Ruptured Abdominal Aortic Aneurysms
November 15, 2023
Related Articles
-
Echocardiographic Estimation of Left Atrial Pressure in Atrial Fibrillation Patients
-
Philadelphia Jury Awards $6.8M After Hospital Fails to Find Stomach Perforation
-
Pennsylvania Court Affirms $8 Million Verdict for Failure To Repair Uterine Artery
-
Older Physicians May Need Attention to Ensure Patient Safety
-
Documentation Huddles Improve Quality and Safety
AUTHORS
Zoe Glick, MD, Department of Emergency Medicine, University of Maryland Medical Center, Baltimore
R. Gentry Wilkerson, MD, Associate Professor, Department of Emergency Medicine, University of Maryland School of Medicine, Baltimore
PEER REVIEWER
Catherine A. Marco, MD, FACEP, Professor, Department of Emergency Medicine, Penn State Health, Hershey Medical Center, Hershey, PA
EXECUTIVE SUMMARY
- Mortality for ruptured abdominal aortic aneurysm (AAA) can be as high as 80%.
- Risk factors associated with AAA include advanced age, smoking, hypertension, family history, and hypercholesterolemia.
- The classic triad for ruptured AAA is hypotension, flank pain/back pain, and a pulsatile abdominal mass, although only 25% to 50% of patients will present with all three.
- Repair generally is recommended for men with AAA measuring 5.5 cm or greater. For women, it may be lower.
- To date, there are no medications proven to be effective at preventing the growth and rupture of AAA.
- Short-term mortality is lower with endovascular repair; however, long-term mortality after endovascular repair compared to open surgical repair is similar and, thus, both approaches should be considered.
Recently the American College of Emergency Physicians (ACEP) created a quality measure, just adopted by the Centers for Medicare and Medicaid Services, that suggests early ultrasound for patients presenting with new abdominal or back pain and hypotension who have not been screened for an abdominal aneurysm at age 55-65 years or older.
— Sandra Schneider, MD, FACEP, Editor
Introduction
The word “aneurysm” is derived from the Greek word “aneurysma,” which means “widening.” An abdominal aortic aneurysm (AAA) is a pathologic dilation of the aorta that can grow over time and progress to rupture. Although screening and surveillance have decreased morbidity and mortality related to AAAs, clinical decision-making, including diagnosis and treatment, can be difficult, and the risk of rupture and misdiagnosis leads to poor health outcomes. Vessel walls rely on continual maintenance and regeneration to maintain structural integrity. When these processes become disrupted, portions of vessel walls can weaken, leading to dilation. Aneurysms rupture when the stress of blood flow exceeds the vessel wall strength and results in catastrophic outcomes.1
Although normal size varies with age, sex, and body habitus, dilation of the aorta greater than 3.0 cm generally is considered aneurysmal.2,3 Ruptured AAAs are associated with significant morbidity and mortality and can be difficult to diagnose in a timely fashion. Given the prevalence and devastating impacts if left unrecognized, leading to subsequent rupture, it is imperative for emergency physicians to understand the disease process, as well as diagnosis, management, and definitive treatment. This article will review current information and recent literature on AAAs, including epidemiology, risk factors, common and uncommon presentations, pathophysiology, diagnosis, and treatment.
Epidemiology
The epidemiology of AAAs has changed in recent years in part due to implementation of screening programs, surveillance, risk modification, and repair. An increase in the prevalence of AAAs was seen throughout the 20th century; however, the prevalence over the past 20 years has been declining in several countries, including the United Kingdom, Sweden, New Zealand, and Denmark. This decline mainly is attributed to the decline of smoking, a known risk factor for AAA.4 The prevalence of AAA ranges from 2% to 12%. The incidence of AAA increases 6% per decade for men older than 65 years of age.5 In the United States, it is estimated that 1.4% of individuals 50-84 years of age have an AAA, or approximately 1.1 million adults.3 Mortality rates attributable to AAA in the United States are approximately 15,000 per year, and AAA is the 15th most frequent cause of mortality in the United States.6,7 The prevalence of AAA is approximately six times higher in men than women and is estimated to be up to 8% for men older than 65 years of age.1,2,6 The prevalence is much lower for women of similar age, estimated as low as 1% to 2%.8,9
Ruptured AAAs carry a significant mortality rate, estimated at 80%.2,4,10 The majority of patients with AAA are asymptomatic until the aneurysm ruptures. It is estimated that nearly one-third of patients who experience a ruptured AAA die before reaching a hospital.2 Although morbidity and mortality from ruptured AAA remains high, the incidence from death has declined 50% in the past 20 years. This is due to multiple factors, including a decline in cigarette smoking, increased surveillance and public awareness, improved surgical techniques, and management of risk factors. Aneurysm rupture remains high in countries with high or increasing cigarette use.3,6,11,12
Classification
Aneurysms occur in all parts of the body, commonly in the thoracic aorta, abdominal aorta, and circle of Willis. The pathogenesis of aneurysm formation in parts of the body has distinct features and proposed mechanisms. For example, smooth muscle cells in the thoracic aorta are derived from neural crest cells and somitic mesoderm, compared to splanchnic mesoderm in the abdominal aorta. This discussion will focus on aneurysms in the abdominal aorta.1,13
The abdominal aorta starts under the diaphragm around the 12th thoracic vertebra and is located in the retroperitoneum to the left of the vertebral column. With age, the aorta elongates and enlarges, making aneurysm location variable. At the level of the umbilicus around the fourth lumbar vertebra, the aorta bifurcates into the right and left iliac arteries.3 The intrarenal aorta is by far the most common location for AAA formation. It is involved in 80% of AAAs and represents 30% of all aneurysms.14
Although more rare, complex aneurysms can occur in other parts of the abdominal aorta and typically require a more intricate reconstruction and repair. Complex aneurysms include juxta-renal (those occurring near the renal arteries), supra-renal (those occurring above the renal arteries), thoraco-abdominal (aneurysms that are more extensive and involving the thoracic and abdominal aorta), and pararenal (involving the renal arteries).15 Approximately 25% of AAAs extend into one or more of the common iliac arteries, and 7% into the internal iliac arteries. The exact cause for why some locations have higher predisposition to aneurysm formation is not completely understood.16
Etiology
The etiology of AAA remains a topic of continued investigation. Until the 20th century, atherosclerotic disease and syphilis were seen as the two most important causes of aneurysms.1 Further research has shown that contributing causes are more nuanced and diverse. Known causes include trauma, infection, connective tissue disorders, and inflammatory disorders.1,4 The development of atherosclerotic disease and AAA have similar risk factors. Research suggests plaque deposition can alter membrane integrity, leading to thinning of the middle membrane and predisposition to aneurysm formation; however, the extent of causality between atherosclerosis and AAA formation remains controversial.1,17
Infection can lead to breakdown of vessel membrane and affect wall integrity. Syphilis, tuberculosis, fungal infections, and other bacterial infections can lead to the development of AAA. Infection can cause an arteritis in the middle layer, which causes a breakdown of elastic fibers, leading to dilation or formation of cystic hematomas. Inflammatory conditions also can predispose to AAA formation. Conditions such as Takayasu’s arteritis, giant cell arteritis, and Behçet’s disease can cause an inflammatory arteritis. Connective tissue disorders such as Marfan syndrome and Ehlers-Danlos Type IV are associated with a higher risk of aneurysm formation. Marfan syndrome, for example, is a mutation in the extracellular matrix protein fibrillin 1. Individuals with this condition have reduced elastin content in the aorta, which can result in aneurysm formation and dissection if the fibers become compromised.1
Risk Factors
The most important risk factors for the development of AAA include male sex, cigarette smoking, advanced age, and having a first-degree relative with AAA.1,4 (See Table 1.) Sex plays a significant role when evaluating the risk of AAA. Older men are at highest risk, and the prevalence of AAA is six times higher in men than in women.18 Age is a significant contributing factor for both women and men. One study found age as the strongest predictive factor for development of an AAA.3 After 65 years of age, the risk of developing an AAA increases 40% every five years.18
Table 1. Risk Factors Associated with Development of Abdominal Aortic Aneurysm (AAA)3 |
||
Risk Factor |
Odds Ratio |
P Value |
Male (compared to female) |
5.71 |
< 0.0001 |
Age (vs. < 55 years) |
||
55-59 |
2.76 |
< 0.0001 |
60-64 |
5.35 |
< 0.0001 |
65-69 |
9.41 |
< 0.0001 |
70-74 |
14.46 |
< 0.0001 |
75-79 |
20.43 |
< 0.0001 |
80-84 |
28.37 |
< 0.0001 |
Smoking, 0.5-1 pack/day |
||
Less than 10 years |
3.19 |
< 0.0001 |
11-20 |
5.79 |
< 0.0001 |
21-35 |
7.99 |
< 0.0001 |
> 35 |
11.19 |
< 0.0001 |
High blood pressure |
1.25 |
< 0.0001 |
Family history of AAA |
3.80 |
< 0.0001 |
High cholesterol |
1.34 |
< 0.0001 |
Cerebrovascular disease |
1.18 |
< 0.0001 |
Diabetes |
0.75 |
< 0.0001 |
Smoking is one of the strongest modifiable risk factors associated with prevalence, growth, and rupture of AAAs. An “ever smoker” is defined as an individual who has smoked 100 cigarettes or more in their lifetime. Stronger smoking history and AAA have a dose-dependent relationship, and each year of smoking increases the relative risk of AAA development by 4%.1,3,4 One retrospective analysis found smoking was the most significant risk factor for AAA and carries a relative risk of 5.9-7.6 when compared to nonsmokers.1,3,5 Former smokers still were three times more likely to develop aneurysms when compared to never smokers.1 Smoking not only is associated with development of AAA but also increases the rate of growth by 35% and carries double the rate of rupture in current smokers.3,19
Cigarette smoking is associated with different aneurysm risks when comparing women and men. One study found that when compared to never smokers, the hazard ratio for AAA in current smokers with more than 20 pack-years was 10.97 for women and 6.55 for men.20 The risk of AAA from inhaled, vaporized nicotine compared to other modalities of consumption is not well understood; however, studies have concluded that exposure to nicotine alone is associated with development of AAA regardless of consumptive method.3
Family history is an important risk factor for developing an AAA. Having a first-degree relative with AAA increases a person’s risk of developing AAA by two-fold,2,4,21 although more conservative estimates are closer to 20%.3,22,23 More research is required to detect the specific genes and genetic factors that confer this heritable risk.1
Other risk factors include coronary artery disease, cerebrovascular disease, atherosclerosis, hypercholesterolemia, and hypertension. Hypertension, hypercholesterolemia, and known coronary artery disease carry a relative risk of < 1.5.5 Elevated mean blood pressure has an independent risk for rupture, demonstrating the involvement of flow dynamics and wall integrity in the pathophysiology.1,19 Several factors are noted to have a decreased risk of AAA, including African-American race, Hispanic ethnicity, Asian ethnicity, and, interestingly, diabetes.4 The mechanism behind the inverse relationship between diabetes and AAA is incompletely understood; however, one proposed mechanism involves changes with glycosaminoglycans in the aorta, alteration of extracellular matrix (ECM) remodeling, and subsequent structure of the aortic wall.1
Pathophysiology
Although a variety of proposed mechanisms exist regarding the exact cause of AAAs, a singular mechanism has not yet been identified. Current research shows a complex interaction between environmental exposures and dysfunction at the cellular level. Pathologic responses, including matrix remodeling, inflammatory and immune responses, oxidative stress, and smooth muscle cellular abnormalities, all have been cited as contributing factors in the formation of AAA.24
Extracellular Matrix Remodeling
The formation of AAAs occurs at the cellular level within each aortic layer. Arterial walls consist of three layers: tunica intima (innermost layer consisting of endothelial cells); tunica media (middle layer made up of mostly smooth muscle cells with ECM support proteins such as elastin and collagen); and the adventitia (external layer of blood vessels containing many cells, including fibroblasts, macrophages, adipocytes, and pericytes). Stem cells within these layers can aid in endothelial repair and regeneration.13
The ECM is comprised mostly of elastin, collagens, glycoproteins, and proteoglycans, all of which contribute to vascular wall strength and stability.24 An initial arterial wall insult causes subsequent inflammation and triggers ECM protein breakdown by proteinases, leading to arterial wall weakening.2
Inflammatory Processes and Targeted Medications
Early infiltration of myeloid cells, such as neutrophils, monocytes, macrophages, and dendritic cells, into the aortic wall leads to its destruction. Neutrophils are highly abundant in AAA walls and are recruited early on during the inflammatory process. Other cells are involved in the activation of pro-inflammatory molecules, which trigger immune mechanisms and contribute to wall weakening.7
Although inflammation and dysfunction of the arterial wall leads to weakening and aortic aneurysm formation, medications and treatments targeting these cells have not been shown to be effective in preventing aneurysm formation, growth, and rupture.5,25-27 Medications such as statins, beta blockers, and other antihypertensives are used to reduce cardiovascular risk but do not reduce AAA growth.25 Randomized clinical trials have even studied antibiotics, such as doxycycline, to reduce aneurysm growth but have not been shown to reduce aneurysm size.28
Oxidative Stress
There is increased oxidative stress in aneurysms, which contributes to the pathogenesis of AAA. Reactive oxygen species (ROS) damage cellular components such as deoxyribonucleic acid (DNA), lipids, and proteins, which cause cellular dysfunction. Some of the known pathological effects of ROS are seen in AAAs, as excess ROS leads to activation of matrix metalloproteinases, induction of pro-inflammatory genes, and apoptosis.5
Genetics
Given that family history is a risk factor for AAA, studies have attempted to identify the exact genes involved in AAA formation. Meta-analysis of genome-wide association studies have identified nine AAA risk loci on various chromosomes.23 (See Table 2.) Although associated genes have been identified, these are seen only in a small portion of AAA, which does not fully account for the strong family association seen in AAA. Further research is needed in this area.
Table 2. Loci Associated with Abdominal Aortic Aneurysm23 |
|
Chromosome |
Nearby Gene(s) |
1 |
PSRC1-CELSR2-SORT1 |
1 |
IL6R |
1 |
SMYD2 |
9 |
DAB2IP |
9 |
CDKN2BAS1/ANRIL |
13 |
LINC00540 |
19 |
LDLR |
20 |
PCIF1/MMP9/ZNF335 |
21 |
ERG |
Clinical Presentation
Individuals with AAAs remain largely asymptomatic until rupture.19 Symptomatic but unruptured AAA can present with a variety of symptoms, including pulsatile abdominal pain and/or back pain.18 The most common presenting symptoms can be found in Table 3.2 The primary risk associated with AAAs is rupture leading to hemorrhagic shock and death.25 Other intraabdominal pathologies, such as cholecystitis, nephrolithiasis, pancreatitis, and appendicitis, can mimic a symptomatic AAA.3 Complications of unruptured AAAs can include embolization, acute thrombosis, hydronephrosis, and pain caused by compression of nearby structures. For example, AAA erosion into the vertebral column can cause back pain as the presenting symptom.1
Table 3. Most Common Presenting Symptoms in Patients with Unruptured Abdominal Aortic Aneurysm |
|
Symptom |
Frequency (%) |
Abdominal pain |
27 |
Back pain |
25 |
Flank pain |
5 |
Groin pain |
5 |
Chest pain |
2 |
Reprinted from Progress in Cardiovascular Diseases, volume 65, Anagnostakos J, Lal BK. Abdominal aortic aneurysms. Pages 34-43, copyright 2021, with permission from Elsevier. |
|
Mortality rates remain high for ruptured AAAs. Early identification is crucial because this is a surgical emergency. Unfortunately, up to 50% of patients will have rupture as their first presentation of AAA. Rupture can occur spontaneously or in the setting of trauma. The classic triad of a ruptured AAA is hypotension, flank or back pain, and a pulsatile abdominal mass.18 Similar to many “classic presentations” in medicine, only 25% to 50% of patients will present with this triad.1,10
An emergency provider should have a high suspicion for this pathology in patients older than 50 years of age with acute onset back pain or abdominal pain that cannot be relieved, especially in the setting of hypotensive shock. Other associated symptoms can include shortness of breath, tachycardia, syncope, flank pain, and flank or periumbilical ecchymosis (Grey Turner or Cullen signs, respectively).18 Less common presenting symptoms can include chest pain, groin pain, dizziness, nausea, and vomiting.29 Uncommon presentations include gastrointestinal bleeding caused by aortoenteric fistulas, painless testicular ecchymosis, and even lower extremity edema caused by compression of the inferior vena cava.30-32
Physical Exam
Physical exam is only moderately sensitive for detecting AAA. Some of the difficulties arise from diverse patient presentations, location, and size of the AAA. A full physical examination should be performed in patients with a suspected AAA. Although patients with pain and tenderness upon palpation of the abdomen have a greater risk of rupture, physical exam has not been attributed as a cause for rupture in patients with AAA.3,18
A pulsatile mass can sometimes be felt; however, the absence of a pulsatile mass does not rule out the diagnosis. Physical exam can be even less reliable in detecting AAA in patients with smaller aneurysms and those with higher body mass index (BMI). Palpation of the supraumbilical region has a sensitivity of 61% for aneurysms 3.0 cm to 3.9 cm, 69% for aneurysms 4.0 cm to 4.9 cm, and 82% for aneurysms 5.0 cm and larger.10 There is an association with popliteal aneurysms and AAA. In patients with popliteal aneurysms, approximately 37% to 40% also will have AAA.3
Shock is a presenting symptom in those with a ruptured AAA secondary to blood loss. The degree of shock is variable. Rupture of the anterior wall into the peritoneum often is more severe, whereas a posterior rupture into the retroperitoneum can sometimes be contained or temporarily sealed off. An initial small posterior tear will evolve into a larger rupture within hours.1
Misdiagnosis
Ruptured AAAs have a high misdiagnosis rate, up to 33% on initial presentation. The most common misdiagnosis for ruptured AAA is renal colic.18 Misdiagnosis of ruptured AAA is associated with increased mortality. One multicenter, retrospective cohort study of 455 patients in Sweden showed a misdiagnosis rate of 38.9%. A mortality rate of 74.6% was seen in patients who were initially misdiagnosed compared to 62.9% in correctly diagnosed patients. This included both patients who underwent surgery and those who did not. Rates of misdiagnosis were similar between women and men. Presenting symptoms were found to have either a positive or negative effect on accurate diagnosis. Abdominal pain, back pain, and syncope were more commonly described in correctly diagnosed patients, whereas vomiting and dyspnea were more commonly associated with misdiagnosis. No significant association with correct or incorrect diagnosis was seen with nausea, groin pain, flank pain, dizziness, or chest pain as a presenting symptom. Patients with an initial systolic blood pressure (SBP) less than 90 mmHg were less frequently misdiagnosed (P = 0.001), although this was not associated with a significant change in mortality. Independent risk factors for increased mortality included misdiagnosis, age, female sex, and lower serum creatinine level.29 It is important to note that in this study, ultrasound was not routinely used in the emergency department (ED), which limits its generalizability to other ED settings.
Growth and Rupture
AAAs are defined as having an aortic diameter greater than 3.0 cm. Growth can occur silently and in the absence of symptoms. For every 0.5 cm increase in AAA diameter, growth rates increase by 0.5 mm a year and have double the rupture rate. Understanding factors that carry an increased risk of rupture is important in managing a patient with known AAA. The Small Aneurysm Trial (UKSAT) in the United Kingdom found various risk factors demonstrating an independent risk for aneurysm rupture. These included female sex, large diameter, low forced expiratory volume in one second, current smoking, and elevated mean arterial blood pressure.3
In the Aneurysm Detection and Management (ADAM) trial, rupture risk was 9% per year for patients with AAAs between 5.5 cm and 5.9 cm, 10% for AAAs 6.0 cm and 6.9 cm, and 33% for those greater than 7.0 cm. More recent comprehensive studies have shown this risk may be lower than previously thought, showing an annual rupture risk of 5.3% for AAAs between 5.5 cm and 7.0 cm and 6.3% for AAAs greater than 7.0 cm.3,33,34
Women have a lower prevalence of AAA; however, rupture rates are four times higher in women.19 One study showed the average risk of rupture per year for aneurysms 5.0 cm to 5.9 cm in men was 1.0%, compared to 3.9% in women. The annual risk of rupture for aneurysms 6.0 cm or greater increased significantly to 14.1% for men and 23.3% in women.35 This difference suggests there may be utility in having a lower threshold for aneurysmal repair for women.
Smoking increases rates of AAA in both women and men; however, cessation of smoking in women has an accelerated decline in the risk of developing AAA compared to men. The risk was halved after 11 years for women and 23 years for men. Other populations who have a greater risk of rupture include patients on immunomodulatory therapy after organ transplants.20
Diagnosis
A variety of modalities should be used in the evaluation and diagnosis of patients with a suspected AAA. Abdominal ultrasound is the gold standard and imaging modality of choice for diagnosis. Ultrasound is the preferred method because of its low cost, lack of ionizing radiation, noninvasive nature, and high sensitivity and specificity for detection of AAA.36
Ultrasound provides an additional potential benefit in that it is readily available in most EDs in the United States. Aortic diameter can be measured either from outer-to-outer (OTO) aortic wall (adventitia) or from inner-to-inner (ITI) maximum anterior-posterior aortic diameter. Some research suggests measuring ITI can underestimate the size of the aneurysm and, therefore, OTO is usually recommended.37,38 This modality provides quick, reliable information with a sensitivity of 96.3% and a specificity of 100% for ruptured AAAs.18 Other literature has noted that although ultrasound has a high sensitivity in identifying AAA, there is less consistency with diagnosing ruptured AAA by ultrasound because of the frequency of rupture into the retroperitoneal space.10
Ultrasound is the modality of choice for detection and screening; however, computed tomography (CT) angiography of the abdomen is helpful to better characterize the anatomical location and aneurysm characteristics, predict prognosis, and can aid in surgical planning. (See Figures 1 and 2.) Understanding the exact location of the aneurysm in relationship to the renal arteries and other vascular structures can help with repair approach and feasibility. For example, aneurysms with shorter necks have poorer outcomes. In stable patients, it also can help identify impending rupture and the extent of aortic wall involvement. It should be noted that unstable patients should not be sent to the CT scanner prior to stabilization.18
Figure 1. Computed Tomography Showing Abdominal Aortic Aneurysm with Intramural Thrombus, Transverse Plane View |
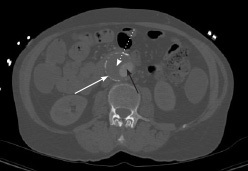 |
White arrow: aortic wall; black arrow: aortic lumen; white dotted arrow: thrombus Source: Image courtesy of J. Stephan Stapczynski, MD. |
Figure 2. Computed Tomography Showing Abdominal Aortic Aneurysm with Intramural Thrombus, Sagittal Plane View |
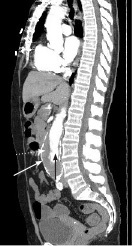 |
White arrow: aortic wall; black arrow: aortic lumen; white dotted arrow: thrombus Source: Image courtesy of J. Stephan Stapczynski, MD. |
Understanding the resources available in each clinical setting is important, since surgery is the definitive management for ruptured AAA. There is no single blood test that can be used to diagnose AAA, but bloodwork can be used to obtain baseline information about organ function and acid base status, and can provide information to help guide surgical candidacy and pre-surgical optimization.18
Differential Diagnosis
Because of the varying presenting symptoms, diagnosing an AAA can be difficult. As discussed previously, many patients remain asymptomatic until rupture. Acute causes of severe back pain and abdominal pain should be considered highest on the differential. It is important to assess hemodynamic stability because this can aid in the differential. Top diagnoses include, but are not limited to, acute appendicitis, acute coronary syndrome, perforated viscus, pulmonary embolism, acute pancreatitis, acute cholecystitis, pyelonephritis, nephrolithiasis, and intestinal ischemia.39 The full differential for AAA is vast, since patients can present with a variety of symptoms and should include causes of intraabdominal, renal, pulmonary, cardiac, vascular, and pelvic pathologies.
Management
After the diagnosis is made, management and treatment should be tailored to the individual when taking into account the AAA size, growth rate, risk factors, and symptomatology. Individuals with asymptomatic AAA between 3.0 cm and 5.4 cm diagnosed in the ED should be referred to a vascular surgeon and seen within 12 weeks. Patients with an AAA 5.5 cm or larger should be referred to a vascular surgeon and seen within two weeks.38 Those with smaller aneurysms who do not meet the threshold for repair should be encouraged to initiate secondary prevention of cardiovascular disease. This includes, but is not limited to, smoking cessation, diet, nutrition and exercise optimization, medicine optimization, lipid modification and statin therapy, diabetes management, and hypertension management.
Unfortunately, evidence has not shown medication to reduce the growth rate or risk of rupture in individuals with known AAA. One multicenter, single-blind, randomized placebo-controlled trial failed to show any significant effect of angiotensin-converting enzyme inhibitor or the calcium channel blocker amlodipine on aneurysm growth rate or rupture.37
Management of Ruptured AAA
Ruptured AAA carries a high mortality rate and remains a catastrophic diagnosis made by emergency physicians. Ruptured AAA always should be on the differential for a life-threatening diagnosis, especially when a patient presents > 50 years of age with acute onset abdominal pain or back pain. The presence of hemodynamic instability and/or a pulsatile mass makes this diagnosis more likely.3 According to the European Society for Vascular Surgery (ESVS), the following should be completed within the first 30 minutes of presentation: initial evaluation, diagnosis, immediate management, and consideration of transfer to a regional center with vascular surgery capabilities.40 Evaluation of a patient in whom a ruptured AAA is suspected should begin with an assessment of airway, breathing, and circulation (ABCs) as well as a general assessment and obtaining vital signs for hemodynamic monitoring. Two large-bore intravenous (IV) lines should be placed.3 The workup for a patient with suspected AAA should be tailored depending on their stability but generally can include bloodwork, such as a complete blood count, electrolytes including liver function tests, coagulation studies, and a type and screen. An electrocardiogram (ECG) should be taken to assess for cardiovascular collapse and arrhythmias. In the event that a ruptured AAA is suspected, blood should be made available because massive transfusion is required in 71% of ruptured AAAs.18 As discussed earlier, imaging can include either ultrasound or CT. Imaging should be used mainly to confirm diagnosis, keeping in mind that patients who are unstable should not be sent to the CT scanner prior to stabilization.
According to the Society for Vascular Surgery (SVS), the following should be completed within the first 30 minutes of presentation: initial evaluation, diagnosis, immediate management, and consideration of transfer to a regional center with vascular surgery capabilities. Initial assessment should include ABCs and placing the patient on the monitor. Two large-bore IVs should be placed. Massive transfusion protocols should be initiated early if there is a high suspicion of rupture or impending rupture. If possible, labs should be drawn, including a type and screen. As discussed earlier, imaging can include either ultrasound or CT. Imaging should be used mainly to confirm diagnosis, keeping in mind that patients who are unstable should not be sent to the CT scanner prior to stabilization.
Upon identification of a ruptured AAA, permissive hypotension should be allowed, with acceptable SBP readings between 70 mmHg and 90 mmHg as long as the patient is conscious. This is recommended to prevent excessive hemorrhage.3 Whenever possible, emergency physicians should obtain a history, including medical comorbidities, and goals of care should be discussed. Vascular surgery consults should be placed early, and transfer should be considered if appropriate services cannot be provided. If the patient is deemed a surgical candidate for elective repair, the SVS recommends a door-to-intervention time of < 90 minutes.41
Surgical Repair
Surgical repair involves either traditional open surgical repair (OSR) or endovascular repair (EVAR). EVAR is a minimally invasive, endovascular technique using X-ray guidance that involves accessing the groin and deploying an expandable stent into the AAA. OSR, on the other hand, involves accessing the AAA through an open abdominal incision, clamping the aorta, and replacing the AAA with a prosthetic graft.41 Although improvements in surgical technique and cardiovascular risk management have been made, five-year survival after successful completion of aneurysm repair remains below 70%.3 Since the development of EVAR in 1991, rates of endovascular repair have increased dramatically, and now more than 80% of repairs are done by this approach.25 Although advancements have been made, the in-hospital mortality rate after surgical repair for ruptured AAA remains high at 35.4%.41
Unruptured AAA
Randomized controlled trials have not shown a benefit to early surgical repair compared to close surveillance for asymptomatic individuals with AAA less than 5.5 cm when growth is less than 1 cm/year. Guidelines vary slightly, but repair should be considered for people with unruptured AAA who are symptomatic, asymptomatic individuals with a diameter greater than 4.0 cm that has grown more than 1 cm in one year, and asymptomatic male patients with AAA 5.5 cm or larger. For women, surgery could be considered starting at 5.0 cm due to the increased incidence of aneurysm rupture in women.18,38
Different organizations have made recommendations slightly favoring one approach over the other. The National Institute for Health and Care Excellence (NICE) recommends offering OSR for patients who meet the criteria mentioned earlier unless they have more complex medical conditions or comorbidities.38 According to the ESVS, EVAR is recommended in those with appropriate anatomy and reasonable life expectancy, whereas OSR is recommended in those with a long life expectancy. Patients with a limited life expectancy are not recommended for either type of elective unruptured AAA repair.41,42 The SVS appears to favor the EVAR approach for patients, depending on patient characteristics.3,41,42
Differences exist between patients who undergo OSR vs. EVAR. Patients who undergo repair by the endovascular approach tend to be older with fewer comorbidities.15 In the setting of unruptured elective AAA repair, in-hospital mortality for both approaches remained low according to the National Vascular Surgery Registry in 2019. For EVAR, the majority of patients returned to a floor level of care with a median length of stay of two days. The in-hospital mortality for EVAR was 0.4%. For OSR, 98% of patients required intermediate or intensive care unit (ICU) level of care and had a total median hospital stay of seven days. These patients had higher rates of immediate renal, cardiac, and respiratory complications. Patients undergoing open repair also had higher rates of returning to the operating room. In-hospital mortality for open repair was higher than for EVAR but remained low at 3.2%.15
To date, four randomized controlled trials (EVAR 1, DREAM, OVER, and ACE) have been conducted comparing EVAR to OSR. Although pooled analysis showed lower in-hospital mortality with EVAR, results were mixed regarding immediate and long-term follow-up outcomes. Some studies showed that mortality benefits with EVAR dissipate after four years, while others noted convergence with mortality rates from OSR much earlier.40
Long-term studies have come to a similar conclusion. Compared to OSR, EVAR is associated with lower immediate post-operative mortality and morbidity but does not show long-term mortality benefits and is associated with higher re-intervention rates.43 Another recent meta-analysis comparing EVAR and OSR for patients 80 years of age or older saw a similar mortality trend as other studies. EVAR had lower mortality in the short term, but similar mortality risk at both 36 and 60 months.44
EVAR also is associated with higher long-term complications and the need for reintervention, although many of these were minor. OSR has higher rates of laparotomy interventions.41,45 EVAR appears to show more benefit with older individuals and those with more complex comorbidities and is associated with decreased blood loss and total length-of-stay. For complex repairs of unruptured AAA, mortality rates for open and endovascular repair were five times higher than the rates for infrarenal repair. For open repairs of complex aneurysms, the need for repeat surgery and return to the operating room was nearly 20%.15
Ruptured AAA Repair
The in-hospital mortality associated with repair in ruptured AAA is much higher than compared to unruptured AAA. The in-hospital mortality rates for ruptured AAA were 40.9% for open repair and 22.6% for EVAR. A randomized, controlled, multicenter trial comparing OSR to EVAR for ruptured AAA demonstrated a similar 30-day mortality (35.4% vs. 37.4%) for EVAR and OSR, respectively. There was no statistically significant difference in mortality at one year, but results did show faster discharge, better health quality-of-life measures, and improved cost effectiveness with the endovascular strategy.46
NICE reports a benefit with EVAR for ruptured AAA, particularly with men older than 70 years of age. For women, NICE recommends EVAR at all ages because women have been shown to have more benefit from EVAR when compared to men. NICE notes that there could be a benefit to OSR in men younger than 70 years of age. The SVS shows preference to EVAR over OSR when possible given a patient’s anatomy, similar to their recommendation with respect to unruptured AAA.41
No single preoperative finding or characteristic can accurately predict the outcome for AAA repair. Many scoring systems have been developed over the years in the hope of better predicting outcomes. For example, the Glasgow Aneurysm Score (GAS) was developed in 1994 for predicting outcomes of AAA repair. Unfortunately, this scoring system did not reliably predict outcomes for ruptured AAA.47 NICE does not recommend the use of risk assessment tools when determining if a patient should undergo elective surgery for asymptomatic, unruptured AAA. Similarly, NICE does not recommend the use of risk assessment tools to decide if a patient should undergo repair for ruptured AAA.38
Complications
Both immediate and late complications are common in patients who have a history of AAA repair. Cardiac-related death occurred in 8% of elective EVAR patients and 7.1% of OSR patients. Acute kidney injury is more common in patients who undergo OSR, which is thought to be due to clamping of the aorta. Renal failure also can occur after EVAR repair and is associated with a significantly higher mortality. Ischemic complications after surgery are associated with significant morbidity and mortality and can occur in 9% of patients, with mortality rates up to 73% for colonic ischemia. Pulmonary complications are relatively common as well. Complications specific to the surgical site can include graft infection, endoleaks, re-bleeding, graft thrombosis, graft infection, and pseudoaneurysms. The need for repeat intervention and repair has been demonstrated in both groups.41
Screening and Surveillance
Implementation of nationwide screening programs for AAA have decreased the morbidity and mortality associated with the disease in recent decades. Sweden developed a program in 2006, England in 2009, and the United States in 2008.8 Early detection, surveillance, and elective repair reduces the risk of rupture and the need for emergent surgery.48 Current guidelines for small asymptomatic aneurysms (< 5.5 cm for men and < 5.0 cm for women) recommend surveillance and conservative management at intervals based on symptomatology and aortic diameter size.49 Randomized controlled trials have not shown a benefit with early repair in these patients.49,50
A meta-analysis of population-based randomized controlled trials showed that an invitation to screen for men 65 years of age and older was associated with a statistically significant 35% reduction in AAA-related mortality. It also was associated with a decrease in AAA-related ruptures and AAA-related emergent surgical procedures. Pooled data did not show a significant effect on all-cause mortality, although some individual studies did.19,29,48
Initial screening programs enrolled only men aged 65 years or older for a single abdominal ultrasound to look at the intrarenal abdominal aorta.8 Screening programs vary slightly and have been modified over time. For example, the U.S. Preventive Services Taskforce (USPSTF) now recommends screening men 65-75 years of age who have ever smoked. (See Table 4.) This recommendation varies slightly across organizations. For example, the American College of Cardiology and the American Heart Association have a similar recommendation, but they also include a one-time screening for men 60 years or older for siblings or offspring of someone with AAA. Based on the USPSTF guidelines, there are insufficient data to recommend universal screening procedures for women with a smoking or family history.4
Table 4. U.S. Preventive Services Task Force Recommendations on Abdominal Aortic Aneurysm Screening4 |
|
Clinical Recommendation |
Evidence Rating |
One-time screening with ultrasound in men aged 65 to 75 years who have ever smoked |
B |
Selective screening with ultrasound in men aged 65 to 75 who have never smoked |
C |
Recommendation against routine screening for women who have never smoked and have no family history |
D |
Insufficient evidence to provide a recommendation for women aged 65 to 75 years who have ever smoked or have a family history |
I |
The SVS, on the other hand, recommends a one-time screening for AAA in men and women 65-75 years of age with a history of tobacco use, men 55 years of age or older with a family history of AAA, and women 65 years of age or older who have smoked or have a family history of AAA. (See Table 5.) SVS has time-based recommendations for surveillance alone depending on aneurysm size outlined in Table 6.3 Lastly, the American College of Preventive Medicine recommends a one-time screen in men 65-75 years of age who have ever smoked and specifically does not recommend universal screening in women.4
Table 5. Society for Vascular Surgery Screening Recommendations for Abdominal Aortic Aneurysm3 |
|
Clinical Recommendations |
Evidence Rating |
One-time ultrasound screening in men or women 65 to 75 years of age with a history of tobacco use |
A (High) |
Ultrasound screening in first-degree relatives of patients with a AAA who are between 65 and 75 years of age or in those older than 75 years of age who are in good health |
C (Low) |
One-time ultrasound screening for AAAs in men or women older than age 75 years with a history of tobacco use in otherwise good health who have not previously received an ultrasound exam |
C (Low) |
AAA: abdominal aortic aneurysm |
|
Table 6. Society for Vascular Surgery Guidelines on Surveillance Based on Abdominal Aortic Aneurysm Size3 |
|
Initial AAA Diameter |
Rescreening Time |
> 2.5 cm but < 3 cm |
10 years |
Between 3.0 cm and 3.9 cm |
Three-year intervals |
Between 4.0 cm and 4.9 cm |
12-month intervals |
Between 5.0 cm and 5.4 cm |
Six-month intervals |
AAA: abdominal aortic aneurysm |
|
Although aneurysm rupture is more common in women, the overall prevalence is much lower when compared to men. One randomized controlled trial comparing screening and controls for women showed the incidence of rupture was the same over five- and 10-year follow-up intervals, thus making universal recommendations for women more nuanced.51
Universally screening all men regardless of smoking history is not currently recommended from any of the previously mentioned organizations because of a lack of evidence demonstrating significant benefit. Additional recommendations can be made depending on a patient’s risk factors. For example, there is special consideration for men with a family history of AAA.4,48
Several large studies have looked the effectiveness of screening programs. The collective data of four population-based randomized controlled trials (n = 134,271) with predominantly men aged 65 years or older evaluated the effectiveness of one-time screening for AAA. The prevalence of AAA was between 4.0% and 7.6%, with most of the aneurysms being small, and only 0.3% to 0.6% were 5 cm or greater. Analysis of AAA-associated mortality in the four trials showed a statistically significant 35% reduction with invitation to screen. The number needed to screen was 305 men to prevent one AAA-associated death. Invitation to screening also was associated with a reduced rate of rupture, with 246 screenings needed to prevent one AAA rupture. Invitation to screen was associated with reduction in emergency surgery. There was no effect on all-cause mortality of the pooled data.48
A meta-analysis comparing early repair for small asymptomatic aneurysms (between 4.0 cm and 5.5 cm) and routine imaging surveillance showed no long-term survival benefit to early repair. This conclusion was similar for both open repair and EVAR. Costs were lower short term in the surveillance groups, but over time showed no difference. Overall, early repair is not shown to be beneficial in the current literature.50
‘Incidentalomas’
AAA can be found incidentally when a patient presents to the ED for an unrelated complaint. CT scans are a common modality used in hospitals as part of a diagnostic workup. One study found incidental AAAs detected with a prevalence of 5.8% on routine CT in a cohort of 3,246 patients. Out of the 186 AAAs detected, only 65% were reported by radiologists. The likelihood of reporting increased with size: 100% reporting rate in aneurysms ≥ 5 cm and 52% reporting rate with aneurysms ≥ 3.0 cm to 3.9 cm.52 It is important to note incidental findings and give accurate and specific follow-up recommendations based on aneurysm size and risk factors. (See Table 6.)
Disposition
Disposition will vary depending on the clinical setting and resources available. The decision to discharge with outpatient follow-up, transfer, or admission will depend on aneurysm size, symptoms, and follow-up capabilities. Asymptomatic individuals with unruptured AAA 5.5 cm or larger should be referred to and seen at a regional vascular surgery service within two weeks. For smaller aneurysms 3.0 cm to 5.4 cm, patients should be seen at a vascular surgery service within 12 weeks.38 For larger asymptomatic aneurysms greater than 5.5 cm, the decision to follow up as an outpatient or undergo hospital admission for evaluation will vary depending on the center. This decision should be made in conjunction with the vascular surgery team. Given their high risk of rupture, the ESVS recommends consideration of inpatient management and immediate repair for aneurysms greater than 9 cm.53
Although mortality rates for AAA have improved over the past 20 years, mortality for ruptured AAA remains high. Ruptured AAA requires evaluation by a vascular surgeon for operative intervention. Patients with ruptured AAAs who are surgical candidates will require disposition to the operating room and subsequent admission. Symptomatic but unruptured AAAs require admission and emergent surgical evaluation. Symptomatic but unruptured AAA may be admitted for medical optimization prior to repair. For these patients, the SVS suggests admission to an ICU for monitoring. Blood products should be made available in case of rupture.3 Keep in mind that individuals with a confirmed ruptured AAA who have a cardiac arrest or persistent loss of consciousness have an exceedingly small chance of surviving a surgical repair. Special consideration should be taken when transferring patients to a regional vascular service. It has been recommended that if a patient with a known ruptured or symptomatic AAA has been accepted for transfer to a center with vascular surgery capabilities, they should be transferred to the center within 30 minutes.38
Summary
Abdominal aorta diameter greater than 3.0 cm is considered aneurysmal. Despite advances in detection and treatment, ruptured AAAs are still associated with significant morbidity and mortality. Risk factors include both genetic and environmental factors. Age is the most significant nonmodifiable risk factor, whereas smoking is the strongest modifiable risk factor. Surveillance is appropriate for small aneurysms; however, repair generally is considered for aneurysms 5.5 cm or greater (with some exceptions).
Differing imaging modalities can be used to diagnose AAA, but ultrasound remains the imaging of choice for screening because of the effectiveness, lack of radiation, low cost, and noninvasive nature of the test. Ultrasound has a high sensitivity (94% to 100%) and high specificity (98% to 100%) for detecting AAA.4,48 To date, there are no medications proven to decrease aneurysm growth and likelihood of rupture. Both endovascular and open repair have different risk profiles and should be considered on an individual basis, with endovascular repair being used more frequently as technological advancements continue.18,25,41
Given its high mortality rate, the emergency physician should always keep AAA on the differential when patients present with acute onset back pain or abdominal pain, especially in the setting of hemodynamic instability and a pulsatile mass.
REFERENCES
- Gao J, Cao H, Hu G, et al. The mechanism and therapy of aortic aneurysms. Signal Transduct Target Ther 2023;8:55.
- Anagnostakos J, Lal BK. Abdominal aortic aneurysms. Prog Cardiovasc Dis 2021;65:34-43.
- Chaikof EL, Dalman RL, Eskandari MK, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 2018;67:2-77.e2.
- US Preventive Services Task Force; Owens DK, Davidson KW, Krist AH, et al. Screening for Abdominal Aortic Aneurysm: US Preventive Services Task Force Recommendation Statement. JAMA 2019;322:2211-2218.
- Quintana RA, Taylor WR. Cellular mechanisms of aortic aneurysm formation. Circ Res 2019;124:607-618.
- Stather PW, Sidloff DA, Rhema IA, et al. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur J Vasc Endovasc Surg 2014;47:240-242.
- Márquez-Sánchez AC, Koltsova EK. Immune and inflammatory mechanisms of abdominal aortic aneurysm. Front Immunol 2022;13:989933.
- Sprynger M, Willems M, Van Damme H, et al. Screening program of abdominal aortic aneurysm. Angiology 2019;70:407-413.
- Davis FM, Daugherty A, Lu HS. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol 2019;39:e83-e90.
- Snow DC, Colbenson K. Avoiding misdiagnosis of abdominal vascular catastrophes. Emerg Med Clin North Am 2021;39:769-780.
- Schermerhorn ML, Bensley RP, Giles KA, et al. Changes in abdominal aortic aneurysm rupture and short-term mortality, 1995-2008: A retrospective observational study. Ann Surg 2012;256:651-658.
- Nelissen BGL, Herwaarden JA, Pasterkamp G, et al. Shifting abdominal aortic aneurysm mortality trends in the Netherlands. J Vasc Surg 2015;61:642-647.e2.
- Zhang L, Issa Bhaloo S, Chen T, et al. Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res 2018;122:1608-1624.
- Accarino G, Giordano AN, Falcone M, et al. Abdominal aortic aneurysm: Natural history, pathophysiology and translational perspectives. Transl Med UniSa 2023;24:30-40.
- Waton S, Johal A, Heikkilă K, et al. National Vascular Registry: 2019 Annual report. London: The Royal College of Surgeons of England, November 2019. https://www.vsqip.org.uk/content/uploads/2019/12/NVR-2019-Annual-Report.pdf
- Norman PE, Powell JT. Site specificity of aneurysmal disease. Circulation 2010;121:560-568.
- van de Luijtgaarden KM, Bastos Gonçalves F, Hoeks SE, et al. Lower atherosclerotic burden in familial abdominal aortic aneurysm. J Vasc Surg 2014;59:589-593.
- Hellawell HN, Mostafa AMHAM, Kyriacou H, et al. Abdominal aortic aneurysms part one: Epidemiology, presentation and preoperative considerations. J Perioper Pract 2021;31:274-280.
- Sweeting MJ, Thompson SG, Brown LC, Powell JT; RESCAN collaborators. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 2012;99:655-665.
- Stackelberg O, Björck M, Larsson SC, et al. Sex differences in the association between smoking and abdominal aortic aneurysm. Br J Surg 2014;101:1230-1237.
- Joergensen TMM, Houlind K, Green A, Lindholt JS. Abdominal aortic diameter is increased in males with a family history of abdominal aortic aneurysms: Results from the Danish VIVA-trial. Eur J Vasc Endovasc Surg 2014;48:669-675.
- van Vlijmen-van Keulen CJ, Pals G, Rauwerda JA. Familial abdominal aortic aneurysm: A systematic review of a genetic background. Eur J Vasc Endovasc Surg 2002;24:105-116.
- Jones GT, Tromp G, Kuivaniemi H, et al. Meta-analysis of genome-wide association studies for abdominal aortic aneurysm identifies four new disease-specific risk loci. Circ Res 2017;120:341-353.
- Li Z, Kong W. Cellular signaling in abdominal aortic aneurysm. Cell Signal 2020;70:109575.
- Schanzer A, Oderich GS. Management of abdominal aortic aneurysms. N Engl J Med 2021;385:1690-1698.
- Cheng J, Zhang R, Li C, et al. A targeting nanotherapy for abdominal aortic aneurysms. J Am Coll Cardiol 2018;72:2591-2605.
- Golledge J, Moxon JV, Singh TP, et al. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med 2020;288:6-22.
- Baxter BT, Matsumura J, Curci JA, et al. Effect of doxycycline on aneurysm growth among patients with small infrarenal abdominal aortic aneurysms. JAMA 2020;323:2029-2038.
- Smidfelt K, Nordanstig J, Davidsson A, et al. Misdiagnosis of ruptured abdominal aortic aneurysms is common and is associated with increased mortality. J Vasc Surg 2021;73:476-483.e3.
- Marine L, Mertens R, Torrealba I, et al. [Rupture of abdominal aortic aneurysm into the duodenum: Uncommon cause of massive gastrointestinal bleeding]. [Article in Spanish]. Rev Med Chil 2021;149:132-136.
- Dargin JM, Lowenstein RA. Ruptured abdominal aortic aneurysm presenting as painless testicular ecchymosis: The scrotal sign of Bryant revisited. J Emerg Med 2011;40:e45-e48.
- Barry I, Tosenovsky P. Symptomatic abdominal aortic aneurysm (AAA) presenting as unilateral lower limb swelling: A case report. Int J Surg Case Rep 2020;73:315-318.
- Parkinson F, Ferguson S, Lewis P, et al. Rupture rates of untreated large abdominal aortic aneurysms in patients unfit for elective repair. J Vasc Surg 2015;61:1606-1612.
- Lederle FA, Johnson GR, Wilson SE, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA 2002;287:2968-2972.
- Brown PM, Zelt DT, Sobolev B, et al. The risk of rupture in untreated aneurysms: The impact of size, gender, and expansion rate. J Vasc Surg 2003;37:280-284.
- Haque K, Bhargava P. Abdominal aortic aneurysm. Am Fam Physician 2022;106:165-172.
- Kiru G, Bicknell C, Falaschetti E, et al. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: A randomised placebo-controlled trial (AARDVARK). Health Technol Assess 2016;20:1-180.
- Abdominal aortic aneurysm: Diagnosis and management. NICE guideline [NG156]. National Institute for Health and Care Excellence. Published March 19, 2020. www.nice.org.uk/guidance/ng156
- Moxon JV, Parr A, Emeto TI, et al. Diagnosis and monitoring of abdominal aortic aneurysm: Current status and future prospects. Curr Probl Cardiol 2010;35:512-548.
- Paravastu SCV, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev 2014;1:CD004178.
- Kyriacou H, Mostafa AMHAM, Sumal AS, et al. Abdominal aortic aneurysms part two: Surgical management, postoperative complications and surveillance. J Perioper Pract 2021;31:319-325.
- Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur J Vasc Endovasc Surg 2019;57:8-93.
- Giannopoulos S, Kokkinidis DG, Armstrong EJ. Long-term outcomes of endovascular vs open surgical repair for abdominal aortic aneurysms: A meta-analysis of randomized trials. Cardiovasc Revasc Med 2020;21:1253-1259.
- Wang G, Sun Y, Lin Z, Fei X. Elective endovascular vs open repair for elective abdominal aortic aneurysm in patients ≥80 years of age: A systematic review and meta-analysis. Vasc Endovasc Surg 2023;57:386-401.
- Rahim AA, Ibrahim R, Yao L, et al. Re-intervention rate in endovascular vs open surgical repair for abdominal aortic aneurysms. Ann Med Surg 2021;69:102703.
- Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: One-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-2069.
- Improve Trial Investigators. Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: One-year outcomes from the IMPROVE randomized trial. Eur Heart J 2015;36:2061-2069.
- Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019;322:2219-2238.
- Golledge J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat Rev Cardiol 2019;16:225-242.
- Ulug P, Powell JT, Martinez MAM, et al. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2020;7:CD001835.
- Scott RAP, Bridgewater SG, Ashton HA. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg 2002;89:283-285.
- Claridge R, Arnold S, Morrison N, van Rij AM. Measuring abdominal aortic diameters in routine abdominal computed tomography scans and implications for abdominal aortic aneurysm screening. J Vasc Surg 2017;65:1637-1642.
- Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg 2011;41(Suppl 1):S1-S58.
Recently the American College of Emergency Physicians (ACEP) created a quality measure, just adopted by the Centers for Medicare and Medicaid Services, that suggests early ultrasound for patients presenting with new abdominal or back pain and hypotension who have not been screened for an abdominal aneurysm at age 55-65 years or older.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
