Preparing for Fresh Concerns of Poliovirus and Acute Flaccid Myelitis
Authors
Trahern W. ("TW") Jones, MD, Assistant Professor, Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City
Arnel Besic, B.S. Student, Department of Chemistry, University of Utah, Salt Lake City
Peer Reviewer
Catherine A. Marco, MD, FACEP, Emergency Medicine, Milton S. Hershey Medical Center, Penn State Health, Hershey, PA
Executive Summary
- Globally, three wild serotypes of poliovirus have been identified: type 1, type 2, and type 3. Because of the efforts of global vaccination campaigns, type 2 was declared eradicated in September of 2015, with the last detected case in 1999. Type 3 was subsequently declared eradicated in October of 2019 and was last detected in 2012. Type 1 poliovirus remains endemic in Afghanistan and Pakistan.
- Vaccine-derived poliovirus (VDPV) is a strain of poliovirus that stems from the weakened live virus contained in the oral poliovirus vaccine (OPV). This weakened form of the virus can revert to a form that causes illness and paralysis if allowed to circulate in populations that are unvaccinated or undervaccinated. In July 2022, a case of VDPV type 2 was detected in an unvaccinated individual in Rockland County, New York, leading to flaccid lower limb weakness.
- Poliovirus is transmitted primarily through fecal-oral contact, droplet transmission, or, more rarely, oral-oral spread or contact with contaminated food and drink. Following transmission, poliovirus replicates within the oropharynx and gastrointestinal tract. During this initial replication phase, poliovirus may invade local lymphatic tissue and enter the bloodstream. From there, it may infect cells of the central nervous system, subsequently leading to destruction of motor neurons of the anterior horn of the spinal cord, resulting in paralysis.
- Most individuals infected with poliovirus remain asymptomatic. Around 25% of infected individuals will experience abortive nonparalytic polio with symptoms unrelated to the central nervous system. Meanwhile, 1% to 5% of infected patients will experience symptoms of meningitis, and about 0.5% may experience the characteristic paralysis of the disease.
- Paralysis typically is asymmetrical, involving one or more limbs and muscle groups, with more severe paralysis in the proximal muscle groups of the limbs. This often is associated with reduced or absent deep tendon reflexes, yet with intact sensation. Paralysis of the respiratory muscles may lead to respiratory failure and hypoxia, which is estimated to lead to a 2% to 10% mortality rate. Weakness or paralysis that persists for 12 months after onset usually is permanent.
- Paralytic polio may be classified into three types: spinal, bulbar, and bulbospinal. Spinal polio, which accounted for 79% of paralytic cases between 1969 and 1979, is characterized by asymmetric paralysis and usually involves the legs. Bulbar polio accounts for 2% of cases, and includes weakness of facial, oropharyngeal, and respiratory muscles innervated by cranial nerves. Nearly 20% of cases manifest as a combination of the two, which is known as bulbospinal polio.
- There are no approved antiviral medications available for acute poliovirus infections, although there are several under development. A number of other medications have been examined through randomized controlled trials in managing the symptoms and hypothesized inflammatory reactions of post-polio syndrome. These have included amantadine, high-dose prednisone, pyridostigmine, modafinil, and coenzyme Q-10, among others. None of those medications have demonstrated benefit, but lamotrigine was found to have a beneficial effect on pain, fatigue, and quality of life. Intravenous immunoglobulin has been found to reduce proinflammatory cytokines in the cerebrospinal fluid, although clinically meaningful response remains doubtful.
- Acute flaccid myelitis (AFM) is a poliomyelitis-like syndrome of unknown etiology. The syndrome is characterized by gastrointestinal or respiratory illness, followed by the onset of limb myalgia, with subsequent asymmetric flaccid weakness affecting one or more limbs in any distribution. Upper limbs and proximal muscle groups also may be affected. Limb paralysis also may be accompanied by hyporeflexia, areflexia, and paresthesias in the affected extremities. However, numbness or sensory deficit would be unusual and may require a reconsideration of the differential diagnosis. Respiratory failure affects many patients, and nearly one-third of patients will require intubation and ventilation. Bowel and bladder dysfunction also may develop in the acute phase, as well as autonomic dysfunction characterized by labile blood pressures and irregular heartbeat.
- Magnetic resonance imaging is a mainstay of diagnosis in AFM. Classically, providers can expect to find T2 hyperintense lesions of spinal cord gray matter. These lesions often are longitudinally extensive, although cervical cord involvement is most common.
- Optimal treatment of AFM remains controversial and incompletely understood. At the time of this writing, there are no antiviral medications known to provide any clinical benefit. Intravenous immunoglobulin, corticosteroids, and therapeutic plasma exchange have been considered in the treatment of AFM.
What will be the next health crisis? Could it be wild-type poliovirus, vaccine-derived poliovirus, or the similar condition of acute flaccid myelitis? Are you prepared to recognize and anticipate the complications? The authors prepare clinicians for the acute management of each of these conditions.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Poliovirus is the causative agent of poliomyelitis. Historically and globally, poliovirus has been responsible for millions of deaths and countless cases of paralysis among the children of the world. Rolling outbreaks throughout the United States and other countries in the 20th century were eventually curtailed and eliminated through the success of vaccination campaigns. These campaigns ultimately culminated in the 1988 World Health Organization resolution for the global eradication of the disease. These efforts cornered the virus in remote regions of Afghanistan and Pakistan, and eventual eradication seemed all but certain. However, new outbreaks of wild poliovirus type 1 and vaccine-derived poliovirus (VDPV) have brought these successes into question. Moreover, ongoing disinformation campaigns and incomplete vaccination have rendered the U.S. and global populations increasingly vulnerable to a return of poliovirus.
Acute flaccid myelitis (AFM) is a condition typified by a clinical presentation and neurologic findings similar to poliomyelitis. Epidemiologically, geographic surges in cases of AFM have led to the suspicion it is caused by an infectious agent, with a preponderance of evidence pointing toward enterovirus D68 (EV-D68) as the main etiologic pathogen. While AFM cases throughout the population remain rare, there is concern that a broader outbreak or epidemic could occur. Additionally, its similarity to poliomyelitis demands careful diagnostic attention from emergency medical providers, primary care providers, public health authorities, and infectious disease specialists.
This review will explore the epidemiology, virology, pathophysiology, clinical manifestations, and important diagnostic and therapeutic considerations for providers who could encounter these clinical syndromes.
Poliomyelitis
Epidemiology
Poliomyelitis likely has plagued humanity for millennia. The earliest depiction of an individual with possible poliomyelitis dates from between 1400 and 1300 BCE, and it is found in the funerary stele or gravestone of the Egyptian priest Ruma.1,2 (See Figure 1.) The stele depicts an individual with a shortened, withered leg, a finding characteristic of paralytic polio. Since then, poliomyelitis survived quietly as an endemic disease until major outbreaks occurred in the early 20th century.
Figure 1. Possible Depiction of Post-Polio Syndrome in Ancient Egypt |
 |
Believed to be the earliest depiction of post-polio syndrome in human history, the funerary stele of Egyptian priest Ruma depicts an individual with a unilaterally shortened, withered leg. Source: Kreuz DG. Polio Egyptian stele. Published Jan. 9, 2006. https://commons.wikimedia.org/wiki/File:Polio_Egyptian_Stele.jpg |
Humans are the only known animal reservoir for poliovirus.3 Transmission typically is mediated through respiratory droplets or the oral-fecal route.1 Because of these reasons, crowded and unsanitary living conditions are believed to encourage the spread and transmission of poliovirus.
Historically, poliomyelitis primarily affected children younger than 5 years of age, although unvaccinated or undervaccinated older children and adults also were at risk.4 Based on historical data, poliovirus infections characteristically peaked in the summer months in temperate climates, but they had no seasonal pattern in tropical climates.5
Prior to the introduction of routine childhood poliovirus vaccinations, the United States reported tens of thousands of annual cases, with an average of 35,000 people disabled by the virus each year in the late 1940s.1 Once the trivalent inactivated poliovirus vaccine (IPV) was introduced in 1955 and the trivalent oral poliovirus vaccine (OPV) was developed in 1963, cases rapidly fell to fewer than 100 annual cases in the 1960s and fewer than 10 annual cases in the 1970s.1 The United States has since been polio-free for more than four decades, and the Western hemisphere has likewise been polio-free since 1991. There have been no wild type cases originating in the United States since 1979, and no wild type cases from travelers have been reported since 1993.
Globally, three wild serotypes of poliovirus (WPV) have been identified: type 1, type 2, and type 3. Because of the efforts of global vaccination campaigns, type 2 was declared eradicated in September of 2015, with the last detected case in 1999. Type 3 was subsequently declared eradicated in October of 2019 and was last detected in 2012.6 Type 1 poliovirus remains endemic in Afghanistan and Pakistan. Overall, the dramatic success of global vaccination campaigns may be best illustrated by case numbers: The number of all reported worldwide polio cases has decreased from an estimated 350,000 cases in 1988 to only six reported cases in 2021.7 (See Figure 2.)
Figure 2. Cases of Paralytic Polio by World Region, 1980 to 2020 |
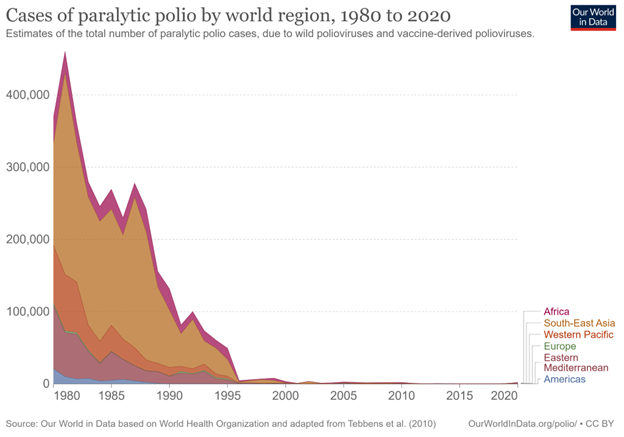 |
Estimates of the total number of paralytic polio cases caused by wild polioviruses and vaccine-derived polioviruses. Source: Our World in Data. Cases of paralytic polio by world region, 1980 to 2020. https://ourworldindata.org/grapher/number-of-estimated-paralytic-polio-cases-by-world-region |
While the eradication of type 1 poliovirus appears to be close, success is not guaranteed. Disinformation and misinformation spread through social media and alternative news outlets have led to a growing anti-vaccination movement, the results of which are growing numbers of unvaccinated and undervaccinated children and adults.8 As a consequence, communities in the United States and globally are becoming increasingly vulnerable to new outbreaks of disease. In fact, recent Centers for Disease Control and Prevention (CDC) data suggest that many U.S. states have lower than 90% vaccine coverage among all assessed kindergartners.9 (See Figure 3.) Overseas, political instability and conflict in regions around the world have led to undervaccination of pediatric populations. Nigeria’s efforts to eradicate polio in the northern provinces of the Borno state were long hampered by attacks by Boko Haram, an Islamist militant group. As such, Nigeria remained the last country in Africa to be declared polio-free in 2020.10,11 The success of Africa’s polio-free declaration was recently tarnished by the discovery of a new case of paralytic poliomyelitis in Lilongwe, Malawi, which appeared to have been introduced into the country from circulating wild type 1 poliovirus from the Sindh province in Pakistan.11,12
Figure 3. Polio Vaccination Rates Among U.S. States |
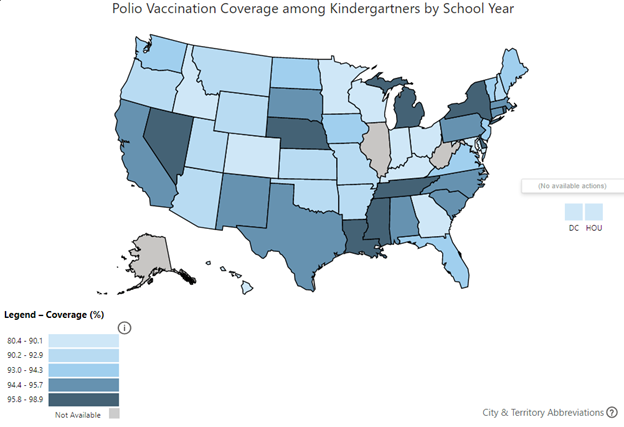 |
Source: Centers for Disease Control and Prevention. Polio vaccination coverage among kindergartners by school year. https://www.cdc.gov/vaccines/imz-managers/coverage/schoolvaxview/data-reports/index.html |
Vaccine-derived poliovirus (VDPV) is a strain of poliovirus that stems from the weakened live virus contained in the OPV.13 This weakened form of the virus can revert to a form that causes illness and paralysis if allowed to circulate in populations that are unvaccinated or undervaccinated. It also may be shed by an immunodeficient individual and infect an unvaccinated individual. VDPV characteristically causes disease only in undervaccinated communities. In July 2022, a case of VDPV type 2 was detected in an unvaccinated individual in Rockland County, New York, leading to flaccid lower limb weakness.14 Additional samples of wastewater confirmed likely community transmission of VDPV, which was attributed to poor vaccination rates among the local population.
Virology and Pathophysiology
Poliovirus is a member of the Picornaviridae family and can be further classified into the genus Enterovirus.15 It is a positive-strand ribonucleic acid (RNA) virus and consists of around 7,500 bases. These bases are enclosed within a non-enveloped capsid that consists of 60 copies of a four-protein conglomerate.16 These four proteins are designated VP1, 2, 3, and 4. They are arranged in an icosahedral (20-faced) structure, with a diameter of about 30 nm, which gives the virus its characteristic shape.
Poliovirus has a rapid replication cycle, with approximately eight hours elapsing between infection and the release of virions once the host cell is lysed. Synthesis of up to 10,000 virions per affected cell can lead to massive dissemination to nearby cells.17 The virus’s genome itself acts as an mRNA molecule with a single long reading frame encoding viral polyprotein between two untranslated regions of RNA. There also is a polyadenosine tail of variable length at the 3’ end and a small viral protein of 22 amino acids called VPg (3B) covalently bound to the 5’ end. After translation, the polyprotein is cleaved into the four aforementioned designations (VP1, 2, 3, and 4).17,18
Poliovirus is transmitted primarily through fecal-oral contact, droplet transmission, or, more rarely, oral-oral spread or contact with contaminated food and drink.5 Following transmission, poliovirus replicates within the oropharynx and gastrointestinal tract. During this initial replication phase, poliovirus may invade local lymphatic tissue and enter the bloodstream. From there, it may infect cells of the central nervous system, subsequently leading to destruction of motor neurons of the anterior horn of the spinal cord, resulting in paralysis. Hosts usually experience a maximum viral load beginning two to three days before the onset of symptoms and lasting until one week after. Viral shedding from the oropharynx and nasopharynx may continue for one to two weeks after illness onset, and viable viruses can be shed in the stool for several weeks after.
Clinical Manifestations
Most individuals infected with poliovirus remain asymptomatic. Around 25% of infected individuals will experience abortive nonparalytic polio with symptoms unrelated to the central nervous system.
Meanwhile, 1% to 5% of infected patients will experience symptoms of meningitis, and about 0.5% may experience the characteristic paralysis of the disease.1,5 Abortive nonparalytic polio is typified by nonspecific flu-like symptoms that may include:
- fever;
- sore throat;
- headache;
- stomach pain;
- nausea;
- fatigue and malaise.
Patients with abortive nonparalytic polio usually do not demonstrate any clinical or laboratory evidence of invasion of the central nervous system. These symptoms often last between two to five days and usually resolve on their own, with complete recovery in less than a week.1,5
Patients with nonparalytic polio meningitis may demonstrate:
- the previously mentioned flu-like symptoms;
- stiffness in the neck, back, or legs;
- pain in the neck, back, or legs;
- increased or abnormal sensation;
- vomiting.
In nonparalytic polio meningitis, the onset of flu-like symptoms will persist for several days and will be followed by the more severe signs and symptoms of aseptic meningitis. These symptoms typically will last two to 10 days, followed by a complete recovery.5
As mentioned, only a small minority of individuals will experience the characteristic flaccid paralysis or weakness of the arms and/or legs known as paralytic polio. This manifestation may be biphasic in children, with an initial onset of flu-like symptoms for several days, followed by a remission period of one to three days without symptoms, and finally followed by paralysis, muscle pain, and fever.5 Paralysis of the affected limb(s) and muscle groups typically will progress within three days. In unimmunized adolescents and adults, the initial onset of flu-like symptoms often is absent, with more severe pain and paralysis and increased rates of fatality.
Paralysis typically is asymmetrical, involving one or more limbs and muscle groups, with more severe paralysis in the proximal muscle groups of the limbs.1,5 This often is associated with reduced or absent deep tendon reflexes, yet with intact sensation. Paralysis of the respiratory muscles may lead to respiratory failure and hypoxia, which is estimated to lead to a 2% to 10% mortality rate. Weakness or paralysis that persists for 12 months after onset usually is permanent. An estimated two out of three people experience such lifelong sequelae of the disease, although some may experience partial or total recovery through compensation by unaffected muscles. The majority of individuals will lose function of the affected limb or muscle group, followed by denervation-induced skeletal muscle atrophy and limb-length discrepancy. (See Figure 4.) Paralytic disease with similar manifestations may be caused by VDPVs that have reverted to and have re-acquired virulence and transmissibility.
Figure 4. Denervation-Induced Skeletal Muscle Atrophy in a Young Poliomyelitis Survivor |
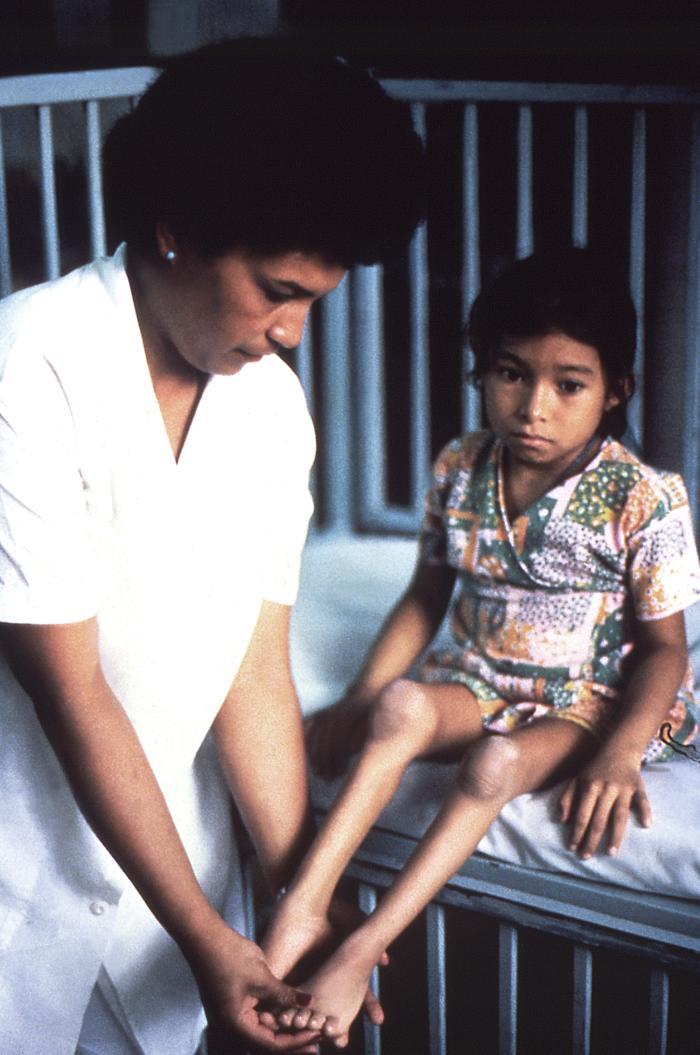 |
Source: Centers for Disease Control and Prevention. Poliomyelitis. https://phil.cdc.gov/Details.aspx?pid=13211 |
Paralytic polio may be classified into three types: spinal, bulbar, and bulbospinal. Spinal polio, which accounted for 79% of paralytic cases between 1969 and 1979, is characterized by asymmetric paralysis and usually involves the legs.5 Bulbar polio accounts for 2% of cases, which includes weakness of facial, oropharyngeal, and respiratory muscles innervated by cranial nerves. Nearly 20% of cases manifest as a combination of the two, which is known as bulbospinal polio.
Post-polio syndrome (PPS) is a phenomenon that usually occurs decades after initial infection and recovery. PPS is non-communicable and affects only polio survivors, although not all survivors of poliomyelitis will experience PPS. It is believed that PPS occurs because of degradation of motor neurons over several years, which leads to muscle weakness, dysfunction, and atrophy.19 Symptoms include:
- slow and progressive muscle weakness after the initial infection;
- muscle atrophy and loss of function;
- joint pain and degradation;
- fatigue;
- increased skeletal issues, such as scoliosis.
Polio survivors who experience PPS most often start to experience gradually worsening weakness in muscle groups that were previously affected by polio. Symptoms can range from minor to severe. Those who experience more acute polio with longer recovery may experience more severe PPS. PPS is rarely life-threatening but may impose significant reduction in the quality of life.19
Diagnosis
The virologic diagnosis of poliomyelitis classically is accomplished through viral cultures of stool and throat specimens.3 To increase diagnostic yield, two separate stool cultures should be performed 24 hours apart.20 These specimens should be obtained at the earliest clinical suspicion for poliovirus infection to optimize recovery of the etiologic virus on culture, and ideally within 14 days after illness onset; however, viral shedding has been observed in the stool for as long as three to six weeks afterward.3 Therefore, even patients who appear to have transitioned from the acute illness to convalescence may warrant virologic testing for diagnosis. In situations of doubt, consultation with an infectious disease specialist or public health department may be necessary.
Polymerase chain reaction (PCR) affords a sensitive and rapid approach to diagnosis. As a member of the genus Enterovirus, poliovirus can be detected through commercial real-time reverse transcriptase PCR multiplexed assays performed upon oropharyngeal/nasopharyngeal swabs and cerebrospinal fluid (CSF).3 While these assays do not differentiate between poliovirus and other enteroviruses, a positive result may be a necessary clue that more specific testing is needed.
Serologic testing is less useful in the general population, since circulating antibodies to polioviruses 1 and 2 are expected in those with normal humoral response to routine childhood immunization.20 However, if an individual is known or confirmed to have never previously received immunization, acute and convalescent serological testing (performed three weeks following onset of illness) may aid in the diagnosis. Differentiation of wild-type polioviruses 1 and 2 vs. VDPV typically is not available commercially, but specimens of confirmed cases should be reported and forwarded to the CDC for intratype differentiation.20 From there, further genome sequencing is used to confirm poliovirus genotype and potentially determine the geographic origin of the strain. CSF profiles in acute paralytic poliomyelitis may demonstrate lymphocytic pleocytosis, with cell counts ranging from 50/mm3 to as high as 2,000/mm3.21,22 Early in the course of disease, some authors from the pre-vaccine era noted occasional increases in neutrophil counts as well. Opening pressure often is normal. CSF protein may be mildly elevated, while CSF glucose often is normal. Magnetic resonance imaging (MRI) findings in acute paralytic poliomyelitis may demonstrate enhancement on T2-weighted images of the anterior horn, concordant with the known pathophysiology of the disease.23
Treatment
There are no approved antiviral medications available for acute poliovirus infections, although there are several under development.22 A number of other medications have been examined through randomized controlled trials in managing the symptoms and hypothesized inflammatory reactions of PPS. These have included amantadine, high-dose prednisone, pyridostigmine, modafinil, and coenzyme Q-10, among others.23 None of those medications have demonstrated benefit, but lamotrigine was found to have a beneficial effect on pain, fatigue, and quality of life.24 Intravenous immunoglobulin has been found to reduce proinflammatory cytokines in the CSF, although clinically meaningful response remains doubtful.23
Otherwise, supportive care is indicated in all cases of acute poliomyelitis and PPS.24 Patients should be hospitalized in the acute phase of illness, and strict bedrest historically has been recommended in the hopes of reducing the degree of paralysis. Hot packs are traditionally used to relieve muscle aches and spasms. Patients require close observation in the acute phase to determine the progression of paralysis and monitor for hypoventilation. Mechanical ventilation should be initiated when vital capacity falls to less than 50%. Bulbar paralysis may lead to pooling of secretions and aspiration; in severe cases of bulbar paralysis, patients may require intubation.
While detailed long-term management of the sequelae of poliomyelitis and PPS are beyond the scope of this review, several considerations may be noted. Hypoventilation can require long-term invasive mechanical ventilation, which may necessitate tracheostomy placement.24,25
Historically, prior to modern tracheostomy and positive pressure ventilation, patients with hypoventilation were managed through whole-body negative pressure ventilatory devices, the best known of which was the Drinker “iron lung.”26 After the progression of paralysis ceases, patients should begin evaluation and treatment with occupational, speech, and physical therapy to regain function and adapt to any residual neurologic deficits. Orthoses and braces may be necessary to assist mobility. Restless leg syndrome commonly is reported among patients with PPS, who may respond to dopamine-agonist therapy.25
Immunization
Currently, only inactivated polio vaccine (IPV) is available in the United States, but many countries still use OPV.3 IPV contains all three wild polio serotypes, while most low- and middle-income countries use bivalent live attenuated OPV containing serotypes 1 and 3. OPV has served as the predominant immunization in the global eradication of polio for several reasons: It is cost-effective, its administration requires no special training (there is no need for injections or sterile syringes), and attenuated vaccine-type poliovirus will be excreted in the feces that, in poor-hygiene situations, may provide “collateral” vaccination of those in close contact with the vaccinated individual.27 In communities with high vaccine coverage, this usually results in the successful eradication of wild type poliovirus and the interruption of any further transmission of vaccine-type virus. However, as mentioned previously, communities with poor immunization coverage may experience ongoing circulation of vaccine-type virus that eventually leads to mutation and the regaining of neuroinvasive characteristics. Ultimately, global health authorities plan to withdraw OPVs upon global polio eradication.
In the United States, children are recommended to undergo a four-dose series of IPV given at 2 months of age, 4 months of age, 6-18 months of age, and 4-6 years of age.3,28 Efficacy is estimated at 90% protection following the first two doses of IPV, and three doses is estimated to provide 99% protection.28 Immunity is believed to be lifelong following the primary immunization series.3
Children and adults who are unvaccinated/undervaccinated and are at increased risk of poliovirus exposure should be advised to receive immunization against poliovirus. High-risk situations and individuals include:
- travel to countries where there is risk of polio exposure;
- laboratory workers who handle specimens that may contain poliovirus;
- healthcare workers who are in close contact with an infected individual;
- individuals who are unvaccinated and live in communities where poliovirus is circulating.
Acute Flaccid Myelitis
Epidemiology
Between 2012 and 2015, public health officials and infectious disease specialists in California became aware of a growing number of patients with a poliomyelitis-like syndrome of unknown etiology.29 The syndrome was characterized by gastrointestinal or respiratory illness, followed by the onset of limb myalgia, paralysis, and the appearance of T2 signal hyperintensity in gray matter on MRI. Nearly 60 cases initially were identified in this first known outbreak of the disease.
Since then, ongoing investigation has revealed AFM outbreaks throughout North America, South America, Europe, Asia, Africa, and Australasia.30 While there are no estimates for total global cases, 2016 surveillance data in California revealed that, among children 1 to 18 years of age, the disease incidence was 1.43 cases per 100,000 person-years.31 Indeed, the disease typically strikes younger children, with a median age of 6 years, but with a range of 3 months to 21 years of age.32 Less than 15% of cases have been recorded in adults.30
Outbreaks frequently are clustered geographically. Additionally, cases in temperate regions are observed to surge every two years, with distinct case number spikes in 2014, 2016, and 2018.33 (See Figure 5.) The expected spike in 2020 presumably was disrupted by the public health interventions and travel restrictions associated with the COVID-19 pandemic, although this is conjectural.
Figure 5. Acute Flaccid Myelitis Cases by Month, 2014-2022 |
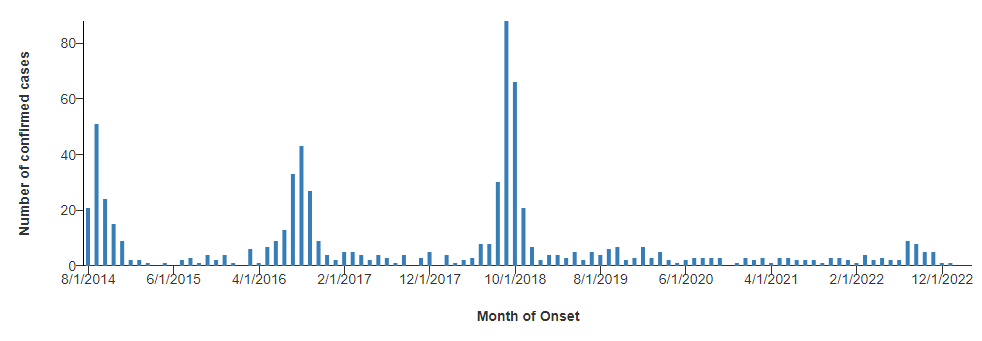 |
Source: Centers for Disease Control and Prevention. AFM cases and outbreaks. Reviewed April 4, 2023. https://www.cdc.gov/acute-flaccid-myelitis/cases-in-us.html |
Proposed Virology and Pathophysiology
The exact cause of AFM is not yet well understood, but EV-D68 is thought to be the culprit in most AFM cases, particularly because its circulation has been observed to surge concurrently with the same biennial pattern observed in AFM cases.30 EV-D68 is a member of the Picornaviridae family and the genus Enterovirus. Like all picornaviruses, EV-D68 contains a positive single-stranded RNA genome, which is carried in a non-enveloped icosahedral capsid. Much like poliovirus, the genome of EV-D68 consists of an open reading frame with an untranslated region on either side. Post-translational proteolytic events lead to the formation of several structural and non-structural proteins. The four structural proteins, VP1, 2, 3, and 4, form the structure of the virus, which consists of 60 copies of each protein.34
The capsid must recognize and bind to a host cell membrane to begin infection. Binding of the capsid to the cell membrane causes an uncoating event that creates a pore through the membrane. Once inside of the host cell, the viral proteins undergo reorganization to release the genome into the cytoplasm. The genome then is replicated by the host cell and leads to the formation of virions. These virions then leave the cell either by cell lysis or exocytosis and subsequently go on to infect other cells.34 There may be other pathogens at play, including coxsackievirus A16 and enterovirus A71, but these have been detected in only a small number of patients with AFM. If poliovirus is detected in a stool sample, it is then considered a polio case, not an AFM case.35
Ultimately, however, the pathophysiology of AFM remains poorly understood. While there is ample precedent among enteroviruses (such as poliovirus) for neurotropic behavior, EV-D68 is primarily recognized as a respiratory virus, and the mechanisms underlying any possible neuroinvasion have not been discovered yet.31 Mouse models with EV-D68 have successfully demonstrated neuronal infection and spread, with additional correlation to paralysis.36 In human populations, however, EV-D68 has not been reliably implicated in spinal cord or brain tissue from AFM patients. In part, this may be because of a lack of direct tissue sampling. Further clouding this picture is the fact that deep metagenomic sequencing from CSF specimens has failed to recover EV-D68 viral nucleic acid.37 However, such difficulty in confirming viral neurotropism is not unusual when compared to other viruses with similar occasional spread to the CNS (such as poliovirus).
Clinical Manifestations
Families typically recount a preceding prodromal illness, characterized by fever and respiratory symptoms like cough, rhinorrhea, and pharyngitis.30 Gastrointestinal symptoms, such as vomiting and diarrhea also are encountered occasionally, although this is less typical. It is common for more than one member of the household to experience a similar respiratory or gastrointestinal illness. Such prodromal symptoms often will improve before the onset of neurological symptoms.
Neurological symptoms may present anywhere from one to 10 days following the onset of prodromal illness.30 Early symptoms can include meningismus, headache, and a return of fever. Pain may develop in the soon-to-be affected limb as well as the neck and back. Subsequently, flaccid weakness presents asymmetrically, affecting one or more limbs in any distribution. Upper limbs and proximal muscle groups also may be affected. Limb paralysis also may be accompanied by hyporeflexia, areflexia, and paresthesias in the affected extremities.
However, numbness or sensory deficit would be unusual and may require a reconsideration of the differential diagnosis. Manifestations in the affected limb(s) may span the spectrum of mere moderate weakness in a single limb to complete paralysis of all limbs, as well as axial and bulbar muscles.
Indeed, some patients may experience weakness in the neck, trunk, diaphragm, and muscles related to the cranial nerves, including unilateral facial weakness or loss of extraocular motion.30 Respiratory failure affects many patients, and nearly one-third of patients will require intubation and ventilation.32 Bowel and bladder dysfunction also may develop in the acute phase, as well as autonomic dysfunction characterized by labile blood pressures and irregular heartbeat.
Altered mental status is rare in AFM, and its presence may require a reconsideration of the differential diagnosis. Similarly, seizures or upper motor neuron signs (such as spasticity, clonus, or hyperreflexia) would not typically be expected in AFM. These manifestations would require further investigation for other causes.
Patients may progress to neurological nadir within hours to days, during which paralysis and neurologic deficits are at their maximum extent of severity.30 This phase of illness subsequently may last days to weeks, after which the patient may spontaneously recover some function. However, less than 10% of patients will make a full recovery; many more will experience lifelong deficits, ranging from partial paralysis to complete quadriplegia.30
Diagnosis
Clinicians must entertain a broad differential diagnosis when encountering the stated clinical manifestations. Poliomyelitis (either wild-type or vaccine-acquired) remains an important consideration, and much of the evaluation recommended by the CDC is targeted at ruling out this entity. Additional considerations include Guillain-Barré syndrome, spinal cord infarction, epidural abscess or mass, demyelinating myelitis, other infectious myelitides (such as West Nile virus, Japanese encephalitis, or varicella), botulism, or orthopedic syndromes leading to pseudoparalysis in young infants or neurodevelopmentally delayed children.
Pathogenic diagnosis may be elusive, but specimens should be collected for stool and respiratory viral cultures to help rule out poliovirus and to potentially culture EV-D68. Similarly, stool and respiratory multiplex and enterovirus PCRs should be undertaken. Serum enterovirus PCR also is recommended. As of this writing, all suspected AFM cases should have specimens of stool, respiratory swabs, serum, and CSF forwarded to the CDC for further surveillance testing.38
MRI is a mainstay of diagnosis in AFM. Classically, providers can expect to find T2 hyperintense lesions of spinal cord gray matter.30 These lesions often are longitudinally extensive, although cervical cord involvement is most common.
Mild CSF pleocytosis also is commonly encountered, typically with a lymphocytic predominance.30 Other syndromes under consideration for diagnosis may be differentiated by this finding, such as Guillain-Barré syndrome, in which pleocytosis would not be expected. When AFM or similar syndromes are suspected, providers in the emergency department should consider obtaining MRI, as described. Neurology and infectious disease specialists should be contacted to help coordinate studies when performing a lumbar puncture. Because of that, this procedure may be more logistically feasible as an inpatient consideration. Likewise, ensuring pathogenic studies are completed correctly (e.g., stool and respiratory viral cultures) may be more feasible as an inpatient consideration in conjunction with infectious disease specialist consults.
Treatment
Optimal treatment of AFM remains controversial and incompletely understood. At the time of this writing, there are no antiviral medications known to provide any clinical benefit. Intravenous immunoglobulin (IVIG), corticosteroids, and therapeutic plasma exchange have been considered in the treatment of AFM.30,37 Most suggestively, IVIG has been demonstrated to contain neutralizing antibodies against EV-D68, and murine studies have suggested that experimental mice experienced a lesser degree of paralysis when treated with IVIG.39,40 As a result, its use is relatively common in U.S. cases of AFM. In contrast, test mice subjected to dexamethasone experienced worse outcomes when treated with empiric steroids, although current recommendations do not wholly exclude dexamethasone’s use.40,41
Supportive care with careful attention to ventilation, nutrition, and management of dysautonomic symptoms remains essential. The vast majority (96%) of all AFM patients will require hospitalization for management. Of those patients, 58% will require intensive care.42 As mentioned, long-term management of AFM and its sequelae is beyond the scope of this review. However, patients will experience the greatest benefit with early and aggressive physical, occupational, and speech therapy to encourage recovery.
Conclusion
The eradication of poliovirus through global vaccination campaigns is tantalizingly close. Tens of thousands of cases of paralytic poliomyelitis have been successfully averted through the diligent immunization of millions of children since the introduction of IPV and OPV. However, disruptions to public health infrastructure, political instability, and anti-vaccination disinformation and misinformation campaigns have placed the health of the world’s children at risk, and the victories of the last century could be easily lost. Ongoing vigilance against wild-type and vaccine-derived type poliovirus remains essential for all pediatric providers.
AFM is a more recently discovered disease entity with manifestations similar to those of paralytic poliomyelitis. While definitive identification of the causative organism and pathophysiology is a work in progress, EV-D68 is heavily implicated in most cases. Definitive treatment remains to be optimally defined. Proper recognition and diagnosis of AFM is critical to rule out wild-type or vaccine-derived poliovirus, as well as to arrange for immediate supportive care for patients with the disease.
References
- Centers for Disease Control and Prevention. What is polio? Reviewed Aug. 11, 2022. https://www.cdc.gov/polio/what-is-polio/index.htm
- Henry R. Etymologia: Poliomyelitis. Emerg Infect Dis 2019;25:1611.
- Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Poliovirus infections. In: Red Book: 2021 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;2021:601-607.
- World Health Organization. Poliomyelitis. Published July 4, 2022. https://www.who.int/news-room/fact-sheets/detail/poliomyelitis
- Centers for Disease Control and Prevention. Pinkbook: Poliomyelitis. Reviewed Aug. 18, 2021. https://www.cdc.gov/vaccines/pubs/pinkbook/polio.html
- Centers for Disease Control and Prevention. Polio disease and poliovirus. Reviewed Dec. 2, 2022. https://www.cdc.gov/orr/polioviruscontainment/diseaseandvirus.htm
- Centers for Disease Control and Prevention. What we do. Updated Oct. 19, 2022. https://www.cdc.gov/polio/what...
- Hussain A, Ali S, Ahmed M, Hussain S. The anti-vaccination movement: A regression in modern medicine. Cureus 2018;10:e2919.
- Centers for Disease Control and Prevention. Vaccination coverage and exemptions among kindergartners. Reviewed May 14, 2021. https://www.cdc.gov/vaccines/imz-managers/coverage/schoolvaxview/data-reports/index.html
- Adebisi YA, Eliseo-Lucero Prisno D, Nuga BB. Last fight of wild polio in Africa: Nigeria’s battle. Public Health Pract (Oxf) 2020;1:100043.
- Chunda P, Chisema MN, Mwale A, et al. The 2022 Malawi polio outbreak. Malawi Med J 2022;34:223-224.
- Global Polio Eradication Initiative. GPEI statement on WPV1 in Malawi. Published February 17, 2022. https://polioeradication.org/news-post/gpei-statement-on-wpv1-in-malawi/
- Polio vaccine: Vaccine-derived poliovirus. https://www.cdc.gov/vaccines/v...
- Link-Gelles R, Lutterloh E, Schnabel Ruppert P, et al. Public health response to a case of paralytic poliomyelitis in an unvaccinated person and detection of poliovirus in wastewater — New York, June–August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1065-1068.
- Tuthill TJ, Groppelli E, Hogle JM, Rowlands DJ. Picornaviruses. Curr Top Microbiol Immunol 2010;343:43-89.
- Minor PD. An introduction to poliovirus: Pathogenesis, vaccination, and the endgame for global eradication. In: Martín J, ed. Poliovirus. Methods in Molecular Biology. Springer Science+Business Media;2016:1-10.
- Burrill CP, Westesson O, Schulte MB, et al. Global RNA structure analysis of poliovirus identifies a conserved RNA structure involved in viral replication and infectivity. J Virol 2013;87:11670-11683.
- Ypma-Wong MF, Filman DJ, Hogle JM, Semler BL. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at gln-gly pairs. J Biol Chem 1988;263:17846-17856.
- U.S. Department of Health and Human Services. Post-polio syndrome. National Institute of Neurological Disorders and Stroke. Reviewed March 13, 2023. https://www.ninds.nih.gov/health-information/disorders/post-polio-syndrome
- Centers for Disease Control and Prevention. Diagnostic methods. Reviewed Sept. 28, 2021. https://www.cdc.gov/polio/what-is-polio/lab-testing/diagnostic.html
- Cummings JN. The cerebrospinal fluid in poliomyelitis. Postgrad Med J 1949;25:19.
- Fraser FR. A study of the cerebrospinal fluid in acute poliomyelitis. J Exp Med 1913;18:242-251.
- Haq A, Wasay M. Magnetic resonance imaging in poliomyelitis. Arch Neurol 2006;63:778.
- Romero JR. Poliovirus. In: Bennett JE, ed. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020:2220-2226.
- Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol 2010;9:634-642.
- Lyons WS. Negative-pressure ventilation. JAMA 2003;289:983; author reply 983.
- Global Polio Eradication Initiative. Oral poliovirus vaccine. https://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/
- Centers for Disease Control and Prevention, Polio vaccination: What everyone should know. Reviewed Oct. 12, 2022. https://www.cdc.gov/vaccines/vpd/polio/public/index.html
- Van Haren K, Ayscue P, Waubant E, et al. Acute flaccid myelitis of unknown etiology in California, 2012-2015. JAMA 2015;314:2663-2671.
- Murphy OC, Messacar K, Benson L, et al; AFM Working Group. Acute flaccid myelitis: Cause, diagnosis, and management. Lancet 2021;397:334-346.
- Kane MS, Sonne C, Zhu S, et al. Incidence, risk factors, and outcomes among children with acute flaccid myelitis: A population-based cohort study in a California health network between 2011 and 2016. Pediatr Infect Dis J 2019;38:667-672.
- Ayers T, Lopez A, Lee A, et al. Acute flaccid myelitis in the United States: 2015-2017. Pediatrics 2019;144:e20191619.
- Centers for Disease Control and Prevention. AFM cases and outbreaks. Reviewed April 4, 2023. https://www.cdc.gov/acute-flaccid-myelitis/cases-in-us.html
- Elrick MJ, Pekosz A, Duggal P. Enterovirus D68 molecular and cellular biology and pathogenesis. J Biol Chem 2021;296:100317.
- Centers for Disease Control and Prevention. Causes of acute flaccid myelitis (AFM). Reviewed June 21, 2022. https://www.cdc.gov/acute-flaccid-myelitis/causes.html
- Hixon AM, Yu G, Leser JS, et al. A mouse model of paralytic myelitis caused by Enterovirus D68. PLoS Pathog 2017;13:e1006199.
- Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak Enterovirus D68 strain associated with acute flaccid myelitis cases in the U.S.A. (2012-14): A retrospective cohort study. Lancet Infect Dis 2015;15:671-682.
- Centers for Disease Control and Prevention. Diagnostic studies for AFM. Reviewed July 1, 2022. https://www.cdc.gov/acute-flaccid-myelitis/hcp/clinicians-health-departments/testing.html
- Zhang Y, Moore DD, Nix WA, et al. Neutralization of Enterovirus D68 isolated from the 2014 U.S. outbreak by commercial intravenous immune globulin products. J Clin Virol 2015;69:172-175.
- Hixon AM, Clarke P, Tyler KL. Evaluating treatment efficacy in a mouse model of Enterovirus D68-associated paralytic myelitis. J Infect Dis 2017;216:1245-1253.
- Centers for Disease Control and Prevention. Acute flaccid myelitis (AFM): Clinical guidance for the acute medical treatment of AFM. Reviewed July 1, 2022. https://www.cdc.gov/acute-flaccid-myelitis/hcp/clinical-management.html
- Board of Scientific Counselors. National overview of acute flaccid myelitis — United States, 2014–2018. Reviewed Dec. 14, 2018. https://www.cdc.gov/ddid/bsc/afm-overview-2018.html
What will be the next health crisis? Could it be wild-type poliovirus, vaccine-derived poliovirus, or the similar condition of acute flaccid myelitis? Are you prepared to recognize and anticipate the complications? The authors prepare clinicians for the acute management of each of these conditions.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
