
Metabolic Dysfunction-Associated Steatotic Liver Disease
September 1, 2024
AUTHORS
Sarah Perrin Dudenhoeffer, DO, Family Medicine Physician and Diabetology Fellow 2024, Touro University California, College of Osteopathic Medicine, Vallejo, CA
Kim Pfotenhauer, DO, Assistant Professor and Diabetologist, Michigan State University College of Osteopathic Medicine, East Lansing, MI
Jay H. Shubrook, DO, Professor and Diabetologist, Department of Clinical Sciences and Community Health, Touro University California, College of Osteopathic Medicine, Vallejo, CA
PEER REVIEWER
Alfred C. Gitu, MD, FAAFP, Program Director and Associate Professor of Family Medicine, The Florida State University COM Family Medicine Residency Program at Lee Health, Fort Myers, FL
EXECUTIVE SUMMARY
Metabolic dysfunction-associated liver disease (MASLD) is the most prevalent liver disease worldwide.
- It is defined by the presence of hepatic steatosis greater than 5% caused by metabolic disease, such as obesity, type 2 diabetes, and metabolic syndrome.
- Metabolic dysfunction-associated steatohepatitis (MASH) is the more severe disease and is defined histologically by the presence of lobular and portal inflammation and hepatocyte injury, including ballooning.
- It is estimated that 38% of the global population has MASLD and 5.3% has MASH, with rates of MASH higher in the United States at 14%.
- The spectrum includes steatohepatitis linked to bridging fibrosis, cirrhosis, and hepatocellular cancer.
- MASH typically is staged by the level of fibrosis, giving the most accurate predictor of disease progression of overall morbidity and mortality.
- Screening for advanced fibrosis is recommended in patients with type 2 diabetes, obese patients with metabolic complications, and those with a family history of cirrhosis or significant alcohol use.
- The most common noninvasive screening test is the Fibrosis-4 (FIB-4) Index for Liver Fibrosis.
- Treatment is multifaceted and should be focused on weight loss, abstention from alcohol, and diabetic control. Pharmacologic options include resmetirom, pioglitazone, and incretin-based agents.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic metabolic disease that you may never have heard of. MASLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is the most prevalent liver disease worldwide.1 MASLD affects 30% of the world’s population, more than half of those people with obesity, and more than 70% of people with type 2 diabetes.1 While many clinicians may see patients with slightly elevated transaminases and assume it is fatty liver, MASLD is not benign and often begins well before laboratory changes. It is associated with a significantly increased risk of cirrhosis, hepatocellular carcinoma, non-liver cancers, and even cardiovascular disease. Although the longstanding established treatment for this condition has been weight loss, there is ample evidence that some off-label treatments have significantly improved MASLD. In 2024, the first U.S. Food and Drug Administration (FDA) approved the first treatment available for this condition. This article reviews the pathogenesis, diagnosis, and natural history of MASLD; known treatments; and future therapies.
Background
MASLD is a spectrum of metabolic liver disease that includes metabolic dysfunction-associated steatotic liver (MASL) and metabolic dysfunction-associated
steatohepatitis (MASH). MASL is defined by the presence of hepatic steatosis (> 5%) that is caused by metabolic disease (obesity, type 2 diabetes, metabolic syndrome).2 MASLD precludes the excessive ingestion of alcohol, which also causes similar pathophysiologic changes and other known causes of liver disease. MASH, formerly known as non-alcoholic steatohepatitis (NASH), is the more severe disease and puts the patient at greater risk for liver fibrosis. MASH is defined histologically by the presence of lobular and portal inflammation and hepatocyte injury, including ballooning.2,3 The definition of MASLD, like its predecessor (NAFLD), still excludes liver disease attributable to other causes, including alcohol, but is more descriptive of the pathophysiology, and it is more patient-centric.4
MASLD and MASH are the hepatic manifestations of metabolic syndrome and a cardiovascular risk equivalent. (See Table 1.) There is a bidirectional relationship between MASLD and type 2 diabetes mellitus (T2DM). MASLD increases the risk of developing T2DM, and having T2DM increases the risk of MASLD. More than two-thirds of people with T2DM have coexisting MASLD and 37% have MASH.5 Further, although MASLD is closely associated with being overweight and obesity, MASLD can occur in people with a normal body mass index (BMI) within the ethnicity-specific recommended range, but these individuals typically have other phenotypic abnormalities, such as an increase in waist circumference and visceral adiposity, further confirming adipose distribution and metabolism are central to MASLD.6 In one study of more than 4,000 asymptomatic participants, waist circumference, insulin resistance, alanine transaminase (ALT), triglycerides, and BMI were the five most relevant variables for differentiation between patients with and without hepatic steatosis across all subgroups.7
Table 1. Metabolic Risk Factors for MASLD2 |
|
Elevated BMI |
BMI ≥ 25 kg/m2 (≥ 23 kg/m2 for Asian race) |
Increased waist circumference |
> 94 cm in men, > 80 cm in women |
Dysglycemia |
Fasting serum glucose ≥ 100 mg/dL or two-hour post-load glucose level ≥ 140 mg/dL or HbA1c ≥ 5.7% or on specific drug treatment |
Hypertension |
BP ≥ 130/85 mmHg or on hypertension treatment |
Hypertriglyceridemia |
≥ 150 mg/dL or specific drug treatment |
Low HDL cholesterol |
< 40 mg/dL for men and < 50 mg/dL for women or specific drug treatment |
MASLD: metabolic dysfunction-associated steatotic liver disease; BMI: body mass index; HbA1c: hemoglobin A1c; BP: blood pressure; HDL: high-density lipoprotein |
|
One important factor in the diagnostic criteria of MASLD is the distinction between patients who have alcohol-induced steatohepatitis and those who have both metabolic liver and alcohol-induced liver disease (MetALD). This would include those people who drink 140 g/week to 350 g/week (for men) and 210 g/week to 420 g/week (for women).4 These other classifications of steatotic liver disease are beyond the scope of this review but are included in the American Association for the Study of Liver Disease (AASLD) consensus paper.4
This article reviews the epidemiology, pathogenesis, diagnosis, and natural history of MASLD as well as known treatments and future therapies.
Epidemiology
It is estimated that 38% of the global population has MASLD and 5.3% has MASH.3 Rates of MASH are higher in the United States (14%).3 The true prevalence of MASLD/MASH is difficult to determine because of its asymptomatic nature earlier in the disease and under-screening. MASLD/MASH rates are higher in people with metabolic disease. Compared to people whose BMI is in the normal range, people who are overweight (70% vs. 33.5%) or are obese (75.3% vs. 33.7%) have higher rates of MASLD and MASH, respectively.3,8 To see a pictorial distribution of the natural history of MASLD to MASH and its complications, please see Figure 2 at https://www.cell.com/cell/fulltext/S0092-8674(21)00494-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867421004943%3Fshowall%3Dtrue
Many people may progress without obtaining a specific diagnosis and will discover they have liver disease only when they decompensate from cirrhosis.1 The prevalence of MASLD is growing rapidly and has tripled in the last 20 years.1,9 It is the most rapidly growing indication for liver transplantation in the United States.10
In the general population, females have a lower risk than males for MASLD but are more at risk for MASH and advanced fibrosis.11 Sexual dimorphism exists with regard to prevalence by age in males vs. females, and the severity of disease progression is increasing and, in some studies, surpassing prevalence in females after menopause.11 A positive linear relationship with other risk factors for MASLD and cardiometabolic disease is present in both sexes and includes BMI, low-density lipoprotein (LDL) cholesterol, triglyceride levels, and insulin resistance.12
MALSD is the most common cause of chronic liver disease found in children ages 2 to 19 years.13 From the first case of NAFLD in a child reported in 1983 to the last large systematic review and meta-analysis (2015) focused on the pediatric population, the estimated worldwide prevalence of MASLD is at least 7.6% in the general pediatric population and 34% in the pediatric population with obesity. Autopsy results reflect up to 38% MASLD prevalence in the pediatric population with obesity. Prevalence increases with age, with the most common time of diagnosis during puberty (between 11 and 13 years of age).13
With increases in childhood obesity and more than two-thirds of adults currently being overweight or obese, the burden of MASLD and its complications unfortunately will increase over the next generation. This likely will make MASH and its complications the next obesity-related epidemic, putting many more people at risk for developing cirrhosis, hepatocellular cancer, other cancers, and cardiovascular disease.2,14
There are some identifiable genetic variances that play a role in the development and progression of MASLD and MASH, and the progression to fibrosis or cirrhosis. Some are known to confer a predisposition for or protection from MASLD, MASH, and fibrosis. Identifying these genetic variances enables potential therapeutic exploitation and the evaluation of dietary effects on expression.15-18 (See Table 2.)
Table 2. Genetic Polymorphism Associated with MASH15-17 |
||
Genetic Polymorphism |
Normal Function |
Outcome |
Patatin-like phospholipase domain-containing protein 3 (PNPLA3) |
Regulates intracellular lipid droplet metabolism |
Reduces lipidation of VLDL/steatosis, increases risk of cirrhosis and hepatocellular carcinoma |
Transmembrane 6, superfamily member 2 (TM6F2) |
Endoplasmic reticulum protein, involved in liver fat metabolism |
Increases hepatocyte content and VLDL, increases risk of cirrhosis and hepatocellular carcinoma |
Glucokinase regulatory protein (GCKR) |
Involved with regulation of de novo lipogenesis, glycolysis |
Increases heptatic fat storage and reduces beta-oxidation/fat catabolism, enhances rate of glycolysis |
Membrane bound O-acyltransferase domain-containing 7 (MBOAT7) |
Remodeling of phosphatidylinositol (lipid and cell signaling) and membrane protein regulation |
Promotion of liver fibrosis independent of inflammatory mediators |
Hydroxysteroid 17-b dehydrogenase 13 (HSD17B13) |
Catalysis of redox reaction involved in lipid metabolism |
Loss of function mutation confers protection against liver disease progression |
MASH: metabolic dysfunction-associated steatohepatitis; VLDL: very low density lipoprotein |
||
There are ethnic differences in the rates of MASLD and its complications. MASLD has been observed to be higher in the Hispanic population in the United States and slightly lower in the Black American population compared to the non-Hispanic white population, but fibrosis prevalence is similar between ethnic groups.19
Despite Hispanics having high rates of MASLD, they are less likely to progress to advanced disease.19 Specifically, Hispanics who are homozygous for the patatin-like phospholipase domain-containing protein 3 rs73809 (PNPLA3) allele have twice the hepatic fat content. It also appears that Asians are more likely to experience advanced disease and complications from MASLD.19
Pathogenesis
The spectrum of MASLD can be steatosis in the liver to steatohepatitis, which has been linked to bridging fibrosis, cirrhosis, and hepatocellular cancer. MASLD involves abnormal lipid accumulation in hepatocytes and lipotoxicity to the parenchyma, leading to inflammation and, eventually, fibrosis. Ongoing tissue remodeling provides more opportunities for the development of cirrhosis and carcinoma.14 Excessive triglyceride synthesis in hepatocytes results from de novo lipogenesis and secondary consumption of a high-fat and -sugar diet. Insulin resistance promotes this process, since insulin has inhibitory effects on lipolysis and an enhancing effect on fatty acid storage.20
Researchers agree that there are genetic and environmental contributions to MASLD and multiple abnormal processes in play in this disease. This is a “multi-hit hypothesis” that appears to be most plausible. First, there is an increase in hepatic intracellular fat seen in insulin resistance, which contributes to more insulin resistance. This will increase the inflammatory response, which may lead to hepatocellular damage and further contribute to insulin resistance. The lipid oxidation and release of reactive oxidative species induce further damage. Later, ballooning and fibrosis can occur as part of the repair process.20-22 (See Figure 1.)
Figure 1. Pathogenesis of MASL/MASH22 |
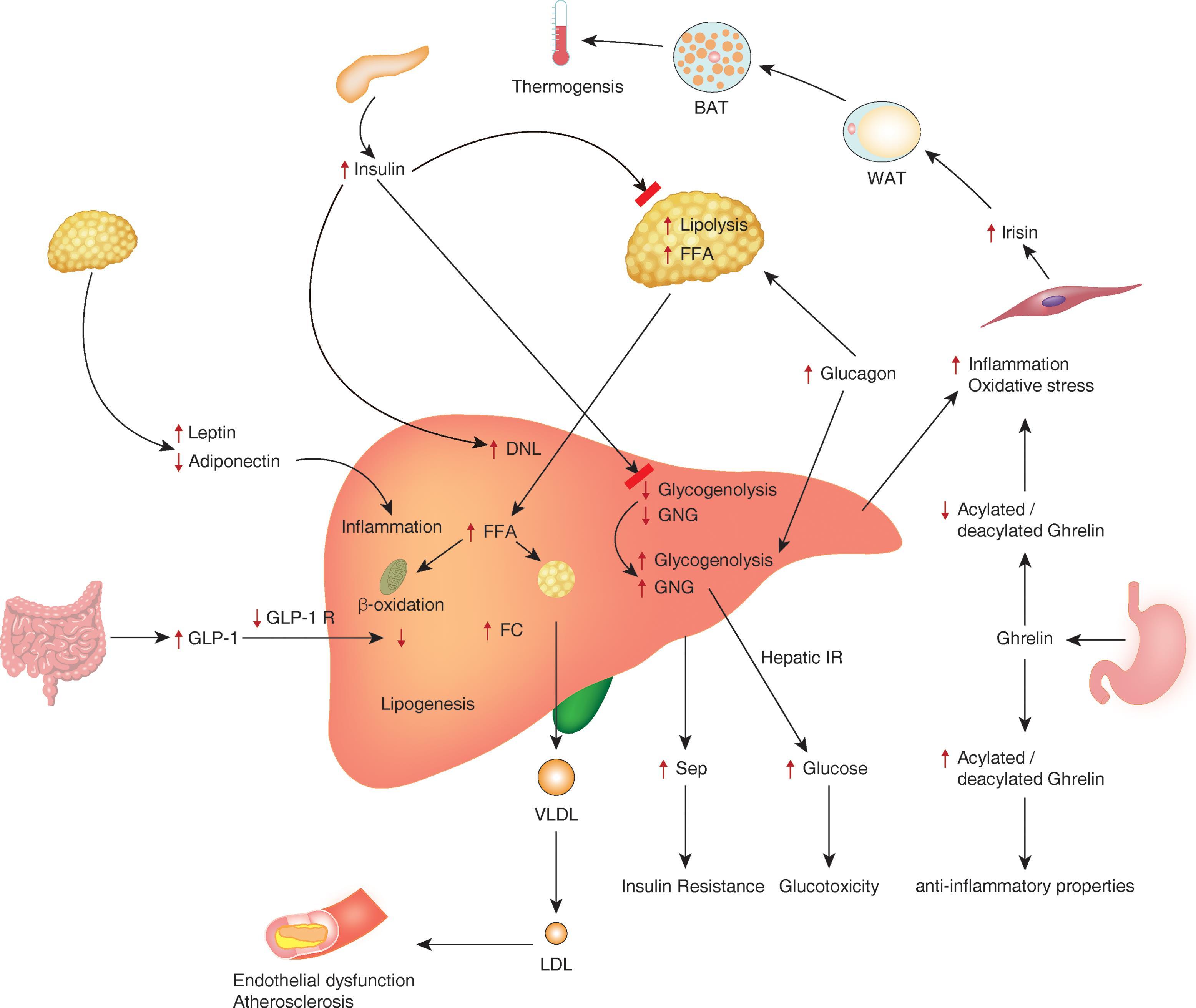 |
MASL: metabolic dysfunction-associated steatotic liver; MASH: metabolic dysfunction-associated steatohepatitis Source: Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic fatty liver disease: A clinical update. J Clin Transl Hepatol 2017;5:384-393. Reprinted with permission. |
Several new developments have been elucidated in the pathophysiology of MASLD. Now it is believed that for some people the initial development of MASLD can begin in utero.23 This particularly is true in mothers with obesity or those with T2DM.
The PNPLA3 allele is tied most closely to the development of MASLD and is seen in times of excessive food intake, particularly in Hispanic patients. Specifically, the presence of the PNPLA3 allele was associated with a higher likelihood of fibrosis.16
The gut microbiota and its metabolites also have been implicated as part of the multi-hit hypothesis of MASLD pathogenesis. Dysbiosis and impaired mucosal barrier function and their downstream inflammatory effects are being explored as contributors and possible therapeutic opportunities.24,25
Not all people diagnosed with MASH will progress to fibrosis and cirrhosis. It is estimated that up to 20% of these people actually will see a regression of their disease.26 However, 33% will progress to fibrosis and cirrhosis. The severity of fibrosis is the most significant predictor of advanced liver outcomes.26
Although there is a focus on liver-related complications, the main increased risk of death comes from cardiovascular disease and non-liver cancer.1 MASLD is a systemic disease and is a risk factor for other diseases, such as colorectal cancer, chronic kidney disease, cardiovascular disease, T2DM, and psoriasis.24
MASLD and Metabolic Syndrome
MASLD is a liver manifestation of the metabolic syndrome and most often develops in the presence of aberrant glucose and lipid metabolism. People with MASLD are more likely to have prediabetes or T2DM, polycystic ovarian syndrome (PCOS), and obstructive sleep apnea.23 People with T2DM typically have 80% more hepatic fat than those without diabetes, even when matched for sex, age, and weight. Further, they are two to four times more likely to experience fatty liver-induced complications.27
MASLD and T2DM
This risk of developing T2DM is increased almost five-fold in the presence of MASLD and the risk of progressing from prediabetes to T2DM is increased two- to three-fold.28,29
It has been more difficult to determine if those with T2DM have a higher risk of MASLD. However, there is an increased risk of chronic kidney disease, retinopathy, and increased insulin requirements in the setting of both MASLD and T2DM.30 The presence of type 2 diabetes in people who develop MASLD puts them at the highest risk of progression to liver fibrosis.26 The mortality from cirrhosis was increased significantly in those with MASLD and T2DM in one large cohort study.31
Mechanisms in MASLD and Cardiovascular Disease
Numerous trials have shown that MASLD is an independent predictor of adverse cardiovascular events. Shared mechanisms include increased production of inflammatory cytokines, endothelial dysfunction, and insulin resistance. In more severe hepatic and cardiovascular disease, an abundance of proinflammatory gram-negative gut microbiota have been observed.32
Natural History
Most people are asymptomatic early in the disease progression. Staging of MASLD is based on the percentage of hepatic steatosis. Less than 5% steatotic involvement is considered physiological. Pathologic steatosis is divided into three stages: 5% to 33% (mild), 35% to 66% (moderate), and > 66% (severe).21 Not all people with hepatic steatosis will have disease progression. When ongoing steatosis is present, especially in those with additional risk factors, the active inflammation can lead to hepatocyte injury and portal inflammation, leading to MASH, an increase in liver complications, and increased cardiovascular risk.
The progression rate is, on average, one stage per 14 years, but it is not yet predictable on an individual patient basis. MASH typically is staged by the level of fibrosis (F0-F4); it is the most accurate predictor of disease progression and the most important predictor of overall morbidity and mortality in MASH.33,34 A pictorial representation of this progress can be viewed in Figure 3 at https://www.gastrojournal.org/article/S0016-5085(12)00160-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
In a meta-analysis including 150 patients with MASLD, fibrosis progression occurred in 39%, 53% remained stable in fibrosis level, and 8% improved over a 14-year period. Approximately 25% of patients with MASH may develop severe fibrosis and cirrhosis. Like MASLD, MASH can have periods of stability, progression, or regression, with up to 27% of patients regressing in fibrosis level in a 14-year time span.34 A small percentage of individuals with MASLD will progress to cirrhosis (~ 3%), but that percentage becomes much higher (approximately 20% in two years) when F3 or F4 hepatic fibrosis is reached.35 Cardiovascular disease, non-hepatic cancers, and liver disease are the most common causes of death for those with MASH.36 F2 hepatic fibrosis needs to be confirmed, and patients with F3 and F4 hepatic fibrosis must be monitored for complications such as esophageal varices and hepatocellular carcinoma.35
Decompensation in the context of cirrhosis from MASLD is associated with worse outcomes than cirrhosis from other causes. This could be the result, in part, of MASLD being a multi-systemic disease wherein other organ systems already are compromised. Hepatocellular carcinoma is linked with MASLD in both cirrhotic and pre-cirrhotic stages.35
Risk Factors
MASLD is the hepatic manifestation of metabolic syndrome and is, thus, likely to be co-incident with cardiovascular disorders, obesity, insulin resistance, hypertension, dyslipidemia, and T2DM.1
Cholecystectomy may be a risk factor for MASLD. A study of 12,232 patients from the third U.S. National Health and Nutrition Examination Survey, 1988-1994, looked at patients who had ultrasonography for gallstone disease. Those with a history of cholecystectomy had a higher prevalence of MASLD than those with gallstones.37
Factors that are associated with progression to advanced fibrosis include male sex, age > 40 years, Caucasian ethnicity, myotonic dystrophy type 2 (DM2), hypertension, a lower hip-to-waist ratio, and also aspartate aminotransferase (AST)/ALT ratio > 1 and elevated gamma-glutamyl transferase (GGT).38 Class 3 obesity (BMI > 40) is strongly associated with MASLD. In patients undergoing bariatric surgery, histologic evidence of MASLD was as high as 90%, with 5% having unsuspected cirrhosis.39
Clinical Manifestations
The vast majority of patients with MASLD are asymptomatic. Patients with MASH are more likely to have complaints of fatigue and right upper abdominal discomfort. Other less specific complaints may be more common in patients with MASLD, including sleep disturbances, anxiety, thirst sensation, bloating, and bowel movement disturbances.40
On physical examination, signs of insulin resistance and liver disease may be observed. Features of insulin resistance may include lipodystrophic body fat distribution and acanthosis nigricans. Features of advanced liver disease may include hepatomegaly, splenomegaly, caput medusae, ascites, gynecomastia, spider angiomata, and palmar erythema.40
Screening, Stratification, and Diagnosis
Screening for advanced fibrosis in high-risk individuals now is a clear recommendation. The AASLD 2023 Practice Guidance recommends screening for advanced fibrosis in patients with T2DM, obesity with metabolic complications, a family history of cirrhosis, or significant alcohol use.40
The American Diabetes Association (ADA) provides guidance that adults with type 2 diabetes or prediabetes, particularly those with cardiometabolic risk factors or established disease, should be screened/risk-stratified for significant fibrosis using a calculated Fibrosis-4 (FIB-4) Index. Those individuals whose results are indeterminate or high-risk for significant liver fibrosis should have additional risk stratification by liver stiffness measurements using transient elastography or biomarker enhanced liver fibrosis measurement.41
Risk stratification with MAFLD starts with identifying high-risk individuals and applying noninvasive tests. (See Table 3.) Direct markers of liver metabolism interrupted by fibrosis and indirect markers from routine lab tests can be used in surrogate calculations. Although liver biopsy for the identification of fibrosis is the historical and current gold standard, its practicality as an assessment tool is limited by its invasiveness. Ultrasound alone, though often used, is limited in its sensitivity and cannot be used to rule out steatohepatitis.
Table 3. Noninvasive Tests in MASLD42 |
|||
NIT |
Low Risk |
Intermediate Risk |
High Risk |
FIB-4 |
< 1.3: FIB-4 every one to two years |
1.3 to 2.67: Consider referral to GI/hepatology and further stratify with ELF or VCTE |
≥ 2.67 or persistently elevated AST and AST: Refer to GI/hepatology care |
ELF |
< 7.7: FIB-4 every one to two years |
7.7 to 9.8: Refer to GI/hepatology care |
> 9.8: Refer to GI/hepatology care |
VCTE |
< 8.0: FIB-4 every one to two years |
8 to 12: Refer to GI/hepatology care |
> 12: Refer to GI/hepatology care |
*Consider candidacy for treatments with steatohepatitis benefit as appropriate |
*Consider candidacy for treatments with steatohepatitis benefit as appropriate |
||
MASLD: metabolic dysfunction-associated steatotic liver disease; NIT: noninvasive fibrosis test; FIB-4: Fibrosis-4 Index for Liver Fibrosis; ELF: enhanced liver fibrosis; AST: aspartate aminotransferase; GI: gastroenterology; VCTE: vibration-controlled transient elastography |
|||
Initial risk assessment in high-risk individuals (those with T2DM, medically complicated obesity, or a family history of significant cirrhosis) can be done with noninvasive tests. The FIB-4, which uses AST and age in the numerator and ALT and platelets in the denominator, is the most commonly used indirect noninvasive test to identify fibrosis risk. The screening should be done every one to two years for high-risk individuals with a low-range fibrosis risk score. The fibrosis risk ranges are low (< 1.3), indeterminate (1.3 to 2.66), and high (≥ 2.67). Patients in the indeterminate risk zone need further stratification and/or consideration of a referral to gastroenterology/hepatology specialty care.42
Calculation of the FIB-4 Score
The FIB-4 score uses the patient’s age, AST, ALT, and platelet count to estimate the level of fibrosis and helps determine whether a patient should undergo further testing, referral, and treatment.
Formula for FIB-4 Score
The FIB-4 score is calculated using the following formula:
FIB-4 score = age (years) × AST (U/L)/Platelet count (× 10^9/L) × [ALT (U/L)]
Where:
• Age is the patient’s age in years;
• AST is the AST level in units per liter (U/L);
• ALT is the ALT level in units per liter (U/L);
• Platelet count is the number of platelets in the blood, measured in 109/L.
Interpretation of FIB-4 Score
• FIB-4 score < 1.3: Low risk of advanced fibrosis (< 2.0 in those > 60 years of age)
• FIB-4 score 1.45 to 2.66: Indeterminate risk
• FIB-4 score ≥ 2.67: High risk of advanced fibrosis
Patient Cases
Case 1: Man with T2DM
Clinical Details
• Patient: 52-year-old male
• Medical history: T2DM, no history of liver disease
• Lab results: AST: 34 U/L; ALT: 29 U/L; Platelet count: 280 × 10^9/L
FIB-4 Calculation
52 (years) × 34 (U/L)/280 (× 10^9/L) × [29 (U/L)] =1.17
Clinical Interpretation: FIB-4 score < 1.45: Low risk of advanced fibrosis
Recommendation: Future screening: Repeat the FIB-4 score annually, since this patient does have a cardiometabolic risk factor (T2DM). Continue to manage cardiometabolic risk factor(s).
Case 2: Woman with History of PCOS and Multiple Comorbidities
Clinical Details
• Patient: 48-year-old female
• Medical history: PCOS, hypercholesterolemia, hypertension, BMI of 37
• Lab results: AST: 70 U/L; ALT: 55 U/L; Platelet count: 180 × 10^9/L
FIB-4 Calculation
48 (years) × 72 (U/L)/160 (× 10^9/L) × [55 (U/L)] = 2.52
Clinical Interpretation: FIB-4 score 1.45 to 3.25: Indeterminate risk of advanced fibrosis
Recommendation: Further evaluation: Given the indeterminate risk, it is advisable to perform a liver stiffness measurement using elastography (e.g., FibroScan), enhanced liver fibrosis (ELF) test, or magnetic resonance elastography (MRE). Based on the results, the patient may remain in the indeterminate risk category or be recategorized into the high-risk group. Close monitoring and lifestyle modifications (diet, exercise) and, possibly, intensified medical management are recommended to address obesity, PCOS, and hypertension.
Case 3: Man with Multiple Comorbidities and High FIB-4 Score
Clinical Details
• Patient: 60-year-old male
• Medical history: T2DM, hypertension, mixed hyperlipidemia, BMI of 39, obstructive sleep apnea
• Lab results: AST: 90 U/L; ALT: 70 U/L; Platelet count: 110 × 10^9/L
FIB-4 Calculation
60 (years) × 90 (U/L)/110 (× 10^9/L) × [70 (U/L)] = 5.87
Clinical interpretation: FIB-4 score > 3.25: High risk of advanced fibrosis
Further investigation and referral: A referral to hepatology is indicated. Advanced imaging (such as MRE) is strongly recommended to confirm the degree of fibrosis and to guide treatment. Aggressive management of the patient’s comorbidities (diabetes, hypertension, obesity, dyslipidemia) is crucial. A diagnostic work-up and management plan for liver-related outcomes and intensified management of comorbidities within an interdisciplinary team is necessary.
Further stratification can be done with vibration-controlled transient elastography (VCTE) or a direct noninvasive test such as ELF. Patients with indeterminate or high risk VCTE or ELF scores require specialty referral for increased NASH or cirrhosis risk.42 (See Figure 2.)
Figure 2. Recommended Screening for Advanced Fibrosis43 |
FIB-4: Fibrosis-4 Index for Liver Fibrosis; ELF: enhanced liver fibrosis Source: American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Care in Diabetes — 2024. Diabetes Care 2024;47(Supplement_1):S52-S76. Reprinted with permission of the American Diabetes Association, Inc. Copyright 2024. |
ELF uses three serum markers of matrix turnover to provide a fibrosis risk score (tissue inhibitor of matrix metalloproteinase-1, hyaluronic acid, and amino-terminal peptide of pro-collagen III). It has a predictive ability for liver-related outcomes, including hospitalization, death, and liver cancer, especially in those with risk factors including diabetes, obesity, and elevated ALT. 44
VCTE measures liver stiffness by measuring the velocity of a mechanical shear wave and the degree of steatosis with ultrasound. VCTE has been compared to histology and has been found reliable in delineating earlier and advanced fibrosis stages.44 VCTE also has been compared to MRE, which remains the most accurate but resource-intensive noninvasive imaging test.45
Several other noninvasive tests are available but are not currently recommended by the clinical guidelines for widespread use, including the indirect AST to Platelet Ratio Index (APRI) score; the hepatic steatosis index, which uses the ALT:AST ratio and considers BMI, sex, and diabetes status; and the NAFLD fibrosis score (which uses age, BMI, hyperglycemia, AST, ALT, platelets, and albumin). These noninvasive tests are evolving, but the risk-identification to FIB-4 to referral and blood biomarker/direct or VCTE-based stratification pathway currently is valid and evidence-based. Of note, FIB-4 is less sensitive in individuals younger than 35 years of age, so using other means of stratification, such as ELF and VCTE, initially should be considered if there is a high suspicion of fibrosis risk.46
Treatment
Treatment of MASLD and MASH should be multifaceted and always include lifestyle measures aimed at hepatic protection. The primary focus on lifestyle management for MASLD should be focused on weight loss and abstention from alcohol.
For those who are overweight or obese, a modest amount of weight loss (5%) can improve steatosis, but > 10% weight loss can improve MASH and fibrosis.47 Greater weight loss has been shown to reverse disease progression in those who have not yet reached cirrhosis. Many dietary patterns could be helpful, but the current recommendations focus on a Mediterranean-style diet centering on non-processed whole foods. The Mediterranean diet has been shown in multiple studies to provide liver-specific and cardiovascular health benefits even in the absence of weight loss.48-52 (See Table 4.)
Table 4. Dietary Plans and Effects on MASLD |
|||||
Dietary Plan |
Weight Loss |
Lower ALT |
Improves Insulin Resistance |
Improves MASLD (Biopsy) |
Improves MASH (Biopsy) |
Low-Calorie |
Yes |
Yes |
Yes |
Yes |
|
Low-Carbohydrate |
Yes |
Yes |
Yes |
Yes |
|
Low-Fat |
Yes |
Yes |
Yes |
Yes |
|
DASH |
Yes |
Yes |
Yes |
Yes |
|
Mediterranean |
Yes |
Yes |
|||
Intermittent Fasting |
Yes |
Yes |
|||
MASLD: metabolic dysfunction-associated steatotic liver disease; ALT: alanine transaminase; DASH: Dietary Approaches to Stop Hypertension |
|||||
Measures that are aimed at improving insulin sensitivity and reducing cardiovascular risk are applicable since insulin resistance is a driver in the pathogenesis of MASLD, and cardiovascular disease still is the most common cause of death in patients with MASLD. Diets high in ultra-processed foods/refined carbohydrates, saturated fats, and especially high sucrose-containing diets are associated with MASLD progression.53,54 Moderate to vigorous exercise is beneficial in decreasing new steatosis and improving chances of regression of steatosis.54
Pharmacologic Treatment Options
Resmetirom
Resmetirom, the first drug to receive FDA approval for treatment of MASH, was approved in March 2024. Resmetirom, a once-daily oral liver-directed thyroid hormone receptor beta-selective agonist, was studied in Phase III in an international multicentered, double-blind, randomized controlled trial for 966 adult patients.
At week 52, the primary endpoint of NASH/MASH resolution with no worsening of fibrosis of the MASLD activity score occurred in 26% of the 80-mg group and 30% of the 100-mg group, compared to 10% of the placebo group. Additionally, fibrosis decreased by one stage at both the 80-mg and 100-mg doses compared to placebo in patients with biopsy-confirmed MASH.56
The most common side effects were nausea and diarrhea. The incidence of severe adverse outcomes was similar among treatment and placebo groups.56 Resmetirom currently is approved for use in adults with stage F2 or F3 fibrosis MASH by biopsy or imaging-based diagnosis.57
Other treatments, many of which are used in the treatment of diabetes and/or obesity, have gained attention for their potential effectiveness in improving steatohepatitis. (See Figure 3.)
Figure 3. Therapeutic Options for MASLD/MASH in 202455 |
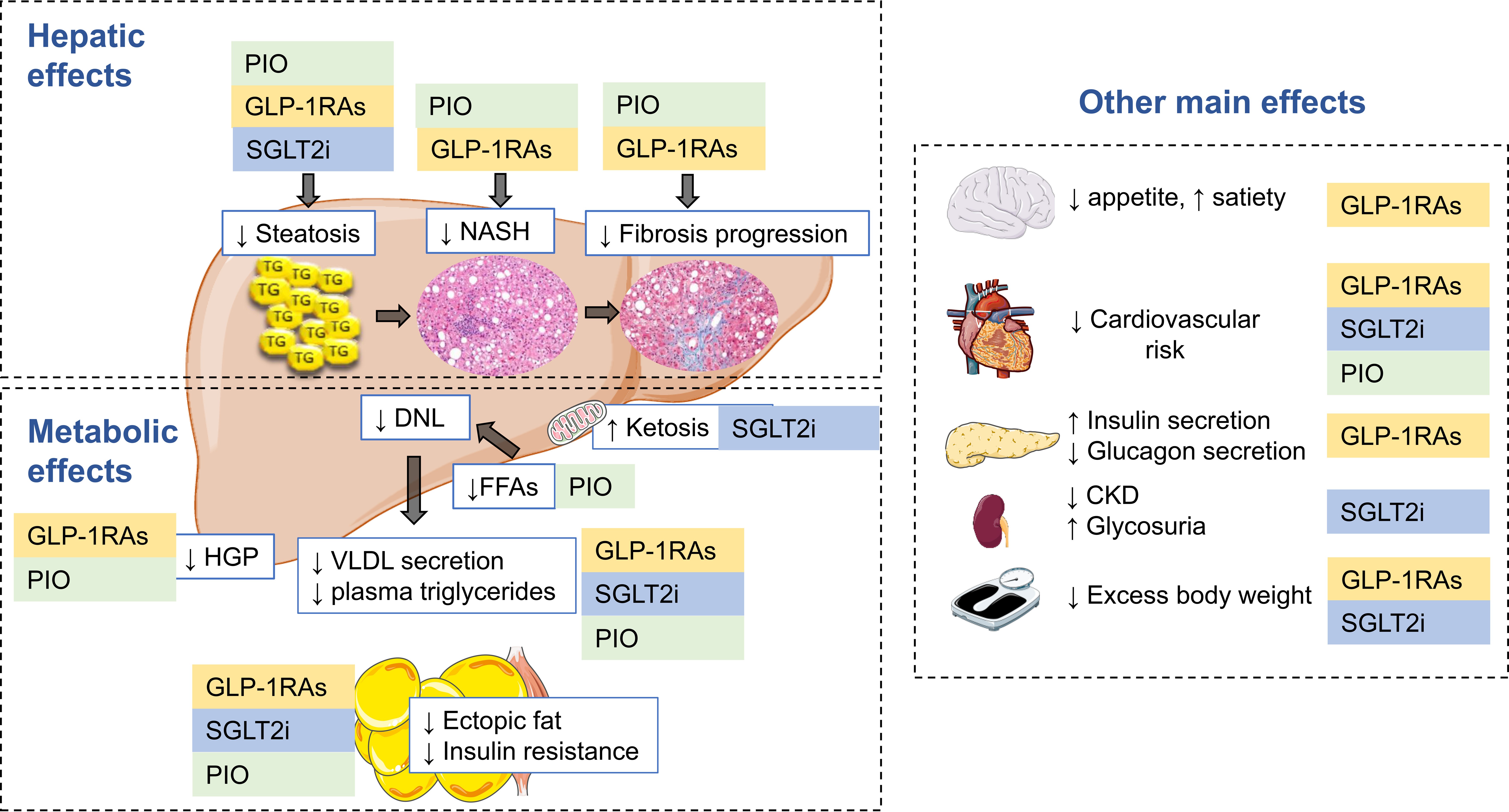 |
MASL: metabolic dysfunction-associated steatotic liver; MASH: metabolic dysfunction-associated steatohepatitis; PIO: pioglitazone; GLP-1RA: glucagon-like peptide-1 receptor agonist; SGLT2i: sodium-glucose cotransporter-2 inhibitor; NASH: nonalcoholic steatohepatitis; DNL: de novo lipogenesis; FFA: free fatty acids; HGP: hepatic glucose production; VLDL: very low density lipoprotein Source: Genua I, Cusi K. Pharmalogical approaches to nonalcoholic fatty liver disease: Current and future therapies. Diabetes Spectr 2024;37:48-58. Reprinted with permission of the American Diabetes Association, Inc. Copyright 2024. |
Pioglitazone
Pioglitazone is a peroxisome proliferator-activated receptor (PPAR) agonist that currently is used to treat T2DM. Pioglitazone was the first glucose-lowering medication shown to reverse MASH in a randomized controlled trial.58 Although it is not FDA approved for MASLD, it has been shown to reduce MASH and fibrosis.59-61
Pioglitazone improves steatohepatitis by improving glucose and lipid metabolism and reducing insulin resistance, reducing free fatty acid levels.62 Further, pioglitazone increases plasma adiponectin and reduces visceral fat, which contributes to reversing steatohepatitis.63 Clinically, pioglitazone reduces hemoglobin A1c (HbA1c) (1% to 1.5%), triglycerides (40%), ALT, AST, and gamma-glutamyl transferase (GGT).62 The pioglitazone dose that improved steatohepatitis is
30 mg to 45 mg.59-61
Potential side effects have limited the use of this medication. Pioglitazone can increase fluid retention and should not be used in patients with clinically significant heart failure. The most common side effects are weight gain and edema. The weight gain associated with pioglitazone often has been overestimated. It can lead to a dose-dependent weight gain of 1% to 2% at 15 mg/day and 3% to 5% at 45 mg/day.64 Cardiovascular risk was a concern in the past for thiazolidinediones after the release of a report in 2008.65 Pioglitazone has been shown to decrease cardiovascular risk, focused on the reduction of stroke risk in people with and without T2DM.66-68
As a result of these collective studies, pioglitazone now is recommended by the ADA 2023 standards of care to lower the risk of cerebrovascular events and myocardial infarction in people with a history of stroke.69 Finally, since pioglitazone has been shown to be effective in both preventing the progression of prediabetes to T2DM and stopping the progression of MASH, this would be an ideal agent to use in patients who have prediabetes.70
Vitamin E
Vitamin E has shown benefit in patients with MASH but without diabetes. A randomized controlled trial with vitamin E 800 IU/day demonstrated a 36% resolution in steatohepatitis compared to 21% with placebo (P = 0.05).71
A more recent randomized controlled trial with vitamin E in people with diabetes did not show benefit in the outcome of a > 2-point reduction in MASLD activity without worsening of fibrosis. At this point, vitamin E can be an option for those people without diabetes but is not recommended in those with diabetes.
Incretin-Based Agents (Semaglutide, Liraglutide, Tirzepatide)
Glucagon-like peptide-1 (GLP-1) agonists, including liraglutide and semaglutide, have been shown to improve steatosis. Semaglutide may slow the progression of fibrosis and has indications for both diabetes and weight loss.72,73 In a randomized controlled trial of liraglutide, 48 weeks of treatment yielded a 39% improvement in MASH both in people with and without diabetes.74 Semaglutide showed a 59% resolution of MASH at 72 weeks.74 These agents are beneficial through multiple pathways — largely by weight loss, but they do not directly affect hepatocytes. Neither of these agents improved fibrosis, but fewer people had fibrosis progression.
The GLP-1/glucose-dependent insulinotropic polypeptide (GIP) receptor agonist tirzepatide has been shown to reduce liver fat content and the volume of visceral adipose tissue. In the SURPASS-3 trial, all doses of tirzepatide significantly reduced magnetic resonance imaging measured visceral fat (P < 0.001) by 40% to 47% compared to 11% with placebo.75
SGLT2 Inhibitors
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, which have indications in T2DM, heart failure, atherosclerotic cardiovascular disease, and chronic kidney disease, also have shown benefit in MASLD.76
A randomized controlled trial using dapagliflozin and empagliflozin has been associated with significant MASLD regression.77-80 The benefits have shown a reduction in liver steatosis, but it is unknown if they improve liver histologic changes.
Future Agents
There are a number of agents currently being investigated for the prevention and treatment of MASLD/MASH. These include a pan-peroxisome proliferator-activated receptor (PPAR) agonist (lanifibranor), glucagon agonists, and a triple incretin receptor agonist (GLP-1, GIP, glucagon) (retruatide), as well as fibroblast growth factor 21 analogs.56
Metabolic Surgery
Metabolic surgery is another effective treatment option for MASLD regarding reducing histologic progression and reducing adverse outcomes, as well as positively affecting common comorbidities, including obesity and T2DM.
A retrospective cohort study of 1,158 patients with fibrosis stages 1-3 (650 patients who underwent bariatric surgery and 508 patients in the non-surgical group) revealed that bariatric surgery was associated with significantly lower rates of major adverse liver outcomes (-12.4%) and major adverse cardiovascular events (-13.9%) over 10 years. There were rare surgical complications leading to death within the first year (four patients [0.6%]).80
In a multicenter, open-label, randomized trial, 288 patients were assigned in a 1:1:1 fashion to lifestyle modification plus best medical care, Roux-en-Y gastric bypass, histological resolution of NASH without worsening of fibrosis, or sleeve gastrectomy. The primary endpoint was met in 54 (70%) of 77 participants in the Roux-en-Y gastric bypass group and 55 (70%) of 79 participants in the sleeve gastrectomy group, compared with 15 (19%) of 80 in the lifestyle modification group (P < 0.0001).
Ten patients (6% total) had adverse events in the two bariatric-metabolic surgery groups and required medical or endoscopic intervention; there were no deaths.81
An inpatient sample analysis of 45,462 patients diagnosed with NAFLD and morbid obesity from 2004 to 2012 compared outcomes of patients with obesity and MASLD who had undergone bariatric surgery with patients who had not. Among them, 18,618 patients (41.0%) had prior bariatric surgery. The primary outcome, in-hospital mortality, was significantly lower in patients with prior bariatric surgery compared to those without (incidence risk ratio = 0.08; 95% confidence interval, 0.03-0.20; P < 0.001). Additionally, prior bariatric surgery was associated with reduced incidence risk ratios for cirrhosis, myocardial infarction, stroke, and renal failure (all P < 0.001). 82
Metabolic surgery can be effective for patients with MASLD in preventing adverse outcomes over time and in the acute setting, and may be more effective for certain patients than lifestyle or medical interventions.
Overall Goals of Therapy
The treatment approach must be multifactorial to be successful in this silent but serious chronic disease. First, when used in high-risk patients, evidence-based screening with noninvasive tests, such as FIB-4, will identify people eligible for early intervention for liver-focused complications. For those with a high-risk FIB-4 score, immediate referral to hepatology is suggested. (See Table 5.) Secondary testing is needed for those with indeterminate risk scores: either an ELF blood test or VCTE. If this testing results in indeterminate or high risk, referral to hepatology is suggested.
Table 5. Treatments Based on Risk Scoring42 |
||
Risk Score |
Low risk: FIB-4 1.3 (or less than 2.0 for age 60 years or older) OR LSM < 8.0 kPA |
Indeterminate/high risk: FIB-4 > 1.3 (age 60 years or younger) or > 2.0 (age 60 years or older) OR LSM > 8.0 kPA |
Primary Management |
Primary care, diabetology, endocrinology |
Primary care, diabetology, endocrinology + hepatology |
Primary Treatment Goal |
Weight loss (lifestyle, pharmacologic, or metabolic surgery) |
Weight loss (structured programs plus all options for low-risk) |
Secondary Goal |
Cardiovascular risk reduction (evidence-based care of diabetes, hypertension, dyslipidemia) |
Primary care, diabetology, and endocrinology focus on cardiovascular risk and hepatology focus on liver-related complications |
Specific Pharmacotherapy |
No |
Resmetirom for stage F2; for patients with diabetes, consider GLP-1RA, pioglitazone, and SGLT-2 |
MASLD: metabolic dysfunction-associated steatotic liver disease; FIB-4: Fibrosis Index-4; LSM: liver stiffness measurement; GLP-1RA: glucagon-like peptide-1 receptor agonists; SGLT-2: sodium-glucose cotransporter-2 |
||
If the person has a low-risk FIB-4 or secondary test, the focus should be on reducing overweight/obesity-related comorbidities and cardiovascular risk. As a component of the cardiometabolic syndrome, patient education is key in that truncal/visceral obesity and excessive intake of processed foods and fructose can have adverse effects on the liver. A Mediterranean dietary plan and regular physical activity have shown benefits. The most impactful treatment is weight loss in these patients, and > 5 % weight loss should be the first priority.
There is a new FDA-approved treatment for early-stage MASH. Multiple diabetes agents have been shown to benefit MASLD/MASH and are recommended by all the major MASLD guidelines. Now that those who are high-risk can be identified, there are affordable screening tests, and there is clarity in treatment algorithms, what is preventing us from stopping MASLD/MASH?
Conclusions
MASLD is a growing public health issue. Advancements in noninvasive testing aid in identifying patients at risk for fibrosis and cirrhosis in time for disease course-modifying intervention. The new nomenclature can help clinicians see MASLD for what it is: a disease of fatty acid metabolism and ectopic storage driven by insulin resistance and a risk factor for cardiovascular morbidity and mortality.
Although lifestyle measures remain a cornerstone of treatment, there is hope for more robust and clinically meaningful treatment of steatohepatitis and fibrosis from current diabetes medications and the newly FDA-approved medication resmetirom.
References
- Younossi ZM, Golabi P, Paik JM, et al. Global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023;77:1335-1347.
- Chan WK, Chuah KH, Rajaram RB, et al. Metabolic dysfunction-associated steatotic liver disease (MASLD): A state-of-the-art review. J Obes Metab Syndr 2023;32:197-213.
- Allen AM, Charlton M, Cusi K, et al. Guideline-based management of metabolic dysfunction-associated steatotic liver disease in the primary care setting. Postgrad Med 2024;136:229-245.
- Kanwal F, Neuschwander-Tetri BA, Loomba R, Rinella ME. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024;79:1212-1219.
- Kasper P, Martin A, Lang S, et al. NAFLD and cardiovascular diseases: A clinical review. Clin Res Cardiol 2021;110:921-937.
- Wong VW, Wong GL, Chan RS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349-1356.
- Semmler G, Balcar L, Wernly S, et al. Insulin resistance and central obesity determine hepatic steatosis and explain cardiovascular risk in steatotic liver disease. Front Endocrinol (Lausanne) 2023;14:1244405.
- Quek J, Chan KE, Wong ZY, et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2023;8:20-30.
- Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184:2537-2564.
- Battistella S, D’Arcangelo F, Grasso M, et al. Liver transplantation for non-alcoholic fatty liver disease: Indications and post-transplant management. Clin Mol Hepatol 2023;29(Suppl):S286-S301.
- Balakrishnan M, Patel P, Dunn-Valadez S, et al. Women have a lower risk of nonalcoholic fatty liver disease but a higher risk of progression vs men: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;19:61-71.e15.
- Nagral A, Bangar M, Menezes S, et al. Gender differences in nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol 2022;12(Suppl 1):S19-S25.
- Goldner D, Lavine JE. Nonalcoholic fatty liver disease in children: Unique considerations and challenges. Gastroenterology 2020;158:1967-1983.e1.
- Phoolchund AGS, Khakoo SI. MASLD and the development of HCC: Patho-genesis and therapeutic challenges. Cancers (Basel) 2024;16:259.
- Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018;68:268-279.
- Mahdessian H, Taxiarchis A, Popov S, et al. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proc Natl Acad Sci U S A 2014;111:8913-8918.
- Jonas W, Schürmann A. Genetic and epigenetic factors determining NAFLD risk. Mol Metab 2021;50:101111.
- Pirola CJ, Garaycoechea M, Flichman D, et al. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. J Lipid Res 2019;60:176-185.
- Iqbal U, Perumpail BJ, Akhtar D, et al. The epidemiology, risk profiling and diagnostic challenges of nonalcoholic fatty liver disease. Medicines (Basel) 2019;6:41.
- Guo X, Yin X, Liu Z, Wang J. Non-alcoholic fatty liver disease (NAFLD) pathogenesis and natural products for prevention and treatment. Int J Mol Sci 2022;23:15489.
- Tilg H, Adolph TE, Moschen AR. Multiple parallel hits hypothesis in nonalcoholic fatty liver disease: Revisited after a decade. Hepatology 2021;73:833-842.
- Pappachan JM, Babu S, Krishnan B, Ravindran NC. Non-alcoholic fatty liver disease: A clinical update. J Clin Transl Hepatol 2017;5:384-393.
- Doycheva I, Ehrmann DA. Nonalcoholic fatty liver disease and obstructive sleep apnea in women with polycystic ovary syndrome. Fertil Steril 2022;117:897-911.
- Mikolasevic I, Milic S, Wensveen TT, et al. Nonalcoholic fatty liver disease — a multisystem disease? World J Gastroenterol 2016;22:9488-9505.
- Ji Y, Yin Y, Li Z, Zhang W. Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD). Nutrients 2019;11:1712.
- Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559-1569.
- Non-alcoholic Fatty Liver Disease Study Group; Lonardo A, Bellentani S, Argo CK, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis 2015;47:997-1006.
- Kim Y, Chang Y, Ryu S, et al. NAFLD improves risk prediction of type 2 diabetes: With effect modification by sex and menopausal status. Hepatology 2022;76:1755-1765.
- Cusi K. Time to include nonalcoholic steatohepatitis in the management of patients with type 2 diabetes. Diabetes Care 2020;43:275-279.
- Hazlehurst JM, Woods C, Marjot T, et al. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016;65:1096-1108.
- de Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care 1999;22:756-761.
- Huang DQ, Downes M, Evans RM, et al. Shared mechanisms between cardiovascular disease and NAFLD. Semin Liver Dis 2022;42:455-464.
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-397.e10.
- Lekakis V, Papatheodoridis GV. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med 2024;122:3-10.
- Loomba R, Adams LA. The 20% rule of NASH progression: The natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology 2019;70:1885-1888.
- Yanai H, Adachi H, Hakoshima M, et al. Metabolic-dysfunction-associated steatotic liver disease – its pathophysiology, association with atherosclerosis and cardiovascular disease, and treatments. Int J Mol Sci 2023;24:15473.
- Kichloo A, Solanki S, Haq KF, et al. Association of non-alcoholic fatty liver disease with gallstone disease in the United States hospitalized patient population. World J Gastrointest Pathophysiol 2021;12:14-24.
- Parameswaran M, Hasan HA, Sadeque J, et al. Factors that predict the progression of non-alcoholic fatty liver disease (NAFLD). Cureus 2021;13:e20776.
- Soresi M, Cabibi D, Giglio RV, et al. The prevalence of NAFLD and fibrosis in bariatric surgery patients and the reliability of noninvasive diagnostic methods. Biomed Res Int 2020;2020:5023157.
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-1835.
- American Diabetes Association Professional Practice Committee. 4. Comprehensive medical evaluation and assessment of comorbidities: Standards of Care in Diabetes — 2024. Diabetes Care 2024;47 (Suppl 1):S52-S76.
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-1669.
- Cusi K, Budd J, Johnson E, Shubrook JH. Making sense of the nonalcoholic fatty liver disease clinical practice guidelines: What clinicians need to know. Diabetes Spectr 2024;37:29-38.
- Saarinen K, Färkkilä M, Jula A, et al. Enhanced liver fibrosis test predicts liver-related outcomes in the general population. JHEP Rep 2023;5:100765. Erratum in: JHEP Rep 2023;5:100884.
- Nogami A, Yoneda M, Iwaki M, et al. Diagnostic comparison of vibration-controlled transient elastography and MRI techniques in overweight and obese patients with NAFLD. Sci Rep 2022;12:21925.
- Sanyal AJ, Castera L, Wong VWS. Noninvasive assessment of liver fibrosis in NAFLD. Clin Gastroenterol Hepatol 2023;21:2026-2039.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367-378.e5; quiz e14-15.
- Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36:1470-1478.
- Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol 2019;71:379-388.
- Tsaban G, Yaskolka Meir A, Rinott E, et al. The effect of green Mediterranean diet on cardiometabolic risk; a randomised controlled trial. Heart 2021;107:1054-1061.
- Properzi C, O’Sullivan TA, Sherriff JL, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: A randomized controlled trial. Hepatology 2018;68:1741-1754.
- Viveiros K. The role of life style modifications in comprehensive non-alcoholic fatty liver disease treatment. Clin Liver Dis (Hoboken) 2021;17:11-14.
- Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J Hepatol 2018;68:1063-1075.
- Sung KC, Ryu S, Lee JY, et al. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol 2016;65:791-797.
- Genua I, Cusi K. Pharmacological approaches to nonalcoholic fatty liver disease: Current and future therapies. Diabetes Spectr 2024;37:48-58.
- Harrison SA, Bedossa P, Guy CD, et al.; MAESTRO-NASH Investigators. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis.
N Engl J Med 2024;390:497-509. - Rezdiffra prescribing information. March 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217785s000lbl.pdf
- Zhao Y, Zhao W, Wang H, et al. Pioglitazone on nonalcoholic steatohepatitis: A systematic review and meta-analysis of 15 RCTs. Medicine (Baltimore) 2022;101:e31508.
- Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297-2307.
- Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: A randomized trial. Ann Intern Med 2016;165:305-315.
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-1685.
- DeFronzo RA, Inzucchi S, Abdul-Ghani M, Nissen SE. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diab Vasc Dis Res 2019;16:133-143.
- Gastaldelli A, Sabatini S, Carli F, et al. PPAR-γ-induced changes in visceral fat and adiponectin levels are associated with improved steatohepatitis in patients with NASH. Liver Int 2021;41:2659-2670.
- Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: A 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000;23:1605-1611.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356(24):2457-2471. Erratum in: N Engl J Med 2007;357:100.
- Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321-1331.
- Zhou Y, Huang Y, Ji X, et al. Pioglitazone for the primary and secondary prevention of cardiovascular and renal outcomes in patients with or at high risk of type 2 diabetes mellitus: A meta-analysis. J Clin Endocrinol Metab 2020;105:dgz252.
- Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005;366:1279-1289.
- ElSayed NA, Aleppo G, Aroda VR, et al. 3. Prevention or delay of type 2 diabetes and associated comorbidities: Standards of Care in Diabetes — 2023. Diabetes Care 2023;46(Suppl 1):S41-S48.
- DeFronzo RA, Tripathy D, Schwenke DC, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med 2011;364:1104-1115.
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-1685.
- Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-690.
- Newsome PN, Buchholtz K, Cusi K, et al; NN9931-4296 Investigators. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 2021;384:1113-1124.
- Flint A, Andersen G, Hockings P, et al. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2021;54:1150-1161.
- Gastaldelli A, Cusi K, Fernández Landó L, et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol 2022;10:393-406
- Jang H, Kim Y, Lee DH, et al. Outcomes of various classes of oral antidiabetic drugs on nonalcoholic fatty liver disease. JAMA Intern Med 2024;184:375-383.
- Latva-Rasku A, Honka MJ, Kullberg J, et al. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: A randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care 2019;42:931-937.
- Kahl S, Gancheva S, Straßburger K, et al. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: A randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care 2020;43:298-305.
- Gaborit B, Ancel P, Abdullah AE, et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc Diabetol 2021;20:57.
- Aminian A, Al-Kurd A, Wilson R, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA 2021;326:2031-2042.
- Verrastro O, Panunzi S, Castagneto-Gissey L, et al. Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): A multicentre, open-label, randomised trial. Lancet 2023;401:1786-1797.
- McCarty TR, Echouffo-Tcheugui JB, Lange A, et al. Impact of bariatric surgery on outcomes of patients with nonalcoholic fatty liver disease: A nationwide inpatient sample analysis, 2004-2012. Surg Obes Relat Dis 2018;14:74-80.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic metabolic disease that you may never have heard of. MASLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is the most prevalent liver disease worldwide. MASLD affects 30% of the world’s population, more than half of those people with obesity, and more than 70% of people with type 2 diabetes. While many clinicians may see patients with slightly elevated transaminases and assume it is fatty liver, MASLD is not benign and often begins well before laboratory changes. This article reviews the pathogenesis, diagnosis, and natural history of MASLD; known treatments; and future therapies.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
