Selected Orthopedic Emergencies
September 15, 2022
AUTHORS
John P. Detherage III, MD, PGY-2 Emergency Medicine, Allegheny General Hospital, Pittsburgh, PA
Moira Davenport, MD, Allegheny General Hospital, Pittsburgh, PA
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Brookdale University Medical Center, Brooklyn, NY
EXECUTIVE SUMMARY
- Hip dislocations should be reduced within six hours of injury, ideally in the emergency department (ED).
- Posterolateral corner injuries of the knee are underdiagnosed; perform tests of ligamentous stability and consult with an orthopedic surgeon if laxity is found.
- Posterior sternoclavicular dislocations can injure mediastinal structures, and imaging with computed tomography (CT) using contrast is recommended for diagnosis since plain radiographs often are nondiagnostic.
- Acute compartment syndrome would be suspected in an adult with pain out of proportion to the visible injury or in a child with increasing anxiety and agitation after initial splinting and analgesia administration.
Knee Dislocations
Knee dislocations (KDs), also referred to as multiligament knee injuries (MLKI), are rare, limb-threatening injuries, occurring at rates between 0.02% to 0.2% of all orthopedic injuries.2 Most KDs can be caused by high-energy trauma, such a motor vehicle collision. KDs from low-energy injuries, such as falls, are more common in females and obese patients. A KD results in the loss of articulation of the tibiofemoral joint, along with the complete disruption of at least three of the four major ligaments of the knee (anterior cruciate ligament [ACL], posterior cruciate ligament [PCL], lateral collateral ligament [LCL], and medial collateral ligament [MCL]).
KDs can be classified by the Schenck system (according to the ligaments disrupted, see Table 1) or the Kennedy system (according to the position of the tibia relative to the femur, see Table 2).3 The Schenck system is based on the six planes of motion in the knee — anterior and posterior translation in the sagittal plane, varus and valgus stress in the coronal plane, and internal and external rotation in the axial plane — and the ligaments that restrict these movements.
Table 1. Schenck Classification of Knee Dislocations |
|
Classification |
Description |
KD I |
KD plus ACL or PCL disruption |
KD II |
KD plus ACL and PCL disruption |
KD III |
KD plus both cruciate ligaments plus either MCL or LCL disruption |
KD IIIM |
KD plus both cruciate ligaments plus MCL disruption |
KD IIIL |
KD plus both cruciate ligaments plus LCL disruption |
KD IV |
KD plus both cruciate and collateral ligament disruption |
KD V |
KD plus fracture |
KD: knee dislocation; ACL: anterior cruciate ligament; PCL: posterior cruciate ligament; MCL: medial collateral ligament; LCL: lateral collateral ligament |
|
Table 2. Kennedy Classification of Knee Dislocations |
|||
Direction |
Approximate Percentage |
Mechanism |
Injury Pattern |
Anterior |
40% |
Hyperextension |
Posterior capsule, PCL, and ACL tears |
Posterior |
33% |
Dashboard impact |
PCL tear |
Lateral |
4% |
Valgus, flexion/adduction |
MCL, LCL, ACL, and PCL tear |
Medial |
18% |
Varus/rotation |
MCL, LCL, ACL, and PCL tear |
Rotatory |
5% |
Rotation around the PCL |
MCL and ACL tear, posterior medial corner of the tibial plateau injury |
ACL: anterior cruciate ligament; PCL: posterior cruciate ligament; MCL: medial collateral ligament; LCL: lateral collateral ligament |
|||
The KD may occur in different directions described by the Kennedy system based on the tibia’s relation to the femur. Anterior KDs typically occur from hyperextension, with the proximal tibia driven anterior to the distal femur, while posterior KDs are more common in high-energy trauma, such as “dashboard” injuries with direct force to the proximal tibia pushing it posterior to the distal femur.4
A unique injury is a posterolateral rotary dislocation, where the medial femoral condyle buttonholes through a tear in the adjacent soft-tissue structures, including the retinaculum, vastus muscle, MCL, and capsule. These KDs present with a noticeable dimple at the medial joint line of the knee. This type of KD should not be reduced in the ED unless neurovascular compromise is present because reduction attempts can cause further soft-tissue damage; they require urgent open reduction in the operating room.5
During the initial evaluation, Advanced Trauma Life Support (ATLS) guidelines should be followed; address any problems posing a threat to the patient’s airway, breathing, or circulation before evaluating the knee. If the knee is noticeably dislocated and neurovascular compromise (pale skin, absent pulses in foot and ankle, absent active movement in the foot and ankle) is present, the joint should be reduced immediately by using gentle traction-countertraction.
A thorough neurovascular exam should include palpation of the dorsalis pedis and posterior tibial pulses and an ankle-brachial index (ABI) should be calculated as well. If the distal pulses are asymmetric with the unaffected limb, or the ABI is less than 0.9, vascular imaging of the limb with computed tomography angiography (CTA) or magnetic resonance angiography (MRA) should be performed. If there are gross signs of vascular compromise after reduction, an expanding pulsatile hematoma in the popliteal space, or active hemorrhage, emergent surgery consultation is warranted.
The neurologic status should be assessed by performing a full motor and sensory exam of the injured extremity. Between 14% and 40% of KD patients experience neurological injury, with the common peroneal nerve (CPN) most commonly affected.6 Tibial nerve injury is rare but may be seen with any posterior KD with vascular compromise. The CPN is examined by assessing active dorsiflexion, toe extension, and eversion of the foot, and the tibial nerve by plantar flexion.
If the patient is neurovascularly intact, pre-reduction X-rays (anteroposterior [AP] and lateral views) should be obtained prior to reduction attempts.7 (See Figure 1.) Procedural sedation may not be required given the significant laxity associated with KDs. Once the knee has been reduced successfully, the patient should be placed in a long leg splint with the knee flexed 15-20 degrees, and post-reduction X-rays should be performed. Repeat neurovascular testing should be performed every two hours to ensure that delayed neurovascular injury does not develop.
Figure 1. Anterior Knee Dislocation Before Reduction |
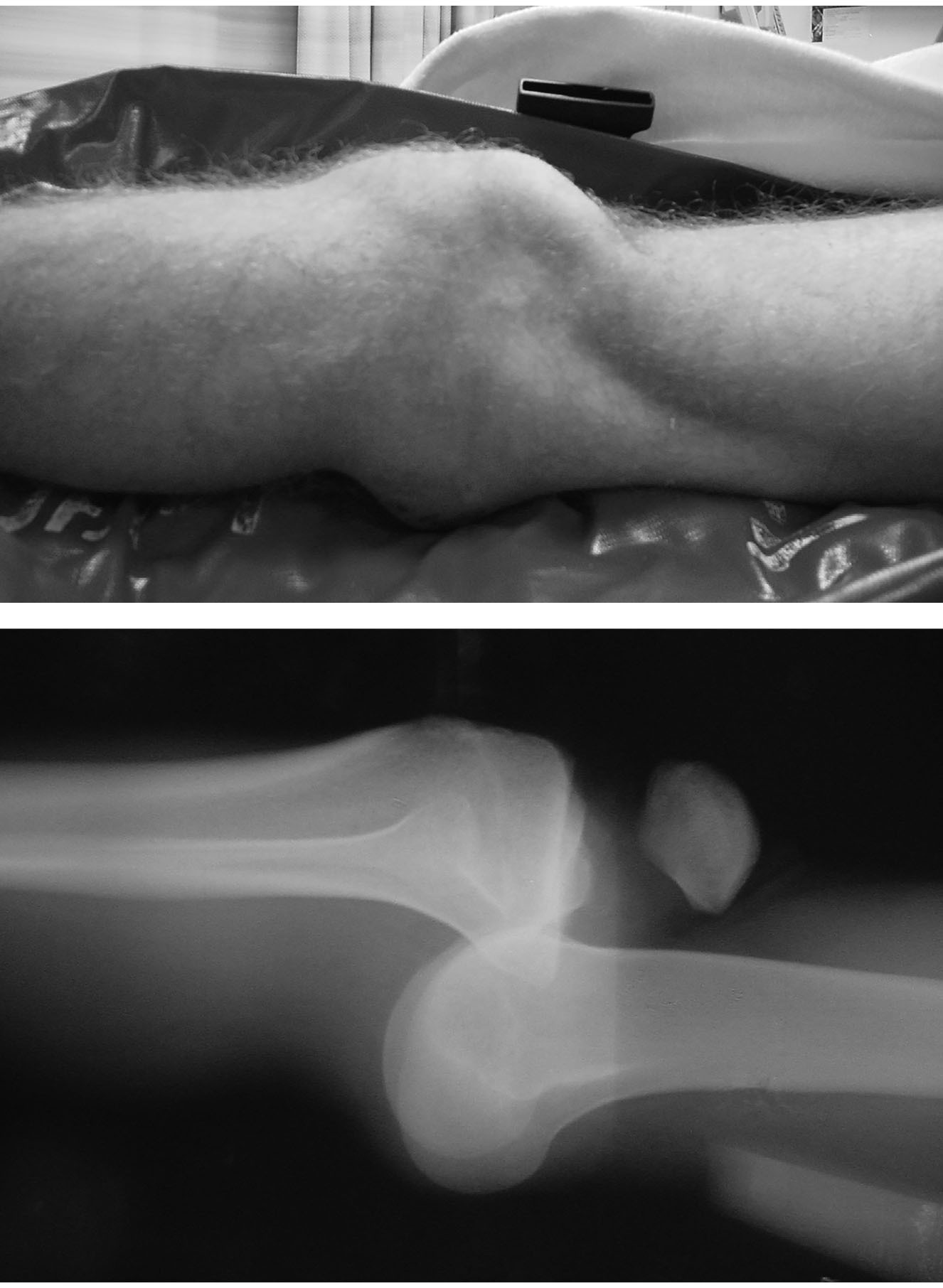 |
Images used with permission from Dr. J. Stephan Stapczynski. |
All patients with knee dislocations should be admitted, ensuring adequate, regular neurovascular examinations, additional advanced imaging, and surgical management (if necessary).8 Some KDs once were treated conservatively with immobilization and rehabilitation; however, in recent years aggressive ligament reconstruction and rehabilitation have proved to provide superior outcomes.9
Clinicians should maintain an index of suspicion for knee dislocation in high-energy trauma because up to 50% of KDs spontaneously reduce before hospital presentation.10 It is critical to make the diagnosis of knee dislocation because of the potential for vascular injury associated with this condition. Popliteal artery injury occurs in 12% to 80% of KDs, while 20% to 30% of these vascular injuries can result in limb amputation. Amputation rates as high as 80% have been reported with prolonged ischemic times.11 Hyperextension injuries resulting in anterior dislocations are the most common cause of popliteal artery injury in KDs.
Neurovascular deficits may present on initial exam pre-reduction, manifest post-reduction, or develop up to 48 hours after the initial injury. Patients with evolving deficits while in the ED require emergent surgery and decompression, since this can be caused by either compartment syndrome, vascular injury, or an expanding hematoma. Nerve injury may be assessed during hospitalization by modalities such as electromyography, ultrasound, or magnetic resonance imaging (MRI). Outcomes from KD with neurologic deficits have a worse prognosis than those with no neurological injury.
Open Long Bone Fractures
An open fracture is defined as any fracture coinciding with a break in the skin, allowing the bone to communicate with the outside environment. (See Figure 2.) Open long bone fractures are commonly caused by high-energy trauma, such as motor vehicle collisions. Long bone open fractures occur at an annual incidence of 11.5 per 100,000 persons per year. Approximately 70% of these injuries involve the lower extremity, with the tibia the most involved bone.12,13 Up to 9% of fractures in children presenting to tertiary care centers are open fractures.14
Figure 2. Open Long Bone Fracture |
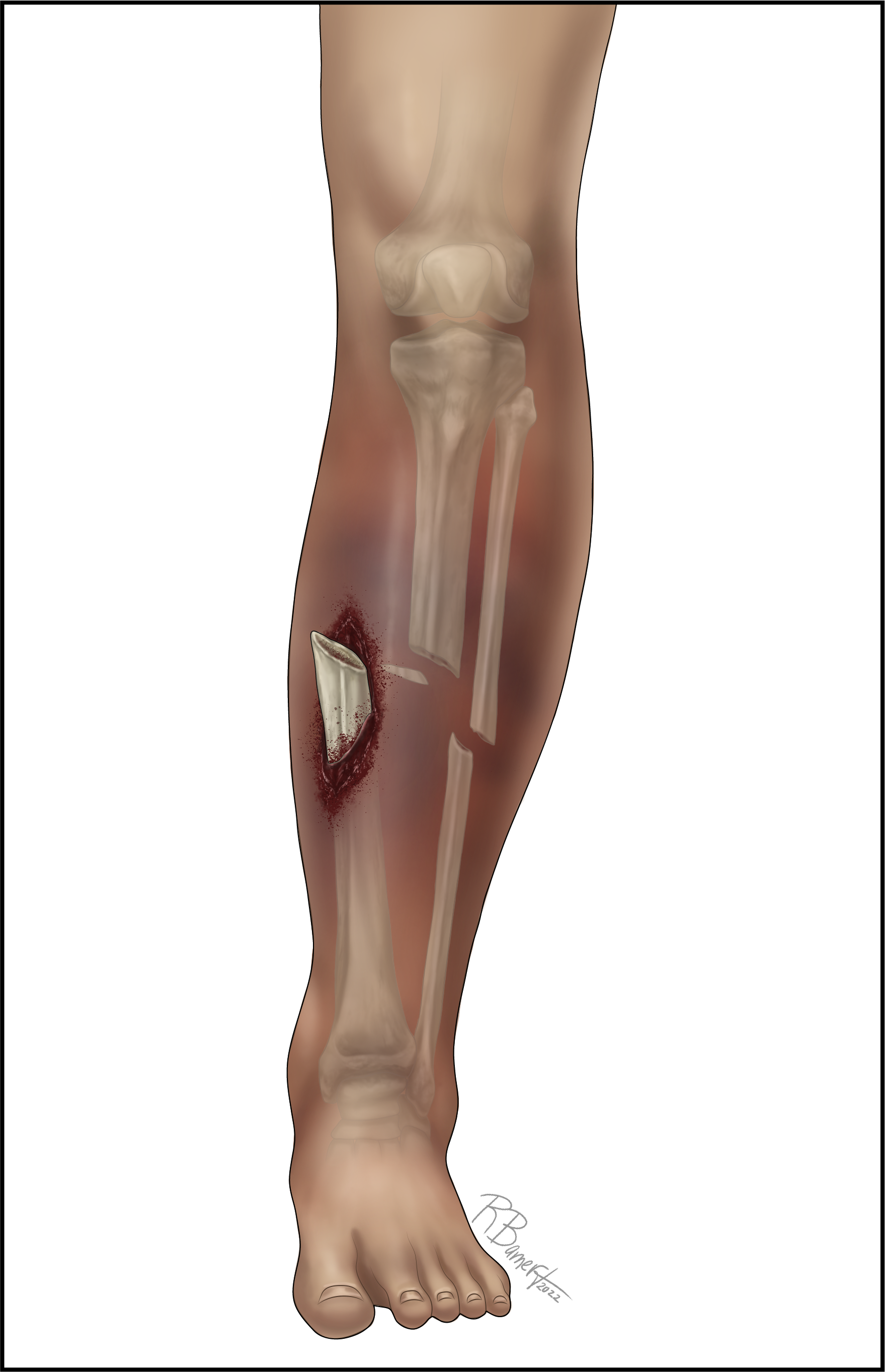 |
The Gustilo and Anderson classification scheme is the most widely used system to describe open fractures; this tool is helpful in guiding management and determining prognosis.15 (See Table 3.) Open fractures are orthopedic emergencies because of the high risk of deep infection and neurovascular injury, which can lead to an increased risk of amputation.
Table 3. Gustilo-Anderson Classification of Open Fractures |
|
Classification |
Description |
I |
Wound < 1 cm and clean |
II |
Laceration > 1 cm without extensive soft-tissue damage, flaps, or avulsions |
IIIA |
Adequate soft-tissue coverage of fracture, has extensive soft-tissue laceration or flaps, high-energy trauma |
IIIB |
Extensive soft-tissue injury with periosteal stripping and bone exposure, typically has massive contamination |
IIIC |
Arterial injury requiring repair |
ATLS protocol should be followed during the assessment of the injured patient. The secondary survey should include a thorough examination of the injured extremity, looking for signs of neurovascular damage or compartment syndrome. Adequate anesthesia should be provided to optimize patient comfort while obtaining plain films.16 If neurovascular compromise is identified, immediate reduction should be performed. If no neurovascular deficits are identified (and emergent orthopedic surgery coverage is available), it is reasonable to defer reduction until an orthopedic surgeon is present. This allows for fracture realignment, wound irrigation, extremity splinting, and, in some cases, traction pinning.17
The general consensus is to administer prophylactic antibiotics within one hour of presentation; tetanus immunization also should be updated.18,19 However, there is no strong evidence-based research dictating superior time frames of surgical treatment or duration of antibiotics.20
Antibiotic prophylaxis recommendations vary based on the type of fracture. The literature supports using cefazolin for gram-positive coverage for Gustilo type I and II fractures and broadening coverage to incorporate gram-negative organisms for type III fractures. This can be done by adding a fluoroquinolone or aminoglycoside to cefazolin, or alternatively by using ceftriaxone and vancomycin or piperacillin/tazobactam. If there is any concern of significant contamination or vascular injury, penicillin, clindamycin, or metronidazole should be added to the previously mentioned regimens for anaerobic coverage.21,22
For severely contaminated wounds or fractures in contact with soil or a marine environment, most of the literature recommends broad-spectrum antibiotic administration as well as the addition of high-dose penicillin for fecal or potential clostridial contamination, which are common in farm-related injuries.23
Initial wound cleaning in the ED should be limited; remove gross debris from the wound, do not remove any bone fragments, place sterile saline-soaked dressing on the wound, and avoid aggressive irrigation or irrigation with antiseptic solution in the ED, since this can push debris further into the wound.
Patients with higher infection rates after open fracture include males, patients with diabetes, polytrauma patients, those with lower extremity fractures, those with grade III open fractures, and those with contaminated wounds. All patients with long bone open fractures should be hospitalized for definitive management, which includes wound debridement, irrigation, and closure in the operating room.
Native Hip Dislocations
Traumatic hip dislocation (HD) accounts for 5.2% of all traumatic dislocations. Posterior HDs are most common, accounting for approximately 90% of injuries.24 Anterior HD is a rare injury accounting for 7% to 13% of HDs, with the majority of these occurring concurrently with either femoral head and/or acetabular fractures. Inferior HD is even more rare.25,26
The most common mechanism of injury for an HD is a “dashboard injury,” which occurs when the hip is flexed and adducted, the knee is flexed, and a posterior force is applied to the knee. Perform a physical exam by following ATLS with special attention to the neurovascular status of the affected extremity.
HD should be suspected in the patient presenting with the hip in flexion, internal rotation, and adduction (posterior HD) or the patient with the hip in external rotation with a small amount of flexion and abduction (anterior HD).27 AP plain and lateral films should be obtained to diagnose HD and rule out fracture. (See Figure 3.) If there is a high degree of suspicion of HD, specifically an anterior dislocation, CT should be performed since AP radiographs are unable to diagnose most anterior HDs.28
Figure 3. Posterosuperior Right Femoral Head Dislocation with Acetabular Fracture |
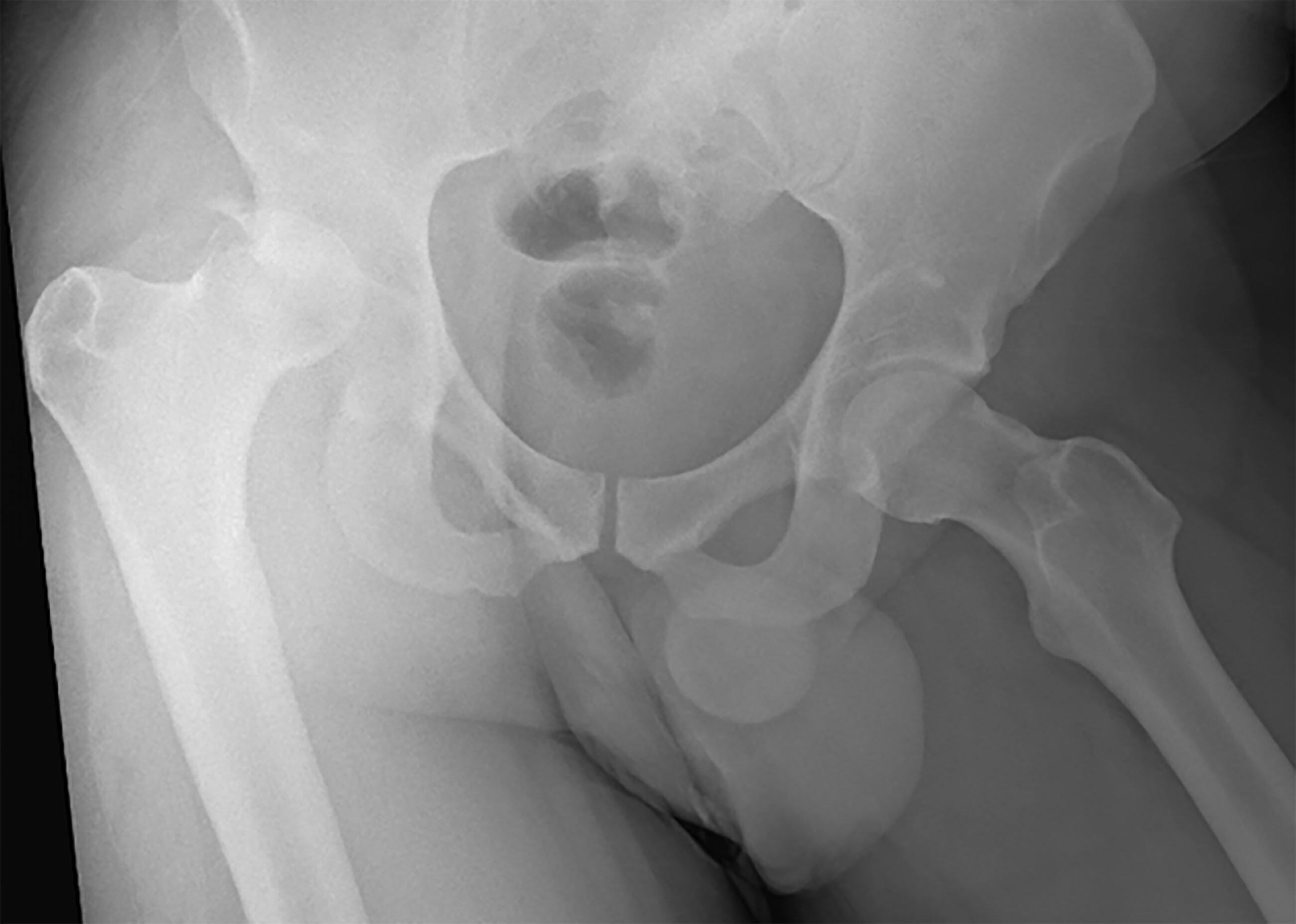 |
Image used with permission from Dr. Dennis Hanlon. |
Femoral neck or acetabular fractures combined with HDs are extremely rare, occurring mainly in young adults from high-speed motor vehicle collisions.29 Do not attempt reduction in the ED since they require reduction and fixation in the operating room. Those with acetabular fractures and dislocation have a higher rate of requiring total hip arthroplasty in the future at 26%, and a higher incidence of developing avascular necrosis (AVN) around 9.2% when compared to those with only acetabular fracture.30
Native HDs are orthopedic emergencies because of the associated long-term morbidity from AVN and posttraumatic osteoarthritis. The rate of AVN for HD is between 1% and 50%, but it decreases to < 10% if reduction is performed within the first six hours. Based on this significantly reduced morbidity, reduction within six hours of injury is recommended.31
More than 90% of all HDs can be reduced in the ED. It is important for EPs to be competent with several different hip reduction techniques, since morbidity is reduced when HDs are successfully reduced in the ED since waiting for the operating room can lead to delays in treatment. If the first reduction attempt is unsuccessful, ensure that adequate sedation has been achieved. If the patient is sufficiently sedated and the attempt is not successful, a different reduction method should be performed. No method is proven to be superior, and some common reduction techniques are explained in Figures 4-6.32
Figure 4. Allis Hip Reduction Technique |
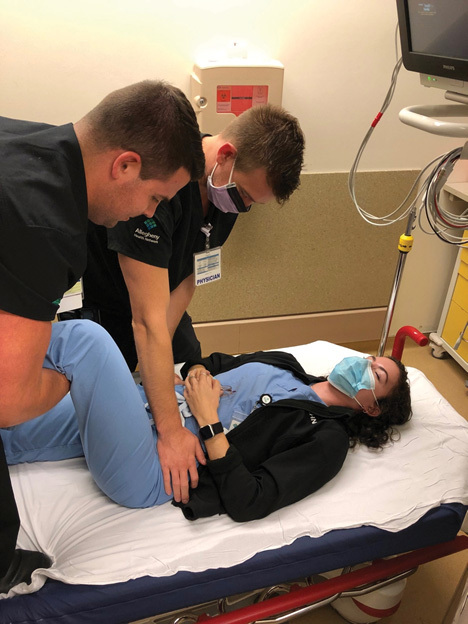 |
While patient is supine, grasp the affected leg with 90 degrees of hip and knee flexion while pulling traction along the long axis of the femur. |
Figure 5. Captain Morgan Hip Reduction Technique |
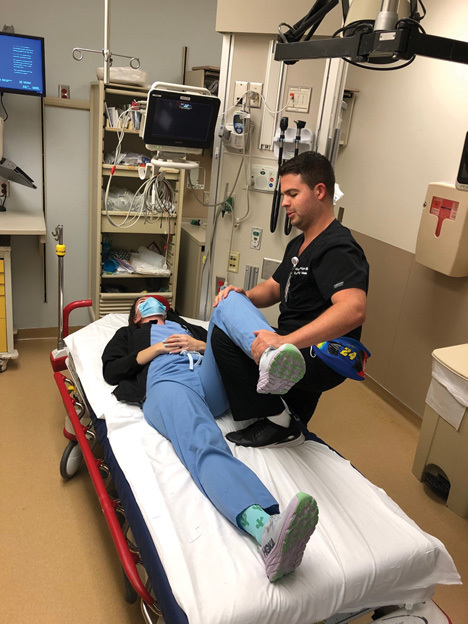 |
While the patient is supine, place your knee in a vertical position into the patient’s popliteal space with their hip and knee flexed. Press down on the patient’s foot while plantar flexing your own to use your knee as a fulcrum. |
Figure 6. Tulsa/Rochester/Whistler Hip Reduction Technique |
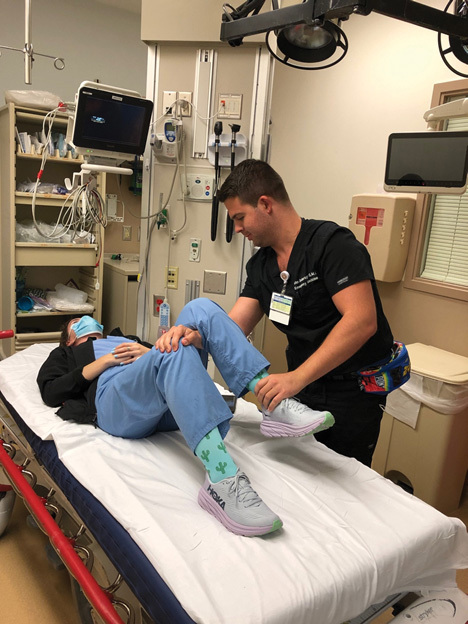 |
Stand lateral to the patient on the affected side. Place your hand on top of the unaffected knee by putting your forearm under the popliteal space of the affected limb while both hips and legs are flexed. Use your forearm as a fulcrum while pressing downward on the affected foot and internally and externally rotating the affected hip. |
Pain management is an important part of HD reduction and can be achieved by various methods. Conscious sedation is used commonly and usually is sufficient to allow reduction. Rarely, some patients require deeper sedation and require operating room-based rapid sequence intubation (RSI) and paralysis to achieve reduction. It is recommended that RSI be performed for HD reduction if the patient requires intubation for another injury or reason, the HD has been present for greater than six hours, or the reduction failed on initial attempts.33
There also is a case report describing successful reduction and analgesia achieved with a fascia iliaca block in a resource-limited setting, and this is a reasonable alternative for the EP trained in these blocks.34 Once the HD is reduced, or reduction is failed after attempts, obtain CT imaging to rule out fracture and to aid in surgical planning.
Soft tissue injuries (nerve, labral, ligamentous tears, muscle tears/avulsions, and hematomas) commonly are associated with HD, and the presence of these injuries can be assessed during post-reduction with advanced imaging, particularly MRI. Nerve injuries occur in 10% of adult HDs and 5% of pediatric HDs. The sciatic nerve is the most commonly injured with posterior HDs.27 The femoral nerve and lateral femoral cutaneous nerve also can be affected. Definitive management of HD-associated nerve injury is performed by both the trauma and orthopedic trauma surgeons. In conclusion, it is critical that EPs reduce HDs as soon as associated fractures have been ruled out and adequate sedation is achieved.
Posterolateral Corner Injuries of the Knee
The posterolateral corner (PLC) of the knee has a complex anatomical makeup and plays a large role in the overall stability of the knee joint. The PLC consists of the LCL and the popliteus complex, which consists of the popliteus tendon and arcuate complex, formed by the popliteofibular ligament, the fabellofibular ligament, and the popliteomeniscal fibers. The PLC structures provide static stabilization against tibial rotation and dynamic stabilization against external rotation, protect against varus forces, and prevent posterior tibial translation.
PLC injuries are underdiagnosed but are believed to be present in 16% to 28% of ligamentous knee injuries, 70% of PCL injuries, and multiligament knee injury.35,36 A high index of suspicion is needed to make this diagnosis. Orthopedic surgeon evaluation should be obtained in the ED if there is suspicion of a PLC injury to expedite surgical management. Significant long-term morbidity and knee instability result if PLC injuries are not corrected within 10-14 days of injury.37
PLC injury most commonly occurs from high-energy trauma to the knee directed to the anteromedial aspect with forced hyperextension. Non-contact PLC injuries can occur when a flexed knee is forcibly hyperextended and externally rotated. Careful attention should be given to the neurovascular assessment, since peroneal nerve damage occurs in 12% to 29% of PLC injuries. Additionally, popliteal artery damage can occur, as previously discussed.
Ligamentous stability should be assessed by the standard knee exam as well as varus rotation, posterolateral drawer test, reverse pivot-shift test, external rotation recurvatum, and dial test. (See Table 4.) If the patient can ambulate, the gait should be assessed.38
Table 4. Tests for Posterolateral Corner Injury |
|
Test |
Description |
Varus rotation |
Varus stress with the knee at 0 and 30 degrees flexion; isolated PLC injury will show more laxity at 30 degrees |
Posterolateral drawer test |
Apply posterior force to the knee in 80 degrees flexion with foot external rotated to 15 degrees; will show both external rotation and posterior displacement of tibia |
Reverse pivot-shift test |
Patient is supine with knee at 90 degrees flexion. Apply continuous valgus force to the knee while the foot is externally rotated and while passively extending the knee. At 20-30 degrees flexion, passive elastic tension of the IT band will cause sudden reduction of posteriorly subluxed tibia. |
External rotation recurvatum |
Patient lies supine while EP grasps the bilateral hallux and pulls. This will cause the injured knee to hyperextend, externally rotate, and become varus angulated. |
Dial test |
Compare external rotation of knee at both 30 and 90 degrees of knee flexion with the contralateral knee. Greater than 10 degrees of difference is positive for PLC injury. |
PLC: posterolateral corner; IT: iliotibial; EP: emergency physician |
|
In the acute setting, plain films and CT imaging may be completed to assess for fracture. Ultrasound also can be used to assess soft tissue structures. However, MRI is the gold standard in the diagnosis of PLC injuries.39 ABIs should be performed in all suspected PLC injuries. If the ABI is less than 0.9, vascular imaging studies should be performed (CTA of the affected extremity). Nerve conduction studies should be considered in the presence of neurologic dysfunction.40
Once a PLC injury is confirmed, surgical planning can proceed. Patients with PLCs are likely to undergo reconstruction as opposed to repair, since reconstruction has demonstrated better long-term results.41 Multiple surgical procedures usually are required with PLC injuries based on surgical protocols of the individually injured ligaments.42
In conclusion, the EP must maintain a high index of suspicion for PLC injury based on the mechanism of injury, physical exam, and imaging studies. Emergent orthopedic evaluation is necessary to ensure that the PLC is treated operatively within seven to 10 days of injury.
Posterior Sternoclavicular Dislocation and Fracture
Posterior sternoclavicular joint (SCJ) fracture-dislocations are rare but potentially life-threatening emergencies. These injuries account for less than 1% of all shoulder girdle injuries and are nine times less common than anterior SCJ dislocations, which are not considered emergencies.43 Posterior SCJ dislocations have a mean age of 25 years and are more common in males.
This injury results from sports or motor vehicle collisions, most commonly producing compression in a lateral to medial direction along with anterior motion of the shoulder girdle relative to the sternum, or less commonly by direct force in an anterior to posterior direction to the SCJ.44 Physeal fracture also can occur in younger patients because the medial physis of the clavicle is the last bone to ossify in the body around 25 years of age. This injury presents similarly to SCJ dislocation.
Posterior SCJ dislocation-fractures are particularly concerning because of the proximity of the injury to the mediastinum. Although rare, there are documented cases of injury to the ribs, vocal cords, great vessels (specifically the brachiocephalic vein), trachea, and lungs.45 These injuries are easy to overlook, so a high index of suspicion should be maintained throughout the history and physical exam.
The exam should begin with observation and palpation of the bilateral SCJs. There may be pain, tenderness, swelling, or ecchymoses over the affected joint. It is sometimes possible for a step-off to be palpated or a small deformity to be noted. The patient may have pain with, or decreased, active and passive range of motion of the arm and shoulder. Less commonly, but more concerning, the patient may have peripheral edema or distal ischemia of the ipsilateral upper extremity. If the patient presents in a non-acute setting, radiculopathy, dysphagia, or respiratory symptoms may be the predominant feature.46
Initial evaluation should include radiographs of the chest and shoulder. Because of overlapping structure, anteroposterior and lateral chest radiographs commonly miss SCJ dislocation. The serendipity view of the clavicles — in which the beam is directed from the front and angled 40 degrees caudally — or the Hobbs view — with the beam directed from the back angulated 45 degrees superiorly — potentially can aid in diagnosis. However, these views are not commonly performed, and most EPs are not familiar with their interpretation, limiting the utility of these views.
No radiographs can definitively rule out SCJ dislocation, and CT with contrast should be obtained to assess soft tissue structures around the injury for signs of compression or trauma. A CTA should be ordered if there is any suspicion for vascular injury. Point-of-care ultrasound (POCUS) with a linear probe also can be used as an imaging modality and has been shown to be capable of diagnosing the injury.47
Once diagnosed, the route of treatment depends on the severity of the dislocation and the neurovascular status of the patient. If there is any sign of neurovascular or airway compromise, reduce the SCJ emergently in the ED.48 Closed reduction can be attempted by hooking the clavicle with either the fingers (the preferred method) or a sterile towel clip piercing through the skin and pulling the clavicle anteriorly.46
During reduction, place the patient supine with folded towels between the scapula to improve joint leverage. Place the injured shoulder in 90 degrees of abduction with slight extension into the anterior plane, apply lateral traction with counter-traction, and extend the shoulder.49 After reduction, a repeat CT with contrast should be obtained. Also after reduction, place the patient in a sling until repeat examination and imaging during follow-up with an orthopedic surgeon.
If the patient is clinically stable with no signs of airway, hemodynamic, or vascular compromise, an orthopedic surgeon should be consulted immediately. The orthopedic surgeon may attempt closed reduction first and will plan operative intervention along with a cardiothoracic surgeon if open reduction is needed. If this injury remains undiagnosed and untreated, there are expected poor clinical outcomes, including damage to the mediastinal and vascular structures already mentioned, persistent pain, and persistent instability. Both open and closed reductions generally have good long-term outcomes.50
In summary, it is important for EPs to maintain a high index of suspicion for SCJ dislocation. If neurovascular compromise is identified with SCJ dislocation, immediate closed reduction should be performed while initiating orthopedic and cardiothoracic surgery consultation.
Acute Compartment Syndrome
Acute compartment syndrome (ACS) is a rise in intracompartmental pressure leading to tissue ischemia, typically resulting from a change in the arteriovenous pressure gradient.51 (See Figure 7.) ACS is a true orthopedic emergency since delayed diagnosis and treatment can lead to limb- or life-threatening outcomes for patients.52
Figure 7. Compartment Syndrome |
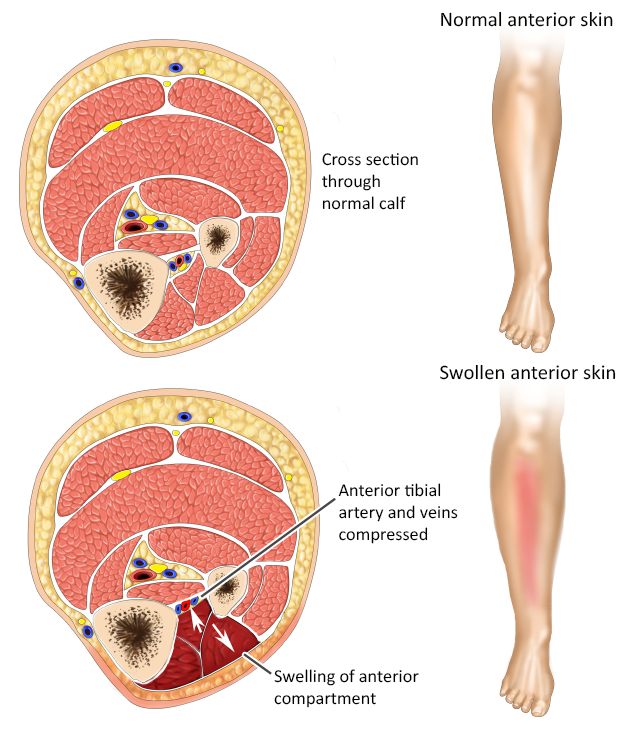 |
ACS occurs with an incidence of 3.1 per 100,000, with men, specifically those younger than 35 years of age, accounting for 10 times as many cases as women.53 ACS can occur in any myofascial compartment of the body, with tibial fractures resulting in lower leg ACS the most common. ACS also has been reported in the upper limb, thigh, abdomen, and gluteal compartments and the foot.51,54,55
ACS most commonly occurs as a result of trauma, but non-traumatic causes have been reported. Substance abuse resulting in overdose and a prolonged period of unresponsiveness is a well-established cause of compartment syndrome. This sequence of events is like that seen in crush injuries.56
Identifying ACS in overdose patients poses a challenge to EPs since these patients typically are minimally responsive and have limited ability to participate in the physical examination. It is imperative to palpate the extremities and perform passive range of motion testing in these patients, since these maneuvers often elicit non-spoken evidence of pain.
Lumbar paraspinal ACS is less common but has increased in incidence recently due to the increasing popularity of and participation in weightlifting and CrossFit. This diagnosis should be considered in the differential diagnosis of severe lower back pain in any high-intensity athlete.57 Acute exertional compartment syndrome is described in case reports in young, high-intensity athletes and military recruits. This subtype of ACS affects the lower extremity primarily and should be considered during the evaluation of the actively exercising patient.58-60
Patients with coagulation disorders, both medication-induced and genetic, are at increased risk of developing ACS.61 Non-traumatic causes of ACS are rare but include reperfusion injuries, intravenous fluid infiltration, splint or casting use, snake envenomation, and insect bites.62
The most well-known presenting signs and symptoms of ACS are commonly known as the 5 Ps: pain out of proportion to exam, pallor, paresthesias, paralysis, and pulselessness. These symptoms are not as reliable in the pediatric population. The recommended guideline for diagnosing suspected pediatric ACS is the 3 As: increasing anxiety, agitation, and analgesic requirement.63 Absent pulses, pallor, and reduced capillary return typically are seen later during the course of ACS. Once these findings are present, the patient is at risk for permanent neurovascular damage, significant morbidity, and potential amputation.64
The most reliable clinical signs of ACS are increasing pain out of proportion to exam and pain on passive stretching of the muscles within the compartment. These findings, along with invasive compartment pressure measurement, are used to diagnose ACS.65
There is no gold standard method for invasive compartment pressure measurement, nor is there an agreed upon gold standard pressure reading for the diagnosis of ACS. Adult compartment pressures typically are around 10 mmHg, with a commonly used threshold to diagnose ACS of >> 30 mmHg, or a ΔP (mean diastolic blood pressure – compartment pressure) of < 30.64 The most important step in treatment for ACS from an EP perspective is prompt recognition of ACS and immediate surgical consultation.
Fasciotomy is the definitive treatment for ACS, and time to fasciotomy is the best predictor of morbidity. The EP’s role in ACS is to resuscitate the patient, elevate the limb to the level of the heart, remove external compressive devices, reduce displaced fractures, and provide analgesia.66
Currently there is debate over whether regional nerve blocks mask ACS and lead to delayed diagnosis and, thus, worse outcomes; however, no strong evidence exists to support either side.67-70 Therefore, nerve blocks currently are not recommended for the treatment of suspected ACS pain without prior multidisciplinary agreement with both the surgical and anesthesia teams. Intravenous (IV) opioids or IV ketamine still is preferred pain control.
The laboratory workup for ACS should include creatine kinase (CK) levels, since levels > 2,000 U/L should raise suspicion for ACS. A basic metabolic panel (BMP) should be performed to assess kidney function because rhabdomyolysis commonly is seen in ACS, and acute kidney injury (AKI) is seen in 10% to 55%. Complete blood count (CBC), type and cross, and coagulation studies also should be ordered in anticipation of possible operative intervention.71
Complications as a result of ACS are common and include amputation (6% to 13% of patients), chronic pain, sensory abnormalities (> 50% of patients), and infection and chronically decreased range of motion (up to 69% of patients).71 Pediatric ACS patients have significantly better clinical outcomes than their adult counterparts, including better long-term function and lower infection rates, even with delayed diagnosis and treatment.72-74
REFERENCES
- Weinick RM, Burns RM, Mehrotra A. Many emergency department visits could be managed at urgent care centers and retail clinics. Health Aff 2010;29:1630-1636.
- Ockuly AC, Imada AO, Richter DL, et al. Initial evaluation and classification of knee dislocations. Sports Med Arthrosc Rev 2020;28:87-93.
- Goebel CP, Domes C. Classifications in brief: The Schenck classification of knee dislocations. Clin Orthop Relat Res 2020;478:1368-1372.
- Trasolini NA, Lindsay A, Gipsman A, Rick Hatch GF. The biomechanics of multiligament knee injuries. Clin Sports Med 2019;38:215-234.
- Hussin P, Mawardi M, Ab Halim AH. A rare variant of knee dislocation. G Chir 2016;37:71-73.
- Hoit G, Farag J, Whelan DB. Neurologic assessment and management of the multiple ligament injured knee: A review and synthesis of current evidence. J Knee Surg 2020;33:339-345.
- Rihn JA, Groff YJ, Harner CD, Cha PS. The acutely dislocated knee: Evaluation and management. J Am Acad Orthop Surg 2004;12:334-346.
- Maslaris A, Brinkmann O, Bungartz M, et al. Management of knee dislocation prior to ligament reconstruction: What is the current evidence? Update of a universal treatment algorithm. Eur J Orthop Surg Traumatol 2018;28:1001-1015.
- James EW, Wolfe I, Marx RG. Results of treatment of the multiple ligament injured (dislocated) knee. Sports Med Arthrosc Rev 2020;28:116-119.
- Anazor FC, Baryeh K, Davies NC. Knee joint dislocation: Overview and current concepts. Br J Hosp Med 2021;82:1-10.
- Matthewson G, Kwapisz A, Sasyniuk T, MacDonald P. Vascular injury in the multiligament injured knee. Clin Sports Med 2019;38:199-213.
- Whitehouse MR, McDaid C, Kelly MB, et al. The effect of timing of antibiotic delivery on infection rates related to open limb fractures: A systematic review. Emerg Med J 2017;34:613-620.
- Myatt A, Saleeb H, Robertson GAJ, et al. Management of Gustilo–Anderson IIIB open tibial fractures in adults—a systematic review. Br Med Bull 2021;139:48-58.
- Elia G, Blood T, Got C. The management of pediatric open forearm fractures. J Hand Surg Am 2020;45:523-527.
- Yim GH, Hardwicke JT. The evolution and interpretation of the Gustilo and Anderson classification. J Bone Joint Surg Am 2018;100:e152.
- Loh B, Lim JA, Seah M, Khan W. Perioperative management of open fractures in the lower limb. J Perioper Pract 2022;32:100-107.
- British Orthopaedic Association Standard for Trauma (BOAST): Open fracture management. Injury 2020;51:174-177.
- Rupp M, Popp D, Alt V. Prevention of infection in open fractures: Where are the pendulums now? Injury 2020;51(Suppl 2):S57-S63.
- Samai K, Vilella A. Update in therapeutics: Prophylactic antibiotics in open fractures. J Trauma Nurs 2018;25:83-86.
- Chan JK-K, Aquilina AL, Lewis SR, et al. Timing of antibiotic administration, wound debridement, and the stages of reconstructive surgery for open long bone fractures of the upper and lower limbs. Cochrane Database Syst Rev 2022;4:CD013555.
- Mauffrey C, Hak DJ, Rojas D, et al. Prevention of the infected fracture: Evidence-based strategies for success! J Orthop Trauma 2019;33(Suppl 6):S1-S5.
- Garner MR, Sethuraman SA, Schade MA, Boateng H. Antibiotic prophylaxis in open fractures: Evidence, evolving issues, and recommendations. J Am Acad Orthop Surg 2020;28:309-315.
- Chang Y, Bhandari M, Zhu KL, et al. Antibiotic prophylaxis in the management of open fractures: A systematic survey of current practice and recommendations. JBJS Rev 2019;7:e1-e1.
- Ahmed G, Shiraz S, Riaz M, Ibrahim T. Late versus early reduction in traumatic hip dislocations: A meta-analysis. Eur J Orthop Surg Traumatol 2017;27:1109-1116.
- Wojahn RD, Kleweno CP, Agel J, Githens MF. Anterior hip dislocation: Characterization of a rare injury and predictors of functional outcome. Injury 2021;52:2327-2332.
- Syam K, Saibaba B, Aggarwal S, et al. Update review and clinical presentation in adult inferior dislocation of hip. Eur J Orthop Surg Traumatol 2017;27:1039-1044.
- Mandell JC, Marshall RA, Weaver MJ, et al. Traumatic hip dislocation: What the orthopedic surgeon wants to know. Radiographics 2017;37:2181-2201.
- Pfeifer K, Leslie M, Menn K, Haims A. Imaging findings of anterior hip dislocations. Skeletal Radiol 2017;46:723-730.
- Liu J, Li Z, Ding J, et al. Femoral neck fracture combined with anterior dislocation of the femoral head: Injury mechanism and proposed novel classification. BMC Musculoskelet Disord 2021;22:810.
- Nicholson JA, Scott CEH, Annan J, et al. Native hip dislocation at acetabular fracture predicts poor long-term outcome. Injury 2018;49:1841-1847.
- Ahmed G, Shiraz S, Riaz M, Ibrahim T. Late versus early reduction in traumatic hip dislocations: A meta-analysis. Eur J Orthop Surg Traumatol 2017;27:1109-1116.
- Gottlieb M. Hip dislocations in the emergency department: A review of reduction techniques. J Emerg Med 2018;54:339-347.
- Bommiasamy AK, Opel D, McCallum R, et al. Conscious sedation versus rapid sequence intubation for the reduction of native traumatic hip dislocation. Am J Surg 2018;216:869-873.
- West C, Ranganath Y, Willey M. Fascia iliaca block for reduction of anterior native hip dislocation: A case report. Iowa Orthop J 2017;37:19-21.
- Weiss S, Krause M, Frosch KH. Posterolateral corner of the knee: A systematic literature review of current concepts of arthroscopic reconstruction. Arch Orthop Trauma Surg 2020;140:2003-2012.
- Chenard KE, Jazrawi LM, Alaia MJ. Posterolateral corner injury evolution of diagnosis and treatment. Bull Hosp Jt Dis (2013) 2020;78:6-11.
- Davenport M, Oczypok MP. Knee and leg injuries. Emerg Med Clin North Am 2020;38:143-165.
- Nannaparaju M, Mortada S, Wiik A, et al. Posterolateral corner injuries: Epidemiology, anatomy, biomechanics and diagnosis. Injury 2018;49:1024-1031.
- Khodarahmi I, Alizai H, Alaia E, Gyftopoulos S. MR imaging of the knee posterolateral and posteromedial corner injuries. Magn Reson Imaging Clin N Am 2022;30:215-226.
- Chahla J, Murray IR, Robinson J, et al. Posterolateral corner of the knee: An expert consensus statement on diagnosis, classification, treatment, and rehabilitation. Knee Surg Sports Traumatol Arthrosc 2019;27:2520-2529.
- Shon OJ, Park JW, Kim BJ. Current concepts of posterolateral corner injuries of the knee. Knee Surg Relat Res 2017;29:256-268.
- Porrino J, Sharp JW, Ashimolowo T, Dunham G. An update and comprehensive review of the posterolateral corner of the knee. Radiol Clin North Am 2018;56:935-951.
- McAleese T, Curtin M, Collins D. Posteriorly displaced salter halter fracture-dislocation at the sternoclavicular joint with associated thoracic outlet syndrome: A case report. Int J Surg Case Rep 2020;72:245-250.
- Kendal JK, Thomas K, Lo IKY, Bois AJ. Clinical outcomes and complications following surgical management of traumatic posterior sternoclavicular joint dislocations. JBJS Rev 2018;6:e2.
- Fournier MN, Sinclair MR, Zheng ET, et al. The frequency of mediastinal injury in acute posterior sternoclavicular dislocations: A multicenter study. J Pediatr Orthop 2020;40:e927-e931.
- Carius BM, Long B, Gottlieb M. Evaluation and management of sternoclavicular dislocation in the emergency department. J Emerg Med 2021;61:499-506.
- Bengtzen RR, Petering RC. Point-of-care ultrasound diagnosis of posterior sternoclavicular joint dislocation. J Emerg Med 2017;52:513-515.
- Chen X, Shafer D, Neeki AS, et al. Emergent management of traumatic posterior sternoclavicular joint dislocation: A case report and literature review. Cureus 2021;13:e18996.
- Honeycutt MW, Cox K, Michaeli D, et al. Pediatric posterior sternoclavicular dislocation closed reduction and management. J Orthop Trauma 2021;35(Suppl 2):S11-S12.
- Sernandez H, Riehl J. Sternoclavicular joint dislocation: A systematic review and meta-analysis. J Orthop Trauma 2019;33:e251-e255.
- McMillan TE, Gardner WT, Schmidt AH, Johnstone AJ. Diagnosing acute compartment syndrome—where have we got to? Int Orthop 2019;43:2429-2435.
- Guo J, Yin Y, Jin L, et al. Acute compartment syndrome: Cause, diagnosis, and new viewpoint. Medicine (Baltimore) 2019;98:e16260.
- Ahluwalia A, Tiwari K, Somashaker N. Acute compartment syndrome in the limb. Br J Hosp Med (Lond) 2020;81:1-6.
- Chen JS, Tejwani NC. Compartment syndrome of the foot. Orthop Clin North Am 2022;53:83-93.
- Adib F, Posner AD, O’Hara NN, O’Toole RV. Gluteal compartment syndrome: A systematic review and meta-analysis. Injury 2022;53:1209-1217.
- Mortensen SJ, Smith RDJ, von Keudell GR, et al. Substance-related found-down compartment syndrome: A systematic review. J Orthop Trauma 2021;35:e247-e253.
- Alexander W, Low N, Pratt G. Acute lumbar paraspinal compartment syndrome: A systematic review. ANZ J Surg 2018;88:854-859.
- McKinney B, Gaunder C, Schumer R. Acute exertional compartment syndrome with rhabdomyolysis: Case report and review of literature. Am J Case Rep 2018;19:145-149.
- Livingston KS, Meehan WP 3rd, Hresko MT, et al. Acute exertional compartment syndrome in young athletes. Pediatr Emerg Care 2018;34:76-80.
- Guenther TM, Sherazee EA, Curtis BC, Riojas RA. Acute exercise induced compartment syndrome in an 22-year-old active-duty man and review of the literature. Mil Med 2020;185:e1829-e1832.
- Inagaki N, Udaka J, Nishiwaki K, et al. Acute compartment syndrome of the upper extremity in acquired hemophilia A: A case report and literature review. JBJS Case Connect 2021;11. doi: 10.2106/JBJS.CC.21.00304
- Ogrodnik J, Oliver JD, Cani D, et al. Clinical case of acute non-traumatic hand compartment syndrome and systematic review for the upper extremity. Hand (N Y) 2019;16:285-291.
- Gresh M. Compartment syndrome in the pediatric patient. Pediatr Rev 2017;38:560-565.
- Duckworth AD, McQueen MM. The diagnosis of acute compartment syndrome: A critical analysis review. JBJS Rev 2017;5:e1.
- Mortensen SJ, Vora MM, Mohamadi A, et al. Diagnostic modalities for acute compartment syndrome of the extremities: A systematic review. JAMA Surg 2019;154:655.
- Long B, Koyfman A, Gottlieb M. Evaluation and management of acute compartment syndrome in the emergency department. J Emerg Med 2019;56:386-397.
- Marhofer P, Halm J, Feigl GC, et al. Regional anesthesia and compartment syndrome. Anesth Analg 2021;133:1348-1352.
- Tran AA, Lee D, Fassihi SC, et al. A systematic review of the effect of regional anesthesia on diagnosis and management of acute compartment syndrome in long bone fractures. Eur J Trauma Emerg Surg 2020;46:1281-1290.
- Klucka J, Stourac P, Stouracova A, et al. Compartment syndrome and regional anaesthesia: Critical review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:242-251.
- Nathanson MH, Harrop‐Griffiths W, Aldington DJ, et al. Regional analgesia for lower leg trauma and the risk of acute compartment syndrome: Guideline from the Association of Anaesthetists. Anaesthesia 2021;76:1518-1525.
- Schellenberg M, Chong V, Cone J, et al. Extremity compartment syndrome. Curr Prob Surg 2018;55:256-273.
- Livingston KS, Glotzbecker MP, Shore BJ. Pediatric acute compartment syndrome. J Am Acad Orthop Surg 2017;25:358-364.
- Lin JS, Samora JB. Pediatric acute compartment syndrome: A systematic review and meta-analysis. J Pediatr Orthop B 2020;29:90-96.
- Gottlieb M, Adams S, Landas T. Current approach to the evaluation and management of acute compartment syndrome in pediatric patients. Pediatr Emerg Care 2019;35:432-437.
