los Angeles, CA
Jackson D. Kloor, MD, Department of Emergency Medicine, UCLA Ronald Reagan Medical Center, Los Angeles, CA
PEER REVIEWER
Catherine A. Marco, MD, FACEP, Professor, Department of Emergency Medicine, Penn State Health – Milton S. Hershey Medical Center, Penn State College of Medicine
EXECUTIVE SUMMARY
- Spontaneous subarachnoid hemorrhage (SAH) accounts for about 1% or less of acute headache presentations to the emergency department (ED).
- Only half of SAH patients present describing the classic thunderclap headache.
- The Ottawa Subarachnoid Hemorrhage Rule has essentially 100% sensitivity and can be used to rule out SAH in a portion of ED patients presenting with headache.
- Modern multidetector computed tomography (CT) scanners, with cuts < 5 mm, detect SAH with 100% sensitivity, if obtained within six hours of headache onset.
- CT angiography (CTA) is 100% specific and 98% sensitive for the detection of cerebral aneurysms > 3 mm.
- The three key treatments for SAH in the ED are to elevate the head of the bed 30-45 degrees to prevent elevated intracranial pressure, use titratable intravenous antihypertensives to reduce systolic blood pressure below 160 mmHg, and to reverse antiplatelet and anticoagulant medications.
- About 15% of patients with SAH expire before reaching the hospital, and of those who do reach the hospital, 25% will die during the first 24 hours and a total of 50% will die by six months.
Introduction
Headache is the sixth most common chief complaint in the emergency department (ED), accounting for 2% to 3% of all visits and 5 million visits annually.1-5 Migraines and other benign conditions are responsible for most headaches.5 However, spontaneous subarachnoid hemorrhage (SAH), most commonly due to bleeding from cerebral aneurysms, accounts for up to 1% of headaches in ED patients.6 Considering its 50% mortality rate at six months, diagnosing and treating aneurysmal SAH is therefore essential.6-9
However, as a result of atypical presentations, diagnosis of aneurysmal SAH often is delayed or missed in the ED.10,11 Patients who complain of sudden severe headaches and have altered consciousness, focal neurologic deficits, or meningismus are likely to receive head imaging. In contrast, patients who are neurologically intact often pose diagnostic challenges. The complaint of “thunderclap headache” is neither specific nor sensitive for SAH, and approximately half of patients with SAH initially have normal neurologic examinations.8,12
Clinical decision-making tools with high sensitivity for ruling out SAH have been developed but lack specificity. As a result, to prevent the overuse of imaging studies and other healthcare resources, emergency providers must decide which of the patients who screen “positive” with the tools are truly suspected of having SAH.1,7,10,13,14
This paper aims to review the typical and atypical presentations of spontaneous SAH and its complications. In addition, this article will discuss how two recently developed, externally validated decision-making rules, the Ottawa Subarachnoid Hemorrhage Rule and 6-hour CT rule, can help to guide the diagnostic approach to patients presenting with thunderclap headaches. Management of spontaneous SAH and its complications, with a focus on treatment in the emergency setting, also will be discussed.
Pathophysiology and Epidemiology
Most (85%) spontaneous SAH is caused by bleeding from cerebral aneurysms, which form as weakened portions of the arterial wall bulge into the arachnoid space.8-10,15 Most cerebral aneurysms are saccular, occur in the anterior circulation, and never rupture.8,15 Half of aneurysmal SAH occurs in patients younger than 55 years of age, leading to a disproportionate increase in years of productive life lost compared to ischemic strokes, which typically occur in older patients.7,16,17
The incidence of aneurysmal SAH, estimated to be seven to 15 people per 100,000, recently has been decreasing worldwide, likely as a result of decreased tobacco use and improved blood pressure management.16,18,19 Countries with high rates of smoking, such as Japan, however, continue to experience an increasing incidence of SAH.16
Risk Factors for Aneurysmal SAH
Approximately 20% of patients with aneurysmal SAH report a family history of cerebral aneurysms; thus, most cerebral aneurysms are not familial.8,9 Modifiable risk factors for aneurysmal SAH include preexisting hypertension (present in 25% of SAH patients), heavy alcohol intake, and the use of tobacco or sympathomimetic drugs.7,8,20 Some genetic conditions, such as polycystic kidney disease and Ehlers-Danlos syndrome, also are associated with cerebral aneurysm formation.21
Gender
The incidence of unruptured intracranial aneurysms is higher in women, who also are more likely to have multiple aneurysms.9,18,22,23 Aneurysms are more likely to form and progress in women after the onset of menopause.24 Gender differences in aneurysm formation are thought to be related to hormonal influences on vascular remodeling, hemodynamics, and inflammation.9,25,26 However, there are inconsistent data on the influence of gender on the risk of aneurysm rupture or functional outcomes after SAH.9,27,28
Aspirin Use
The role of aspirin use in the development and rupture of cerebral aneurysms is not fully understood. Some studies have demonstrated a decreased risk of aneurysm formation and growth in individuals who take aspirin daily, thought to be because of its anti-inflammatory effects.29-32 Upon aneurysmal rupture, antecedent aspirin use is not associated with worse outcomes or higher morality in SAH.29 However, aspirin use has been shown to increase the risk of rebleeding and should not be administered after patients are diagnosed with ruptured cerebral aneurysms.29
Presentation
Thunderclap Headache
Approximately 70% of patients with SAH only complain of headaches.12 Sentinel headaches, caused by the transient leakage of blood from cerebral aneurysms, can last hours to days and may occur weeks before an aneurysm ruptures.8,12 In 30% of patients, the headache is unilateral.8
The classic thunderclap headache, described as an acute-onset headache that rapidly reaches maximum severity (typically within one hour), occurs in only half of SAH patients (i.e., low sensitivity).12,33,34 In addition, less than 10% of thunderclap headaches are caused by aneurysmal SAH (i.e., low specificity).21,35 Table 1 lists the differential diagnosis for patients presenting with thunderclap headaches.
Table 1. Conditions Associated with Thunderclap Headaches |
|
Other Nonspecific Symptoms
Nausea and vomiting are common in patients with SAH.8 At headache onset, up to 50% of patients lose consciousness and 20% have a seizure.8 Contrary to traditional teaching, patients with aneurysmal SAH may present with headaches that are mild, occur after non-strenuous activities, and resolve with nonopioid analgesics.33
Physical Examination
Initial neurologic examination at the time of SAH diagnosis is the most important early predictor of mortality and functional outcomes.36 Therefore, it is important for emergency providers to document thorough physical examinations, including the Glasgow Coma Scale (GCS), on patients with SAH, as this information is incorporated into grading systems and is predictive of outcomes.37 (See Table 2.)
Table 2. Subarachnoid Hemorrhage Grading Systems Based on Initial Emergency Department Presentations8,17,38 |
In both scales, higher grades are associated with worse functional outcomes. |
World Federation of Neurologic Surgeons (WFNS) scale Grade 1: GCS 15, no motor deficits Grade 2: GCS 13-14, no motor deficits Grade 3: GCS 13-14, motor deficit present Grade 4: GCS 7-12 Grade 5: GCS 3-6 |
Hunt and Hess Severity scale Grade 1: mild headache or asymptomatic Grade 2: moderate/severe headache and no focal deficits except for cranial nerve palsy Grade 3: drowsiness/confusion or mild focal neurologic deficit Grade 4: stupor, moderate/severe hemiparesis, or early decerebrate posturing Grade 5: comatose or decerebrate posturing |
GCS: Glasgow Coma Scale |
Altered consciousness or focal neurologic deficits at presentation, seen in half of the patients with SAH, are associated with high mortality rates.8,36 Meningismus, characterized by neck pain and stiffness caused by irritation from blood in the subarachnoid space, occurs in 35% of patients, although it may take hours to develop.8 Retinal hemorrhages occur in 10% of SAH patients.7
Diagnosis
Missed or delayed diagnosis occurs in 25% to 50% of patients with aneurysmal SAH and is associated with worse outcomes.10,11 It is more common in patients who initially present with mild symptoms and unremarkable neurologic examinations.11 Given the serious consequences of missing SAH, clinical decision-making rules have been created to help emergency providers rule out SAH with high sensitivity.
Ottawa Subarachnoid Hemorrhage Rule
The Ottawa Subarachnoid Hemorrhage Rule is a set of six criteria that, if absent, rule out SAH with 100% sensitivity in patients with headaches, without the need for computed tomography (CT) imaging.39 (See Table 3.) If any of the rule’s six criteria are present, then SAH cannot be ruled out. Numerous studies have demonstrated the rule’s 100% sensitivity, with only one study, performed in a Chinese population, showing a lower (94%) sensitivity.39-45
Table 3. Ottawa Subarachnoid Hemorrhage Rule39 |
If any of the criteria are present, then subarachnoid hemorrhage cannot be ruled out without computed tomography imaging:
|
The rule can only be applied to alert, neurologically intact patients, ages 15 years or older, who lack preceding trauma or a history of a prior cerebral aneurysm, SAH, or brain tumor. Given the low specificity of the rule (7% to 13%), emergency providers must decide which of the many patients who screen “positive” are at enough risk of having an SAH to apply the rule and proceed with head imaging.39-44
Non-Contrast Head CT
Because of its high sensitivity and ready availability, non-contrast head CT is the most common imaging modality used in the ED to diagnose SAH.7 SAH appears as hyperdense material in the subarachnoid space on non-contrast CT. (See Figure 1A.) Given that most (70%) cerebral aneurysms occur at the anterior, middle, and posterior cerebral arteries, aneurysmal bleeding often surrounds the basal cisterns.7,21 (See Figure 1B.) With time, as blood proteins diffuse or become absorbed or degraded, SAH becomes isodense to brain tissue, and non-contrast head CT becomes less sensitive.7,14
Early generation, single-detector CT scanners were 93% sensitive for SAH.8,14 As a result, if clinical suspicion for SAH remained high after a negative head CT, a lumbar puncture (LP) was typically recommended.46 However, modern multidetector CT scanners, with cuts < 5 mm, detect SAH with 100% sensitivity, if obtained within six hours of headache onset.6-8 Sensitivity decreases to 90% if the CT is obtained 24 hours after headache onset.8
Figure 1. Left Middle Cerebral Artery Aneurysm |
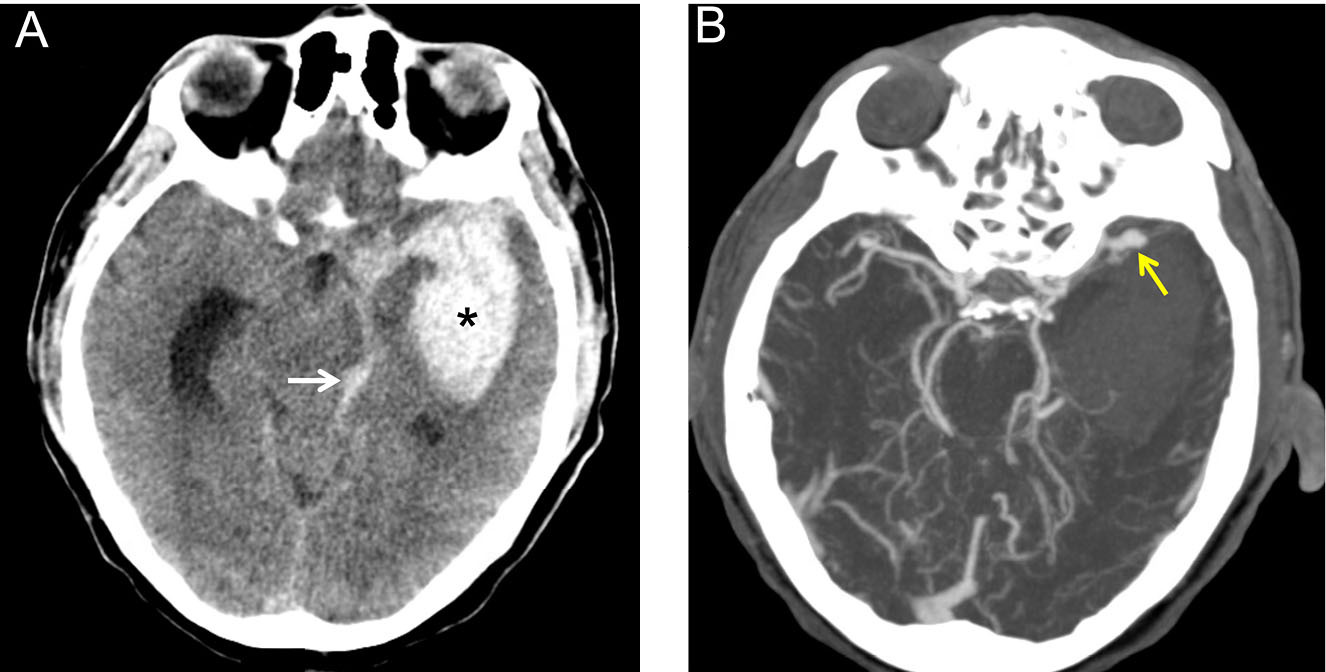 |
(A) Non-contrast head computed tomography of a 52-year-old man presenting with a thunderclap headache after jogging. Hyperdense blood (star) is seen in the left basal ganglia and internal capsule, with intraventricular extension (arrow). |
The diagnostic approach to SAH in the ED is thereby evolving, as CT technology advances and more is learned about its sensitivity at various time intervals.
Six-Hour CT Rule
If a patient presents within six hours of headache onset and has a normal head CT, the American College of Emergency Physicians (ACEP) recommends against further workup for SAH (level B evidence).14 Known as the “6-hour CT rule,” this recommendation assumes that the patient is neurologically intact, not anemic, and not pregnant or postpartum. In addition, the CT must be performed on a modern, multidetector CT and read by an experienced radiologist (level B evidence).8,14,47 Multiple neurologic and neurosurgical societies support ACEP’s recommendation.5 Factors that decrease the sensitivity of non-contrast CT and can lead to a false negative study are listed in Table 4.7,48
Table 4. Factors that Decrease the Sensitivity of Non-Contrast Computed Tomography in Detecting Subarachnoid Hemorrhage7 |
|
Lumbar Puncture
Analysis of cerebrospinal fluid (CSF) still plays a role in the diagnostic workup of many patients with thunderclap headaches, including those presenting more than six hours after headache onset.49-51 Xanthochromia, the yellow hue that results from hemoglobin breakdown in CSF, is diagnostic for SAH and can be detected six hours after headache onset and disappears after 12 hours.7,47,52,53 The presence of red blood cells (RBCs) in CSF is suggestive of SAH and can be detected as early as three hours after headache onset.
However, traumatic LPs, occurring in up to 30% of patients from local trauma or inadvertent puncture of the venous plexus during the procedure, also can contaminate the CSF with RBCs and cause a false positive.7,14 No evidence supports the theory that a decreasing number of RBCs observed in consecutively collected tubes (“RBC clearing”) is proof of a traumatic LP.7 Some literature does recommend continuing the diagnostic workup for SAH if the CSF in the fourth tube contains > 2,000 × 106 RBCs/L.7,53
In addition to the high rate of traumatic LPs, disadvantages of LPs include the risks of causing patient discomfort or complications that require additional invasive interventions (e.g., epidural hematomas, post-LP headaches). LPs also can be difficult to perform in certain patients (e.g., young, obese, or those with altered mental status).52
CT Angiography
If suspicion for SAH remains high after a normal non-contrast head CT, CT angiography (CTA) is another diagnostic study to consider. It is 100% specific and 98% sensitive for the detection of cerebral aneurysms > 3 mm.21,52 Sensitivity is lower for aneurysms < 3 mm, although most aneurysms do not rupture until > 5 mm.8 Given that CTA is rapid, noninvasive, and painless, it is preferred over LP by most patients.14 CTA also is able to evaluate for ischemic strokes and other causes of thunderclap headaches, such as cerebral venous thrombosis and arteriovenous malformations.54
However, CTA does not detect subarachnoid blood itself, which may lead to missed diagnosis in patients with non-aneurysmal SAH, such as perimesencephalic bleeding.17 In addition, CTA may detect incidental aneurysms, which are present in 2% to 3% of the general population.56 This may lead to increased patient anxiety and unnecessary follow-up testing and interventions.18,49,53,55 CTA also exposes patients to radiation and the risk, albeit low, of contrast-induced nephropathy.14
Additional Diagnostic Considerations
Other imaging studies, including magnetic resonance imaging and digital subtraction angiography (the criterion standard for diagnosis of cerebral aneurysms), are highly sensitive for SAH but not readily available or routinely performed in the ED.7,14 Transcranial Doppler sonography, although not typically performed in the ED, is commonly used to detect vasospasm in hospitalized patients with SAH.56 Research into the correlation of biomarkers, such as copeptin (a hypothalamic stress hormone), with spontaneous SAH has shown promising results but is not yet in clinical use.57
History of Unruptured Cerebral Aneurysms
Most incidentally detected cerebral aneurysms never rupture or cause symptoms.58
However, knowledge of their presence is associated with increased patient anxiety and may lead to unnecessary imaging performed for trivial symptoms out of precaution.59 As a general rule, any headaches with new or worsening features in patients with known cerebral aneurysms should be presumed to be aneurysmal SAH until proven otherwise.58,60
In addition, the presence of cranial nerve palsies (most commonly cranial nerve III), seizures, or focal neurologic deficits can result from the mass effects of expanding aneurysms (typically > 7 mm) and should be investigated.58,60 Given the risks of missing aneurysmal SAH, emergency providers should err on the side of caution and consider imaging in all patients with known cerebral aneurysms who present with headaches or neurologic symptoms.60
Complications of SAH
Spontaneous SAH causes early brain injury by increasing intracranial pressure, which leads to decreased cerebral perfusion pressure and cerebral ischemia.36 Subarachnoid blood itself also damages brain tissue.38 The acute complications that develop from aneurysmal SAH are responsible for a significant proportion of morbidity and mortality.
Rebleeding
Rebleeding occurs in 15% to 20% of patients with aneurysmal SAH and has 70% mortality, making its prevention a top priority in the ED.13,36,56 Up to 90% of rebleeding occurs within six hours of the initial SAH and may present as worsening headaches, mental status changes, posturing, seizures, or cardiac arrest.8,21,61 It is more common in patients with large aneurysms, poor initial neurologic status, uncontrolled hypertension, and delays in definitive management.56,62 Therefore, early blood pressure control and neurosurgical consultation is essential.63,64
Acute Hydrocephalus
Acute hydrocephalus occurs in up to 30% of patients with aneurysmal SAH and results from decreased CSF absorption at subarachnoid granulations, increased CSF secretion, and obstruction of CSF circulation caused by blood products and adhesions.8,21,56 CSF diversion, typically achieved by placing an external ventricular drain (EVD), is the only treatment proven to improve outcomes in patients with acute hydrocephalus.56
Up to 30% of patients with poor-grade SAH improve neurologically after EVD placement, often achieving functional outcomes similar to patients with good-grade SAH.36 An EVD also may be placed to relieve increased intracranial pressure caused by space-occupying intracranial hemorrhage, often seen in patients with ruptured middle cerebral artery aneurysms.21
EVD placement does not increase the risk of rebleeding, although overly aggressive CSF drainage does.36 An estimated 25% of SAH patients will develop chronic hydrocephalus requiring continuous CSF diversion, commonly via placement of a ventriculoperitoneal shunt.65
Cardiac and Pulmonary Complications
Spontaneous SAH can cause increased autonomic vascular tone and surges in catecholamines that lead to cardiac complications, including left ventricular dysfunction, stress-induced heart failure (i.e., Takotsubo cardiomyopathy), and cardiac arrest.8,66 Patients with SAH may develop electrocardiogram abnormalities (e.g., deep “cerebral” T-wave inversions, ST elevations/depressions), increases in cardiac biomarkers, or signs of cardiogenic shock.36 Cardiac dysfunction can lead to decreased cerebral perfusion and worse neurologic outcomes.
Pulmonary edema following SAH may be cardiogenic or neurogenic in etiology.21 Acute respiratory distress syndrome, seen in nearly 30% of patients with SAH, is especially difficult to treat, since traditionally used diuretics increase the risk of hypovolemia-induced cerebral ischemia.36 Other common pulmonary complications of SAH include pneumonia, aspiration pneumonitis, and pulmonary embolism.21
Vasospasm and Delayed Cerebral Ischemia
Approximately 30% to 50% of initial survivors of aneurysmal SAH will develop cerebral vasospasm, a major contributor to poor functional outcome.21 Symptomatic vasospasm is a poorly understood phenomenon characterized by transient narrowing of the cerebral arteries that leads to neurologic deterioration (e.g., decreased consciousness, new focal neurologic deficits) lasting greater than one hour.21,36,56,67,68
The risk of vasospasm begins three days after SAH, peaks at seven to 10 days, and usually resolves after 21 days.52,56 Vasoconstriction is likely not the only contributor to poor outcomes, since the administration of vasodilators does not prevent neuronal death.56 Delayed immune cell activation and central nervous system (CNS) inflammation also are thought to play important roles in delayed cerebral ischemia.56,68
Nimodipine, a calcium-channel blocker that crosses the blood-brain barrier, is the only Food and Drug Administration (FDA)-approved medication shown to improve neurologic outcomes and decrease mortality in patients with aneurysmal SAH.36,56 As a result, many guidelines recommend initiating nimodipine as soon as possible, typically given as 60 mg orally every four hours for 21 days.56 In addition to its vasodilatory effect, nimodipine is thought to have neuroprotective effects, possibly by reducing microthrombi formation and resisting calcium-mediated excitotoxicity, although its exact mechanism of benefit is unknown.56 Nimodipine has been shown to be more beneficial than other calcium-channel blockers, including nicardipine, which often is used for blood pressure control following SAH.56
Acute Management of SAH
Treatment advancements have led to a 17% decrease in the case fatality rate of aneurysmal SAH over the past 30 years, although the overall mortality still remains high.36 As with all critically ill patients, emergency providers should initially focus on the patient’s airway, breathing, and circulation (the ABCs). Many patients with aneurysmal SAH will not require endotracheal intubation emergently, although it may be considered in patients at risk of deterioration during transport to specialized treatment centers. Given the importance of blood pressure control and the risk of cardiac stunning, all SAH patients should have continuous cardiac monitoring.
Emergency providers also should consider measures to prevent increased intracranial pressure, such as keeping the head of the patient’s bed at 30-45 degrees (the optimal angle for cerebral venous drainage) and giving medications to prevent pain, agitation, and nausea.36 Hyperosmolar agents, such as mannitol and hypertonic saline, are not routinely given in the ED to patients with SAH.36
Blood Pressure Control
The risk of rebleeding from unsecured cerebral aneurysms increases with systolic blood pressure (SBP) > 160 mmHg.8 Given that 40% of ED patients with aneurysmal SAH have SBP > 185 mmHg, emergency providers should be prepared to start titratable antihypertensive medications, such as nicardipine, labetalol, esmolol, or clevidipine. However, sudden hypotension also should be avoided, given its associated risk of cerebral hypoperfusion.
There is no consensus on optimal blood pressure goals in aneurysmal SAH.7 The Neurocritical Care Society, American Heart Association, and American Stroke Association recommend a goal SBP < 160 mmHg and mean arterial pressure < 110 mmHg in patients with unsecured cerebral aneurysms.7,36,69 Neurosurgeons often aim for even lower blood pressure targets.7 Of note, these parameters do not apply after the aneurysm is treated, when spontaneously high blood pressure may be beneficial.36
Antiplatelet and Anticoagulant Reversal
All antiplatelet and anticoagulant medications should be immediately reversed in patients with suspected aneurysmal SAH.29,70 Institutional protocols often depend on the availability of reversal agents. For patients on vitamin K antagonists (e.g., warfarin), administration of prothrombin complex concentrate (PCC) is preferred over vitamin K or fresh frozen plasma (FFP), given its rapid onset, small infusion volume, and compatibility with all blood types.53 Platelet transfusion may be recommended for patients on aspirin or adenosine diphosphate inhibitors prior to aneurysm clipping.29,53 Common anticoagulants and reversal agents are presented in Table 5.71
Table 5. Common Anticoagulants and Their Reversal Agents71 |
||
Anticoagulant |
Mechanism of Action |
Reversal Agent |
Dabigatran |
Direct thrombin inhibitor |
Idarucizumab |
Rivaroxaban |
Factor Xa inhibitor |
3 factor and 4 factor PCC, andexanet alfa |
Apixaban |
||
Edoxaban |
Andexanet alfa |
|
Betrixaban |
Andexanet alfa |
|
Warfarin |
Vitamin K antagonist |
PCC, vitamin K, fresh frozen plasma |
PCC: prothrombin complex concentrate |
||
Figure 2. Perimesencephalic Subarachnoid Hemorrhage |
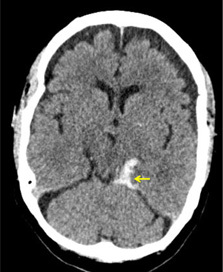 |
A non-contrast head computed tomography of a 48-year-old woman presenting with a thunderclap headache after lifting a heavy suitcase. Hyperdense blood (arrow) is seen in the quadrigeminal cistern, extending inferiorly into the cerebellopontine angle. After computed tomography angiography revealed no source of bleeding, the patient was diagnosed with a perimesencephalic subarachnoid hemorrhage. |
Body Temperature Regulation
Approximately 40% of patients with SAH develop a fever in the first 48 hours, which is associated with higher rates of delayed cerebral ischemia, poor functional outcomes, and mortality.36,72,73 Therefore, core body temperature should be monitored, and fevers should be treated aggressively. The role of therapeutic hypothermia, which has been shown to decrease cerebral edema and bleeding complications associated with ischemic stroke, is not well-established in the management of hemorrhagic stroke.74,75
There is some evidence that early hypothermia decreases rates of vasospasm and delayed cerebral ischemia in patients with poor-grade SAH, although it has not been shown to improve mortality or functional outcomes.36,75,76 Based on the current evidence, therapeutic hypothermia is not routinely initiated in patients with SAH in the ED.77
Antifibrinolytics
The administration of antifibrinolytics, such as tranexamic acid (TXA), when combined with standard treatment, has not been shown to improve outcomes or mortality in patients with SAH secondary to unsecured aneurysms.61,78 There is even some evidence that TXA may increase the risks of delayed cerebral ischemia and thromboembolic events following SAH.56,61 However, the role of antifibrinolytics has not been well-elucidated in resource-limited areas where definitive aneurysm treatment is delayed or unavailable.52
Seizure Prevention and Treatment
Approximately 20% of patients with SAH experience seizures prior to ED arrival, and an additional 5% to 10% of patients develop seizures while hospitalized.69 However, the prophylactic administration of antiepileptic medications following SAH is a controversial topic, as some evidence suggests it is associated with worse functional and cognitive outcomes.52,79,80 Therefore, consultation is recommended prior to the administration of prophylactic antiepileptics in the ED. In patients who are actively seizing, benzodiazepines are the first-line treatment and should be given immediately.
Additional Treatment Considerations
Emergency providers should aim to maintain euvolemia in patients with SAH, as hypervolemia is associated with systemic complications (e.g., pulmonary edema) and does not improve cerebral perfusion.36 Anemia in patients immediately following aneurysmal SAH is not associated with mortality or functional outcomes and has little clinical relevance.81 However, there is growing evidence that anemia that develops during hospitalization leads to worse outcomes and should be addressed.81,82 Hypomagnesemia frequently occurs following aneurysmal SAH and should be treated. However, prophylactic magnesium infusion has not been shown to decrease rates of vasospasm or improve outcomes in SAH.36,56 Prophylactic administration of statins similarly has not shown benefit.36
Definitive Management of Aneurysmal SAH
Treatment of ruptured cerebral aneurysms depends on multiple factors, including the patient’s age and comorbidities and the location and size of the aneurysm.56 There are limited data on the optimal timing of aneurysm repair, although the American Heart Association and American Stroke Association recommend that patients undergo endovascular coiling or surgical clipping as early as possible to prevent rebleeding.36,83 Given difficulties in predicting outcomes after aneurysmal SAH, it is recommended that most patients undergo definitive repair.21 Exceptions may include patients with advanced age, significant comorbidities, impaired brainstem reflexes, or posturing not improved with placement of an EVD.21
Endovascular Coiling
Most cerebral aneurysms in the United States are repaired with endovascular coiling, which has higher survival rates and better functional outcomes than surgical clipping.36,56,84 During the procedure, coils are deployed intravascularly into the aneurysm sac, causing it to thrombose and become obliterated.56 Flow-diverting devices and embolic agents also may be used.56 Endovascular coiling typically is recommended for treatment of posterior circulation aneurysms and ruptured aneurysms in patients older than 70 years of age, due to the risks associated with surgery.56
Surgical Clipping
Clipping is a neurosurgical technique in which a clip is placed across the neck of a cerebral aneurysm, cutting off its blood supply. It often is the preferred treatment for middle cerebral artery aneurysms and aneurysms associated with large intraventricular hematomas.56 Young patients also may prefer surgical clipping over endovascular coiling, because of its lower rates of rebleeding and aneurysm recurrence.21,56,85
Disposition and Prognosis
Patients with spontaneous SAH should be admitted to an intensive care unit for close hemodynamic and neurologic monitoring. Often, patients require temporizing EVDs to relieve acute hydrocephalus and monitor intracranial pressure.56 Early transfer of patients with aneurysmal SAH to centers with a high volume of aneurysm repair cases (> 35 cases per year) is associated with decreased mortality and improved outcomes, regardless of the initial grade of SAH.21,36,56 Multidisciplinary teams involved in SAH treatment often include neurosurgeons, endovascular interventionalists, and neurocritical care intensivists.7
An estimated 15% of patients with spontaneous SAH die prior to hospital arrival.7,8,86 Among those who initially survive, an estimated 25% die within 24 hours and 50% die within six months.14,86,87 Major contributors to poor outcomes include advanced patient age, the severity of the initial bleed, and subsequent episodes of rebleeding and vasospasm.88 Ruptured posterior circulation aneurysms also have high rates of mortality, due to brainstem proximity and hemorrhage into the fourth ventricle.77 Approximately one-third of patients who survive aneurysmal SAH remain functionally dependent long-term, and half develop chronic cognitive deficits that impair daily living.11,13,86-90
Non-Aneurysmal Spontaneous SAH
Perimesencephalic Non-Aneurysmal SAH
Perimesencephalic non-aneurysmal SAH is thought to be caused by transient increases in intracranial pressure, leading to the rupture of aberrant deep cerebral veins, typically around the midbrain.91,92 (See Figure 2.) Cases have been reported after swimming, golfing, and heavy lifting.91 Compared to aneurysmal SAH, patients with perimesencephalic SAH have milder symptoms, lower rates of hydrocephalus and other SAH-related complications, and better prognoses.8,91-95
The diagnosis of perimesencephalic SAH traditionally is made after CTA or digital subtraction angiography fails to identify a source of bleeding in patients with SAH.91,96 Similar to aneurysmal SAH, patients with perimesencephalic SAH usually are admitted for close neurologic monitoring, although clinical courses usually are benign, and outcomes are excellent. Rebleeding and death are uncommon, and patients who develop acute hydrocephalus rarely require an EVD.91,93,97
Reversible Cerebral Vasoconstriction Syndrome
Reversible cerebral vasoconstriction syndrome is characterized by recurring thunderclap headaches, occasionally with focal neurologic deficits, caused by transient, multifocal, segmental vasoconstriction of the cerebral arteries.98-100 In some patients, the cortical pial vessels tear as the cerebral arteries vasodilate, leading to non-aneurysmal SAH.101 The exact mechanism underlying the syndrome is unknown, and it has been linked to vasoactive medication use and certain comorbidities (e.g., systemic lupus erythematosus, sickle cell disease).99
In adults, it most affects middle-aged women, and, in children, it most affects adolescent boys, suggesting that sex hormones also may play roles.99 In the absence of SAH, patients with reversible cerebral vasoconstriction syndrome usually have unremarkable head CTs and CSF analyses in the ED. If SAH is identified on CT, it is characteristically located in the high convexity sulci (“convexal SAH”), in contrast to the diffuse pattern seen with aneurysmal SAH.100,102
CNS Infection
Patients with bacterial meningitis also can develop SAH, which may be secondary to infectious vasculitis or mycotic aneurysms.103 The majority of patients present with altered consciousness, and 50% have focal neurologic deficits.103 In patients with bacterial meningitis, the development of concurrent SAH is associated with higher rates of mortality and worse functional outcomes.103
Additional causes of spontaneous SAH not reviewed here include vascular malformations, intracranial arterial dissection, pituitary apoplexy, cerebral venous thrombosis, cerebral amyloid angiopathy, vasculitides, and cocaine use.8,52
Conclusion
Aneurysmal SAH is a “can’t miss” diagnosis that, unfortunately, often is missed. Emergency providers should appreciate the wide spectrum of presentations associated with aneurysmal SAH, since most patients do not complain of thunderclap headaches and many have normal neurologic examinations. By recognizing risks for aneurysmal SAH and applying the Ottawa Subarachnoid Hemorrhage Rule to the appropriate patients, emergency providers can resist the urge to perform unnecessary head CTs while remaining vigilant for SAH.
Emergency providers also should be aware that, with advancements in CT technology, the diagnostic approach to aneurysmal SAH has evolved. If a patient presents within six hours of headache onset and has a negative CT, then SAH is ruled out (i.e., an LP is not needed). Of course, emergency providers need to understand the assumptions and limitations of this recommendation. In addition, if clinical suspicion for SAH or other dangerous causes of headaches remains high, the decision to perform an LP or CTA often is made with consideration of the patient preferences and the risks and benefits of each study. As these diagnostic studies continue to improve and clinical decision-making tools become more specific, we can expect the diagnostic approach to aneurysmal SAH in the ED to continue to evolve.
Finally, although definitive management of cerebral aneurysms with coiling or clipping has improved outcomes, mortality remains high following aneurysmal SAH. Arguably, the most important role of emergency providers is early diagnosis to prevent rebleeding events. Focused interventions targeting SAH risk factors, such as smoking and hypertension, also may be effective at preventing aneurysmal SAH. Once aneurysmal SAH has occurred, emergency providers should remember to document neurologic examinations (given the prognostic implications) and focus on rapidly controlling blood pressure, reversing anticoagulant and antiplatelet medications, and admitting patients to a neurologic intensive care unit.
REFERENCES
- Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2019 emergency department summary tables. Available at: https://www.cdc.gov/nchs/data/nhamcs/web_tables/2019-nhamcs-ed-web-tables-508.pdf
- Godwin SA, Cherkas DS, Panagos PD, et al. Clinical policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2019;74:e41-74.
- Granato A, D’Acunto L, Morelli ME, et al. Lost diagnoses in not otherwise specified headache in emergency department. Acta Neurol Belg 2022;122:129-134.
- Munoz-Ceron J, Marin-Careaga V, Peña L, et al. Headache at the emergency room: Etiologies, diagnostic usefulness of the ICHD 3 criteria, red and green flags. PLoS One 2019;14:e0208728.
- Peretz AP, Dujari S, Cowan R, Minen M. ACEP guidelines on acute nontraumatic headache diagnosis and management in the emergency department, commentary on behalf of the Refractory, Inpatient, Emergency Care Section of the American Headache Society. Headache 2020;60:643-646.
- Perry JJ, Sivilotti MLA, Emond M, et al. Prospective implementation of the Ottawa subarachnoid hemorrhage rule and 6-hour computed tomography rule. Stroke 2020;51:424-430.
- Dubosh NM, Edlow JA. Diagnosis and initial emergency department management of subarachnoid hemorrhage. Emerg Med Clin North Am 2021;39:87-99.
- Long B, Koyfman A, Runyon MS. Subarachnoid hemorrhage: Updates in diagnosis and management. Emerg Med Clin North Am 2017;35:803-824.
- Fuentes AM, Stone McGuire L, Amin-Hanjani S. Sex differences in cerebral aneurysms and subarachnoid hemorrhage. Stroke 2022;53:624-633.
- Ogunlaja OI, Cowan R. Subarachnoid hemorrhage and headache. Curr Pain Headache Rep 2019;23:44.
- Ois A, Vivas E, Figueras-Aguirre G, et al. Misdiagnosis worsens prognosis in subarachnoid hemorrhage with good Hunt and Hess Score. Stroke 2019;50:3072-3076.
- Long D, Koyfman A, Long B. The thunderclap headache: Approach and management in the emergency department. J Emerg Med 2019;56:633-641.
- Carpenter CR, Hussain AM, Ward MJ, et al. Spontaneous subarachnoid hemorrhage: A systematic review and meta-analysis describing the diagnostic accuracy of history, physical examination, imaging, and lumbar puncture with an exploration of test thresholds. Acad Emerg Med 2016;23:963-1003.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Headache; Godwin SA, Cherkas DS, Panagos PD, et al. Clinical Policy: Critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2019;74:e41-e74.
- Karceski S. Subarachnoid hemorrhage: The long and short of it. Neurology 2020;95:e1915-e1917.
- Etiman N, Chang H, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta-analysis. JAMA Neurol 2019;76:588-597.
- Jaja BNR, Saposnik G, Lingsma HF, et al; SAHIT collaboration. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: The SAHIT multinational cohort study. BMJ 2018;360:j5745.
- Cras TY, Bos D, Ikram MA, et al. Determinants of the presence and size of intracranial aneurysms in the general population: The Rotterdam study. Stroke 2020;51:2103-2110.
- Korja M, Lehto H, Juvela S, Kapiro J. Incidence of subarachnoid hemorrhage is decreasing together with decreasing smoking rates. Neurology 2016;87:1118-1123.
- McGurgan IJ, Clarke R, Lacey B, et al. Blood pressure and risk of subarachnoid hemorrhage in China. Stroke 2018;50:STROKEAHA118022239.
- Neifert SN, Chapman EK, Martini ML, et al. Aneurysmal subarachnoid hemorrhage: The last decade. Transl Stroke Res 2021;12:428-446.
- McDowell MM, Zhao Y, Kellner CP, et al. Demographic and clinical predictors of multiple intracranial aneurysms in patients with subarachnoid hemorrhage. J Neurosurg 2018;128:961-968.
- Rosi Junior J, Gomes Dos Santos A, da Silva SA, et al. Multiple and mirror intracranial aneurysms: Study of prevalence and associated risk factors. Br J Neurosurg 2021;35:780-784.
- Turan N, Heider RA, Zaharieva D, et al. Sex differences in the formation of intracranial aneurysms and incidence and outcome of subarachnoid hemorrhage: Review of experimental and human studies. Transl Stroke Res 2016;7:12-19.
- Diagbouga MR, Morel S, Bijlenga P, Kwak BR. Role of hemodynamics in initiation/growth of intracranial aneurysms. Eur J Clin Invest 2018;48:e12992.
- FrÖsen J, Cebral J, Roberston AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus 2019;47:E21.
- Rehman S, Sahle BW, Chandra RV, et al. Sex differences in risk factors for aneurysmal subarachnoid haemorrhage: Systematic review and meta-analysis. J Neurol Sci 2019;406:116446.
- Galea JP, Dulhanty L, Patel HC; UK and Ireland Subarachnoid Hemorrhage Database Collaborators. Predictors of outcome in aneurysmal subarachnoid hemorrhage patients: Observations from a multicenter data set. Stroke 2017;48:2958-2963.
- Florez WA, García-Ballestas E, Maeda F, et al. Relationship between aspirin use and subarachnoid hemorrhage: A systematic review and meta-analysis. Clin Neurol Neurosurg 2021;200:106320.
- Can A, Rudy RF, Castro VM, et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: A case-control study. Neurology 2018;91:e1175-e1181.
- Du R. Author response: Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: A case-control study. Neurology 2019;92:1025-1026.
- Huang WY, Saver JL, Wu YL, et al. Frequency of intracranial hemorrhage with low-dose aspirin in individuals without symptomatic cardiovascular disease: A systematic review and meta-analysis. JAMA Neurol 2019;76:906-914.
- MacGrory B, Vu L, Cutting S, et al. Distinguishing characteristics of headache in nontraumatic subarachnoid hemorrhage. Headache 2018;58:364-370.
- Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet 2017;389:655-666.
- Edlow JA. Managing patients with nontraumatic, severe, rapid-onset headache. Ann Emerg Med 2018;71:400-408.
- de Oliveira Manoel AL, Goffi A, Marotta TR, et al. The critical care management of poor-grade subarachnoid haemorrhage. Crit Care 2016;20:21.
- Mahta A, Murray K, Reznik ME, et al. Early neurological changes and interpretation of clinical grades in aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 2021;30:105939.
- Szeder V, Tateshima S, Duckwiler GR. Intracranial aneurysms and subarachnoid hemorrhage. In: Daroff RB, Jankovic J, Mazziotta JC, et al. Bradley’s Neurology in Clinical Practice. 7th ed. Elsevier;2016:983.
- Perry JJ, Sivilotti MLA, Sutherland J, et al. Validation of the Ottawa Subarachnoid Hemorrhage Rule in patients with acute headache. CMAJ 2017;189:E1379-E1385.
- Ramachandran S, Foley RW, Venkatesh H. Can the Ottawa subarachnoid haemorrhage rule help reduce investigation rates for suspected subarachnoid haemorrhage? Am J Emerg Med 2019;37:155.
- Bellolio MF, Hess EP, Gilani WI, et al. External validation of the Ottawa subarachnoid hemorrhage clinical decision rule in patients with acute headache. Am J Emerg Med 2015;33:244-249.
- Kimura A, Kobayashi K, Yamaguchi H, et al. New clinical decision rule to exclude subarachnoid haemorrhage for acute headache: A prospective multicentre observational study. BMJ Open 2016;6:e010999.
- Chu KH, Keijzers G, Furyk JS, et al. Applying the Ottawa subarachnoid haemorrhage rule on a cohort of emergency department patients with headache. Eur J Emerg Med 2018;25:e29-e32.
- Foley RW, Ramachandran S, Akintimehin A, et al. Subarachnoid haemorrhage rules in the decision for acute CT of the head: External validation in a UK cohort. Clin Med (Lond) 2021;21:96-100.
- Cheung HY, Lui CT, Tsui KL. Validation and modification of the Ottawa subarachnoid haemorrhage rule in risk stratification of Asian Chinese patients with acute headache. Hong Kong Med J 2018;24:584-592.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Stroke 2016;47:750-755.
- Chakraborty T, Daneshmand A, Lanzino G, Hocker S. CT-negative subarachnoid hemorrhage in the first six hours. J Stroke Cerebrovasc Dis 2020;29:105300.
- Oh SY, Lim YC, Shim YS, et al. Initial misdiagnosis of aneurysmal subarachnoid hemorrhage: Associating factors and its prognosis. Acta Neurochir (Wien) 2018;160:1105-1113.
- Kumar A, Niknam K, Lumba-Brown A, et al. Practice variation in the diagnosis of aneurysmal subarachnoid hemorrhage: A survey of US and Canadian emergency medicine physicians. Neurocrit Care 2019;31:321-328.
- Gill HS, Marcolini EG, Barber D, Wira CR. The utility of lumbar puncture after a negative head CT in the emergency department evaluation of subarachnoid hemorrhage. Yale J Biol Med 2018;91:9.
- Meurer WJ, Walsh B, Vilke GM, Coyne CJ. Clinical guidelines for the emergency department evaluation of subarachnoid hemorrhage. J Emerg Med 2016;50:696-701.
- Patel S, Parikh A, Okorie ON. Subarachnoid hemorrhage in the emergency department. Int J Emerg Med 2021;14:31.
- Marcolini E, Hine J. Approach to the diagnosis and management of subarachnoid hemorrhage. West J Emerg Med 2019;20:203-211.
- Probst MA, Hoffman JR. Computed tomography angiography of the head is a reasonable next test after a negative noncontrast head computed tomography result in the emergency department evaluation of subarachnoid hemorrhage. Ann Emerg Med 2016;67:773-774.
- Scharf E, Pelkowski S, Shahin B. Unruptured intracranial aneurysms and life insurance underwriting. Neurol Clin Pract 2017;7:274-277.
- Daou BJ, Koduri S, Thompson BG, et al. Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci Ther 2019;25:1096-1112.
- Blum CA, Winzeler B, Nigro N, et al. Copeptin for risk stratification in non-traumatic headache in the emergency setting: A prospective multicenter observational cohort study. J Headache Pain 2017;18:21.
- Wójtowicz K, Kunert P, Przepiórka Ł, Marchel A. Warning signs in the era of unruptured intracranial aneurysms: Report on 2 cases of fatal aneurysmal hemorrhage. Cerebrovasc Dis Extra 2021;11:77-80.
- Ignacio KHD, Pascual JSG, Factor SJV, Khu KJO. A meta-analysis on the prevalence of anxiety and depression in patients with unruptured intracranial aneurysms: Exposing critical treatment gaps. Neurosurg Rev 2022;45:2077-2085.
- Thompson BG, Brown RD Jr, Amin-Hanjani S, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association. Guidelines for the management of patients with unruptured intracranial aneurysms: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2368-2400.
- Feng Y, Chen H. Tranexamic acid for subarachnoid hemorrhage: A systematic review and meta-analysis. Am J Emerg Med 2021;50:748-752.
- Lin QS, Ping C, Lin YX, et al. Systolic blood pressure variability is a novel risk factor for rebleeding in acute subarachnoid hemorrhage: A case-control study. Medicine (Baltimore) 2016;95:e3028.
- Minhas JS, Moullaali TJ, Rinkel GJE, Anderson CS. Blood pressure management after intracerebral and subarachnoid hemorrhage: The knowns and known unknowns. Stroke 2022;53:1065-1073.
- Liotta EM, Karmarkar A, Batra A, et al. Magnesium and hemorrhage volume in patients with aneurysmal subarachnoid hemorrhage. Crit Care Med 2020;48:104-110.
- Wilson CD, Safavi-Abbasi S, Sun H, et al. Meta-analysis and systematic review of risk factors for shunt dependency after aneurysmal subarachnoid hemorrhage. J Neurosurg 2017;126:586-595.
- Norberg E, Odenstedt-Herges H, Rydenhag B, Oras J. Impact of acute cardiac complications after subarachnoid hemorrhage on long-term mortality and cardiovascular events. Neurocrit Care 2018;29:404-412.
- Stienen MN, Germans M, Burkhardt JK, et al; Swiss SOS Study Group. Predictors of in-hospital death after aneurysmal subarachnoid hemorrhage: Analysis of a nationwide database (Swiss SOS [Swiss Study on Aneurysmal Subarachnoid Hemorrhage]). Stroke 2018;49:333-340.
- Coulibaly AP, Provencio JJ. Aneurysmal subarachnoid hemorrhage: An overview of inflammation-induced cellular changes. Neurotherapeutics 2020;17:436-445.
- John S, Meurer WJ, Qualls S, Edlow BL. Emergency Neurological Life Support Subarachnoid Hemorrhage Protocol Version 4.0. Neurocritical Care Society. Last updated October 2019. https://higherlogicdownload.s3.amazonaws.com/NEUROCRITICALCARE/fdc4bb32-6722-417b-8839-f68ac1ef3794/UploadedImages/ENLS_Documents/ENLS_V4.0_protocol%20files/ENLS_V_4_0_Protocol_SAH_Final.pdf
- Steiner T, Weitz JI, Veltkamp R. Anticoagulant-associated intracranial hemorrhage in the era of reversal agents. Stroke 2017;48:1432-1437.
- Sweidan AJ, Singh NK, Conovaloff JL, et al. Coagulopathy reversal in intracerebral haemorrhage. Stroke Vasc Neurol 2020;5:29-33.
- Addis A, Gaasch M, Schiefecker AJ, et al. Brain temperature regulation in poor-grade subarachnoid hemorrhage patients — A multimodal neuromonitoring study. J Cereb Blood Flow Metab 2021;41:359-368.
- Kramer CL, Pegoli M, Mandrekar J, et al. Refining the association of fever with functional outcome in aneurysmal subarachnoid hemorrhage. Neurocrit Care 2017;26:41-47.
- Sun YJ, Zhang ZY, Fan B, Li GY. Neuroprotection by therapeutic hypothermia. Front Neurosci 2019;13:586.
- Choi W, Kwon SC, Lee WJ, et al. Feasibility and safety of mild therapeutic hypothermia in poor-grade subarachnoid hemorrhage: A prospective pilot study. J Korean Med Sci 2017;32:1337-1344.
- Rhim JK, Park JJ, Kim H, Jeon JP. Early and prolonged mild hypothermia in patients with poor-grade subarachnoid hemorrhage: A pilot study. Ther Hypothermia Temp Manag 2022;12:229-234.
- Won SY, Kim MK, Song J, Lim YC. Therapeutic hypothermia in patients with poor-grade aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2022;221:107369.
- Post R, Germans MR, Tjerkstra M, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): A randomised controlled trial. Lancet 2021;397:112-118.
- Chen Y, Xia F, Cai C, et al. Duration and choices of prophylactic anticonvulsants in subarachnoid hemorrhage: A systematic review and meta-analysis. Neurosurg Rev 2021;44:2459-2467.
- Fang T, Valdes E, Frontera JA. Levetiracetam for seizure prophylaxis in neurocritical care: A systematic review and meta-analysis. Neurocrit Care 2022;36:248-258.
- Said M, Gümüs M, Rodemerk J, et al. Systematic review and meta-analysis of outcome-relevant anemia in patients with subarachnoid hemorrhage. Sci Rep 2022;12:20738.
- Ayling OGS, Ibrahim GM, Alotaibi NM, et al. Anemia after aneurysmal subarachnoid hemorrhage is associated with poor outcome and death. Stroke 2018;49:1859-1865.
- Rawal S, Alcaide-Leon P, Macdonald RL, et al. Meta-analysis of timing of endovascular aneurysm treatment in subarachnoid haemorrhage: Inconsistent results of early treatment within 1 day. J Neurol Neurosurg Psychiatry 2017;88:241-248.
- Golnari P, Nazari P, Garcia RM, et al. Volumes, outcomes, and complications after surgical versus endovascular treatment of aneurysms in the United States (1993-2015): Continued evolution versus steady-state after more than 2 decades of practice. J Neurosurg 2020;134:848-861.
- Hulsbergen AFC, Mirzaei L, van der Boog ATJ, et al. Long-term durability of open surgical versus endovascular repair of intracranial aneurysms: A systematic review and meta-analysis. World Neurosurg 2019;132:e820-e33.
- Bae IS, Chun HJ, Choi KS, Yi HJ. Modified Glasgow coma scale for predicting outcome after subarachnoid hemorrhage surgery. Medicine (Baltimore) 2021;100:e25815.
- Manella H, Sivasankar S, Perry JJ, et al. A web-based decision tool to estimate subarachnoid hemorrhage risk in emergency department patients. Cureus 2018;10:e2096.
- Darkwah Oppong M, Buffen K, Pierscianek D, et al. Secondary hemorrhagic complications in aneurysmal subarachnoid hemorrhage: When the impact hits hard. J Neurosurg 2019;132:79-86.
- Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med 2017;377:257-266.
- Wenneberg SB, Block L, Sörbo A, et al. Long-term outcomes after aneurysmal subarachnoid hemorrhage: A prospective observational cohort study. Acta Neurol Scand 2022;146:525-536.
- Hou K, Yu J. Current status of perimesencephalic non-aneurysmal subarachnoid hemorrhage. Front Neurol 2022;13:960702.
- Sahin S, Delen E, Korfali E. Perimesencephalic subarachnoid hemorrhage: Etiologies, risk factors, and necessity of the second angiogram. Asian J Neurosurg 2016;11:50-53.
- Mensing LA, Vergouwen MDI, Laban KG, et al. Perimesencephalic hemorrhage: A review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke 2018;49:1363-1370.
- Atchie B, McGraw C, McCarthy K, et al. Comparing outcomes of patients with idiopathic subarachnoid hemorrhage by stratifying perimesencephalic bleeding patterns. J Stroke Cerebrovasc Dis 2019;28:2407-2413.
- Jeon JP, Kim SE, Chai CL, et al. Seizure incidence of angiogram-negative subarachnoid hemorrhage: An updated meta-analysis. J Chin Med Assoc 2020;83:466-470.
- Wallace AN, Vyhmeister R, Dines JN, et al. Evaluation of an anatomic definition of non-aneurysmal perimesencephalic subarachnhoid hemorrhage. J Neurointerv Surg 2016;8:378-385.
- Wang MD, Fu QH, Song MJ, et al. Novel subgroups in subarachnoid hemorrhage and their association with outcomes — A systematic review and meta-regression. Front Aging Neurosci 2020;12:573454.
- Chen SP, Wang SJ. Pathophysiology of reversible cerebral vasoconstriction syndrome. J Biomed Sci 2022;29:72.
- Maldonado-Soto AR, Fryer RH. Reversible cerebral vasoconstriction syndrome in children: An update. Semin Pediatr Neurol 2021;40:100936.
- Spadaro A, Scott KR, Koyfman A, Long B. Reversible cerebral vasoconstriction syndrome: A narrative review for emergency clinicians. Am J Emerg Med 2021;50:765-772.
- Dakay KB, Azher I, Mahta A, et al. Multifocal atraumatic convexity subarachnoid hemorrhage. Cureus 2021;13:e16091.
- Burton TM, Bushnell CD. Reversible cerebral vasoconstriction syndrome. Stroke 2019;50:2253-2258.
- Deliran SS, Brouwer MC, van de Beek D. Subarachnoid hemorrhage in bacterial meningitis patients. Cerebrovasc Dis 2022;51:118-124.
This article reviews the typical and atypical presentations of spontaneous subarachnoid hemorrhage and its complications. It also will discuss management of spontaneous subarachnoid hemorrhage and its complications, with a focus on treatment in the emergency setting.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
