AUTHORS
Natalie Diers, MD, Resident, Emergency Medicine Residency Program, UCF/HCA Florida Healthcare GME (Greater Orlando/Osceola)
Nicole Vuong, MD, Resident, Emergency Medicine Residency Program, UCF/HCA Florida Healthcare GME (Greater Orlando/Osceola)
Shayne Gue, MD, MSMEd, FACEP, Director of Education, Emergency Medicine Residency Program, UCF/HCA Florida Healthcare GME (Greater Orlando/Osceola), Assistant Professor of Emergency Medicine, University of Central Florida College of Medicine
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity, Samaritan Hospital, Troy, NY
EXECUTIVE SUMMARY
- Oncologic emergencies can be due to local tumor effects, metabolic complications, and adverse effects of treatment.
- Bony metastases are more common in breast, prostate, thyroid, and lung cancer.
- Bony metastases typically are treated with bisphosphonates, such as pamidronate.
- Superior vena cava syndrome from malignancy typically involves insidious compression with the development of collateral blood flow to mitigate the hemodynamic effects.
- Malignant pericardial effusion often is asymptomatic until the amount of fluid reaches a large volume.
- The most common location for malignant spinal cord compression is the thoracic spine.
- Excessive secretion of parathyroid hormone-related protein accounts for more than 80% of hypercalcemia of malignancy.
- Hyperviscosity syndrome can be due to an increase in the cellular components or the proteins found in whole blood.
- Venous thromboembolism due to malignancy is best treated with direct-acting oral anticoagulants or low molecular weight heparin.
- Patients with febrile neutropenia deemed at high risk for adverse outcomes should be treated promptly with broad-spectrum empiric antibiotics, ideally within one hour of presentation.
- The most life-threatening electrolyte abnormality in tumor lysis syndrome is hyperkalemia.
Definition of the Problem
The current trajectory in population growth suggests increasing growth in our elderly population over the next decade. In the United States, the population older than the age of 65 years grew nearly five times faster than the total population over the last 100 years, with its greatest growth between 2010 and 2020.1 With a growing elderly population, the incidence of cancer also has increased rapidly.2
Oncologic emergencies cover a variety of conditions and complications that may occur in conjunction with malignancy.3 These include the initial presentations of new cancer diagnoses, complications of metastatic disease, and issues related to the adverse effects of treatment. (See Table 1.) As the name implies, these complications can manifest suddenly and be imminently life-threatening.
This review will cover the most potentially serious oncologic emergencies, but space does not allow discussion of complications due to biologic therapy.
Table 1. Categories of Oncologic Emergencies
Complications Related to Local Tumor Effects | Metabolic Complications | Complications from Cancer Treatment |
|
|
|
Relevancy
More than 15 million patients are living with cancer in the United States, accounting for nearly 4 million annual emergency department visits.4 Up to 20% of intensive care unit (ICU) admissions will have an underlying cancer diagnosis.5 Advancement in technology and new developments in cancer management likely will increase the number of patients who experience these emergencies, requiring familiarity for practitioners across all medical specialties, especially emergency medicine clinicians.6
Epidemiology
In 2022, there were an estimated 1.9 million new cancer diagnoses and more than 600,000 cancer-related deaths in the United States.7 In men, prostate, lung, and colorectal cancers are the most common, accounting for 48% of all incident cases. For women, breast, lung, and colorectal cancer make up 51% of all new diagnoses, with breast cancer alone accounting for 31% of cancer cases.
Experts speculate the dramatic increase in the number of patients with a cancer diagnosis is the result of both the improvement in the performance of screening exams as well as advancements in cancer treatments. The latter also have led to an increased rate of cancer survival. The five-year relative survival rate for all cancers combined increased between the mid-1970s and 2011 through 2017 from 48% to 68% overall. In white individuals, survival rates increased from 50% to 68%; in Black individuals, survival increased from 39% to 63%.7
In men, the five-year survival rate is highest for prostate cancer at 98%. For women, there is a 90% five-year survival rate for breast cancer. Cancers with the lowest five-year survival rates include pancreatic (11%), liver and esophagus (20%), and lung (22%).7 Lung cancer is the leading cause of cancer-related deaths, with an estimated 350 deaths per day, more than breast, prostate, and pancreatic cancer combined, and 2.5 times more than colorectal cancers.7
Although cancer survival is improving globally, there is significant geographic variation in the prevalence and outcomes of cancer. This most often is attributable to the geographic association with well-known risk factors, such as smoking, obesity, and access to healthcare screening.8 The largest geographic variations are observed for the cancer types that are considered most preventable: lung cancer, cervical cancer, and melanoma of the skin.
Overall, the risk of death from cancer in the U.S. population has continued to decline over the last 30 years, with experts suggesting more than 3.5 million deaths have been avoided due to better health education, improvements in screening initiatives and access to care, and advancements in treatment. Specifically, educational campaigns to reduce the rate of tobacco use have led to a significant decline in new cases of lung and other smoking-related cancers; initiatives promoting early cancer screening and detection have led to improved morbidity and mortality in many cases; and newer therapeutic interventions and adjuvant chemotherapies have improved cancer survival.7
Complications Related to Local Tumor Effects
Bone Metastasis and Pathologic Features
Most cancers can metastasize to the bone; however, the incidence of bone metastasis varies between cancer types.9 On postmortem examinations, bone metastasis was seen most often in breast (73%), prostate (68%), thyroid (42%), and lung cancer (36%).10 Malignant cells will enter the vasculature from the primary tumor site and spread into the bony matrix, colonizing the bone microenvironment.11 It is not uncommon for disseminated tumor cells to enter a state of dormancy and remain silent for years before proliferating and causing the effects of metastasis.12 Treatment of bone metastasis rarely is curative, but disease control can help to improve effects and quality of life.13
Etiology. Bone is a specialized connective tissue consisting of mineralized extracellular components as well as active and mobile cells.14 Bone is continuously remodeled throughout an individual’s life to maintain skeletal integrity and shape.15 In healthy bone, the interaction between osteoblasts and osteoclasts is well-balanced with appropriate bone remodeling.16 While osteoblasts and osteoclasts are two of the major cells responsible for bone health, there also are complex signaling mechanisms that take place between other cell types. Osteocytes and the many bone-forming cells respond to a multitude of growth factors and anabolic hormones, such as fibroblast growth factor, insulin-like growth factor, parathyroid hormone, estrogen, and calcitonin.15
Pathophysiology. Cancer is an uncontrolled overgrowth of abnormal cells in the body. These cells are not responsive to normal hormonal checkpoints and, as a result, destructive effects are seen. Primary tumors can release cells to invade surrounding and distant tissue through the production of proteases. These proteases allow malignant cells to enter into the vasculature and disseminate. The migration of cancer cells is a well-orchestrated process, and modulation of the local and tumor microenvironments is critical for the completion of a complex metastatic cascade.
Metastasis to bone is one of the most common sites for several reasons. First, red marrow is highly vascularized and this high blood flow allows for easy migration of cancer cells. There also are numerous adhesive molecules on tumor cells that bind them to particular stromal cells, which are prevalent in bone marrow. Cancer cells also are very responsive to angiogenic factors and bone resorbing hormones, which are present in large quantities in the bone marrow.17
Malignant cells must accomplish a series of complex steps to ensure outgrowth within the bone marrow. Tumor cells must migrate across the vascular wall, invade and survive in the bone marrow stroma, stimulate and grow their own vascular supply, and migrate to the safety of the bone surface. The endosteal bone surface is covered by lining cells or endosteal cells, which form a continuous membrane over the outer trabecular bone surface. Tumor cells will release agents that will stimulate the motility of lining cells, activating bone resorption and providing access for the tumor to the bone surface.
Bone metastasis can be classified as osteolytic (bone destructive) or osteoblastic (bone forming or sclerotic). Osteolytic lesions are seen most commonly with multiple myeloma, primary bone tumors, and breast cancer. Osteoblastic lesions are seen in 25% of patients with breast cancer and in the majority of patients diagnosed with prostate cancer. This is thought to be due to an inflammatory response that osteoblasts undergo in late stages of cancer. Osteoblasts will produce inflammatory cytokines that allow for the maintenance and survival of cancer cells and osteoclasts, leading to increased osteoclast activation, increased cancer proliferation, increased bone resorption, and ultimately bony destruction.18
Clinical Features. Progression to bony metastasis can occur at any time during the disease course. Additionally, malignant cells can undergo dormancy and cancer cell reactivation, leading to macro-metastatic lesion growth. Late or advanced-stage bone metastases are characterized by macro-metastatic lesion formation and extensive tumor cell colonization of bone.18
Patients usually present with bone pain and may develop fractures at the metastatic location (pathologic fractures) and hypercalcemia. In more severe presentations with bony metastasis to vertebrae, patients can present with neurological symptoms of spinal cord compression. Patients with known bony metastasis also can seek help for pain control. In addition to treating pain, it is important to evaluate for complications that can arise from bony metastasis.
Diagnostic Studies. Medical imaging is the initial approach to suspect bony metastases. Plain radiography (X-ray), computed tomography (CT), magnetic resonance imaging (MRI), and single-photon emission computed tomography (SPECT) all are used for the diagnosis of metastasis.19 X-ray is the most cost-efficient and most readily available imaging modality, but it has limited sensitivity, typically only able to diagnose bony lesions greater than 1 cm or destruction of bone trabecular greater than 50%. (See Figure 1.)
CT imaging varies in sensitivity, ranging from 71% to 100%, and is less sensitive for evaluation of metastasis into the bone marrow.19 MRI is preferred for evaluating metastasis to soft tissue. SPECT is the most sensitive and specific for early identification of bony metastasis but typically is more costly and not as easily accessible from the emergency department.
Figure 1. Pathological Fracture of Right Proximal Humerus in a Patient with Metastatic Breast Cancer |
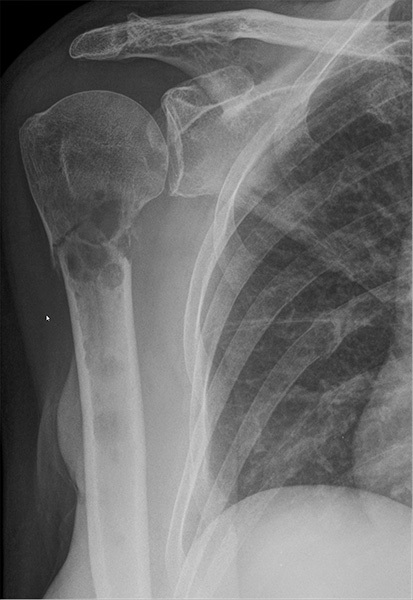 |
Other osteolytic lesions also are seen at the glenoid cavity and several ribs. Pathological fractures also are seen at the ribs (Courtesy of Hellerhoff - CC BY-NC-SA 4.0) - https://commons.wikimedia.org/wiki/File:Pathologische_Fraktur_Humerus_bei_Metastasen_Mammakarzinom_69W_-_CR_ap_-_001.jpg |
Metabolic markers in serum may be abnormal before changes are visualized on imaging.19 During bone remodeling, there is a release of proteins and peptides that reflect the cellular activity of osteoblasts and osteoclasts. Serum testing of peptide or growth products, such as alkaline phosphatase and osteocalcin, or degradation products, such as urinary hydroxyproline and deoxypyridinoline, can indicate potential metastatic disease involving the bone.
Management. Management of bony metastasis typically is initiated with drugs aimed to block osteoclast function, thereby slowing bone resorption. Bisphosphonates work in this manner by suppressing osteoblast-derived hormones and inducing osteoclast apoptosis.18 Pamidronate is a specific agent within the bisphosphonate class that is ingested by osteoclasts during bone resorption and induces apoptosis.
Pamidronate does not inhibit bone mineralization or formation; it primarily works to decrease the rate of bone turnover, stabilize the formed bone matrix, and reduce hypercalcemia. It is important to note that while pamidronate is a treatment to stabilize bony lesions and restrict further progression, it is not curative. Currently there are no treatments that specifically target osteoblasts or promote bone deposition.
For patients presenting with pathologic fractures, management may vary depending on the location of the lesion, the fracture type, patient factors and comorbidities, and specialist availability. In general, orthopedic and oncology consultants should be involved to further guide management.
Disposition. Because of the differences in presentations of bony metastasis and pathologic fractures, disposition varies. Small, stable lesions without fracture may be appropriate for outpatient treatment in coordination with the patient’s oncology team. Typically, those with fractures will require orthopedic consultation and management. Other considerations include comorbid conditions, other patient factors, and overall prognosis.
Superior Vena Cava Syndrome
Etiology. The superior vena cava (SVC) is a large vessel that provides venous drainage from the head, neck, and upper extremities to the right atrium and heart. SVC syndrome is a pathologic condition that results from the compression or obstruction of the SVC. Extrinsic compression occurs most commonly due to mediastinal masses, aortic aneurysms, and thymomas. Historically, aneurysmal disease due to tuberculosis or syphilis was the predominant cause. However, in recent years, the most common etiologies are lung cancer and lymphoma.20 Intrinsic causes usually result from thrombosis of the vessel, which has become more frequent because of the increased use of intravascular devices, such as indwelling catheters and pacemakers.
Pathophysiology. The SVC delivers venous blood from the upper torso, neck, head, and upper extremities to the heart, responsible for approximately one-third of the body’s total venous return to the heart.21 When compression or obstruction occurs, blood will flow through collateral circulation to the inferior torso and into the inferior vena cava or azygos vein.
Dilation and development of adequate collateral circulation can take weeks to months to develop;21 thus, acute obstruction (most often due to thrombosis) often presents differently than insidious compression (in the case of most malignancies). It is possible that malignant SVC obstruction can be slowly progressive and then suddenly manifest due to a provoked thrombosis. Normal central venous pressure (CVP) is between 2 mmHg and 8 mmHg. In SVC syndrome, CVP may increase to 20 mmHg to 40 mmHg.
Clinical Features. The severity of symptoms may vary depending on the rate and degree of narrowing that occurs within the SVC. A patient presenting with an acute obstruction or thrombosis of the SVC likely will present with more severe symptoms. Acute obstruction quickly diminishes cardiac return, leading to decreased cardiac output and potential hemodynamic collapse. A patient with the development of a gradual compressive mass may present with more vague, gradual onset symptoms due to the development of collateral vasculature to mitigate SVC obstruction.
Symptoms of SVC obstruction may include headache or swelling of the face, neck, or arms. Other symptoms can include chest pain, shortness of breath, and altered mental status. Some patients may present with signs of airway obstruction, including the trachea, pharynx, and larynx, which can occur due to direct compression from the enlarging mass or as a result of edema within the mediastinum. These patients also can present with cough, dysphagia, and stridor.
Diagnostic Studies. Evaluation for SVC syndrome requires radiographic investigation. Although plain radiography may be used in the initial presentation of patients presenting with shortness of breath, it is not sensitive to rule out SVC syndrome. X-ray may be useful in identifying a causative lung mass or mediastinal or hilar adenopathy. Other findings may be present; one retrospective review of patients with SVC syndrome found that 66% had pleural effusions on chest X-ray.22
However, because of the low sensitivity of plain radiography, advanced imaging is preferred, typically contrast-enhanced CT of the chest with multiphase imaging.20 If the patient is unable to receive intravenous contrast for CT, MRI or magnetic resonance angiography can be considered, and both are highly sensitive. Finally, contrast venography may be performed after diagnosis to assist in surgical planning.
Management. As with other entities described, therapeutic interventions for SVC syndrome vary depending on presenting symptoms. Those presenting with signs or symptoms of impending airway compromise should undergo endotracheal intubation to protect and stabilize the airway. Patients presenting with signs of hemodynamic compromise (from decreased venous return) should receive intravenous crystalloids to expand intravascular volume and improve cardiac output.
For less urgent management, patients should undergo tissue sampling of the compressive mass to guide treatment. Corticosteroids are used in some tumors, such as certain lymphomas or thymomas, because they are known to be steroid-responsive. Chemotherapy usually is the treatment of choice in lymphomas, germ cell tumors, and small cell lung cancer. In those masses that are not amenable to either, patients may undergo interim intravascular stent placement until more definitive treatment with radiation therapy and/or surgical intervention can be obtained.
Disposition. Most patients with confirmed SVC syndrome will require admission and hospitalization for diagnosis and therapeutic intervention. Emergency clinicians may consider consulting oncology, otolaryngology, cardiothoracic surgery, or vascular surgery specialists depending on institutional availability. Those requiring airway protection or at risk for airway compromise should be admitted to an ICU for further assessment and management.
Malignant Pericardial Effusion
Etiology. Pericardial effusions can be caused by numerous inflammatory and non-inflammatory causes.23 Pericardial effusions can develop from trauma, renal disease, and viral pericarditis. However, malignancy is the leading cause of pericardial effusion, with the most common causative cancers being those of the lung and breast.24
Pathophysiology. The pericardial space lies between the serosal layers of the visceral and parietal pericardium. In normal adults, this space may contain up to 50 mL of plasma ultrafiltrate.25 Pericardial effusions result when the volume of blood or serous fluid accumulation within the pericardial sac exceeds this amount.26
Signs and symptoms are dependent on the effusion volume, rate of accumulation, area of distribution, composition, and hemodynamic impact.27 Malignant effusions usually are exudative and gradual in onset with slow fluid accumulation producing an insidious onset of symptoms. Effusions can develop from direct or metastatic spread of the primary malignancy or as a complication of certain chemotherapeutic agents and other treatments.28
Clinical Features. With pericardial effusions due to malignancy, patients generally are asymptomatic in the early stages due to slow accumulation of fluid without hemodynamic effects. However, as the fluid continues to accumulate, patients develop dyspnea, cough, chest pain, fatigue, and lightheadedness. Although rare, further accumulation of fluid can impair cardiac output, a condition termed cardiac tamponade. In about 25% of patients presenting with cardiac tamponade, malignancy was identified as the most likely underlying etiology.24
Diagnostic Studies. Pericardial effusions can be diagnosed by medical imaging. Although plain radiography of the chest may suggest the diagnosis, echocardiography is the most expeditious modality to make a definitive diagnosis.26 Both transthoracic (TTE) or transesophageal echocardiogram (TEE) can estimate the size of the effusion as well as its distribution and location, should a pericardiocentesis be required. (See Figure 2.)
Figure 2. Hemorrhagic Pericardial Effusion |
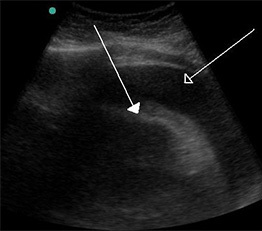 |
A large hemorrhagic pericardial effusion due to malignancy seen on ultrasound. Source: Doc James. https://commons.wikimedia.org/wiki/File:Hemorragic_effusion.jpg |
Cardiac computed tomography (CCT) or cardiac magnetic resonance (CMR) imaging also are considered acceptable alternative imaging modalities if echocardiography is not available.26 These imaging modalities can identify the presence or absence of an effusion, but the diagnosis of a malignant etiology requires positive cytology from analysis of pericardial fluid obtained through pericardiocentesis.
Management. Therapeutic interventions for malignant pericardial effusion vary based on acuity at presentation, symptomatology, overall prognosis, and resource availability. In all cases, emergency clinicians should consult a multidisciplinary team, including oncology and cardiothoracic surgery specialists, to help develop and carry out a management plan.
In emergent cases of hemodynamic instability due to large effusion size or evidence of cardiac tamponade, pericardiocentesis with removal of fluid volume may serve as a temporizing measure.28 The majority of patients requiring pericardiocentesis eventually will undergo pericardiac window as a more permanent therapeutic measure. Treatment also may be targeted to the primary cancer via chemotherapy, radiation therapy, surgical methods, and other therapeutic interventions.
Disposition. As with any pericardial effusion, those patients with trace or small-volume effusions without symptoms or clinical signs may be suitable for outpatient follow-up and observation. Those with larger-volume effusions, significant symptoms, or signs of clinical instability should be admitted to intensive care with consultation of multidisciplinary care teams.
Malignant Spinal Cord Compression
Etiology. Malignant spinal cord compression (MSCC) is a common oncological emergency, affecting up to 40% of cancer patients with known bony metastases.29 Unfortunately, it carries significant morbidity and is increasing in frequency due to improved diagnostic accuracy and increased life expectancy in cancer patients.30
Pathophysiology. MSCC can occur due to primary malignancy of the nervous system but also from metastasis of malignant cells into the vertebrae or the spinal canal. Spinal cord compression occurs when malignant cells within the vertebrae or epidural space compress the thecal sac, causing venous congestion and subsequent vasogenic edema.31 This can lead to spinal cord infarction and neurological damage from compression if not alleviated.
MSCC is seen most commonly with prostate, breast, and lung cancers, which are the most common to metastasize to distant sites. The thoracic spine is the most common site for developing symptoms of cord compression.
Clinical Features. Patients with MSCC present with signs and symptoms similar to other compressive spinal pathologies. Patients can develop sensory loss and motor deficits at or below the level of compression. Typical symptoms include weakness, sensory loss, loss of anal sphincter tone and bowel incontinence, urinary retention, back pain, and numbness below the spinal level involved. Patients also may present with hyperreflexia and other upper motor neuron signs, due to the anatomy of compression within the spinal column.32
Diagnostic Studies. Although X-ray may help distinguish bony destruction from metastatic lesions, contrast-enhanced MRI of the whole spine is the most sensitive imaging modality and is considered the gold standard for the diagnosis of cord compression. Clinicians may consider contrast-enhanced CT imaging if MRI is not available.
Management. Initial management is directed at decreasing vasogenic edema within the spinal canal. Dexamethasone is the preferred agent to be given in spinal cord compression, with a recommended initial intravenous bolus of 10 mg followed by 4 mg intravenously every six hours. Emergent consults with neurosurgery and radiation oncology should be performed concomitantly.
Decompressive surgery, coupled with postoperative radiotherapy, has been shown to improve clinical outcomes if patients are appropriate for surgical intervention. Surgical treatment within 48 hours of onset of neurological deficits can help to prevent loss of limb function and loss of bowel and bladder function.
Surgical intervention is favored for those patients with pathological fracture with an unstable spine, radioresistant tumor, previous radiotherapy at the level of compression, disease localized to a single vertebral level, and good prognostic factors, including age younger than 65 years, life expectancy more than six months, and systemic treatment options.33 Given the rarity of these favorable patient factors, only 10% to 15% of patients will qualify for surgical decompression.31
Additional management includes adequate pain control, appropriate consultation and management for bladder and bowel function, and consideration for rehabilitation services.
Disposition. All patients with diagnosed spinal cord compression require neurosurgical consultation and should be admitted for therapeutic interventions. Patients will require multidisciplinary teams of neurosurgical and oncology specialists, along with case management, physical and occupational therapy, and other rehabilitative services. Prognosis correlates with neurologic status at diagnosis; those who can walk usually retain independent ambulation, while those who cannot walk rarely regain ambulation.
Metabolic Complications
Hypercalcemia
Hypercalcemia is a metabolic abnormality categorized into mild, moderate, or severe presentations based on serum calcium levels. It is considered mild when calcium levels are between 10 mg/dL and 12 mg/dL, moderate when levels are between 12 mg/dL and 14 mg/dL, and severe when levels are greater than 14 mg/dL.34 Serum calcium levels greater than 13 mg/dL should increase suspicion of a malignant cause for hypercalcemia. Hypercalcemia of malignancy occurs in approximately 20% of all cancer patients during their clinical course.35
Etiology. Calcium is the most abundant mineral in the human body and is essential as a second messenger in several physiologic processes.36 Circulating calcium levels are tightly regulated by the interactions between parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D. They have their effect on calcium levels via the bones, kidneys, and gastrointestinal tract.
PTH is secreted by the parathyroid glands and stimulates bone resorption, increases calcium reabsorption at the distal convoluted tubules, and stimulates 1-alpha-hydroxylase, the renal enzyme responsible for converting 25-hydroxyvitamin D to the active 1,25-dihydroxyvitamin D. Active 1,25-dihydroxyvitamin D acts by promoting increased calcium and phosphate reabsorption in the small intestine, decreasing renal excretion, and working synergistically with PTH to increase calcium release from bones.
There are many causes of hypercalcemia, and the initial step in differentiation is determining whether it is due to a benign or malignant etiology. Common benign causes include primary hyperparathyroidism and familial hypocalciuric hypercalcemia.
Pathophysiology. Hypercalcemia of malignancy can be explained through three main mechanisms: secretion of parathyroid hormone-related protein (PTHrP), osteolytic metastases leading to the release of osteoclast-activating factors, and tumor production of ectopic 1,25-dihydroxyvitamin D (calcitriol). Excessive secretion of PTHrP by tumors, also known as humoral hypercalcemia of malignancy (HHM), is the most common cause, accounting for more than 80% of cases of hypercalcemia of malignancy.37
PTHrP shares structural similarities to parathyroid hormone and, thus, mimics PTH by acting at the same receptors on the bones and kidneys. PTHrP acts on osteoblasts to enhance the synthesis of a ligand for receptor activator of nuclear factor-κB (RANK), which stimulates osteoclasts to promote bone remodeling and calcium release into the bloodstream. At the kidneys, PTHrP increases the excretion of phosphate and reabsorption of calcium, resulting in hypophosphatemia and hypercalcemia.
Osteolytic metastases causing excessive calcium release from bones account for approximately 20% of malignancy-related hypercalcemia.37 Local cytokines released from tumors can stimulate local production of PTHrP, thereby inducing the ligand and RANK interaction and leading to excessive osteoclast activation and bone remodeling. This is seen commonly in multiple myeloma and solid organ tumors that metastasize to bones.
The ectopic activity of 1-alpha-hydroxylase is seen commonly in lymphomas. This enzyme converts 25-hydroxyvitamin D to the active 1, 25-dihydroxyvitamin D, leading to hypercalcemia through increased bone resorption and increased intestinal absorption of dietary calcium.
Clinical Features. The presentation of hypercalcemia can range from asymptomatic to multiorgan system involvement, depending on acuity and severity. Signs and symptoms of hypercalcemia commonly are summarized by the mnemonic “stones, bones, groans, and psychiatric overtones.” This is used to describe renal manifestations (varying from nephrogenic diabetes insipidus to nephrolithiasis), musculoskeletal symptoms (such as muscle weakness and bone pain), gastrointestinal symptoms (nausea and constipation), and psychiatric disturbances (anxiety, depression, and even psychosis).
Diagnostic Studies. The first step in the evaluation of suspected hypercalcemia is the measurement of serum calcium levels. In serum, approximately 40% to 45% of calcium is bound to plasma proteins; thus, total calcium fluctuates in relation to serum albumin levels. If albumin levels are abnormal, serum calcium should be corrected using the formula: calcium + 0.8 (4.0 - albumin). However, measuring ionized calcium levels directly is the preferred method and should be measured in cases of suspected hypercalcemia The normal range of ionized calcium is 4.65 mg/dL to 5.25 mg/dL.
Once hypercalcemia is confirmed, further laboratory evaluation is needed, with an initial workup consisting of PTH, PTHrP, phosphorus, 25-hydroxyvitamin D, and 1,25-dihydroxyvitamin D levels. Elevated PTHrP with low-normal levels of PTH indicates humoral hypercalcemia of malignancy. In combination with low to normal phosphorus and 1,25-dihydroxyvitamin D levels, the diagnosis is confirmed.
Local osteolytic hypercalcemia is identified by increased levels of phosphorus with suppressed levels of PTH and 1,25-dihydroxyvitamin D. Laboratory studies pointing toward excess production of 1,25-dihydroxyvitamin D will show elevated 1,25-dihydroxyvitamin D levels in the absence of concomitant elevation in serum 25-hydroxyvitamin D. In these cases, PTH also will be suppressed.
Importantly for the emergency clinician, a 12-lead electrocardiogram (ECG) also should be obtained to evaluate for shortened QT interval, prolonged PR interval, widened QRS, ST changes, flattened or inverted T waves, as well as AV blocks, which could indicate potential for dysrhythmia.
Management. Initial treatment of hypercalcemia is aimed at lowering serum calcium levels through administering intravenous isotonic fluids (normal saline), followed by calcitonin and bisphosphonates.
Normal saline acts immediately, with variable effects lasting for as long as the fluids are continued. Many hypercalcemic patients are dehydrated, and volume expansion with isotonic fluids restores renal perfusion to increase the excretion of calcium.38 The rate of hydration is dependent on the severity of hypercalcemia as well as other patient comorbidities. Usually, a 1-liter to 2-liter bolus can be administered safely, followed by maintenance infusion at a rate of 100 mL/hour to 150 mL/hour, titrated to maintain a urine output of 100 mL/hour.35
Calcitonin (given at a dose of 4 IU/kg) acts within four to six hours, and the effect lasts approximately two days. Calcitonin is a peptide hormone secreted by the parafollicular C cells of the thyroid gland. It acts by inhibiting bone resorption by osteoclasts and renal calcium reabsorption.
Bisphosphonates take effect in two to three days and their effect lasts for two to four weeks. Bisphosphonates are pyrophosphate analogs that bind to hydroxyapatite in mineralized bone to induce apoptosis of osteoclasts, thus blocking bone resorption.39 Conversely, they prevent apoptosis of osteoblasts, thus reducing bone turnover and the release of calcium. Commonly used bisphosphonates include zoledronic acid (4 mg intravenous given over 15-30 minutes) or pamidronate (60 mg to 90 mg intravenous over two hours) in patients without kidney dysfunction.
Targeted treatment of the underlying malignancy is the ultimate therapy for malignant hypercalcemia.
Disposition. Patients presenting with moderate to severe hypercalcemia require hospitalization to initiate therapeutic interventions and observation to ensure there is no further progression. Urinary output should be monitored carefully to ensure adequate calcium excretion, and consultation with nephrology and oncology specialists is recommended.
Hyperviscosity Syndrome
Acute hyperviscosity syndrome is characterized by elevated blood viscosity due to deformities in the shape of red blood cells or an increase in serum proteins or cellular components, leading to decreased tissue perfusion.
Etiology. Hyperviscosity can be caused by a pathologic elevation in any component of blood, including cellular components such as erythrocytes, leukocytes, and platelets, or acellular components such as proteins. Conditions responsible for hyperviscosity syndrome related to cellular components include polycythemia vera, leukemia, and thrombocytosis. Acellular causes of hyperviscosity syndrome can be monoclonal, such as in cases of Waldenstrom macroglobulinemia and cryoglobulinemia, or polyclonal, as is seen in SjÖgren’s syndrome and uncontrolled human immunodeficiency virus (HIV) infections. Deformities in cell shape caused by sickle cell disease or hereditary spherocytosis also can lead to hyperviscosity syndrome.
Pathophysiology. Viscosity is a measurement of a fluid’s resistance to flow based on shear stress. Colloquially, this can be thought of as the thickness of the fluid. Hyperviscosity syndrome is a pathological condition in which blood is thicker than normal, resulting in decreased blood flow. The decrease in blood flow reduces microvascular circulation with hypoperfusion of tissues.40
Clinical Features. Patients may present with a classic triad of mucosal bleeding (nose, mouth, gastrointestinal), visual changes (diplopia, retinal hemorrhages), and neurologic disturbances (confusion, somnolence).41 Dyspnea secondary to heart failure or pulmonary hypertension can be a presenting symptom.
Bleeding is seen most commonly and typically is due to impaired platelet function. Fundoscopic examination may reveal “sausage link” or “boxcar” engorgement of retinal veins, papilledema, flame-shaped hemorrhages, and exudates. Ocular examination is a key component of the physical exam because positive findings can enable early diagnosis and treatment.42 The severity of symptoms is related to the serum viscosity, with more severe symptoms as viscosity increases.
Diagnostic Studies. Complete blood count with peripheral blood smear, serum chemistries, coagulation profile, urinalysis, and immunoglobulin levels should be completed. Rouleaux formation on peripheral blood smear is highly suggestive of serum stasis. Urinalysis with elevated albumin-protein gap and proteinuria is consistent with underlying gammopathy.
Cellular hyperviscosity is identified by increased numbers of one of the cellular components of whole blood. Typical values for cellular hyperviscosity are a red blood cell (RBC) count > 7 million/µL in men or > 6.4 million/µL in women, white blood cell (WBC) count > 100,000/µL to 200,000/µL, or platelet count > 1 million/µL.
Acellular hyperviscosity can be assessed by measuring serum viscosity in relation to water. Serum viscosity relative to water normally is 1.4 to 1.8, and symptoms of hyperviscosity typically arise when serum viscosity exceeds 4 to 5.
Management. The mainstay of treatment includes supportive care, fluid administration, plasma exchange or plasmapheresis (or leukapheresis), and chemotherapy. Patients generally are dehydrated; thus, fluid administration is advised and empiric administration of 1 L to 2 L of normal saline is common practice in cases of suspected hyperviscosity syndrome.
Plasmapheresis reduces immunoglobulin levels, subsequently reducing plasma viscosity. This can be completed daily and can reduce serum viscosity by 20% to 30% per session.43 Plasmapheresis must be followed by specific treatment of the underlying disorder.
With severe neurologic symptoms such as seizures or coma, phlebotomy can be performed as a temporary measure, removing 500 mL of whole blood and replacing it with normal saline.
Disposition. Early identification and timely treatment can prevent thromboembolic complications, such as myocardial infarction or ischemic stroke. Most patients will require hospitalization with oncology consultation to perform plasmapheresis (or leukapheresis) and initiate chemotherapy or other therapeutic interventions for the underlying malignancy.
Venous Thromboembolism
Venous thromboembolism (VTE) commonly presents as deep venous thrombosis of the lower extremities or pulmonary embolism. Causes can be divided into hereditary and acquired, with malignancy considered an acquired cause. VTE can be the first manifestation of cancer, preceding the diagnosis of malignancy, or can arise as a complication during the disease course.44
Etiology. Patients with malignancy are at increased risk of arterial and venous thromboembolism. VTE is a common cause of morbidity and mortality in cancer patients. Approximately 10% to 20% of patients with symptomatic deep venous thrombosis have known active malignancy, with the incidence of VTE in patients with cancer estimated to be four to seven times higher than in patients without cancer.45
Pathophysiology. The theory behind the pathogenesis of VTE suggests that three core factors predispose a patient to develop vascular thrombosis. These components are collectively known as Virchow’s triad and include endothelial/vessel wall damage, stasis of blood flow, and hypercoagulability. The mechanisms behind cancer-associated thrombosis are multifactorial and can be due to the disruption of any component of Virchow’s triad. A hypercoagulable state may be created by the production of substances such as tissue factor or cancer procoagulant. Tumor compression of blood vessels or prolonged immobility can induce stasis and promote endothelial damage.
Clinical Features. Patients can present with VTE such as deep venous thrombosis or pulmonary embolism, or with arterial thromboembolism such as ischemic strokes or myocardial infarctions. Clinical signs and symptoms are the same as would be expected in cases of the same entity in patients without malignancy.
Diagnostic Studies. Risk-stratification tools can aid in determining which diagnostic tests to pursue. Commonly used scoring tools include the Wells’ Criteria for DVT and Wells’ Criteria for Pulmonary Embolism, as well as the PERC Rule for Pulmonary Embolism. A D-dimer can be obtained in low-risk patients with no further testing necessary if negative. Positive D-dimer results should prompt further evaluation with ultrasound to evaluate for deep venous thrombosis or computed tomographic angiography or ventilation-perfusion scanning to evaluate for pulmonary embolism.
Routine evaluation for occult malignancy in patients with provoked or unprovoked VTE is not warranted. For the first unprovoked episode of VTE, recommendations are to limit evaluation to history and physical exam, basic laboratory studies, and age-appropriate cancer screening.46 No survival advantage has been demonstrated with more aggressive testing.47
Management. Low molecular weight heparin (LMWH) has been the preferred anticoagulation option for the management of cancer-related thrombosis.48 LMWH has a shorter half-life and fewer drug-drug interactions when compared to vitamin K antagonists, such as warfarin; however, because of the need for injection administration and higher costs, compliance may be a concern.
Direct oral anticoagulants (DOACs), particularly direct factor Xa inhibitors such as apixaban, edoxaban, and rivaroxaban, have emerged as alternative safer and more convenient options. In comparing DOACs to LMWH, patients were found to have a reduced risk of recurrent VTEs but were at greater risk of experiencing major bleeding episodes.49
Presently, therapeutic alternatives for cancer-associated thrombosis include apixaban, edoxaban, rivaroxaban, and LMWH.50
Anticoagulation therapy should be continued for three to six months, or longer if there is active malignancy or there are ongoing risk factors.51
Disposition. Historically, patients with new VTE due to malignancy were hospitalized to ensure adequate anticoagulation and to observe for any hemodynamic compromise. More recent literature has suggested that stable patients with low clot burden may be candidates for initiation of DOACs with close outpatient follow-up. There is a need for further research specific to VTE patients with underlying malignancy as the culprit etiology.
Complications from Cancer Treatment
Febrile Neutropenia
Febrile neutropenia is defined as a single axillary or oral temperature greater than or equal to 101.3° F (38.5° C) or a sustained temperature greater than or equal to 100.4° F (38° C) for one hour, in addition to an absolute neutrophil count (ANC) less than 500 cells/µL or a decrease of ANC to less than 500 cells/µL over the next 48 hours in a cancer patient receiving chemotherapy.52 ANC can be calculated by multiplying the total WBC count by the percentage of polymorphonuclear cells (PMNs) and band neutrophils. The formula would appear as: ANC = WBC (cells/µL) × percent (PMNs + bands)/100.
Etiology. Neutrophils are a type of white blood cell (leukocyte) and are critical components of the host immune system.53 They are the first cell line recruited to sites of infection and act by creating reactive oxygen species to ingest and digest microorganisms, primarily protecting against bacterial infections. The lower limit of normal for neutrophil count is 1,500 cells/µL, with anything below this level considered neutropenia.
As neutropenia becomes more severe, a host’s immune defense weakens, and the risk of infection, along with the severity of any acquired infection, increases.54 The primary causes of morbidity and mortality in patients who present with fever and neutropenia are infections.55
Pathophysiology. Neutropenic fevers occur when patients who are already neutropenic encounter infectious pathogens. Because of their immunocompromised state, they are unable to mount an appropriate immune response to the infectious agent.
Clinical Features. Fever typically is a presenting sign; however, a reliable body temperature measurement must be obtained. Neutropenic patients may present with nonspecific symptoms and have subtle signs of an inflammatory process as a result of the inability to mount a sufficient immune response. Thus, a high index of suspicion must be based on patient history, chemotherapy treatment, medications, history of infections, underlying malignancy, and vital signs.56
Diagnostic Studies. Initial laboratory studies should include complete blood count (CBC), complete metabolic panel (CMP), lactic acid and procalcitonin levels, urinalysis, and blood cultures obtained from two distinct sites (two peripheral sites or one from a peripheral site and one from a central line, if present), as well as from any sites of infection. Presenting symptoms should direct further testing. For instance, in patients presenting with respiratory symptoms, a chest X-ray should be obtained.
Risk stratification for mortality or other serious complications in patients with febrile neutropenia can be done using the Multinational Association for Supportive Care in Cancer (MASCC) risk index score.57 Different studies have found this risk index score to have sensitivities between 71% and 95% and specificities of 40% to 95% in identifying low-risk patients.
Management. For febrile neutropenic patients at high risk for adverse outcome, such as identified by the MASCC risk index score, prompt administration of empiric antibiotic treatment improves survival.58 Febrile neutropenic protocols used in some emergency departments stress antibiotic administration within one hour of presentation in high-risk patients.
Monotherapy regimens with antipseudomonal coverage include piperacillin-tazobactam, cefepime, meropenem, and imipenem-cilastatin. Aminoglycosides, such as gentamicin, amikacin, and tobramycin, can be added in cases with concern for multidrug-resistant organisms. Vancomycin should be added in cases of known colonization with methicillin-resistant Staphylococcus aureus (MRSA), suspected catheter-related infections, and blood cultures positive for gram-positive bacteria. Persistent fever after four days of antibiotic treatment should prompt initiation of antifungal therapy.
Disposition. Although a feared complication of malignancy and chemotherapy, not all patients presenting with febrile neutropenia will require inpatient admission. Patients at low risk for adverse outcomes, such as those identified by the MASCC and the Clinical Index of Stable Febrile Neutropenia (CISNE) scoring systems, may be appropriate for outpatient management provided they live less an a hour away from the hospital, have a reliable caregiver at home, and have a means of quickly returning for emergency care. Studies comparing the two scoring systems for such purposes show mixed results, but both are considered acceptable.59,60
Tumor Lysis Syndrome
Etiology. Tumor lysis syndrome is a metabolic emergency that arises from rapid cell lysis that leads to acute renal failure and the sequelae of electrolyte derangements, including hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia. This phenomenon is seen most frequently in patients with rapidly proliferating and bulky, chemosensitive tumors, such as leukemias, lymphomas, and large solid tumors. It may arise spontaneously or, more commonly, in response to initiation of cancer treatment.41
Pathophysiology. The lysis of cancerous cells, either spontaneously or in response to treatment with cytotoxic therapies, causes the release of intracellular contents, consisting of deoxyribonucleic acid (DNA), phosphate, potassium, and calcium. DNA is broken down by DNAse, releasing purines and pyrimidines, which are further metabolized into uric acid. The resultant hyperuricemia leads to the deposition of uric acid crystals in the renal tubules, since uric acid is poorly soluble in water, causing acute kidney injury.61
Hyperphosphatemia also contributes to acute kidney injury. Phosphate concentration in malignant cells is up to four times higher than in normal cells. Thus, large amounts of phosphate are released during rapid cell lysis, leading to hyperphosphatemia. Phosphate then binds to calcium to create calcium phosphate salts that deposit in the renal tubules. This risk is increased when the calcium phosphate product (calcium concentration multiplied by phosphate concentration) exceeds 60 mg2/dL2. Of note, calcium phosphate salts also deposit in other soft tissues, such as the conducting system of the heart. As phosphate binds to calcium, serum calcium levels are reduced, leading to hypocalcemia. (See Figure 3.)
Figure 3. Cell Lysis Leads to the Classic Laboratory Abnormalities Seen in Tumor Lysis Syndrome |
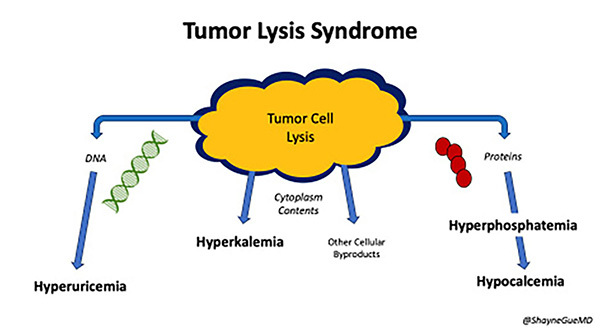 |
Clinical Features. Hyperuricemia, as mentioned previously, will cause acute uric acid nephropathy and acute kidney injury due to the deposition of uric acid crystals in the renal tubules. Hyperphosphatemia causes further injury to the kidneys by binding with calcium to create calcium phosphate salts that deposit in the renal tubules.
Symptoms of hypocalcemia include tetany, paresthesias, seizures, and cardiac dysrhythmias. Pathognomonic physical exam findings in hypocalcemia include Chvostek’s and Trousseau’s signs, described as twitching of the facial muscle in response to tapping on the facial nerve and carpopedal spasm caused by inflation of a sphygmomanometer cuff, respectively.
Hyperkalemia can cause life-threatening cardiac dysrhythmias, such as ventricular tachycardia, which can deteriorate into ventricular fibrillation and even cardiac arrest.
Diagnostic Studies. A 12-lead ECG should be obtained to evaluate for any cardiac manifestations of severe electrolyte disturbances. CBC typically will demonstrate leukocytosis with anemia and thrombocytopenia. The chemistry panel will show hyperkalemia and elevated serum creatinine levels, along with hypocalcemia. Additional laboratory studies, including phosphorous, uric acid, and LDH levels, all likely are to be elevated.41
Management. The initial management priority in tumor lysis syndrome is aggressive intravenous fluid hydration with isotonic crystalloids (normal saline) to induce high urine output (goal of at least 100 mL/hour) and improve renal perfusion. This should be continued until the tumor burden has resolved and/or serum LDH has normalized.62
Hyperkalemia is the most life-threatening electrolyte abnormality secondary to the risk of cardiac dysrhythmias. Management is the same as in other causes of hyperkalemia, with treatment consisting of calcium gluconate to stabilize the cardiac membrane, insulin with glucose and inhaled beta-agonists (albuterol) to shift potassium into the cells, potassium binders (such as sodium polystyrene and patiromer) to aid in excretion of potassium via the gastrointestinal tract, and hemodialysis in severe cases.
Hyperuricemia may be treated with allopurinol, rasburicase, or febuxostat. Allopurinol is preferred as prophylaxis in intermediate-risk patients and acts by reducing the conversion of nucleic acid byproducts to uric acid to prevent urate uropathy. Rasburicase is a recombinant urate oxidase enzyme recommended for high-risk patients or patients with renal impairment. It is the treatment of choice for established diagnoses of tumor lysis syndrome and acts by converting uric acid to the soluble form, allantoin. Rasburicase breaks down uric acid, while allopurinol prevents uric acid formation.62
Hyperphosphatemia and hypocalcemia are treated with intravenous hydration. Phosphate binders, such as sevelamer, will decrease phosphorus levels, which in turn will increase calcium levels.
Despite the aforementioned treatments, if patients are oliguric or anuric, volume overloaded, have refractory hyperkalemia, symptomatic hypocalcemia, or calcium phosphate product exceeds 70 mg2/dL2, continuous renal replacement therapy should be initiated in consultation with nephrology.63
Disposition. Tumor lysis syndrome is a life-threatening condition and should be managed in the ICU by a multidisciplinary team of oncologists, nephrologists, intensivists, and internists. This condition is best prevented rather than managed, since it is very lethal. Mortality rates have improved, but the prognosis remains guarded at best.64
Conclusion
Advancements in the understanding of cancer have resulted in improved screening and treatments for the disease. Oncologic emergencies include various conditions, including complications of local tumor effects, metabolic complications, and complications of cancer treatment. It is important for emergency clinicians to understand how to recognize and evaluate these emergencies to provideappropriate care for these patients.
References
- Caplan Z. U.S. older population grew from 2010 to 2020 at fastest rate since 1880 to 1890. United States Census Bureau. May 25, 2023. https://www.census.gov/library/stories/2023/05/2020-census-united-states-older-population-grew.html
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48.
- Behl D, Hendrickson AW, Moynihan TJ. Oncologic emergencies. Crit Care Clin 2010;26:181-205.
- Hsu J, Donnelly JP, Moore JX, et al. National characteristics of emergency department visits by patients with cancer in the United States. Am J Emerg Med 2018;36:2038-2043.
- Spring J, Munshi L. Oncologic emergencies: Traditional and contemporary. Crit Care Clin 2021;37:85-103.
- Gould Rothberg BE, Quest TE, Yeung SJ, et al. Oncologic emergencies and urgencies: A comprehensive review. CA Cancer J Clin 2022;72:570-593.
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33.
- Dong W, Kim U, Rose J, et al. Geographic variation and risk factor association of early versus late onset colorectal cancer. Cancers (Basel) 2023;15:1006.
- Yu Y, Li K, Peng Y, et al. Animal models of cancer metastasis to the bone. Front Oncol 2023;13:1165380.
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12(20 Pt 2):6243s-6249s.
- Tulotta C, Groenewoud A, Snaar-Jagalska BE, Ottewell P. Animal models of breast cancer bone metastasis. Methods Mol Biol 2019;1914:309-330.
- Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer 2020;1:672-680.
- Coleman RE, Croucher PI, Padhani AR, et al. Bone metastases. Nat Rev Dis Primers 2020;6:83.
- Kamrani P, Marston G, Arbor TC, et al. Anatomy, Connective Tissue. In: StatPearls [Internet]. StatPearls Publishing; March 5, 2023. https://www.ncbi.nlm.nih.gov/books/NBK538534/
- Henry JP, Bordoni B. Histology, Osteoblasts. In: StatPearls [Internet]. StatPearls Publishing; May 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557792/
- Chen X, Wang Z, Duan N, et al. Osteoblast-osteoclast interactions. Connect Tissue Res 2018;59:99-107.
- Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: Mechanisms and therapeutic opportunities. Nat Rev Endocrinol 2011;7:208-218.
- Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Cancer metastases to bone: Concepts, mechanisms, and interactions with bone osteoblasts. Cancers (Basel) 2018;10:182.
- Yang M, Liu C, Yu X. Skeletal-related adverse events during bone metastasis of breast cancer: Current status. Discov Med 2019;27:211-220.
- Holden K, Rao S, White R, et al. Hematologic and oncologic emergencies. Crit Care Nurs Q 2023;46:100-113.
- Wilson LD, Detterbeck FC, Yahalom J. Clinical practice. Superior vena cava syndrome with malignant causes [published correction appears in N Engl J Med 2008;358:1083]. N Engl J Med 2007;356:1862-1869.
- Rice TW, Rodriguez RM, Barnette R, Light RW. Prevalence and characteristics of pleural effusions in superior vena cava syndrome. Respirology 2006;11:299-305.
- Vakamudi S, Ho N, Cremer PC. Pericardial effusions: Causes, diagnosis, and management. Prog Cardiovasc Dis 2017;59:380-388.
- Sánchez-Enrique C, Nuñez-Gil IJ, Viana-Tejedor A, et al. Cause and long-term outcome of cardiac tamponade. Am J Cardiol 2016;117:664-669.
- Hoit BD. Anatomy and physiology of the pericardium. Cardiol Clin 2017;35:481-490.
- Hoit BD. Pericardial effusion and cardiac tamponade pathophysiology and new approaches to treatment. Curr Cardiol Rep 2023;25:1003-1014.
- Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-2964.
- Burazor I, Imazio M, Markel G, Adler Y. Malignant pericardial effusion. Cardiology 2013;124:224-232.
- Klimo P Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol 2005;7:64-76.
- Akanda ZZ, McKay MJ. Narrative review-diagnosing and managing malignant epidural spinal cord compression: An evidence-based approach. Ann Transl Med 2023;11:386.
- Regnier L, Wilkinson AN. Common oncologic emergencies. Can Fam Physician 2023;69:28-32.
- Donnally CJ III, Hanna A, Odom CK. Cervical Myelopathy. In: StatPearls. StatPearls Publishing; Jan. 15, 2023.
- Chi JE, Ho CY, Chiu PY, et al. Minimal invasive fixation following with radiotherapy for radiosensitive unstable metastatic spine. Biomed J 2022;45:717-726.
- Feldenzer KL, Sarno J. Hypercalcemia of malignancy. J Adv Pract Oncol 2018;9:496-504.
- Asonitis N, Angelousi A, Zafeiris C, et al. Diagnosis, pathophysiology and management of hypercalcemia in malignancy: A review of the literature. Horm Metab Res 2019;51:770-778.
- Favus MJ, Goltzman D. Regulation of calcium and magnesium. In: Rosen CJ, Bouillon R, Compston JE, Rosen V, eds. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 8th ed. 2013;171-179.
- Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med 2005;352:373-379.
- McCurdy MT, Shanholtz CB. Oncologic emergencies. Crit Care Med 2012;40:2212-2222.
- Drake MT, Clarke BL, Lewiecki EM. The pathophysiology and treatment of osteoporosis. Clin Ther 2015;37:1837-1850.
- Perez Rogers A, Estes M. Hyperviscosity Syndrome. In: StatPearls [Internet]. StatPearls Publishing; March 13, 2023 Jan-. https://www.ncbi.nlm.nih.gov/books/NBK518963/
- Aliyeva GD. Chapter 7 - Oncologic Emergencies. Rapid Response Situations: Management in Adult and Geriatric Hospitalist Medicine. Elsevier; 2022:147-162. https://www.sciencedirect.com/science/article/pii/B9780323833752000073
- Gertz MA. Acute hyperviscosity: Syndromes and management. Blood 2018;132:1379-1385.
- Ballestri M, Ferrari F, Magistroni R, et al. Plasma exchange in acute and chronic hyperviscosity syndrome: A rheological approach and guidelines study. Ann Ist Super Sanità 2007;43:171-175.
- Falanga A, Marchetti M. Venous thromboembolism in the hematologic malignancies. J Clin Oncol 2009;27:4848-4857.
- Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol 2018;72:89-93.
- Bertoletti L, Robin P, Jara-Palomares L, et al; MVTEP investigators. Predicting the risk of cancer after unprovoked venous thromboembolism: External validation of the RIETE score. J Thromb Haemost 2017;15:2184-2187.
- Piccioli A, Lensing AW, Prins MH, et al; SOMIT Investigators Group. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: A prospective randomized clinical trial. J Thromb Haemost 2004;2:884-889.
- Wang TF, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost 2018;2:429-438.
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017-2023.
- Farge D, Frere C, Connors JM, et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol 2022;23:e334-e347.
- Pavlovic D, Niciforovic D, Markovic M, Papic D. Cancer-associated thrombosis: Epidemiology, pathophysiological mechanisms, treatment, and risk assessment. Clinical Medicine Insights: Oncology 2023;17. doi:10.1177/11795549231220297
- Higdon ML, Atkinson CJ, Lawrence KV. Oncologic emergencies: Recognition and initial management. Am Fam Physician 2018;97:741-748.
- Lehman HK, Segal BH. The role of neutrophils in host defense and disease.
J Allergy Clin Immunol 2020;145:1535-1544. - Patel K, West H. Febrile neutropenia. JAMA Oncol 2017;3:1751.
- Hakim H, Flynn PM, Knapp KM, et al. Etiology and clinical course of febrile neutropenia in children with cancer. J Pediatr Hematol Oncol 2009;31:623-629.
- Klastersky J, de Naurois J, Rolston K, et al, ESMO Guidelines Committee. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol 2016;27(suppl 5):v111-v118.
- Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol 2000;18:3038-3051.
- Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother 2014;58:
3799-3803. - Mohindra R, Mathew R, Yadav S, Aggarwal P. CISNE versus MASCC: Identifying low risk febrile neutropenic patients. Am J Emerg Med 2020;38:2259-2263.
- Coyne CJ, Le V, Brennan JJ, et al. Application of the MASCC and CISNE risk-stratification scores to identify low-risk febrile neutropenic patients in the emergency department. Ann Emerg Med 2017;69:755-764.
- Howard SC. Tumor lysis syndrome. In: Abeloff’s Clinical Oncology. Sixth edition. Elsevier EBooks;2020:572-580.
- Puri I, Sharma D, Gunturu KS, Ahmed AA. Diagnosis and management of tumor lysis syndrome. J Community Hosp Intern Med Perspect 2020;10:269-272.
- Barbar T, Jaffer Sathick I. Tumor lysis syndrome. Advances in Chronic Kidney Disease 2021;28:438-446.e1.
- Gopakumar KG, Seetharam S, Km JK, et al. Risk-based management strategy and outcomes of tumor lysis syndrome in children with leukemia/lymphoma. Pediatr Blood Cancer 2018;65:e27401.
