
Managing Pott’s Puffy Tumor and Sinogenic or Otogenic Intracranial Empyema
Author
Trahern W. ("TW") Jones, MD, Assistant Professor, Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City
Peer Reviewer
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity Health Care, Samaritan, Troy, NY
Executive Summary
- Sinogenic infections are conceived as originating with rhinosinusitis, which typically arises because of viral infection, followed by bacterial superinfection and invasion. Rhinosinusitis itself is a very common, benign, and self-limited condition. Even with bacterial superinfection, rhinosinusitis usually does not require direct procedural intervention or antibiotic therapy.
- Indeed, prior studies looking at the microbiologic composition of abscesses and empyemas have found a predominance of viridans group streptococci, group A Streptococcus, Staphylococcus aureus, and Streptococcus pneumoniae, and, less commonly but still importantly, Fusobacterium species and Eikenella corrodens; more rarely, organisms such as Pseudomonas aeruginosa and Prevotella species also may be found.
- Headache and fever are nearly ubiquitous among patients presenting with sinogenic Pott’s puffy tumor and sinogenic intracranial epidural empyema. Additional symptoms may include facial pain, purulent rhinorrhea, malaise, and vomiting. As frontal bone osteomyelitis develops in Pott’s puffy tumor, patients may experience boggy, tender swelling over the forehead, consistent with sympathetic soft tissue inflammation and edema caused by the underlying infection and subperiosteal abscess formation.
- Prior to starting antibiotics, practitioners should attempt to obtain blood cultures to evaluate for bacteremia. Additional serum studies to obtain include a complete blood cell count and differential (which will provide a baseline for the degree of leukocytosis and thrombocytosis, both of which could be elevated), a complete metabolic panel (to evaluate renal and liver function prior to initiating antibiotics), erythrocyte sedimentation rate, and C-reactive protein (which often will be monitored through the ensuing course of antibiotic therapy, and which can be helpful in determining if new intracranial complications have developed).
- When radiologic data and clinical concern suggest Pott’s puffy tumor or sinogenic/otogenic intracranial empyema are present, providers should not hesitate to contact otorhinolaryngology, neurosurgery, and infectious disease consultation services.
- Typically, initial coverage consists of ceftriaxone (for broad-spectrum upper respiratory gram-negative and gram-positive coverage), vancomycin (for coverage of potential methicillin-resistant S. aureus or penicillin-resistant streptococci), and metronidazole (for coverage of upper respiratory anaerobic species). All three antibiotics achieve excellent cerebrospinal fluid drug levels.
- The overall course of therapy for Pott’s puffy tumor and sinogenic or otogenic intracranial infection requires close inpatient management, continued surgical evaluation, and appropriate long-term outpatient follow-up. Following drainage of any accessible infected fluid collections, empyemas, or abscesses, antibiotic therapy may range from four to six weeks or longer. Follow-up imaging at the end of therapy usually is recommended to ensure full resolution of any remaining abscesses or empyemas.
Following the COVID-19 pandemic, the incidence of sinogenic and otogenic intracranial empyemas increased. The author reviews the presentation, imaging, and treatment for this potentially life-threatening infection, with a reminder to keep this on your differential when evaluating your youngest patients.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Case
John is a previously healthy 15-year-old young man who presents with fever, headache, and swelling over the forehead. His mother reports that, about two weeks prior to presentation, he developed symptoms of a viral upper respiratory infection that included rhinorrhea, cough, and congestion. All the symptoms except for the congestion seemed to pass, but soon after, he began to develop persistent headaches. He has had worsening fevers over the past two to three days. This morning, his mother noted that his forehead appeared to have a boggy, “squishy” swelling in the center. He has felt increasingly worse today, but his mother specifically denies any seizure activity, word-finding difficulty, or confusion.
Upon initial evaluation in the emergency department, his temperature is 38.7°C. He is tired-appearing but in no acute distress. His forehead indeed has an edematous but nonfluctuant swelling in the center that is tender to palpation. He is alert and oriented to person, place, and time. All cranial nerves are intact, and his neurologic exam discloses no defects or abnormalities. His initial laboratory studies are significant for a white blood cell count of 17.6 k/μL with 81% neutrophils. His initial C-reactive protein is 15.1 mg/dL. A blood culture is obtained and pending.
Introduction
Pott’s puffy tumor and sinogenic or otogenic intracranial empyemas represent severe and life-threatening complications of bacterial rhinosinusitis or otomastoiditis. Such infections can lead to cerebritis, brain abscesses, and serious morbidity and mortality. Essentially, these disease entities are considered polymicrobial in nature, with constituent bacterial species assumed or demonstrated to consist of upper respiratory gram-positives, gram-negatives, and anaerobes. However, many cases seem to be driven mostly or in part by viridans streptococcal species, especially Streptococcus anginosus group bacteria. While antibiotic therapy is a mainstay of treatment, many cases will require surgical source control. Therefore, early involvement of otorhinolaryngologists, neurosurgeons, and infectious disease specialists is essential.
While Pott’s puffy tumor and sinogenic or otogenic intracranial empyemas have been described in the pediatric literature for many decades, recent upticks in their incidence following the COVID-19 pandemic have alerted pediatric providers to the hazards they pose and the overarching need for a systematic approach. This review will seek to provide the pediatric emergency medicine practitioner with a thorough background in the pathogenesis and epidemiology of such entities, their clinical manifestations and complications, and key aspects of their diagnosis and treatment.
Pathogenesis
To more accurately discuss the infections under consideration, the terminology of these conditions should be better clarified. “Rhinosinusitis” refers to inflammation (usually caused by viral or bacterial infection) of the nasal cavities and paranasal sinuses, including the frontal sinus. “Pott’s puffy tumor” was originally described in the 18th century by the accomplished London surgeon Sir Percivall Pott and refers specifically to frontal bone osteomyelitis with subperiosteal abscess formation.1 The word “tumor,” in this case, is somewhat misleading, as the entity is fundamentally caused by infection and inflammation rather than neoplasm. In this case “tumor” is quite literally the Latin word for “swelling.” “Sinogenic,” of course, refers to a paranasal sinus-based source of an infection, providing the practitioner a clue as to its microbiologic constituents. Likewise, “otogenic” refers to an infection arising from the middle ear and mastoid air cells. “Empyema” refers to a pus collection contained within a potential anatomic space (e.g., the subdural or epidural space). “Abscess” refers to a pus collection within the parenchyma of a solid organ (e.g., cerebral abscess). While it is not unusual to hear some of these terms used interchangeably or loosely (such as Pott’s puffy tumor when referring to frontal bone osteomyelitis without subperiosteal purulent fluid collection, or sinogenic epidural empyema where the infection has not yet induced a purulent response around the meninges), understanding the terminology helps the practitioner understand the pathophysiology of these infections.
Sinogenic infections are conceived as originating with rhinosinusitis, which typically arises because of viral infection followed by bacterial superinfection and invasion.1 Rhinosinusitis itself is a very common, benign, and self-limited condition. Even with bacterial superinfection, rhinosinusitis usually does not require direct procedural intervention or antibiotic therapy. Indeed, recent surveys estimate that nearly 30 million individuals in the United States may experience an episode of rhinosinusitis at least once per year, the vast majority of whom have no serious sequelae or complications.1
However, when such infections persist with bacterial growth and failure to drain according to normal anatomic pathways, the sinus tissues and surrounding bone will experience localized inflammation and venous congestion, followed by small vessel thrombosis.1
In turn, such conditions lead to increased intraosseous pressure and ischemia of the bony structures of the frontal sinus, inducing avascular necrosis of the trabecular matrix. With a polymicrobial mix of organisms persistently growing in the surrounding sinus cavity, conditions will thereafter become more favorable to opportunistic anaerobes, and the disrupted blood supply impairs the host immune response to infection. The infection subsequently will invade surrounding structures and the intracranial vault, leading to central nervous system complications. In some cases, emissary veins draining the paranasal sinuses may lead to hematologic spread of infection into the intracranial venous sinus system, skipping the calvarial bones altogether.2
Interestingly, such conditions are believed to be fostered more commonly in infection of the frontal sinus than others, such as the ethmoid sinuses, where vascular anastomoses compensate for any venous congestion and thrombosis due to localized inflammation.1
Aside from viral or bacterial rhinosinusitis, other predisposing conditions to frontal bone osteomyelitis and/or invasive sinogenic infections into the cranial vault include direct trauma to the forehead or sinuses, again leading to vascular congestion and potential disruption of normal sinus drainage.1 Other risk factors also include intranasal drug use (insufflation), diabetes mellitus, and odontogenic disease. Examples of comorbidities and clinical symptoms for Pott's puffy tumor can be found in Table 1.
Table 1. Clinical Characteristics of Patients with Pott's Puffy Tumor |
|||
| Comorbidity | Pediatric (n = 151) N (% Total) |
Adult (n = 170) N (% Total) |
P Value |
Sinusitis |
126 (83.4%) |
140 (82.3%) |
0.882 |
Head trauma |
23 (15.2%) |
32 (18.8%) |
0.459 |
Odontogenic disease |
11 (7.3%) |
26 (15.3%) |
0.034* |
Intranasal drug use |
1 (0.7%) |
20 (11.8%) |
< 0.001* |
Diabetes |
7 (4.6%) |
11 (6.5%) |
0.628 |
Rheumatologic disorder |
4 (2.6%) |
4 (2.4%) |
0.857 |
Obesity |
1 (0.7%) |
3 (1.8%) |
0.625 |
Malignancy |
0 (0.0%) |
1 (0.6%) |
1 |
None/unknown |
8 (5.2%) |
5 (2.9%) |
0.284 |
| Clinical Symptoms | Pediatric (n = 151) N (% Total) |
Adult (n = 170) N (% Total) |
P Value |
Forehead swelling |
108 (71.5%) |
131 (77.0%) |
0.305 |
Frontal headache |
110 (72.8%) |
79 (46.4%) |
0.001* |
Fever |
45 (29.8%) |
30 (17.6%) |
0.012* |
Periorbital edema |
83 (54.9%) |
31 (18.2%) |
< 0.001* |
Purulent or non-purulent rhinorrhea |
35 (23.2%) |
27 (15.9%) |
0.119 |
Nasal congestion |
41 (27.1%) |
16 (9.4%) |
< 0.001* |
Facial pain or pressure |
22 (14.6%) |
22 (12.9%) |
0.746 |
Mental status changes |
26 (17.2%) |
10 (5.9%) |
0.001* |
Vision changes |
15 (9.9%) |
13 (7.6%) |
0.554 |
Nausea and/or vomiting |
23 (15.2%) |
3 (1.7%) |
< 0.001* |
Sinocutaneous fistula |
3 (2.0%) |
20 (11.8%) |
0.008* |
Otic (effusion, mastoiditis) |
3 (2.0%) |
0 (0.0%) |
0.103 |
None/unknown |
4 (2.6%) |
42 (24.7%) |
< 0.001* |
*Clinically significant results Reprinted with permission from Rohde RL, North LM, Murray M, et al. Pott's puffy tumor: A comprehensive review of the literature. Am J Otolaryngol 2022;43:103529. |
|||
Otogenic infections and intracranial invasion develop in an analogous fashion. Otitis media may occur following the build-up of congestion and disruption of normal middle ear drainage in the context of recent viral illness. If the infection progresses before medical or surgical intervention can be undertaken, hyperemia and inflammation of the middle ear and mastoid air cell mucosa may follow, which triggers an exudative process and accumulation of pus within the implicated anatomic compartments.3 Normal anatomic drainage through the epitympanic recess of the middle ear subsequently is blocked by the thick, purulent material of this inflammatory process, and pressure builds within the mastoid air cells. As the local environment grows increasingly hypoxic and acidotic, the bony septa of the mastoid are decalcified, resorbed, and osteomyelitis may follow.3 Patients may develop subperiosteal abscess and adjacent tissue inflammation, leading to swelling, fluctuance, and tenderness over the mastoid process. From this stage, the infection may erode through the thin bone of the tegmen tympani, which normally serves as the barrier between the middle ear and the intracranial vault, leading to subsequent central nervous system involvement.4
Etiologic Agents
The origin of such infection provides a clue to the microbes driving their progression. Given the sinogenic etiology of Pott’s puffy tumor and epidural empyemas, opportunistic upper respiratory gram-positive, gram-negative, and anaerobic bacteria usually are implicated.
Indeed, prior studies looking at the microbiologic composition of abscesses and empyemas in such conditions have found a predominance of viridans group streptococci, group A Streptococcus, Staphylococcus aureus, and Streptococcus pneumoniae, and less commonly but still importantly, Fusobacterium species and Eikenella corrodens; more rarely, organisms such as Pseudomonas aeruginosa and Prevotella species also may be found.1,5-9 Overall, it should be fundamentally recognized that such infections are almost always polymicrobial, suggesting that broader empiric antibiotic coverage is reasonable at the outset of therapy.
In particular, the anginosus subgroup of viridans group streptococci has been observed to be more heavily implicated in recent years.10 While the anginosus group includes such species as Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius, the latter organism has most recently shown an exceptional propensity for more extensive, invasive, and aggressive abscess and empyema formation than its cousins. In addition, S. intermedius infection is believed to be more likely to lead to central nervous system invasion. The reasons for the anginosus group’s apparent increasing predilection for such infections is unknown, although certain hypotheses (more virulent strains, more antibiotic resistance) have been ruled out. There is no evidence for genetic relatedness of isolated species, nor is there any evidence for increasing antibiotic resistance in these organisms.11
Antibiotic therapy often is guided by microbiological culture data, which will be discussed later. However, it always should be noted that many anaerobic species that could be present in such infections are only poorly recovered (if recovered at all). Thus, providers always should assume there likely are more species present than are necessarily discovered in surgical cultures.
It also should be noted that skin flora, such as coagulase-negative staphylococci (i.e., Staphylococcus epidermidis), occasionally may find their way into operative cultures. These organisms are best treated as contaminants and almost never need to be considered when selecting antibiotic therapy.7
Epidemiology
Classically, sinogenic intracranial empyemas were thought to occur primarily among adolescent males.6,12-14 Prior cohorts and case series have noted that such patients typically are between 9 and 18 years of age. However, subsequent reports also have demonstrated that children as young as 6 years of age may be identified, with girls equally likely to be affected.5 Overall, the majority of all patients identified in case series and cohorts are 18 years of age or younger, although adults still are susceptible to this pattern of infection.1
Interestingly, the overarching number of cases reported in the medical literature during the past 40 years has been increasing, from a total of five adult and pediatric cases in 1979-1981 to 77 in 2018-2022. However, it is unclear if this phenomenon is because of increased recognition of the condition or another factor.
Both older and more recent datasets suggest that such intracranial complications and frontal bone osteomyelitis usually have been observed to follow a strong seasonal pattern, with cases peaking in the winter months.11,14 This phenomenon is believed to follow patterns of respiratory viral infections leading to typical rhinosinusitis, which provides the pathologic basis for genesis and intracranial extension of disease.
More recently, in 2021 and 2022, pediatric providers noted an apparent increase in cases of intracranial complications of rhinosinusitis and otomastoiditis.11,15 In June 2022, the Centers for Disease Control and Prevention asked providers to report possible cases of this disease process, and further analyzed nationally representative datasets for cases of pediatric brain abscesses and empyema. It was determined that, after a decrease in pediatric hospitalizations caused by these conditions in 2020 concurrent with the onset of the COVID-19 pandemic (and subsequent social distancing measures that led to an overall drop in many pediatric communicable diseases), cases of sinogenic and otogenic intracranial abscesses and empyemas began to rise in the summer of 2021 to a peak in March 2022. (See Figure 1.)
Figure 1. Pediatric Brain Abscesses, Epidural Empyemas, and Subdural Empyemas Associated with Streptococcus Species |
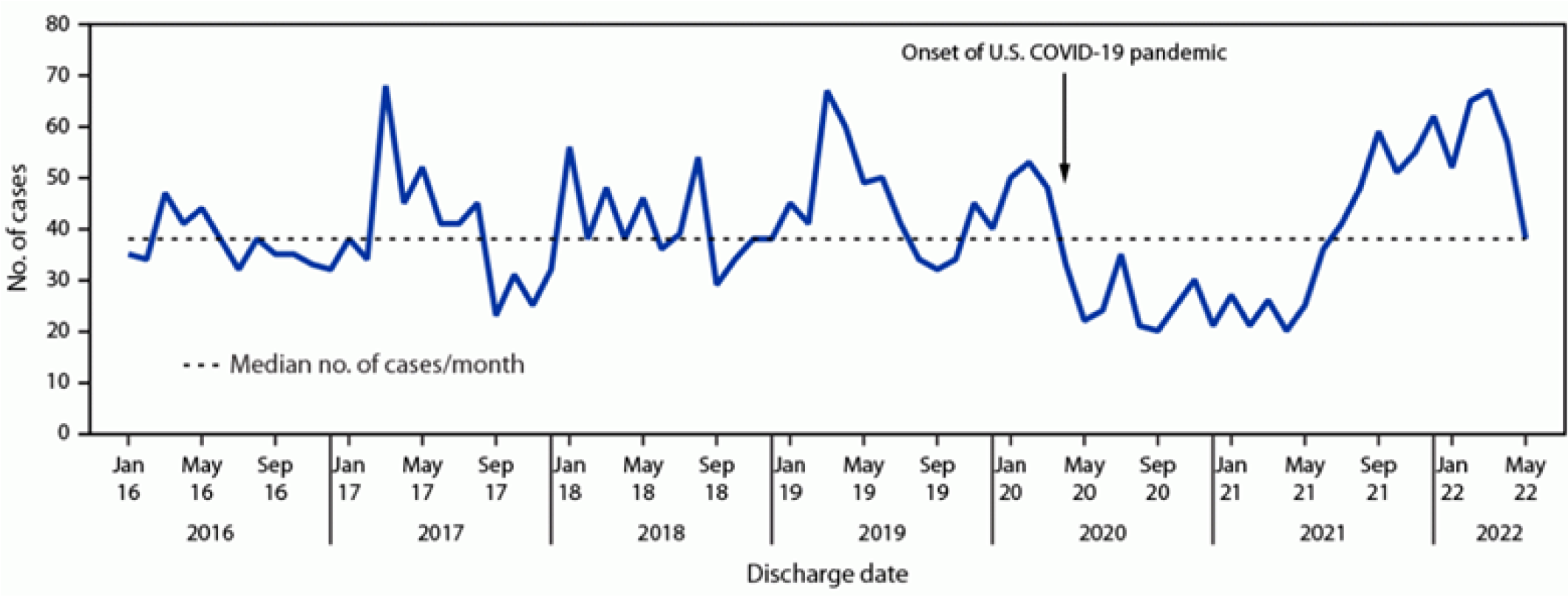 |
Source: Accorsi EK, Chochua S, Moline HL, et al. Pediatric brain abscesses, epidural empyemas, and subdural empyemas associated with Streptococcus species — United States, January 2016–August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1169-1173. |
However, this peak generally was consistent with historical, seasonal fluctuations of the disease, and the perceived increase in cases was believed to be merely an effect of suppression and redistribution of cases over time because of the COVID-19 pandemic and associated social distancing measures.11
Clinical Manifestations and Complications
Headache and fever are nearly ubiquitous among patients presenting with sinogenic Pott’s puffy tumor and sinogenic intracranial epidural empyema.2,5,16 Additional symptoms may include facial pain, purulent rhinorrhea, malaise, and vomiting. As frontal bone osteomyelitis develops in Pott’s puffy tumor, patients may experience boggy, tender swelling over the forehead, consistent with sympathetic soft tissue inflammation and edema caused by the underlying infection and subperiosteal abscess formation. (See Figure 2.) Patients with otomastoiditis may present with classic signs of otalgia, otorrhea, and proptosis of the ear on the affected side, as well as drainage and more nonspecific signs of fever and headache. (See Figure 3.) An algorithm to determine the symptoms of sinusitis and treatment can be seen in Figure 4.
Figure 2. Swelling Indicative of Pott's Puffy Tumor |
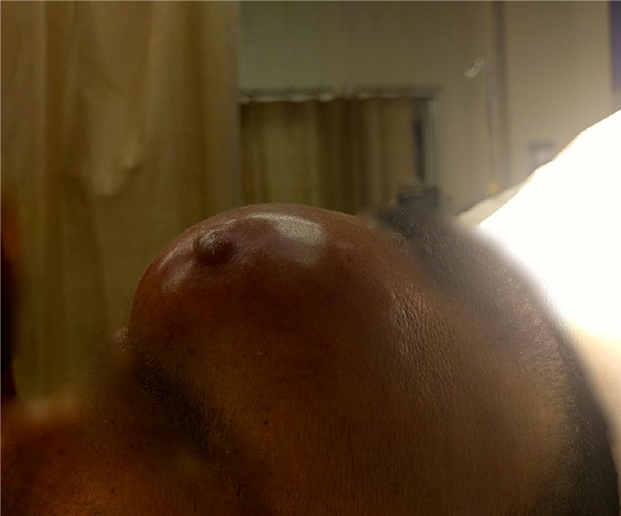 |
Source: Fythrion. Potts puffy tumor detail. Published Jan. 1, 2020. https://commons.wikimedia.org/wiki/File:Potts_puffy_tumor_detail.jpg CC BY-SA 4.0 |
Figure 3. Mastoiditis with Subperiostal Abscess |
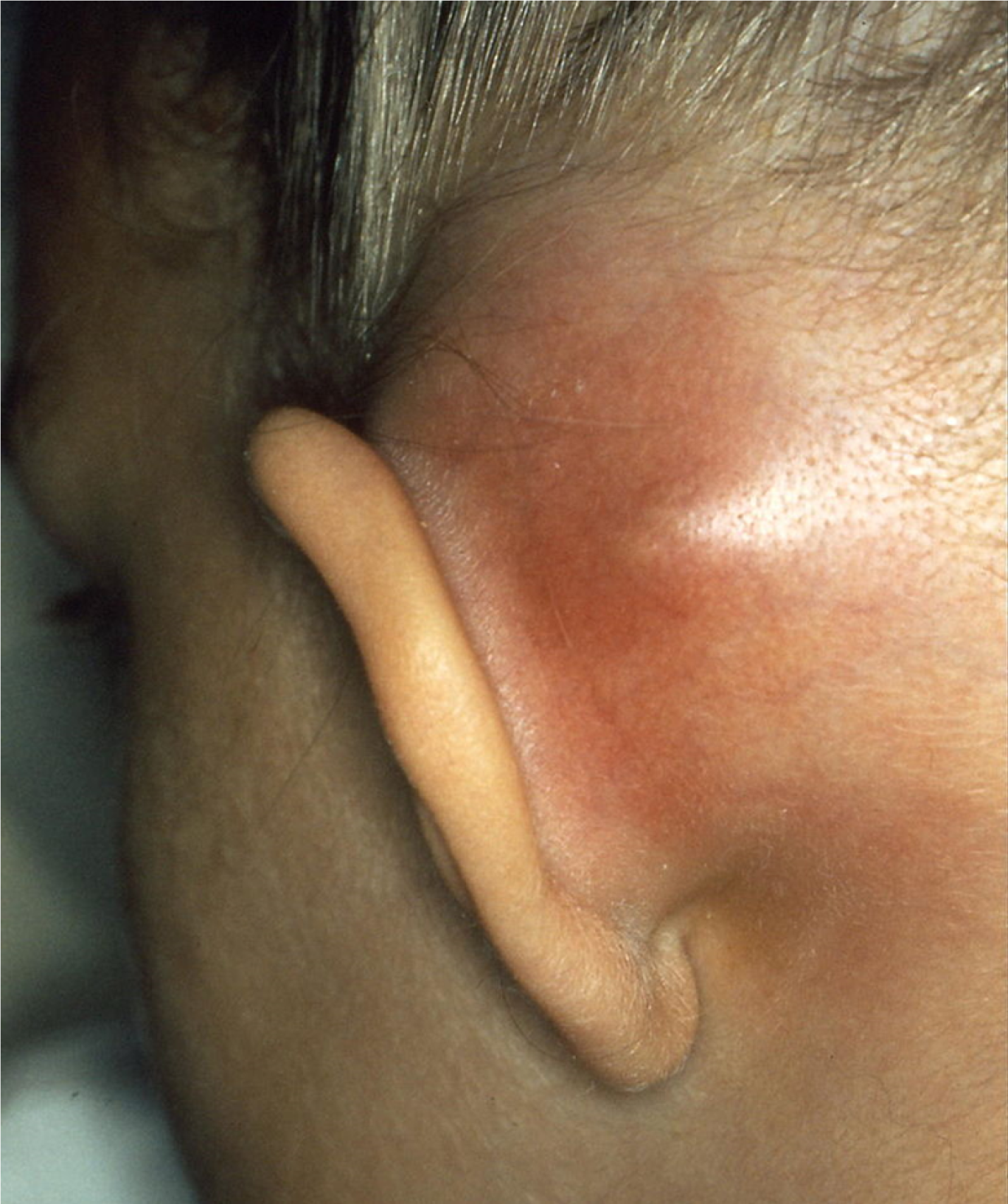 |
Source: Welleschik B. [Subperiosteal abscesses in mastoiditis]. Published December 2006. https://upload.wikimedia.org/wikipedia/commons/f/f9/Mastoiditis1.jpg CC BY-SA 3.0 |
Figure 4. Recommendations of a Clinical Advisory Committee on Pediatric Sinusitis |
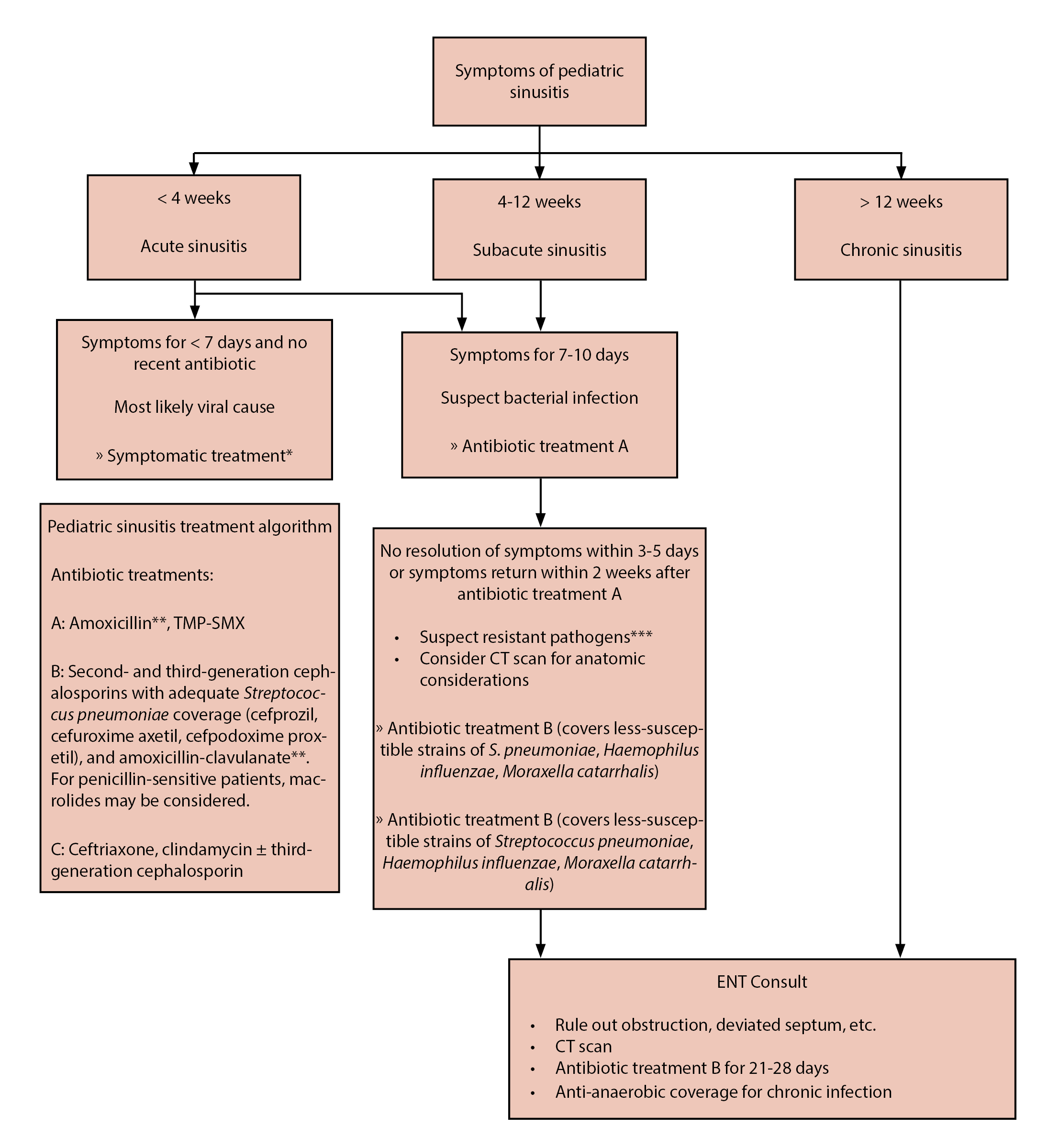 |
TMP-SMX: trimethoprim/sulfamethoxazole; CT: computed tomography *Topical or systemic decongestants, nonsteroidal anti-inflammatory drugs **In areas of high drug-resistant S. pneumoniae prevalence, amoxicillin dose should be increased ***Resistant pathogens suspected in children in day care, immunity-impared children, etc. Reprinted with permission from Brooks IB, Gooch 3rd WM, Reiner SA, et al. Medical management of acute bacterial sinusitis - Recommendations of a Clinical Advisory Committee on Pediatric and Adult Sinusitis. Ann Otol Rhinol Laryngol Suppl 2000;182:2-20. |
If and when central nervous system invasion occurs, practitioners should be attentive to signs and symptoms like neck stiffness and meningismus, photophobia, seizures, diplopia, numbness, limb weakness, clonus, hyperreflexia, altered mental status, aphasia, and obtundation.2,5 Indeed, specific central nervous system defects that could arise in such infections are contingent on specific foci of the cranial nerves or cerebrum that could be affected.
For example, word-finding difficulty could represent cerebritis or injury to the major language-processing centers of the brain in the dominant frontal or temporal lobes of the patient. Such signs could herald the development of meningitis or a mass-occupying abscess in the epidural space or the brain parenchyma, or cerebritis from adjacent suppuration and invasion. However, many patients with central nervous system invasion may display only nonspecific signs and symptoms, or they simply may experience a persistent headache and fever. In such situations, practitioners must maintain a high index of suspicion and consider further evaluation and imaging in those whose clinical presentation suggests nothing more than osteomyelitis.
Aside from the suppurative complications listed previously, providers also should be on the alert for intracranial vascular complications, such as infarction and venous sinus thrombosis, both of which are noted in case series as less common but serious sequelae of such infections.7,9
Diagnosis and Evaluation
Because of the risk of intracranial extension and the potentially nonspecific symptoms that could accompany empyema formation, abscess development, or cerebritis, practitioners should maintain a high index of suspicion for central nervous system complications. With this in mind, practitioners should obtain three-dimensional imaging for any patients presenting with fever, headache, and forehead swelling or ear proptosis. Contrast is essential for identifying rim-enhancing abscesses or empyemas. Computed tomography generally is favored in the initial characterization of mastoiditis (because of the better definition it provides when evaluating the structures of the temporal bone), while magnetic resonance imaging is preferable for identifying intracranial empyema and abscess formation. (See Figures 5 and 6.)
Figure 5. Pott's Puffy Tumor on Magnetic Resonance Imaging |
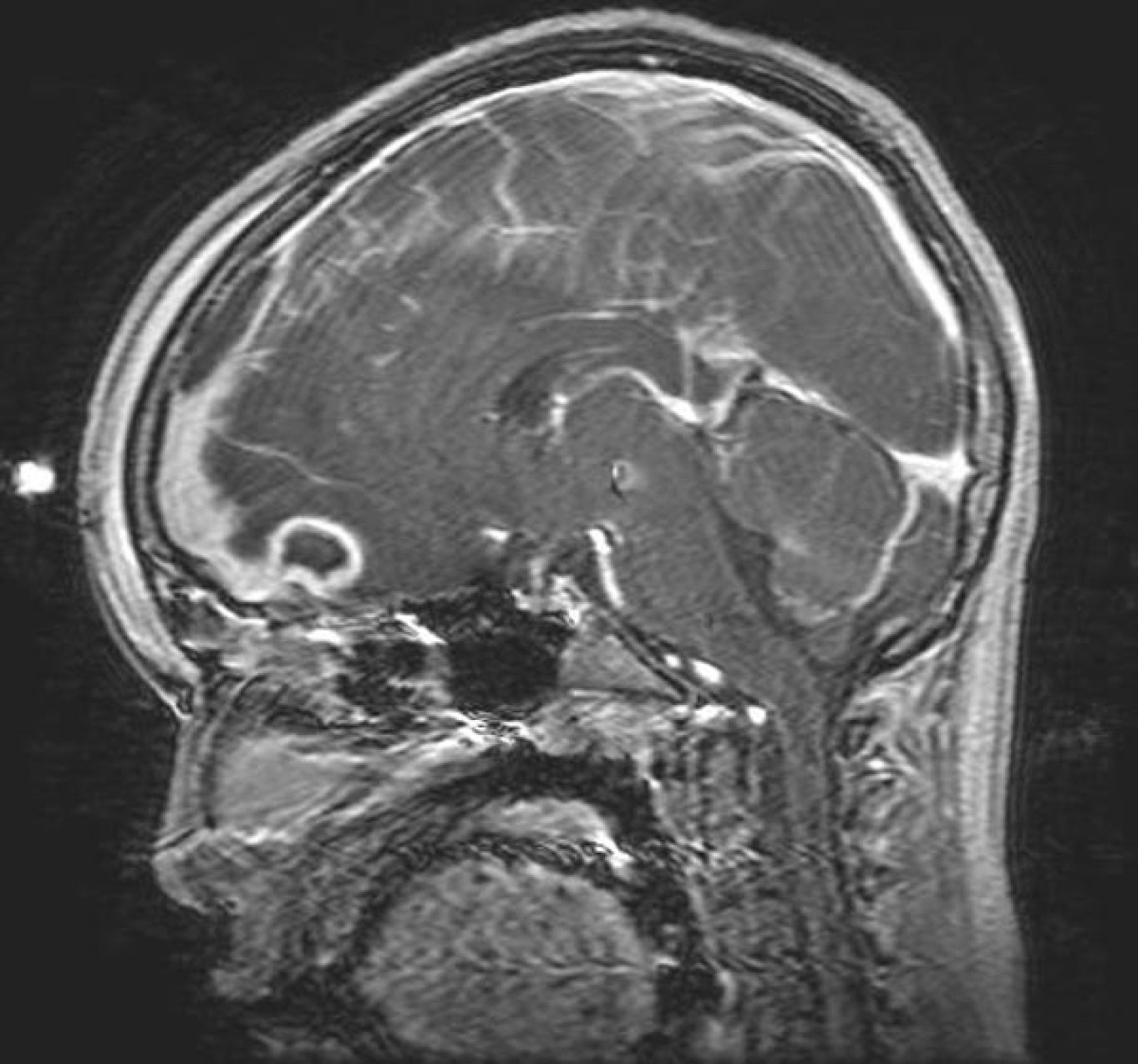 |
Source: Radswiki. Pott's puffy tumor 004. Published May 31, 2008. https://upload.wikimedia.org/wikipedia/commons/0/01/Potts-puffy-tumor-004.jpg CC BY-SA 3.0 |
Figure 6. Early Rocky Mountain Spotted Fever on Arm |
 |
Source: Centers for Disease Control and Prevention. Rocky Mountain spotted fever (RMSF): Signs and symptoms. Last reviewed Feb. 19, 2019. https://www.cdc.gov/rmsf/healthcare-providers/signs-symptoms.html |
Prior to starting antibiotics, practitioners should attempt to obtain blood cultures to evaluate for bacteremia. Additional serum studies to obtain include a complete blood cell count and differential (which will provide a baseline for the degree of leukocytosis and thrombocytosis, both of which could be elevated), a complete metabolic panel (to evaluate renal and liver function prior to initiating antibiotics), erythrocyte sedimentation rate, and C-reactive protein (which often will be monitored through the ensuing course of antibiotic therapy, and which can be helpful in determining if new intracranial complications have developed). Obtaining such laboratory studies in the emergency department or at admission will help smooth the patient’s course for long-term antibiotic therapy and surgical follow-up.
Additionally, microbiologic data are essential for selection of targeted antibiotic therapy. While such infections often are conceived of as polymicrobial and demand broader coverage, it still is important to determine if resistant organisms are present. Methicillin-resistant S. aureus (MRSA) always should be considered, as well as penicillin-resistant streptococci, and, more rarely but still importantly, organisms like Pseudomonas aeruginosa. Practitioners often can obtain a MRSA nasal polymerase chain reaction test to determine whether the patient is colonized, which will help guide empiric therapy. Most critically, aerobic and anaerobic cultures always should be requested if surgical washout is pursued. As mentioned previously, cultures often are inadequate at recovering anaerobic organisms; the lack of growth on an anaerobic culture should never be used as evidence that anaerobic coverage is not needed.
Medical Therapy and Surgical Intervention
When radiologic data and clinical concern suggest Pott’s puffy tumor or sinogenic/otogenic intracranial empyema are present, providers should not hesitate to contact otorhinolaryngology, neurosurgery, and infectious disease consultation services. As discussed previously, sinogenic and otogenic intracranial empyemas and Pott’s puffy tumor are fundamentally treated as polymicrobial infections. Thus, practitioners should start patients on broad-spectrum coverage when such infections are discovered. Central nervous system and blood-brain barrier penetration is essential, as patients with intracranial invasion are at risk for cerebritis and cerebral abscess formation.
Typically, initial coverage consists of ceftriaxone (for broad-spectrum upper respiratory gram-negative and gram-positive coverage), vancomycin (for coverage of potential MRSA or penicillin-resistant streptococci), and metronidazole (for coverage of upper respiratory anaerobic species). All three antibiotics achieve excellent cerebrospinal fluid drug levels. When culture data and molecular diagnostics provide further resolution to the question of which bacterial species are driving the infection, providers may choose to narrow therapy to two agents, typically ceftriaxone and metronidazole.
Most importantly, infected fluid collections (such as subperiosteal abscesses, subdural or epidural empyemas, and parenchymal abscesses) must be evaluated by appropriate surgical services.7,8 The timeless advice, “ubi pus, ibi evacua” (“where there is pus, it must be drained”) remains pertinent; reduction of the bioburden of any invasive bacterial infection relieves pressure in closed spaces like the cranial vault and improves antibiotic penetration into such potential spaces. In many cases, recovery is entirely contingent on appropriate source control. However, neurosurgical services are best equipped to determine the risk/benefit approach of draining intracranial fluid collections, and their expertise is critical in initial evaluation. Even when intracranial fluid collections are not present, otorhinolaryngology services may choose to perform functional endoscopic sinus surgery (FESS), which can be quite helpful in relieving intrasinus pressure and ameliorate drainage of infected contents in cases of sinogenic infection and Pott’s puffy tumor. Likewise, mastoidectomy, tympanostomy tube placement, or tympanocentesis in otogenic cases can provide source control and reduce the inflammatory bioburden of such infections.7
The overall course of therapy for Pott’s puffy tumor and sinogenic or otogenic intracranial infection requires close inpatient management, continued surgical evaluation, and appropriate long-term outpatient follow-up. Following drainage of any accessible infected fluid collections, empyemas, or abscesses, antibiotic therapy may range from four to six weeks or longer. Follow-up imaging at the end of therapy usually is recommended to ensure full resolution of any remaining abscesses or empyemas. As mentioned previously, antibiotic therapy usually is kept broad (i.e., gram-positive, gram-negative, and anaerobic coverage) and chosen for its central nervous system penetration, but if culture data do not demonstrate the presence of MRSA or penicillin-resistant streptococci, vancomycin often is discontinued. Prolonged therapy provides adequate antibiotic penetration and elimination of infection in poorly vascularized bony structures of the skull and sinuses. Typically, infectious disease specialists can help guide and manage this course of antibiotic therapy, particularly if complications are encountered, such as antibiotic side effects or concern for failure of medical therapy.
Conclusion
Providers should maintain a high index of suspicion for intracranial invasion of sinogenic and otogenic infections. In particular, frontal bone swelling/bogginess or proptosis of the ear are highly suggestive of Pott’s puffy tumor and mastoiditis, respectively, and warrant three-dimensional, contrasted imaging. Intracranial invasion may lead to abscess and empyema formation with attendant neurologic defects and long-term brain injury. Neurosurgery, otorhinolaryngology, and infectious disease specialists should be engaged as soon as such cases are identified. With appropriate surgical drainage and long-term antibiotic therapy, such infections often will resolve, and outcomes often can be excellent.
Case Conclusion
The patient undergoes contrasted magnetic resonance imaging of the head and brain, revealing frontal bone osteomyelitis with underlying frontal bone subperiosteal abscess. There is enhancement of the dura mater but no discrete fluid collection and no evidence for intracranial empyema or abscess. Blood cultures are obtained, and he is started on broad-spectrum antibiotic therapy with ceftriaxone, metronidazole, and vancomycin. Neurosurgery and otorhinolaryngology surgical services evaluate the patient, and he undergoes operative incision and drainage of the frontal subperiosteal abscess, as well as functional endoscopic sinus surgery the same day. The infectious diseases service is contacted for their guidance during his inpatient stay. Intraoperative cultures demonstrate the presence of Streptococcus intermedius. No MRSA or other resistant organisms are identified. The vancomycin is discontinued, and he is continued on ceftriaxone and metronidazole therapy.
Following his operative drainage, his fevers abates and his symptoms are significantly relieved. Likewise, his C-reactive protein, formerly elevated to 15.1 mg/dL, falls steadily to 8.0 mg/dL, then to 5.3 mg/dL, then to 2.7 mg/dL, and eventually normalizes over the next several days. A peripherally inserted central catheter line is placed, and he is eventually discharged home for a planned four- to six-week course of intravenous ceftriaxone and oral metronidazole, guided by outpatient follow-up with the infectious diseases team.
References
- Rohde RL, North LM, Murray M, et al. Pott’s puffy tumor: A comprehensive review of the literature. Am J Otolaryngol 2022;43:103529.
- Milinis K, Thompson N, Atsmoni SC, Sharma SD. Sinogenic intracranial suppuration in children: Systematic review and meta-analysis. Otolaryngol Head Neck Surg 2022;167:215-223.
- Cherry JD, Vahabzadeh-Hagh AM, Shapiro NL. Mastoiditis. In: Cherry JD, Harrison GJ, Kaplan SL, eds. Feigin and Cherry's Textbook of Pediatric Infectious Diseases. 8th ed. Elsevier Saunders;2019:169-175.
- Moore C, Deng F, Twarner R. Tegmen tympani. Radiopaedia. Updated Jan. 5, 2020. https://radiopaedia.org/articles/tegmen-tympani?lang=us
- Adame N, Hedlund G, Byington CL. Sinogenic intracranial empyema in children. Pediatrics 2005;116:e461-e467.
- Johnson DL, Markle BM, Wiedermann BL, Hanahan L. Treatment of intracranial abscesses associated with sinusitis in children and adolescents. J Pediatr 1988;113:15-23.
- Raineau M, Crowe AM, Beccaria K, et al. Pediatric intracranial empyema complicating otogenic and sinogenic infection. Int J Pediatr Otorhinolaryngol 2024;177:111860.
- French H, Schaefer N, Keijzers G, et al. Intracranial subdural empyema: A 10-year case series. Ochsner J 2014;14:188-194.
- Migirov L, Duvdevani S, Kronenberg J. Otogenic intracranial complications: A review of 28 cases. Acta Otolaryngol 2005;125:819-822.
- McNeil JC, Dunn JJ, Kaplan SL, Vallejo JG. Complications of otitis media and sinusitis caused by Streptococcus anginosus group organisms in children. Pediatr Infect Dis J 2020;39:108-113.
- Accorsi EK, Chochua S, Moline HL, et al. Pediatric brain abscesses, epidural empyemas, and subdural empyemas associated with Streptococcus species — United States, January 2016–August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1169-1173.
- Rosenfeld EA, Rowley AH. Infectious intracranial complications of sinusitis, other than meningitis, in children: 12-year review. Clin Infect Dis 1994;18:750-754.
- Giannoni C, Sulek M, Friedman EM. Intracranial complications of sinusitis: A pediatric series. Am J Rhinol 1998;12:173-178.
- Piatt JH Jr. Intracranial suppuration complicating sinusitis among children: An epidemiological and clinical study. J Neurosurg Pediatr 2011;7:567-574.
- Angelo SJ, Anderson MG, Sutter PA, et al. Changes in the epidemiology of pediatric sinogenic and otogenic intracranial infections during the COVID-19 pandemic: A single-institution study. J Neurosurg Pediatr 2023;32:231-241.
- Klivitsky A, Erps A, Regev A, et al. Pott’s puffy tumor in pediatric patients: Case series and literature review. Pediatr Infect Dis J 2023;42:851-856.
Following the COVID-19 pandemic, the incidence of sinogenic and otogenic intracranial empyemas increased. The author reviews the presentation, imaging, and treatment for this potentially life-threatening infection, with a reminder to keep this on your differential when evaluating your youngest patients.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
