Reversal of Oral Anticoagulation in the Emergency Department
June 1, 2023
AUTHORS
Emmagene Worley, MD, Assistant Professor of Emergency Medicine, Columbia University Medical Center, New York, NY
Charles Pan, MD, Resident, Department of Emergency Medicine, NewYork-Presbyterian Hospital, New York, NY
PEER REVIEWER
Frank LoVecchio, DO, MPH, FACEP, Vice-Chair for Research, Medical Director, Samaritan Regional Poison Control Center, Emergency Medicine Department, Maricopa Medical Center, Phoenix, AZ
EXECUTIVE SUMMARY
- Patients who take aspirin and experience significant bleeding can receive DDAVP (desmopressin) to reverse the bleeding potential. Those taking dual antiplatelet therapy with significant bleeding will require platelet transfusion.
- Patients with an elevated international normalized ratio (INR) < 10 without significant bleeding should have their next dose or two of warfarin held. Patients whose INR is > 10 should receive vitamin K. Those who have significant bleeding with an elevated INR should receive vitamin K plus fresh frozen plasma.
- Tranexamic acid has been shown to be most useful in reducing bleeding in patients with significant acute trauma and postpartum hemorrhage but has been used in a variety of other scenarios.
- Direct acting oral anticoagulants have specific reversal agents.
Introduction
Hemostasis, or the cessation of bleeding, is a series of steps involving many different proteins, cells, and interactions that ultimately serves to form a stable clot that prevents further blood loss. The physiology of hemostasis is complex, beginning with trauma to local vasculature and ultimately ending with the formation of a fibrin “plug” at the site of bleeding.1 Platelets and the coagulation cascade play a critical role in the achievement of hemostasis.
Hemostasis is a regulated process in equilibrium with fibrinolysis, or the breakdown of clot formation. Abnormalities in both hemostasis and fibrinolysis pose hematologic challenges. Pathological overactivation or underactivation of either process can manifest clinically as conditions associated with high morbidity and mortality, including, but not limited to, ischemia, infarction, hemorrhage, or death. However, pathologies that predispose patients to thromboembolism require initiation and often lifelong maintenance of anticoagulation therapy for both therapeutic and preventive benefits. There is a wide array of conditions that necessitate anticoagulation therapy, such as coronary artery disease, atrial fibrillation, deep vein thrombosis, pulmonary embolism, or antiphospholipid syndrome, to name a few.
Depending on their mechanism of action, certain medications are considered “antiplatelet,” while others are labeled “anticoagulant.” While therapeutic intervention of both classes of medications results in a decreased likelihood of clot formation, an unsurprising common side effect is increased bleeding risk. This review will describe the physiological components of the clotting cascade, highlight common anticoagulant agents in use, and discuss means of oral anticoagulation reversal.
Hemostasis
Hemostasis, by definition, is the physiological process that leads to cessation of bleeding. The formation of what is commonly referred to as a “blood clot” consists of two main components, primary hemostasis and secondary hemostasis. Primary hemostasis is the process through which platelets undergo adhesion, activation, and aggregation to form a platelet plug.2 Secondary hemostasis involves the coagulation cascade, a series of steps involving numerous clotting factors and enzymatic reactions ultimately resulting in the formation of the serine protease thrombin (factor IIa).2 Thrombin is involved in several processes, which all serve to maintain hemostasis, including activation of other clotting factors resulting in propagation of clot formation.3 Thrombin itself also activates platelets, and it cleaves fibrinogen (factor I) to fibrin (factor Ia), ultimately resulting in the formation of a fibrin mesh atop the platelet plug.4
Despite the nomenclature, in vivo studies have demonstrated that primary hemostasis and secondary hemostasis occur concurrently, resulting in the simultaneous formation of both platelet aggregation and fibrin.5
Primary Hemostasis: Platelets
Vascular injury itself triggers the formation of a platelet plug. Damage to the vessel endothelium not only causes local vasoconstriction, but also facilitates a thrombogenic environment due to exposed subendothelial contents, such as von Willebrand factor (vWF), fibronectin, laminin, and collagen.6
Pathophysiologically, the most important protein in the initial recruitment of platelets to the injured vascular site is vWF. It is a large multimeric glycoprotein with severe binding sites for platelet receptors that mediate primary hemostasis. The trigger for initial platelet receptor binding to vWF is elevated shear rates.
Under normal conditions, blood flow is laminar, with maximal velocity in the center of the vessel and decreasing velocities as one moves toward the vessel periphery. This differential change generates a tangential force termed “fluid shear stress.”7 Shear stress increases in certain conditions, such as vascular atherosclerosis, pre-existing thrombi, or local tissue injury. Under these circumstances, the interaction between the A1 domain of vWF and the platelet receptor GP IBα is promoted and creates a transient but critical tethering of circulating platelets to the endothelium.7 The GP IBα receptor is part of the larger GP Ib-IX complex on platelet surfaces, a complex that also binds to other ligands involved in hemostasis, such as thrombin, coagulation cascade factors, and p-selectin.8 Dysfunction in expression of this complex is implicated as the pathogenesis behind Bernard-Soulier syndrome, a hereditary bleeding diathesis.8
The establishment of bonds between platelets and vWF confers the next step in platelet plug formation. The platelet receptor GP VI binds to collagen fibrils on the subendothelial surface, and this is widely regarded as the key mechanism behind the induction of platelet activation.9 Concurrently, platelet integrin αIIbβ3 (GP IIb/IIIa) is activated, and changes in its conformation result in high affinity binding to fibrinogen and vWF.10 This causes a series of intracellular changes within platelet cells, including irreversible adhesion and cytoskeletal reorganization, whereby platelets take on a flat, spreading morphology with a large adhesive shape from their original mobile, discoid states.11 (See Figure 1.) Once activated, these platelets are better programmed to form thrombi. Lack of integrin αIIbβ3 leads to Glanzmann thrombasthenia, another bleeding diathesis. In one study of mice with Glanzmann thrombasthenia, clinical manifestations included absent platelet aggregation, greatly reduced fibrinogen uptake, severely prolonged bleeding times, and spontaneous hemorrhage.12
Figure 1. Hemostasis |
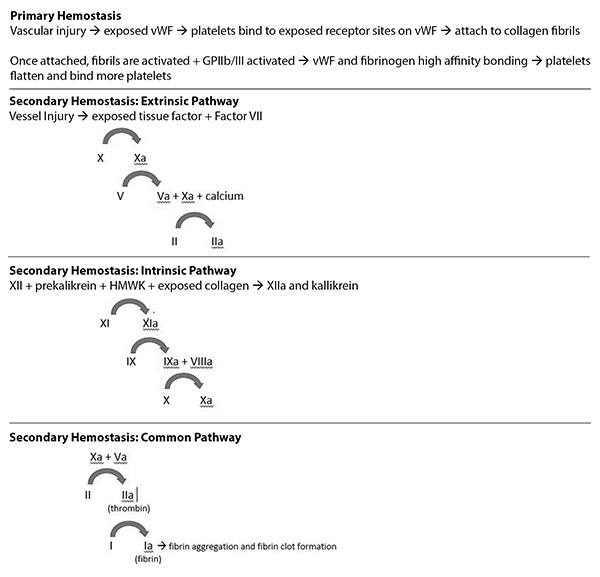 |
vWf: von Willebrand factor; HMWK: high molecular weight kininogen |
Once the primary layer of activated platelets has attached to the endothelium, subsequent accumulation of more platelets via further binding of fibrinogen and vWF to integrin αIIbβ3 secures the thrombus.13 Activated platelets also will further contribute to the hemostatic process via positive feedback. They release adenosine diphosphate (ADP) and serotonin from cytoplasmic granules and synthesize thromboxane A2, all of which have prothrombotic properties.14 Specifically, ADP contributes to potentiation and irreversibility of platelet aggregation via its interactions with different ADP receptors on platelets.15 The three ADP receptors, P2Y1, P2Y12, and P2X1, all induce effects that emphasize their role in maintaining a prothrombotic environment. In particular, the P2Y12 receptor has been extensively studied as a target for antithrombotic therapeutics.
Secondary Hemostasis: Coagulation Cascade
Secondary hemostasis is comprised of the intrinsic and extrinsic pathways. The intricate process of secondary hemostasis is a series of steps involving circulating proteins in response to vascular and endothelial injury. These proteins are zymogens, which become activated by the presence of an upstream coagulation factor, typically via proteolytic cleavage. Once activated, the clotting factor is able to catalyze the next reaction in the cascade. Taken together, the intrinsic and extrinsic pathways ultimately result in the formation of the activated serine protease thrombin (factor IIa), which, among other reactions, in turn converts soluble fibrinogen (factor I) to insoluble fibrin (factor Ia), anchoring the hemostatic clot. The nomenclature of each factor is represented by a Roman numeral, and its respective activated factor is denoted by a lowercase “a.”
Extrinsic Pathway
The extrinsic pathway consists of factor VII and the transmembrane receptor tissue factor (TF). TF is expressed within the subendothelial layers of vessel walls, in cells such as vascular myocytes, pericytes, and adventitial fibroblasts.16 Subsequently, vessel injury exposes tissue factor to the intravascular environment. Circulating plasma factor VII is the ligand for TF, and once the TF-factor VII complex forms, factor VII becomes activated to form factor VIIa, and its enzymatic activity greatly increases.17 Factor VIIa then activates factor X to form factor Xa, which in turn activates prothrombin (factor II) to thrombin (factor IIa). This final reaction requires factor Va and calcium (factor IV); factor Xa itself also catalyzes the formation of factor Va from factor V.17
Intrinsic Pathway
The intrinsic pathway begins with the zymogens factor XII and prekallikrein. In the presence of cofactor high molecular weight kininogen (HMWK) and negatively charged anions, usually collagen exposed from endovascular injury, the aforementioned zymogens become activated to factor XIIa and kallikrein, respectively.18 Factor XIIa activates factor XI to factor XIa, and factor XIa triggers the activation of factor IX to factor IXa. Similar to factor VIIa in the extrinsic pathway, factor IXa is capable of activating factor X to factor Xa.19 This process requires the presence of activated factor VIIIa; mutations in the gene encoding factor VIII results in hemophilia A, the most common hereditary disorder of hemostasis.20
Common Pathway
Factor Xa is an integral activated serine protease that plays multiple roles in the common pathway of the coagulation cascade. It is formed through both the extrinsic and intrinsic pathways. In the presence of factor Va, factor Xa is able to activate factor II (prothrombin) more rapidly by 300,000 fold than isolated Xa.21 This is a key step in the coagulation cascade, since activated factor IIa, or thrombin, cleaves factor I (fibrinogen) into factor Ia (fibrin).22 Fibrin monomers will interface with other fibrin monomers to form oligomers; as this process continues and more fibrin monomers aggregate, the resulting molecule takes the structure referred to as a double-stranded protofibril.23 Protofibrils continue to elongate and strengthen via interactions between individual fibrin monomers both laterally and longitudinally, ultimately forming a three-dimensional matrix that is referred to as the fibrin clot.23
Cross-linking of the fibrin clot further strengthens both its hemostatic integrity and its resistance to thrombolysis. This is achieved by factor XIIIa, which is the resultant activated form of factor XIII in the presence of thrombin and calcium.24 Once this final step has occurred, the fibrin clot maintains hemostasis and prevents further exsanguination, until counterregulatory mechanisms of fibrinolysis begin the process of fibrin clot matrix cleavage.
Tertiary Hemostasis: Fibrinolysis
Fibrinolysis is the process by which the zymogen plasminogen is activated to plasmin, a serine protease that dissolves the previously formed fibrin clot. This activation process is facilitated through the effects of two other serine proteases, tissue plasminogen activator (tPA) and urokinase (uPA).25 Plasmin ultimately cleaves the fibrin clot, forming remnants denoted as fibrin degradation products (FDP).26 Similar to hemostasis, fibrinolysis also is a tightly regulated process since destruction of the fibrin clot will restore blood circulation.27
Epidemiology of Thrombotic Disease
Anticoagulation is indicated for a variety of conditions, and thrombotic disease pathologies can affect almost every organ system. A brief list of indications for anticoagulation include coronary artery disease, arrhythmias, valvular heart disease, myocardial infarction, venous thromboembolism (VTE), congenital or acquired hematologic abnormalities, cerebrovascular disease, and/or vascular abnormalities.
Broadly, anticoagulation can refer to medications with either antiplatelet or anticoagulation properties. These therapeutics commonly are prescribed in oral formations. However, other routes of administration are available for anticoagulation medications; for certain patients, subcutaneous injections of anticoagulants, such as unfractionated heparin, low molecular weight heparin, or fondaparinux, are indicated. The scope of this review article will only focus on the reversal of oral anticoagulants.
The burden of thrombotic disease is not insignificant. In 2003, there were an estimated 2 million cases of VTE in the United States, with an additional estimated mortality of 60,000 from pulmonary embolism.28 According to a 2021 study from the Journal of the American College of Cardiology, cardiovascular and cerebrovascular disease accounted for more than 13 million deaths.29
The magnitude of conditions requiring antiplatelet or anticoagulation medications indicates their widespread prevalence. Despite the relatively safe profile of these medications, increased bleeding risk is a common side effect of all antiplatelet and anticoagulant agents. The benefits of correcting any underlying thrombotic pathology must be weighed against the risks of bleeding before initiation of oral anticoagulants. Especially as the trend of the global population increases in age, predisposing patients to increased risks of bleeding, care and attention should be focused on understanding the options for reversal of each agent. Providers should be well versed in reversal agents for oral anticoagulants if there is evidence of supratherapeutic, prolonged, or life-threatening bleeding.
Oral Antiplatelet and Anticoagulant Medications: Indications and Reversal Agents
Aspirin
Aspirin is one of the most common antiplatelet medications in use today. It has been used for its anti-inflammatory and analgesic properties since the time of Hippocrates. Today, it often is combined with a P2Y12 inhibitor as dual antiplatelet therapy (DAPT) for the treatment of cardiovascular and cerebrovascular disease. It can be in a variety of formations, and comes in two main dosages, 81 mg (“baby” aspirin) and 325 mg (“full strength” aspirin). It inhibits the cyclooxygenase (COX) enzymes in platelet endoplasmic reticulum, which convert arachidonic acid to prostaglandin H2. This leads to the formation of thromboxane A2 (TXA2) and prostaglandin I2 (PGI2). Therefore, taking aspirin leads to decreased levels of TXA2 and PGI2. This has the overall effect of decreasing platelet aggregation. (See Table 1.)
Table 1. Oral Anticoagulants and Reversal Agents |
|||
Drug |
Mechanism of Action |
Reversal Agent |
Adjuncts |
Aspirin |
COX inhibition, decreasing platelet aggregation |
Desmopressin |
|
P2Y12 Inhibitors (examples: clopidogrel, ticagrelor) |
Inhibit P2Y12 reception, decreasing platelet aggregation |
None |
|
Warfarin |
Blocks the activation of vitamin K, reducing coagulation cofactors II, XII, IX, and X, and proteins C and S |
Vitamin K, IV or PO depending on severity |
|
Dabigatran |
Direct thrombin inhibitor |
Idarucizumab |
|
Rivaroxaban Apixaban Edoxaban |
Factor Xa inhibitor |
Andexanet alfa |
|
COX: cyclooxygenase; TXA: tranexamic acid; IV: intravenous; PO: orally; PCC: prothrombin complex concentrate; FFP: fresh frozen plasma |
|||
Aspirin is absorbed readily in the upper gastrointestinal (GI) tract and has a measurable inhibition of platelet function within 60 minutes. Aspirin’s inhibition of the COX enzyme is permanent for the life of the platelet, so while the half-life of aspirin is only 20 minutes, the effects can persist for about 10 days (the average life of the platelet). However, studies have shown that with as little as 20% of COX activity, hemostasis may be normal.30
DDAVP (desmopressin) is the first-line treatment for the reversal of antiplatelet medications. It increases platelet function by increasing vWF and factor VIII on platelets, improving their function. It is given as an intravenous infusion of 0.3 mcg/kg to
0.4 mcg/kg. It can be given every 12 hours, but complications such as hyponatremia and tachyphylaxis increase with each repeated use.31 DDAVP also can be considered in dialysis patients presenting with bleeding, even if they are not on oral anticoagulation. Uremia, a common complication seen in patients with end-stage renal disease, causes platelet dysfunction resulting in subsequent bleeding. On the contrary, platelet transfusions, while seemingly an intuitive solution, are not recommended. In the PATCH trial, platelet transfusions increased the odds of death, dependence, and adverse events in patients who developed spontaneous intracranial hemorrhage (ICH) and were on aspirin.32
Tranexamic acid (TXA) also is used for reversing antiplatelet agents. (See Table 2.) It prevents the conversion of plasminogen to plasmin, thereby inhibiting fibrinolysis. Hyperfibrinolysis often is observed following conditions such as trauma and postpartum hemorrhage, and TXA appears to have the most efficacy in post-traumatic bleeding.33 However, there is emerging literature that suggests TXA also can be administered in a variety of bleeding settings, including if patients are on anticoagulation.
Table 2. Evidence Convincing or Suggesting Use of Tranexamic Acid |
|
Generally, the loading dose of TXA is 1 gram infused over 10 minutes to avoid hypotension, followed by maintenance doses of 1 gram given as infusions over eight hours.34 The CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2) trial, published in 2010, identified that both all-cause mortality and risk of death due to bleeding were significantly reduced in patients receiving TXA within eight hours of injury compared to those receiving placebo.35 Additionally, the occurrence of occlusive vascular events, defined as myocardial infarction, stroke, pulmonary embolism, and deep vein thrombosis, did not differ between the intervention and placebo groups.35
In 2019, the CRASH-3 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 3) trial sought to examine the effects of TXA administration early (within three hours) in ICH due to traumatic brain injuries (TBIs). While there was no significant difference in the primary outcome of 28-day in-hospital head injury-associated mortality in all patients receiving TXA within three hours, there were mortality benefits noted in subgroup analyses: Patients with mild to moderate TBI (defined as having Glasgow Coma Scale [GCS] scores 9-15) had reduced mortality.36
In obstetrics, TXA also is used in cases of postpartum hemorrhage, as evidenced by the WOMAN (WOrld Maternal Antifibrinolytic Trial). Early administration (also within three hours) of TXA after delivery reduced death due to bleeding, although there was no statistical difference in primary composite outcomes of death from all causes or hysterectomy within 42 days.37 Similar to CRASH-2, no statistical difference in thromboembolic events or other adverse medical events were noted.
Despite the promising evidence of early TXA administration in bleeding due to trauma, ICH, or postpartum hemorrhage, the HALT-IT trial, published in 2020, found that TXA does not reduce mortality from GI bleeding, and did not show any difference in subsequent rebleeding rates, thromboembolic events, or need for endoscopic, surgical, and/or radioscopic interventions.38
P2Y12 Inhibitors
The P2Y12 inhibitors (common agents include clopidogrel, prasugrel, ticagrelor, and ticlopidine) refer to a class of medications whose mechanism involves irreversibly inhibiting the P2Y12 receptor on platelets for ADP. This prevents the activation of IIb/IIIa and irreversibly decreases platelet aggregation for the lifetime of the platelet, about seven to 10 days.
Clopidogrel, one of the most commonly used, is a pro-drug and requires a two-step activation process with a variety of CYP enzymes, so there often is patient variability in platelet activity. While the half-life of these agents is about six hours, the receptor inhibition is irreversible, similar to the kinetics of aspirin. Aspirin and P2Y12 inhibitors often are used together, termed DAPT, for the treatment of cardiac, cerebrovascular, and peripheral vascular conditions.39,40
There is no specific reversal agent for these drugs. Desmopressin is not effective, unlike with aspirin. The evidence regarding platelet transfusion for life-threatening bleeding is controversial. The PATCH trial showed that platelet transfusions increased the odds of death in patients with ICH. The use of platelets in other processes, including surgical emergencies, is unclear, and consensus recommendations point to using platelet transfusion in patients undergoing emergent surgical procedures.39,40
Vitamin K Antagonist
Vitamin K is an essential cofactor in the synthesis of clotting factors II, VII, IX, X, and protein C and protein S. Warfarin blocks the vitamin K epoxide reductase complex I. This inhibition then decreases the amount of activated vitamin K and decreases the amount of clotting factors.
Warfarin was one of the first anticoagulants available, and it is used for the treatment of many conditions, most commonly atrial fibrillation, mechanical heart valve replacement, and thrombotic disease, such as pulmonary embolism or deep vein thrombosis. It has to be monitored frequently by checking the patient’s international normalized ratio (INR) and adjusting the dose to maintain a fairly narrow therapeutic window between 2-3 or 2.5-3.5 depending on the underlying indication for anticoagulation. Many external factors influence the metabolization and efficacy of warfarin, such as diet, which can make it difficult to maintain this narrow window.
While INR will fluctuate often, an increase in INR increases a patient’s risk of bleeding. It has been shown that bleeding risk increases substantially with an INR greater than 10. The mainstays of treatment of elevated INR (and therefore elevated bleeding risk) are stopping the warfarin, giving vitamin K itself, and/or additional administration of fresh frozen plasma (FFP) or prothrombin complex concentrate (PCC). These are used in a stepwise fashion, depending on the INR level, risk of bleeding, or severity of bleeding. Many institutions have specific pathways, and clinicians should follow local guidelines for the management of elevated INR.
At lower but still elevated INR levels and with no evidence of bleeding, clinicians usually opt to hold the next warfarin doses and allow the INR to drift back down into acceptable ranges. With higher INR levels, increased risk of bleeding, or impending surgical needs, clinicians can hold warfarin dosing plus give vitamin K.
Supratherapeutic INR values often are common in patients on warfarin, and many patients with supratherapeutic INR values are asymptomatic. In fact, in one observational cohort of 1,104 patients on warfarin with one INR value between 5.0 and 9.0, only 0.96% experienced major hemorrhage within 30 days.41
One algorithm suggests holding warfarin doses for patients with supratherapeutic INR values between 4 and 10 without bleeding, as long as they had close outpatient follow-up.42 The algorithm also suggests giving a dose of oral vitamin K for those with INR values greater than 10 but no signs or symptoms of bleeding; however, if any signs or symptoms of bleeding were noted regardless of INR value, admission to the hospital and reversal of warfarin was indicated.42
The mainstay of warfarin reversal is vitamin K itself, given orally or parenterally. PCC and FFP also are used to help reverse the effects of warfarin. Vitamin K, FFP, or PCC generally is given in a stepwise fashion based on the severity of bleeding and INR level.
Vitamin K can be administered orally or intravenously, but its time to activation of coagulation factors to reverse bleeding takes anywhere from 24 to 36 hours.43 This gradual time frame is too long in cases of emergent or life-threatening bleeding. For these cases, FFP can be considered. FFP contains all proteins found in blood, including coagulation factors. (See Table 3.)
Table 3. Guidelines for Supratherapeutic INR Due to Oral Vitamin K Antagonist (Warfarin) |
||
INR |
Bleeding |
Management |
< 4 |
None |
|
4-10 |
None |
|
> 10 |
None |
|
Any |
Active bleeding, life-threatening hemorrhage, or hemodynamic instability |
|
INR: international normalized ratio; PO: oral; FFP: fresh frozen plasma; PCC: prothrombin complex concentrate |
||
There are both advantages and disadvantages to using FFP to reverse warfarin. FFP has been in clinical practice longer than newer reversal agents, such as PCC, and, thus, the main advantages are that FFP is more widely used, easily accessible (especially in remote hospitals), and has lower costs than newer agents.43 One study also found a higher risk of thromboembolic events in patients receiving PCC over FFP for urgent warfarin reversal.44
The main disadvantages of FFP include the large amount of volume required to achieve therapeutic coagulation, as well as transfusion-associated reactions such as lung injury or volume overload. FFP also appears to require longer time to reverse warfarin than PCC. Additionally, FFP must be thawed prior to use, while PCC can be used immediately since it is stored at room temperature.45 However, there does not seem to be any significant morbidity or mortality benefits or superiority in using PCC over FFP, and thus FFP still is used commonly.43 The decision to give FFP vs. PCC should be a multidisciplinary discussion conducted between providers and pharmacy, and the clinical context must be carefully considered.
Direct Oral Anticoagulants: Direct Xa Inhibitors and Direct Thrombin Inhibitors
The recent rise in prevalence of patients on direct oral anticoagulants (DOACs) makes it particularly important that clinicians understand how to reverse these agents. The most common DOACs currently available either directly inhibit factor Xa (as evidenced by the letters “xa” in the drug name) or inhibit factor IIa. Dabigatran is a direct thrombin inhibitor, whereas rivaroxaban, apixaban, and edoxaban are examples of factor Xa inhibitors.
DOACs often are selected and initiated as therapy over vitamin K antagonists, such as warfarin, because of their more favorable side effect profile. Among the many features afforded by DOACs over warfarin are lower bleeding risks and ease of use. In fact, in 2019 the American College of Cardiology made a class I recommendation favoring DOACs over warfarin in those patients with nonvalvular atrial fibrillation, and in 2020 the American Society of Hematology supported the use of DOACs over warfarin to treat VTE and pulmonary embolism.46 Both rivaroxaban and apixaban additionally have demonstrated noninferiority in preventing stroke and systemic embolism when compared to warfarin.47
A 2021 study in England demonstrated that DOACs represented 62% of total anticoagulants, with an average increase of 87% per year.48 These agents do not typically require routine monitoring of coagulation assays, offering a more convenient option for patients. However, DOACs are avoided in patients with decreased creatinine clearance or end-stage kidney disease; in fact, rivaroxaban, apixaban, and edoxaban are contraindicated if creatinine clearance is less than 15 mL/minute.47 Those patients are anticoagulated with warfarin instead.
Despite the widespread use of DOACs, as of 2019 there were only two Food and Drug Administration (FDA)-approved DOAC reversal agents: idarucizumab and andexanet alfa.49 However, these therapies are expensive, with the estimated charge for each drug of $3,500 and $12,000, respectively.49 Idarucizumab is a monoclonal antibody fragment specifically used in dabigatran reversal, since it binds dabigatran with significantly greater affinity than with thrombin. The onset of iadrucizumab occurs fairly quickly. In a prospective cohort study of 90 patients, those with elevated dilute thrombin times (dTT, a laboratory marker sensitive to the presence of dabigatran) demonstrated normalization of those values within minutes.50
Additionally, unlike other DOACs, dabigatran also can be reversed with hemodialysis in the event of life-threatening bleeding.51 Hemodialysis is not effective for agents such as apixaban or rivaroxaban because they are highly protein bound.52 Unlike dabigatran, the Xa inhibitor DOACs can be reversed with andexanet alfa, a recombinant modified form of the enzymatically inactive factor Xa. Andexanet alfa also has a relatively quick time to onset, with two randomized placebo-controlled trials showing time to effect within two to five minutes.53 However, cost and availability continue to be the main concerns with both reversal agents.
As discussed earlier, FFP often is used to reverse bleeding from vitamin K antagonists. PCC is a newer biological agent that now has FDA-approved indications for urgent reversal of acquired coagulation factor deficiency induced by warfarin-induced anticoagulation in patients with major acute bleeding, or those needing urgent invasive surgeries.54 PCC often is used to reverse newer DOACs as well, although this is technically off-label, and there is a lack of prospective evidence on this practice.55
PCC only consists of specific clotting factors. Three-factor PCC contains coagulation factors II, IX, and X, whereas four-factor PCC contains coagulation factors II, VII, IX, and X.56 Despite the lack of prospective evidence, current literature supports the efficacy of PCC in life-threatening cases of bleeding due to vitamin K antagonists and as emergency use in bleeding due to direct oral coagulation usage.
A recent meta-analysis examining 32 studies including 1,832 patients with ICH found that PCC had overall similar profiles in anticoagulation reversal, mortality, and thromboembolic events when compared with andexanet alfa and idarucizumab, but the authors cautioned that lack of head-to-head comparison warrants cautious interpretation.57
In a study examining the use of PCC in cases of supratherapeutic anticoagulation due to vitamin K antagonists, complete normalization of the INR was noted within 15 minutes.58 Another study demonstrated that mean INR correction time with PCC was 41 minutes, compared to 115 minutes with FFP.59 A recent review of anticoagulation guidance in the American Journal of Hematology states that for patients with dabigatran-, rivaroxaban-, or apixaban-associated major bleeding where a reversal agent is warranted, and neither andexanet alfa nor idarucizumab is available, PCC is suggested as a treatment modality.60
Other Reversal Agents to Consider
Finally, in addition to targeted therapies mentioned earlier, other adjunct reversal agents can be considered in patients on oral anticoagulation who require control of bleeding. In patients with high suspicion for coagulopathy, platelet and cryoprecipitate transfusions can be considered to maintain platelet and fibrinogen counts, respectively, within normal limits.61 Additionally, activated charcoal can be considered to reverse ingestions of dabigatran, apixaban, and rivaroxaban if within two to six hours.61
Conclusion
This review discussed the physiology of the clotting cascade as well as means of oral anticoagulation reversal. As the number of patients on oral anticoagulants increases, providers must be aware of the increased risk of bleeding in these patients and be skilled in understanding the reversal agents indicated for oral anticoagulants.
REFERENCES
- LaPelusa A, Dave HD. Physiology, hemostasis. In: StatPearls [Internet]. StatPearls Publishing. Updated May 8, 2022.
- Garmo C, Bajwa T, Burns B. Physiology, clotting mechanism. In: StatPearls [Internet]. StatPearls Publishing; 2023. Updated Sept. 5, 2022.
- Gale AJ. Continuing education course #2: Current understanding of hemostasis. Toxicol Pathol 2011;39:273-280.
- Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood 2005;106:2605-2612.
- Furie B. Pathogenesis of thrombosis. Hematology Am Soc Hematol Educ Program 2009;255-258.
- Periayah MH, Halim AS, Mat Saad AZ. Mechanism action of platelets and crucial blood coagulation pathways in hemostasis. Int J Hematol Oncol Stem Cell Res 2017;11:319-327.
- Rana A, Westein E, Niego B, Hagemeyer CE. Shear-dependent platelet aggregation: Mechanisms and therapeutic opportunities. Front Cardiovasc Med 2019;6:141.
- Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost 2013;11:605-614.
- Jung SM, Moroi M. Platelet glycoprotein VI. Adv Exp Med Biol 2008;640:53-63.
- Huang J, Li X, Shi X, et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol 2019;12:26.
- Shin EK, Park H, Noh JY, et al. Platelet shape changes and cytoskeleton dynamics as novel therapeutic targets for anti-thrombotic drugs. Biomol Ther (Seoul) 2017;25:223-230.
- Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol 2008;28:403-412.
- Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res 2007;120(Suppl 1):S5-S9.
- Rumbaut RE, Thiagarajan P. Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. Morgan & Claypool Life Sciences; 2010. Chapter 4, Platelet Aggregation.
- Murugappa S, Kunapuli SP. The role of ADP receptors in platelet function. Front Biosci 2006;11:1977-1986.
- Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27:1687-1693.
- Green D. Coagulation cascade. Hemodial Int 2006;10(Suppl 2):S2-S4.
- Barmore W, Bajwa T, Burns B. Biochemistry, Clotting Factors. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated May 8, 2022.
- Wheeler AP, Gailani D. The intrinsic pathway of coagulation as a target for antithrombotic therapy. Hematol Oncol Clin North Am 2016;30:1099-1114.
- Salen P, Babiker HM. Hemophilia A. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated July 18, 2022.
- Brown MA, Stenberg LM, Stenflo J. Coagulation Factor Xa. Handbook of Proteolytic Enzymes. 3rd ed. Academic Press; 2013:2908-2915. https://doi.org/10.1016/B978-0-12-382219-2.00642-6
- Göbel K, Eichler S, Wiendl H, et al. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders — A systematic review. Front Immunol 2018;9:1731.
- Weisel JW, Litvinov RI. (2017). Fibrin formation, structure and properties. Subcellular Biochemistry 2017;82:405-456.
- Muszbek L, Bereczky Z, Bagoly Z, et al. Factor XIII: A coagulation factor with multiple plasmatic and cellular functions. Physiol Rev 2011;91:931-972.
- Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood reviews Rev 2015;29:17-24.
- Yatsenko TA, Rybachuk VM, Yusova OI, et al. Effect of fibrin degradation products on fibrinolytic process. Ukr Biochem J 2016;88:16-24.
- Altaf F, Wu S, Kasim V. Role of fibrinolytic enzymes in anti-thrombosis therapy. Front Mol Biosci 2021;8:680397.
- Schafer AI, Levine MN, Konkle BA, Kearon C. Thrombotic disorders: Diagnosis and treatment. Hematology Am Soc Hematol Educ Program 2003;520-539.
- Vaduganathan M, Mensah GA, Turco JV, et al. The global burden of cardiovascular diseases and risk: A compass for future health. J Am Coll Cardiol 2022;80:2361-2371.
- Awtry EH, Loscalzo J. Aspirin. Circulation 2000;101:1206-1218.
- Leissinger C, Carcao M, Gill JC, et al. Desmopressin (DDAVP) in the management of patients with congenital bleeding disorders. Haemophilia 2014;20:158-167.
- Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al; PATCH Investigators. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet 2016;387:2605-2613.
- Pabinger I, Fries D, Schöchl H, et al. Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr 2017;129:303-316.
- Chauncey JM, Wieters JS. Tranexamic acid. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated July 25, 2022.
- CRASH-2 trial collaborators; Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010;376:23-32.
- CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial [published correction appears in Lancet 2019;394:1712]. Lancet 2019;394: 1713-1723.
- Shakur H, Elbourne D, Gülmezoglu M, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): Tranexamic acid for the treatment of postpartum haemorrhage: An international randomised, double blind placebo controlled trial. Trials 2010;11:40.
- HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): An international randomised, double-blind, placebo-controlled trial. Lancet 2020;395:1927-1936.
- Beavers CJ, Naqvi IA. Clopidogrel. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated July 11, 2022.
- Yeung LYY, Sarani B, Weinberg JA, et al. Surgeon’s guide to anticoagulant and antiplatelet medications part two: Antiplatelet agents and perioperative management of long-term anticoagulation. Trauma Surg Acute Care Open 2016;1:e000022.
- Garcia DA, Regan S, Crowther M, Hylek EM. The risk of hemorrhage among patients with warfarin- associated coagulopathy. J Am Coll Cardiol 2006;47:804-808.
- Garcia DA, Crowther MA. Reversal of warfarin: Case-based practice recommendations. Circulation 2012;125:2944-2947.
- Luo Y, Ma C, Yu Y. Application of fresh frozen plasma transfusion in the management of excessive warfarin-associated anticoagulation. Blood Sci 2022;4:57-64.
- Maguire M, Fuh L, Goldstein JN, et al. Thromboembolic risk of 4-factor prothrombin complex concentrate versus fresh frozen plasma for urgent warfarin reversal in the emergency department. West J Emerg Med 2019;20:619-625.
- Zareh M, Davis A, Henderson S. Reversal of warfarin-induced hemorrhage in the emergency department. West J Emerg Med 2011;12:386-392.
- Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open 2021;4:e2137288.
- Skorska J, Uprichard J. Direct oral anticoagulants: A quick guide. Eur Cardiol 2017;12:40-45.
- Afzal S, Zaidi STR, Merchant HA, et al. Prescribing trends of oral anticoagulants in England over the last decade: A focus on new and old drugs and adverse events reporting. J Thromb Thrombolysis 2021;52:646-653.
- Desai NR, Cornutt D. Reversal agents for direct oral anticoagulants: Considerations for hospital physicians and intensivists. Hosp Pract (1995) 2019;47:113-122.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-520.
- Chan KE, Edelman ER, Wenger JB, et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 2015;131:972-979.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors [published correction appears in Am J Hematol 2012;87:748]. Am J Hematol 2012;87(Suppl 1):S141-S145.
- Shih AW, Crowther MA. Reversal of direct oral anticoagulants: A practical approach. Hematology Am Soc Hematol Educ Program 2016;2016:612-619.
- Baskaran J, Lopez RA, Cassagnol M. Prothrombin complex concentrate. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated May 1, 2022.
- Müller M, Eastline J, Nagler M, et al. Application of prothrombin complex concentrate for reversal of direct oral anticoagulants in clinical practice: Indications, patient characteristics and clinical outcomes compared to reversal of vitamin K antagonists. Scand J Trauma Resusc Emerg Med 2019;27:48.
- Faulkner H, Chakankar S, Mammi M, et al. Safety and efficacy of prothrombin complex concentrate (PCC) for anticoagulation reversal in patients undergoing urgent neurosurgical procedures: A systematic review and metaanalysis. Neurosurg Rev 2021;44:1921-1931.
- Chaudhary R, Singh A, Chaudhary R, et al. Evaluation of direct oral anticoagulant reversal agents in intracranial hemorrhage: A systematic review and meta-analysis. JAMA Netw Open 2022;5:e2240145.
- Zareh M, Davis A, Henderson S. Reversal of warfarin-induced hemorrhage in the emergency department. West J Emerg Med 2011;12:386-392.
- Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg 2000;14:458-461.
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol 2019;94:697-709.
- Dhakal P, Rayamajhi S, Verma V, et al. Reversal of anticoagulation and management of bleeding in patients on anticoagulants. Clin Appl Thromb Hemost 2017;23:410-415.
This review will describe the physiological components of the clotting cascade, highlight common anticoagulant agents in use, and discuss means of oral anticoagulation reversal.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
