Pelvic Inflammatory Disease
May 15, 2023
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
AUTHORS
Tiffany Murano, MD, Department of Emergency Medicine, Columbia University Medical College, New York, NY
Marc Berenson, MD, Chief Resident, Department of Emergency Medicine, Rutgers New Jersey Medical School, Newark
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity, Samaritan Hospital, Troy, NY
EXECUTIVE SUMMARY
- Pelvic inflammatory disease (PID) is a clinical diagnosis.
- Potential pathogens in PID include sexually transmitted organisms, vaginal flora, enteric bacteria, and respiratory pathogens.
- A low threshold for diagnosis is encouraged to initiate treatment, reduce complications, and limit spread to sexual
partners. - The minimal criteria for diagnosis include pelvic pain, uterine/adnexal tenderness, and cervical motion tenderness.
- Consider imaging with ultrasound, computed tomography, or magnetic resonance imaging when the patient has characteristics of severe disease or fails to respond as anticipated to therapy.
- Pregnant women with PID should be admitted for parenteral antibiotic therapy.
- Standard outpatient treatment for PID is with a single intramuscular injection of a third-generation cephalosporin plus a 14-day course of both doxycycline and metronidazole.
- Intrauterine device removal is not necessary for successful treatment.
- Follow-up with retesting for gonorrhea and chlamydia in three months is recommended after successful treatment.
Introduction
The term pelvic inflammatory disease (PID) describes a compilation of infections that arise from an ascending infection of the vagina or cervix to the upper genital tract, which is comprised of the uterus, fallopian tubes, and ovaries. These infections include, either alone or in combination, tubo-ovarian abscess (TOA), salpingitis, endometritis, and peritonitis. PID has an estimated prevalence of 4.4% in U.S. women between the ages of 18-44 years.1
The clinical diagnosis of PID is challenging in the emergency department (ED). Nonetheless, making the diagnosis is important, since PID is associated with uterine and fallopian tube scarring leading to tubal factor infertility and ectopic pregnancy, as well as chronic pelvic pain. This article provides an evidence-based review of diagnostic and treatment recommendations for PID.
Epidemiology
The National Health and Nutrition Examination Survey in the 2013-2014 cycle found the prevalence of self-reported PID in women 18-44 years of age is approximately 4.4%.1 Multiple studies also have found that the incidence and prevalence of PID is higher in Black and non-white women than in white women.2,3
There was a decline in the prevalence of PID in the United States between 2006 and 2013. The number of annual ED visits because of PID also has declined. However, as of 2013, the number of annual visits to the ED for PID still was significant — 0.41% of all ED visits, or a total of 7.4 million visits.1 Although the exact reason for the decline in the prevalence of PID is not known, it is thought that increased screening for sexually transmitted infections (STIs) leading to earlier detection and treatment, increased availability of and adherence to antibiotics, and improved diagnostic testing may be contributing factors.4
The cost of PID, including the potential associated complications, is estimated to be $3,200 per patient.5 For the United States, the annual direct expenditures related to PID and its sequelae were estimated to exceed $1.8 billion in 1998.6
The major risk factors associated with PID include age younger than 25 years, younger age at first intercourse, the number of sexual partners (either a large number of total sexual partners or multiple sexual partners within a specific time frame), and a history of STIs.7 (See Table 1.) Other risk factors include failure to use barrier contraceptives, lower education level and socioeconomic status, and vaginal douching.1,7
Table 1. Risk Factors for Pelvic Inflammatory Disease |
|
Pathophysiology
PID is an infection that originates in the lower female reproductive tract (cervix and vagina) and ascends into the upper reproductive tract (uterus, fallopian tubes, and ovaries). Traditionally, the majority of PID cases are due to an untreated STI caused by Neisseria gonorrhoeae and Chlamydia trachomatis. However, in recent years, studies have shown that less than 50% of women diagnosed with acute PID have tested positive for N. gonorrhoeae and C. trachomatis.8,9 Recent studies also have suggested Mycoplasma genitalium as a potential significant cause of PID.10-12 (See Table 2.)
Table 2. Potential Pathogens of Pelvic Inflammatory Disease |
Sexually Transmitted Infection Pathogens
Vaginal Flora
Enteric Bacteria
Respiratory Pathogens
|
Other organisms that are found in normal vaginal flora also have been associated with PID, including Gardnerella vaginalis, Haemophilus influenzae, enteric gram-negative rods, and anaerobic bacteria. Anaerobic bacteria have been isolated from women who have PID and, specifically, Bacteroides fragilis has been shown to cause tubal and epithelial destruction.8 Bacterial vaginosis (BV) is present in many women with PID; however, studies have demonstrated that there is no increased risk of developing PID in women who have BV.13 Respiratory pathogens also are potential etiologies for PID.
Clinical Presentation
There is no single presenting symptom or diagnostic finding that is sensitive and specific for PID. As such, its diagnosis can be imprecise and must be based on a combination of historical and clinical findings.
The symptoms of PID are highly variable and often vague, and at times women may be asymptomatic. This is a concern, since even asymptomatic PID can lead to complications, such as infertility, in up to 40% of women.14 The most common presenting complaint of PID is lower abdominal pain, with up to 90% of individuals complaining of cramping or dull, bilateral discomfort.15 Other nonspecific findings may be abnormal or postcoital bleeding (more than 33% of individuals), vaginal discharge (75% of individuals), dyspareunia, fever, nausea, and vomiting.15 Symptoms most commonly occur at times of low progesterone leading to thinning of the cervical mucosal barrier, which arises at the end of menses to early in the menstrual cycle.
The physical exam should focus on three major areas: vital signs, abdominal exam, and pelvic exam. Vital sign abnormalities can include fever, tachycardia, and hypotension; however, these findings are rare with uncomplicated PID and likely point toward a more progressive disease pattern, including pyosalpinx, TOA, and peritonitis. Most commonly, the abdominal exam is notable for lower abdominal tenderness, which is present in 94% of patients. Upper abdominal pain should alert the provider to possible complications of PID, such as Fitz-Hugh-Curtis syndrome (FHCS), which may present with right upper quadrant and left upper quadrant pain signifying perihepatitis and perisplenitis, respectively.
The pelvic exam should include a bimanual exam and speculum exam. On bimanual exam, cervical motion tenderness, adnexal tenderness, and uterine tenderness each have sensitivities of 92% to 96%.16 A palpable adnexal mass is less common but may be present in up to two-thirds of patients with pyosalpinx or TOA. Concerning findings on speculum exam include yellow mucopurulent cervical discharge and cervical friability, as demonstrated by cervical bleeding with insertion of a cotton swab into the cervical os.16
The pelvic exam can be difficult to perform because of patient discomfort. Although the Centers for Disease Control and Prevention (CDC) recommends a pelvic exam when PID is suspected, there is some research to suggest not all gynecology-related complaints require such an exam. Based on a retrospective chart review of 130 patients, Fischer et al noted abdominal pain, dyspareunia, or abnormal vaginal bleeding were present in 93% of patients diagnosed with PID.17 Therefore, they suggested that it may be possible to avoid a pelvic exam in patients without any of these cardinal symptoms, instead using urine-based STI testing and patient-obtained vaginal swabs to diagnose uncomplicated genitourinary tract infections. However, larger prospective studies are required before this can be recommended for routine use.17
Because of the vague nature of the disease, the CDC has lowered its threshold for empiric treatment. Current CDC recommendations state that empiric treatment for PID should be initiated in sexually active women or women with STI risk factors experiencing lower abdominal or pelvic pain with no other causative diagnosis noted in the presence of one other minimum clinical criterion.8
Minimum physical exam criteria for the diagnosis of PID include cervical motion tenderness, uterine tenderness, and adnexal tenderness. Such criteria have been made in hopes of increased sensitivity. Goyal et al sought to determine the effect of this change by the CDC on the incidence of PID diagnosis rates among adolescent ED patients before and after the revised CDC guidelines.18 Surprisingly, within the studied population, the incidence of PID diagnoses did not rise despite the broadened guidelines.18 The authors raised several possibilities to explain this trend, such as more outpatient STI screening leading to increased treatment and decreased progression of PID. However, missed diagnosis or under-detection by the ED provider also must be considered. In a study by Wood et al, researchers reported that up to 50% of patients diagnosed with cervicitis actually met diagnostic criteria for PID.19
Differential Diagnosis
The differential diagnosis of PID can be divided into gynecologic and non-gynecologic etiologies.
Gynecologic
Uncomplicated cervicitis is a top differential, since the interpretation of cervical motion tenderness and adnexal tenderness is inherently somewhat subjective. Any woman with lower abdominal pain should have the diagnosis of ectopic pregnancy considered; a negative serum beta-human chorionic gonadotropin (HCG) makes this extremely unlikely. In the setting of a positive pregnancy test with signs of sepsis, septic abortion or retained products of conception also could appear in a similar fashion.
Ovarian torsion also must be considered in any woman with lower abdominal pain; however, symptoms usually are more sudden in onset, lack fever, and may be associated with nausea and vomiting. Finally, malignancy with super-infection also can present somewhat similarly, although this is rare; findings on speculum or bimanual exam of an irregular mass would raise concern for this possibility.
Non-Gynecologic
Urinary tract etiologies, such as cystitis or pyelonephritis, also may present with lower abdominal pain and systemic signs of infection. While a urinalysis demonstrating pyuria may be suggestive, similar findings also can be found in the presence of gonorrheal and chlamydial infection. If symptoms are suggestive (prominent dysuria, flank pain, or costovertebral angle tenderness) along with PID symptoms, it may be prudent to proceed with co-treatment for both etiologies based on local resistance patterns.
Intra-abdominal infectious etiologies, such as appendicitis, diverticulitis, colitis, or intra-abdominal abscess, can present similarly with abdominal pain and signs of systemic illness; if the pelvic examination is normal, further investigation should be carried out to assess for these potential surgical emergencies.
Diagnosis
Laboratory Evaluation
Currently, there is no ancillary test for PID that is both highly sensitive and specific. Therefore, to expedite treatment, the diagnosis of PID commonly is made based on clinical criteria with or without laboratory backing. Laboratory tests that may be helpful and should be performed routinely are those such as pregnancy tests, nucleic acid amplification tests (NAAT) for N. gonorrhoeae and C. trachomatis, and vaginal wet mount. Although these tests do not make the diagnosis of PID, results can modify clinician suspicion for PID.16
In the diagnosis of STIs, direct immunofluorescence and enzyme-linked immunoassay testing may exist in lower resourced settings. The sensitivity of these non-NAAT tests is limited when compared to NAATs, with women tested by non-NAATs found to have a 17% higher risk of progressing to PID by 12 months than those tested by NAATs.20 In terms of sensitivity of NAAT for N. gonorrhoeae and C. trachomatis collection sites, cervical or vaginal swabs show the highest sensitivity; however, first-void urine samples appear to be near equivalent.16
Moreover, self-administered vaginal testing by swab has been shown to have similar sensitivity to physician-collected samples for the bacteriologic diagnosis.21,22 It should be noted, however, that PID and TOA are clinical diagnoses, and substituting self-swab for a speculum and bimanual pelvic examination possibly could lead to missed cases. No definitive literature yet exists to guide our practice in that context.
Wet mount testing primarily is used to detect leukorrhea, trichomoniasis, and bacterial vaginosis. The presence of leukorrhea — greater than one polymorphonuclear (PMN) leukocyte per epithelial cell — is highly sensitive but not specific for PID. However, lack of these findings is a negative predictor for PID.8 Of note, unlike N. gonorrhoeae and C. trachomatis NAATs, patient-obtained wet mount samples were found to be less sensitive compared to physician-obtained samples.16
Other tests that should be considered are urinalysis, complete blood count for leukocytosis, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). The CDC recommends concomitant evaluation for both bacterial vaginosis and trichomoniasis.8 A fourth-generation human immunodeficiency virus (HIV) antigen-antibody and a rapid plasma reagent (RPR)/venereal disease research lab (VDRL) also have been recommended, given evidence of rising co-infection rates and increased susceptibility to HIV acquisition during acute gonococcal infection.8
Urinalysis commonly is abnormal, with one study demonstrating 82% of STI-positive women also had above-trace leukocyte esterase or pyuria.23 In this same study, this overlap of findings was shown to lead to overdiagnosis of urinary tract infections and underdiagnosis of STIs.23 To avoid this pitfall, urinalysis interpretation should rely heavily on the patient’s presentation.16 CRP, ESR, and white blood cell count all can be elevated with PID, but may be more predictive of complications of PID, such as TOA. The evidence for the use of procalcitonin in both diagnosis and prognostication in PID and TOA is limited.24,25
Imaging
In the early stages of PID, both computed tomography (CT) and ultrasound commonly are normal. As the disease progresses, the sensitivity of imaging modalities increases.
The sensitivity of ultrasound for inflammation can be highly variable early in the course of illness, ranging from 32% to 85%.26 Later findings of PID on ultrasound include uterine enlargement and endometrial thickening. This thickening of the endometrium may be accompanied by an ill-defined uterus and loss of tissue planes. Findings of salpingitis also may be noted on ultrasound, as the fallopian tubes become dilated with heterogenous fluid and echogenic debris suggesting hydrosalpinx or pyosalpinx. On ultrasound, progression to TOA is marked by further increase in echogenic debris noted in both the fallopian tubes and ovaries, which represents blood, pus, and inflammatory exudates.16,27
Despite ultrasound being the typical initial test of choice in females with lower abdominal and pelvic complaints, the nonspecific presentation of PID often can lead providers to perform CT imaging first. Similar to ultrasound, initial findings of early PID are subtle on CT. Initially, mild pelvic edema is seen as haziness of the pelvic fat planes leading to fat stranding. This is the most sensitive finding for early PID, noted in 60% of patients; however, it is highly nonspecific.26
Free fluid in the cul-de-sac is another early finding commonly seen on ultrasound as well as CT. Again, this is nonspecific and has been identified in up to 68.3% of non-PID cases, including appendicitis, ovarian cyst rupture, gastroenteritis, and ectopic pregnancy.28,29 Lastly, in the early phase of PID, hyperemic thickening of the fallopian tubes may be noted. Fluid within the endometrial or cervical canal, endometrial hyperemia, and uterine swelling all are consistent with endometritis and cervicitis. However, these may be normal variants depending on the extent of the findings and the menstrual cycle, thus limiting specificity.26
As inflammation or infection progresses outward along the female reproductive tract, ovarian stromal swelling and surrounding edema are noted. Because of the thickening of the internal mucosal folds of the distending fallopian tubes, a “cog wheel” or “beads on a string” appearance may be seen. This differentiates PID from appendicitis, which more commonly causes a smooth and featureless inflammation of these nearby structures. These findings are the most characteristic of early PID on CT, with a specificity of more than 90%.26
Findings of PID on MRI are similar to those of CT but with increased resolution, leading to improved sensitivity (91% to 98%) and specificity (81% to 95%).16 On MRI, early PID usually presents as ill-defined hyperintense areas on T2-weighted, fat-suppressed images and T1-weighted, gadolinium-enhanced images. This is similar to the nonspecific fat stranding noted on contrast CT. The MRI findings of pyosalpinx are dilated, tortuous, fluid-filled fallopian tubes that may form a C-shaped or S-shaped structure. A mixture of pus, hemorrhage, and debris in these structures can create a heterogenous signal intensity on both T1- and T2-weighted images.16
Complications of Pelvic Inflammatory Disease
Tubo-Ovarian Abscess
Presenting signs and symptoms of TOA often are indistinct from those of uncomplicated PID; however, the clinician often will see more pronounced and systemic findings of inflammation on both exam and laboratory studies. In a series of patients with ultrasound-confirmed TOA, 60% were noted to be febrile in the ED, 68% had leukocytosis of greater than 10,000 white blood cells per mL, and 26% reported nausea.30
In terms of history, providers should be concerned for the presence of TOA in PID patients who fail to respond to antimicrobial therapy. On exam, a palpable adnexal mass also should raise concern for TOA. Lastly, serum markers for inflammation, such as CRP and ESR, also may be elevated. Although there is limited literature, one study showed procalcitonin elevation to be a positive predictor of PID complicated by TOA, even more than ESR, CRP, and leukocytosis. Procalcitonin has a sensitivity of 62% and specificity of 75% at a cutoff level of 0.330 ng/mL.31
When TOA is suspected, imaging to rule out the condition should be performed promptly. On both ultrasound and contrast-enhanced CT imaging, TOA manifests as a complex fluid collection with internal septations. (See Figures 1 and 2.) Surrounding nonspecific inflammatory changes likely will be seen, such as fat stranding on CT scan and free fluid in the cul-de-sac. Air within the structure is less common but may be present. The differential diagnosis for these findings includes a complex ovarian neoplasm, and the clinician should use systemic findings to help differentiate the two.26
Figure 1. Transvaginal Ultrasound Showing a Sagittal View of the Right Adnexa with a Fluid Collection (between the arrows) |
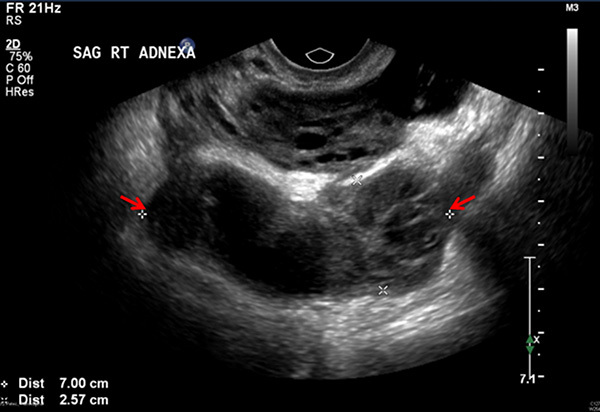 |
Image courtesy of Basil Hubbi, MD, Department of Radiology, Rutgers New Jersey Medical School. |
Figure 2. CT Scan of the Pelvis Shows a Large Loculated Abscess with Air in the Left Pelvis |
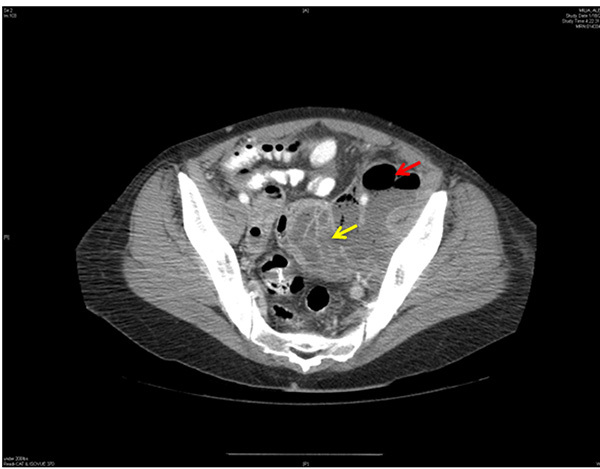 |
Loculated abscess indicated by yellow arrow. Air is shown by red arrow. CT: computed tomography Image courtesy of Basil Hubbi, MD, Department of Radiology, Rutgers New Jersey Medical School. |
Treatment of TOA should use initial parenteral antibiotics, including those with anaerobic coverage, such as clindamycin, metronidazole, or ampicillin/sulbactam.16 Per CDC guidelines, inpatient observation for at least 24 hours is recommended in hemodynamically stable patients to rule out progression to early sepsis and monitor for abscess rupture. Appropriate antibiotics alone have been shown to be effective in 70% to 87.5% of patients.30,32
Early surgical exploration is indicated in patients with an acute abdomen when ruptured TOA is a concern or in those who are septic or hemodynamically unstable. TOA size also is a predictor of the possible need for surgical intervention. In one study, 60% of patients with an abscess larger than 10 cm required surgical management compared to only 20% of those with an abscess 4 cm to 6 cm in diameter.26
Finally, if there has been no clinical improvement within 48-72 hours of antibiotic administration, minimally invasive drainage of the abscess or surgical management should be considered.30 Some authors advocate for ultrasound-guided aspiration within 24 hours of starting antibiotic treatment without waiting for a clinical response, reasoning that early drainage will aid in the diagnosis, identify organisms involved, allow for the patient to have pain relief sooner, and accelerate recovery.33-35
Fitz-Hugh-Curtis Syndrome
FHCS is a rare complication of PID in which the hepatic capsule becomes inflamed by primary genital tract infection spread by several means. Possible paths of inflammatory spread include ascension up the paracolic gutters via ascitic flow, hematogenous spread, translymphatic spread, and exaggerated immune response. This complication can be present in 3% to 37% of patients with PID and typically is highest in adolescent females.30
Right upper quadrant pain is the most common sign and symptom, and traditionally it is pleuritic or positional in nature. The differential diagnosis is broad, with the phenomenon most commonly confused with acute cholecystitis on exam. Other differential diagnoses include pneumonia, pulmonary embolism, and renal colic. Of note, left upper quadrant pain may be seen from left-sided perisplenitis.29,36
A definitive diagnosis is made laparoscopically by visualizing adhesions resembling violin strings or by hepatic capsular biopsy to identify causative organisms. Noninvasive diagnostic modalities, such as ultrasound, may show fluid collections and widening of the subphrenic area between the liver and the diaphragm. On CT scan, hepatic capsular enhancement may be seen. (See Figure 3.) Typically, a CT scan of the abdomen and pelvis is performed using a portal venous phase scan. However, recent studies have found that dynamic/biphasic abdominal CT scan using an additional arterial phase scan demonstrates increased sensitivity than portal venous phase alone by showing much greater depiction of perihepatic enhancement.36 Antibiotic treatment of FHCS involves the same antibiotic regimen as for uncomplicated PID.29
Figure 3. Coronal CT Scan Shows an IUD in the Mid Pelvis with a Left Adnexal Abscess and a Perihepatic Subcapsular Abscess with Air |
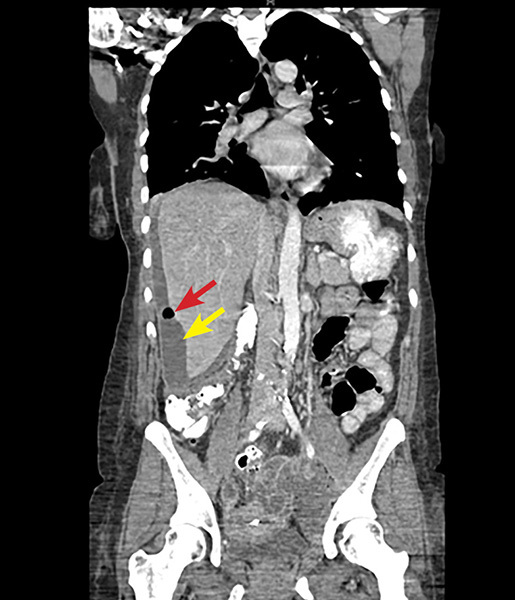 |
Perihepatic subcapsular abscess indicated by yellow arrow. Air indicated by red arrow. CT: computed tomography; IUD: intrauterine device Image courtesy of Basil Hubbi, MD, Department of Radiology, Rutgers New Jersey Medical School. |
Special Populations
Pregnancy
Pregnant women with PID have a higher risk for both preterm delivery and maternal morbidity and, therefore, should be hospitalized for parenteral antibiotics per the CDC’s guidelines.8 Historically it was thought that PID was uncommon after the first trimester due to the presence of a cervical mucous plug. However, a recent meta-analysis demonstrated that PID in pregnancy most commonly occurs in the second trimester.37 Uterine structural anomalies, fallopian tube anomalies, history of STIs, recent pelvic surgery, and in vitro fertilization or oocyte retrieval were cited as risk factors.37
HIV
Little difference in presentation or response to treatment has been noted between HIV-positive and HIV-negative females. Both populations have been shown to respond similarly to antibiotic management of PID, and there are insufficient data to support a more aggressive antibiotic treatment regimen for HIV-positive females.30 CD4 count did not predict any change in severity of clinical presentation; however, those with low CD4 counts were more likely to have bacterial vaginosis as a significant driver of their PID.38 Of note, HIV-positive females with PID are more likely to experience severe disease (pyosalpinx and/or TOA) than HIV-negative women; 28% vs. 24%
(P = 0.02).39
Sexual and Gender Minorities
LGBTQ+ patients have historically faced structural discrimination in healthcare settings, leading to decreased safe access to care and comparatively worse health outcomes.40,41 Data suggest that the prevalence of STIs among transgender patients is higher than among cis-gender patients.42 All sexually active patients with uterine anatomy are at risk for PID and should be assessed as such.43 Best practices include the identification and use of preferred pronouns as well as gender-inclusive and trauma-informed strategies in assessment and treatment.44
The CDC recommends that any transgender man who has received metoidioplasty surgery including urethral lengthening without a vaginectomy should have a cervical swab to assess for bacterial STIs because a urine specimen will be inadequate for detecting cervical infections.8
Treatment
Several treatment regimens targeted toward N. gonorrhoeae and C. trachomatis as well as other likely pathogens have been shown to be clinically effective in treating PID. Treatment should be initiated as soon as the presumptive diagnosis is made because the prevention of long-term sequelae is related to early antibiotic therapy. There are several important factors to consider with respect to selection of treatment options.
The first factor is severity of initial presentation. Patients who present with mild to moderate symptoms, are nontoxic in appearance, and can tolerate oral intake without nausea or vomiting may be treated as outpatients. Conversely, those patients who are pregnant, present with severe symptoms, are septic or toxic-appearing, have associated TOA, or have intractable vomiting are treated most appropriately in the inpatient setting with parenteral antibiotics. In addition, hospitalization should be considered for women in whom other surgical emergencies cannot be definitely excluded.8
Another factor to consider is the patient’s access to treatment and follow-up. Remember to ask patients if they have prescription plans and any associated prohibitive copayments for medications. Patients who are unable to follow an oral regimen because of lack of access to medications or funds to buy their medications may benefit from consultation with a social worker to assist in obtaining prescriptions. Admission to an observation unit is a consideration for these patients.8
Other patients who would benefit from inpatient treatment include those who may be non-compliant with outpatient regimens or who failed clinical response to oral or outpatient regimens.
Intramuscular and Oral Treatment
There are several recommended combination intramuscular and oral regimens for patients who are diagnosed with PID. Practitioners may consider using one of these treatment options for patients who have mild to moderate illness. (See Table 3.)
Table 3. Treatment Regimens and Indications for Pelvic Inflammatory Disease |
||
Route |
Indication |
Regimen |
Oral and intramuscular |
Outpatient treatment for patients with mild to moderate illness |
OR
OR
*For persons weighing > 150 kg, ceftriaxone 1 g should be administered. Treatment of those with cephalosporin allergy **
|
Parenteral |
Inpatient treatment for patients with:
|
OR
OR
Alternative Regimens
OR
|
IM: intramuscular; PO: orally; BID: twice per day; QD: once per day; q: every; IV: intravenous Recommended indications and treatments reflected in this table are compiled from the Centers for Disease Control and Prevention.8 ** Fluoroquinolone use is no longer recommended for treatment of gonorrhea. Use only if cephalosporin allergy, community prevalence of and individual risk for gonorrhea is low, and follow-up is likely. |
||
A single intramuscular (IM) injection of ceftriaxone, cefoxitin, or another third-generation cephalosporin, in addition to both doxycycline 100 mg and metronidazole 500 mg twice daily for 14 days, are recommended regimens.8 If giving IM cefoxitin, also administer a single oral dose of probenecid 1 g to the patient, since probenecid has been shown to sustain the tissue concentration of cefoxitin.45 There are no data to support the use of oral cephalosporins. Given similar efficacy rates between intravenous (IV) and IM therapy, IV dosing can be considered if parenteral access is already available at the time of treatment.8
For those with cephalosporin allergy, a fluoroquinolone and metronidazole regimen can be considered; however, this should be reserved in situations with low community prevalence and low individual risk for gonococcal infection. Follow-up is especially important if these regimens are used.8
Parenteral Treatment
Parenteral treatments have similar clinical efficacy to oral regimens and generally are reserved for those patients who require inpatient therapy either to an inpatient floor or an observation unit. Indications for hospitalization include: TOA, pregnancy, severe illness (such as nausea, vomiting, and fever > 38.5°C), inability to follow an outpatient regimen, inability to tolerate oral intake, outpatient treatment failure or lack of clinical response to oral treatment, and when other surgical emergencies (such as appendicitis) cannot be excluded.8 Treatment regimens that are recommended by the CDC are summarized in Table 3.8
Ceftriaxone, cefotetan, and cefoxitin currently are the only parenteral cephalosporins that have been supported in the literature for treatment of PID. Cefotetan and cefoxitin have activity against anaerobic organisms, obviating the need for the use of metronidazole when these agents are used; given ceftriaxone’s lack of anaerobic coverage, metronidazole should be administered when this agent is selected. There is limited evidence to support the use of other parenteral second- and third-generation cephalosporins. The bioavailability of doxycycline is equivalent orally and intravenously. Since there have been reports of pain associated with IV infusion of doxycycline, consider using the oral form if the patient is able to tolerate oral intake.
Alternative regimens include: ampicillin-sulbactam 3 g IV every six hours plus doxycycline 100 mg IV/orally (PO) every 12 hours; or clindamycin 900 mg IV every eight hours plus gentamicin
2 mg/kg loading dose followed by
1.5 mg/kg every eight hours maintenance. Ampicillin/sulbactam has been shown to be effective in treating N. gonorrhoeae and C. trachomatis, as well as anaerobic bacteria in women with TOA.46
Patients who are in observation or inpatient units eventually may be transitioned to oral regimens and discharged.8
Alternate Regimens
There are limited data regarding alternate medications for the treatment of acute PID. Penicillin allergy is not an indication to use an alternate regimen for fear of cross-reactivity with later-generation cephalosporins, since this is an infrequent occurrence with the recommended cefoxitin and ceftriaxone, second- and third-generation cephalosporins, respectively.47
Other Considerations
Treatment of sexual partners of patients with PID is essential to prevent spread of the disease and reinfection of the patient. It is common for asymptomatic infection to be present in both male and female populations.8 According to the CDC, it is recommended that all partners who have had sexual contact within 60 days be referred for evaluation, gonorrhea and chlamydia testing, and presumptive treatment, regardless of birth sex.8
For patients who have not had sexual contact within 60 days of presentation, it is recommended that their last sexual contact be referred for evaluation and treatment. Use caution when delivering information to patients, as this has the potential to become an emotionally charged situation between sexual partners. In addition, the clinical diagnosis is not 100% precise. Further, non-sexually transmitted bacteria are found in patients with PID, suggesting some cases may not be sexually transmitted.9 Nonetheless, evaluation of partners is highly recommended. It also should be noted that sharing of erotic stimulation devices may lead to the transmission of bacterial vaginosis.48
Intrauterine Devices
The early literature looking at PID with intrauterine device (IUD) use was conflicting and potentially riddled with biases established during early IUD use.49 With the increased popularity of IUD use in the 1960s and 1970s, there were numerous studies that held that the use of IUDs was associated with an increased risk of contracting PID. However, more recent data have suggested that the absolute risk of PID from IUD use is very small.50-52 Moreover, recent literature has called this concept into question, stating that previous studies have had numerous confounding factors and biases, and, therefore, are flawed.49
Risk of infection aside, there also is the question of whether a patient who has an IUD and is diagnosed with PID should have the device removed or left in place. Two studies examined outcomes between these two approaches and found that patients who had their IUDs removed had longer hospitalizations.53,54 Additional studies found no significant difference in outcomes between those who have the IUD removed and those who have it left in place.55,56 According to the committee opinion developed by the American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice, women with an IUD who develop PID may have their IUD left in situ and be treated with antibiotics according to CDC guidelines.57
Discharge Instructions and Follow-Up
It is recommended that all patients treated for PID be tested for HIV as well as N. gonorrhoeae and C. trachomatis using NAAT.8 This is an opportunity to instruct and educate patients on safe sexual intercourse practices. Inform patients that PID often is an STI and that it is important to minimize transmission. Instruct patients to abstain from sexual intercourse until the treatment regimen is complete, all symptoms have resolved, and any/all sexual partners have been screened and treated appropriately.
If it is deemed unlikely that partners will present for testing and treatment, expedited partner therapy (EPT) may be considered. For treatment of chlamydia, use doxycycline. For treatment of N. gonorrhoeae, oral cefixime can be used; however, it is important to note that its use is limited due to decreased efficacy and rising drug resistance, so partner follow-up should be encouraged. Do note that state and/or local laws vary regarding the ability and procedure to provide EPT.
It is important to stress that medication adherence is essential in treating the disease and preventing emergence of drug-resistant organisms. Inform patients who are discharged that their symptoms should improve within three days of treatment initiation. In addition, instruct patients to return to the ED if their symptoms persist or worsen or if new symptoms arise.
Recommend short-term follow-up with the gynecology specialist or adolescent medicine service for retesting in three months.
The CDC now recommends that all sexually active adults and adolescents are informed about the existence of pre-exposure prophylaxis against HIV infection (PrEP).58 The CDC notes that the presence of recent pregnancy or diagnosis of another bacterial STI is evidence of sexual activity and, therefore, constitute a reason to discuss PrEP with the patient. PrEP may be initiated in both the outpatient setting as well as in the ED; ideally, systems for referral to longitudinal care should be in place prior to ED initiation.59,60
Conclusion
PID affects many female patients and accounts for significant gynecologic sequelae if left undiagnosed and untreated. Because of the variability and vagueness of symptoms, the diagnosis of PID can be elusive and, therefore, the emergency physician’s index of suspicion must remain high. There is no one specific test for PID, but NAATs for gonorrhea and chlamydia, vaginal wet mount, and pregnancy tests may be helpful in guiding the diagnosis and management. Imaging modalities, such as ultrasound and CT scan, may be instrumental in diagnosing complications of PID, such as TOA and FHCS. When considering the complications and long-term effects of PID if untreated, initiating early therapy according to CDC guidelines is key. Appropriate follow-up for both the patient and the patient’s sexual partners should be recommended according to CDC recommendations.
Acknowledgment
The authors would like to thank Basil Hubbi, MD, Department of Radiology, Rutgers New Jersey Medical School, for generously sharing the images depicted in this article. The authors also acknowledge the contributions of Dr. Albert Della Fave in research and writing.
REFERENCES
- Kreisel K, Torrone E, Bernstein K, et al. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age — United States, 2013-2014. MMWR Morb Mortal Wkly Rep 2017;66:80-83.
- Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect 2006;82:452-457.
- Cox S, Dean T, Posner SF, et al. Disparities in reproductive health-related visits to the emergency department in Maryland by age and race, 1999-2005. J Womens Health (Larchmt) 2011;20:1833-1838.
- Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: Insights from exploratory time-series analyses. Am J Prev Med 2010;38:652-657.
- Owusu-Edusei K Jr, Chesson HW, Gift TL, et al. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013;40:197-201.
- Rein DB, Kassler WJ, Irwin KL, Rabiee L. Direct medical cost of pelvic inflammatory disease and its sequelae: Decreasing, but still substantial. Obstet Gynecol 2000;95:397-402.
- Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis 2013;40:413-418.
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep 2021;70:1-187.
- Haggerty CL, Totten PA, Tang G, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect 2016;92:441-446.
- Ona S, Molina RL, Diouf K. Mycoplasma genitalium: An overlooked sexually transmitted pathogen in women? Infect Dis Obstet Gynecol 2016;2016:4513089.
- Haggerty CL. Evidence for a role of Mycoplasma genitalium in pelvic inflammatory disease. Curr Opin Infect Dis 2008;21:65-69.
- Haggerty CL, Totten PA, Astete SG, et al. Failure of cefoxitin and doxycycline to eradicate endometrial Mycoplasma genitalium and the consequence for clinical cure of pelvic inflammatory disease. Sex Transm Infect 2008;84:338-342.
- Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol 2004;104:761-769.
- Wiesenfeld HC, Hillier SL, Meyn LA, et al. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012;120:37-43.
- Toth M, Patton DL, Esquenazi B, et al. Association between Chlamydia trachomatis and abnormal uterine bleeding. Am J Reprod Immunol 2007;57:361-366.
- Bugg CW, Taira T, Zaurova M. Pelvic inflammatory disease: Diagnosis and treatment in the emergency department [digest]. Emerg Med Pract 2016;18(12 Suppl Points & Pearls):S1-S2.
- Fisher LD, Fletcher KE, Blake DR. Can the diagnosis of pelvic inflammatory disease be excluded without a bimanual examination? Clin Pediatr (Phila) 2004;43:153-158.
- Goyal M, Hersh A, Luan X, et al. Are emergency departments appropriately treating adolescent pelvic inflammatory disease? JAMA Pediatr 2013;167:672-673.
- Woods JL, Bailey SL, Hensel DJ, Scurlock AM. Cervicitis in adolescents: Do clinicians understand diagnosis and treatment? J Pediatr Adolesc Gynecol 2011;24:359-364.
- Davies B, Turner KME, Benfield T, et al. Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort. PLoS Med 2018;15:e1002483.
- Berwald N, Cheng S, Augenbraun M, et al. Self-administered vaginal swabs are a feasible alternative to physician-assisted cervical swabs for sexually transmitted infection screening in the emergency department. Acad Emerg Med 2009;16:360-363.
- Lunny C, Taylor D, Hoang L, et al. Self-collected versus clinician-collected sampling for chlamydia and gonorrhea screening: A systemic review and meta-analysis. PLoS One 2015;10:e0132776.
- Tomas ME, Getman D, Donskey CJ, Hecker MT. Overdiagnosis of urinary tract infection and underdiagnosis of sexually transmitted infection in adult women presenting to an emergency department. J Clin Microbiol 2015;53:2686-2692.
- Erenel H, Yilmaz N, Oncul M, et al. Usefulness of serum procalcitonin levels in predicting tubo-ovarian abscess in patients with acute pelvic inflammatory disease. Gynecol Obstet Invest 2017;82:262-266.
- Hong IK, Kwon MJ, Nam SH, et al. Value of serum procalcitonin as an early predictor of antibiotic treatment response in inpatients with pelvic inflammatory disease (VALID). Taiwan J Obstet Gynecol 2020;59:660-664.
- Spain J, Rheinboldt M. MDCT of pelvic inflammatory disease: A review of the pathophysiology, gamut of imaging findings, and treatment. Emerg Radiol 2017;24:87-93.
- Roche O, Chavan N, Aquilina J, Rockall A. Radiological appearances of gynaecological emergencies. Insights Imaging 2012;3:265-275.
- Sung MK, Singh S, Kalra MK. Current status of low dose multi-detector CT in the urinary tract. World J Radiol 2011;3:256-265.
- You JS, Kim MJ, Chung HS, et al. Clinical features of Fitz-Hugh-Curtis syndrome in the emergency department. Yonsei Med J 2012;53:753-758.
- Mitchell C, Prabhu M. Pelvic inflammatory disease: Current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am 2013;27:793-809.
- Erenel H, Yilmaz N, Oncul M, et al. Usefulness of serum procalcitonin levels in predicting tubo-ovarian abscess in patients with acute pelvic inflammatory disease. Gynecol Obstet Invest 2017;82:262-266.
- McNeeley SG, Hendrix SL, Mazzoni MM, et al. Medically sound, cost-effective treatment for pelvic inflammatory disease and tuboovarian abscess. Am J Obstet Gynecol 1998;178:1272-1278.
- Granberg S, Gjelland K, Ekerhovd E. The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol 2009;23:667-678.
- Gjelland K, Ekerhovd E, Granberg S. Transvaginal ultrasound-guided aspiration for treatment of tubo-ovarian abscess: A study of 302 cases. Am J Obstet Gynecol 2005;193:1323-1330.
- Perez-Medina T, Huertas MA, Bajo JM. Early ultrasound-guided transvaginal drainage of tubo-ovarian abscesses: A randomized study. Ultrasound Obstet Gynecol 1996;7:435-438.
- Joo SH, Kim MJ, Lim JS, et al. CT diagnosis of Fitz-Hugh and Curtis syndrome: Value of the arterial phase scan. Korean J Radiol 2007;8:40-47.
- Marcinkowski KA, Mehta V, Mercier R, Berghella V. Pelvic inflammatory disease in pregnancy: A systematic review focusing on perinatal outcomes. Am J Obstet Gynecol MFM 2022;4:100643.
- Bukusi EA, Cohen CR, Stevens CE, et al. Effects of human immunodeficiency virus 1 infection on microbial origins of pelvic inflammatory disease and on efficacy of ambulatory oral therapy. Am J Obstet Gynecol 1999;181:1374-1381.
- Mugo NR, Kiehlbauch JA, Nguti R, et al. Effect of human immunodeficiency virus-1 infection on treatment outcome of acute salpingitis. Obstet Gynecol 2006;107:807-812.
- White Hughto JM, Reisner SL, Pachankis JE. Transgender stigma and health: A critical review of stigma determinants, mechanisms, and interventions. Soc Sci Med 2015;147:222-231.
- Radix AE, Lelutiu-Weinberger C, Gamarel KE. Satisfaction and healthcare utilization of transgender and gender non-conforming individuals in NYC: A community-based participatory study. LGBT Health 2014;1:302-308.
- Pitasi MA, Oraka E, Clark H, et al. HIV testing among transgender women and men — 27 states and Guam, 2014-2015. MMWR Morb Mortal Wkly Rep 2017;66:883-887.
- Muzny CA, Harbison HS, Pembleton ES, Austin EL. Sexual behaviors, perception of sexually transmitted infection risk, and practice of safe sex among southern African American women who have sex with women. Sex Transm Dis 2013;40:395-400.
- Coleman E, Radix AE, Bouman WP, et al. Standards of Care for the Health of Transgender and Gender Diverse People, Version 8. Int J Transgend Health 2022;23(Suppl 1):S1-S260.
- Reeves DS, Bullock DW, Bywater MJ, et al. The effect of probenecid on the pharmacokinetics and distribution of cefoxitin in healthy volunteers. Br J Clin Pharmacol 1981;11:353-359.
- McGregor JA, Crombleholme WR, Newton E, et al. Randomized comparison of ampicillin-sulbactam to cefoxitin and doxycycline or clindamycin and gentamicin in the treatment of pelvic inflammatory disease or endometritis. Obstet Gynecol 1994;83:998-1004.
- Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: A meta-analysis. Otolaryngol Head Neck Surg 2007;136:340-347.
- Evans AL, Scally AJ, Wellard SJ, Wilson JD. Prevalence of bacterial vaginosis in lesbians and heterosexual women in a community setting. Sex Transm Infect 2007;83:470-475.
- Hubacher D, Grimes DA, Gemzell-Danielsson K. Pitfalls of research linking the intrauterine device to pelvic inflammatory disease. Obstet Gynecol 2013;121:1091-1098.
- Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000;356:1013-1019.
- Birgisson NE, Zhao Q, Secura GM, et al. Positive testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the risk of pelvic inflammatory disease in IUD users. J Womens Health (Larchmt) 2015;24:354-359.
- Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: An international perspective. Lancet 1992;339:785-788.
- Teisala K. Removal of an intrauterine device and the treatment of acute pelvic inflammatory disease. Ann Med 1989;21:63-65.
- Larsson B, Wennergren M. Investigation of a copper-intrauterine device (Cu-IUD) for possible effect on frequency and healing of pelvic inflammatory disease. Contraception 1977;15:143-149.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol 2011;118:184-196.
- Tepper NK, Marchbanks PA, Curtis KM. U.S. selected practice recommendations for contraceptive use, 2013. J Womens Health (Larchmt) 2014;23:108-111.
- American College of Obstetricians and Gynecologists’ Committee on Gynecologic Practice; Long-Acting Reversible Contraceptive Expert Work Group. Committee Opinion No 672: Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol 2016;128:e69-e77.
- Centers for Disease Control and Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States — 2021 Update: A clinical practice guideline. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- Shull JA, Attys JM, Amutah-Onukagha NN, Hill MJ. Utilizing emergency departments for pre-exposure prophylaxis (PrEP). J Am Coll Emerg Physicians Open 2020;1:1427-1435.
- Stanley K, Lora M, Merjavy S, et al. HIV prevention and treatment: The evolving role of the emergency department. Ann Emerg Med 2017;70:562-572.e3.
The clinical diagnosis of pelvic inflammatory disease (PID) is challenging in the emergency department. Nonetheless, making the diagnosis is important, since PID is associated with uterine and fallopian tube scarring leading to tubal factor infertility and ectopic pregnancy, as well as chronic pelvic pain. This article provides an evidence-based review of diagnostic and treatment recommendations for PID.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
