Identifying and Treating Pediatric Ocular Trauma
Authors
Eric Sasine, MD, Emergency Medicine PGY-3, University of North Carolina Hospitals, Chapel HillPeer Reviewer
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Keller Army Community Hospital, West Point, NY
Executive Summary
- The different aspects of the ocular exam are: visual inspection (assess for periorbital ecchymosis, periorbital swelling, exophthalmos, enophthalmos, eyelid laceration, dysconjugate gaze, chemosis, subconjunctival hemorrhage, distortion of the pupil, hyphema, and presence of foreign body [particularly on lid eversion]; palpating (feeling for instability, tenderness, crepitus, and step-offs of the orbit); measuring pupillary response, shape, and symmetry; examining the range of extraocular movements; examining visual acuity; examining visual field testing; and measuring tonometry.
- The following is a summary of the features that warrant repair by an ophthalmologist: deep lacerations (anything deeper than the epidermis should be referred to ophthalmology — orbicularis oculi, tarsal cartilage, subcutaneous fat, or through-and-through lacerations); involvement or potential involvement of the lacrimal system (medial third of the eye or fluorescein seen coming from the wound); involvement of the lid margin (which, if improperly repaired, can lead to lid deformity and chronic eye irritation); ptosis on exam (suggests severing of the levator palpebrae muscle); and inability to close the eye (suggests severing of the orbicularis oculi muscle).
- Patients with corneal abrasion will present with tearing, photophobia, a foreign body sensation, and intense pain after eye trauma. Nonverbal children may present with fussiness, a closed eye, and eye irritation as the only complaints. Multiple linear abrasions near one another should raise suspicion for a retained foreign body under the eyelid. If linear abrasions are present, evert the eyelids to check for foreign body, and consider irrigation to wash out occult foreign bodies.
- Fluorescein staining of an area with globe rupture will show a positive Seidel sign, in which aqueous humor pours out of a defect and looks like a dark waterfall through the bright fluorescein stain.
- Be especially suspicious for globe rupture in patients with full-thickness eyelid lacerations, displaced orbital fractures, blowout orbital fractures, or traumatic hyphema.
- Signs concerning for globe rupture include distortion of the pupil and iris (“teardrop-shaped” pupil); hyphema; pigmented tissue emanating from the sclera (protrusion of the choroid); significant subconjunctival hemorrhage (particularly if it encompasses all of the sclera); hemorrhagic chemosis.
Pediatric ocular injuries are predominantly minor but may be devastating. The emergency provider must understand the anatomy and injuries that may result in significant damage and a timely critical approach to preserve the child’s vision.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Eye injuries are common in the pediatric population, with 100,000 to 200,000 pediatric emergency department (ED) visits for eye trauma occurring every year in the United States.1,2 While most eye injuries, thankfully, do not lead to permanent vision loss, it is a real possibility, and clinicians must keep this in mind when evaluating children with ocular trauma. Despite public health efforts to reduce the incidence and severity of ocular trauma, 50,000 people per year worldwide have partial or complete vision loss in one eye caused by an injury.3 Certain ocular pathologies require rapid diagnosis and intervention to preserve a child’s vision. Proper ED management of ocular injury has been implicated in improved long-term vision outcomes.4
This article reviews ocular injuries, starting with the surrounding orbital structures and diving deeply to the eye and inner structures. Each section will cover the relevant anatomy and pathophysiology to provide a basis for managing these injuries. However, before jumping into the types of ocular injury that providers may encounter, there are important general considerations when approaching a child with ocular trauma. The first is that trauma to the eye is a type of head trauma. Although this article focuses on the injuries that may occur to the eye and orbit, emergency providers need to consider intracranial trauma when assessing a child with ocular trauma. The second is that eye injuries can co-occur, so when one type of injury is detected, a thorough eye exam still must be done to evaluate for other types of ocular injuries.
To be comfortable managing ocular injuries, emergency providers need to be skilled at performing ocular exams. Eye injuries often are only detected on physical exam (as opposed to history or imaging), so a careful and systematic physical exam is warranted in all cases of ocular trauma. This can be quite difficult with young children; providers always should conduct as much of the physical exam as reasonably can be done. However, some aspects are impossible with young children, such as visual acuity and visual fields. Some tasks may require anxiolysis or multiple assistants to perform, such as fluorescein staining, eyelid eversion, point-of-care ultrasound, or irrigation. As a summary, the different aspects of the ocular exam are:
- Visual inspection: Assess for periorbital ecchymosis, periorbital swelling, exophthalmos, enophthalmos, eyelid laceration, dysconjugate gaze, chemosis, subconjunctival hemorrhage, distortion of the pupil, hyphema, and presence of foreign body (particularly on lid eversion);
- Palpation: Feel for instability, tenderness, crepitus, and step-offs of the orbit;
- Measure pupillary response, shape, and symmetry;
- Examine the range of extraocular movements;
- Examine visual acuity;
- Examine visual field testing;
- Measure tonometry.
For reference throughout this review, an anatomical cross-section of the eye is shown in Figure 1.
Figure 1. Cross-Section of the Eye |
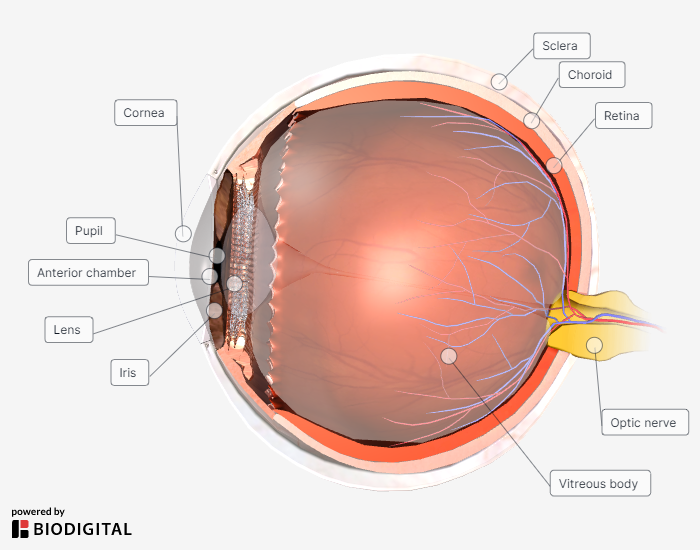 |
Orbital Fractures
Anatomy and Pathophysiology
The orbit is a cone-shaped hole that contains fat, fascia, extraocular muscles, and the eye. It is made of seven different bones. Surrounding those bones are several air-filled sinuses. Trauma to the orbit can cause fractures of the orbital bones either directly or through the hydraulic mechanism. In the hydraulic mechanism, pressure to the globe of the eye (from blunt trauma) can transmit pressure to the deeper structures of the orbit, causing orbital blowout fractures.5 A blowout fracture occurs when one of the sinus walls collapses. The inferior wall is the most common site of wall collapse, followed by the medial wall and, finally, the lateral wall.
The extraocular muscles can become trapped in blowout fractures, leading to limited range of extraocular movement. The most common type of muscle entrapment is an inferior blowout fracture, in which the inferior rectus is trapped in the maxillary sinus and upward gaze is limited. Entrapped extraocular muscle can become ischemic. One clinical sign of ischemia is the oculovagal response, in which light pressure applied to an entrapped eye causes a vagal response (bradycardia, hypotension, presyncope, and nausea). Ischemic muscle is at risk of necrosis. Therefore, the oculovagal response is a warning sign and should prompt expedited surgical correction.6 The injured eye should not have pressure applied to it if there are signs of globe rupture or orbital compartment syndrome (both of which will be discussed in depth), so do not test for the oculovagal reflex in patients who have exophthalmos, distorted pupils, or a positive Seidel sign.
Etiology and Epidemiology
Any type of blunt facial trauma can cause orbital fracture. Common causes include motor vehicle collisions, assaults, falls, and — particularly common in adolescents — sports injuries.7
Presentation and Evaluation
Children with an orbital fracture typically will present after blunt trauma. If they are old enough to speak, they likely will complain of orbital pain and may report diplopia. On exam, the provider may see enophthalmos, exophthalmos, dysconjugate gaze, and limited range of extraocular movement. If signs or symptoms of orbital fracture are present, as described earlier, then a computed tomography (CT) scan of the orbit should be done.8 Classically, a coronal slice of an inferior blowout fracture will show a teardrop-shaped bulge of tissue in the maxillary sinus. Although CT is the preferred imaging modality, it has poor specificity for predicting entrapment.9 Co-occurring injuries with orbital fracture can include globe rupture and corneal abrasion, so a fluorescein stain is warranted in suspected or confirmed cases of orbital fracture.
Treatment and Disposition
The biggest long-term risk of orbital fracture is damage to the patient’s vision. If the eyes are not symmetric, then the patient may have permanent visual impairment. As such, any child with features for high-risk visual impairment needs emergent ophthalmologic evaluation.7 These include diplopia, clinical signs of muscle entrapment, exophthalmos, enophthalmos, oculovagal reflex, and large herniation seen on CT. Any patient without these high-risk features is safe for discharge with urgent ophthalmologic follow-up.
Lastly, if there is a blowout into a sinus, then the patient should be given antibiotics. Sinus blowout fractures are considered open fractures and are at risk of causing maxillofacial osteomyelitis. Five days of cephalexin is the standard recommendation.10 Additionally, sinus precautions should be advised for those with sinus fractures. These include not blowing the nose, using a straw, diving into water, sneezing with the mouth closed, or playing a wind instrument.
Eyelid Laceration
Anatomy and Pathophysiology
Knowing which eyelid lacerations can be repaired by a non-ophthalmologist is all about knowing eyelid anatomy. Each eyelid is composed of several tissue planes. The many intricacies of these planes are beyond the scope of this review. The layers essential to know in evaluating a child with an eyelid laceration in the ED are listed here from superficial to deep:
- epidermis;
- orbicularis oculi muscle;
- tarsus (a firm, fibrous cartilage that connects to the orbital septum and levator aponeurosis);
- subcutaneous fat; and
- palpebral conjunctiva.
Anything deeper than the epidermis is high-risk. The orbicularis oculi muscle wraps around the eye in both the upper and lower eyelids and mediates closing, so lacerations of this layer can cause failure to blink properly. The levator muscle lifts the upper eyelid, so laceration of the upper eyelid can cause ptosis by severing this muscle.
Lacerations of the lacrimal ducts also are high-risk and need to be managed by an ophthalmologist. Tears are produced in the superior-lateral orbit and secreted into the palpebral conjunctiva. Those tears are collected by pores in the medial third of each eyelid margin called puncta (singular punctum) that lead to the canalicular collecting system. Any laceration of the medial third of the upper or lower eyelid potentially can involve the canalicular system. Defects of the collecting system that are not properly repaired can cause chronic tearing.
Etiology
The eyelids are the thinnest epidermis in the body (0.05 mm thick, only one-thirtieth the thickness of the epidermis of the hands).11 They can be lacerated by even minor blunt trauma as well as the full range of other mechanisms of laceration that affect thicker skin.
Presentation and Evaluation
Patients will present after blunt or penetrating trauma. On exam, visually inspect and gently probe the wound. Look for muscle, tarsal cartilage, or fat in the laceration — all of which suggest deep involvement. Have the patient open their eyes and blink several times to check for ptosis or failure to properly close. Invert the eyelids to check for through-and-through penetration. If there is penetrating laceration of the eyelid, perform a fluorescein stain and check for other signs of globe rupture (such as pupillary distortion, hyphema, tenting of the iris, or extrusion of pigmented material from the sclera). If the laceration is in the medial third of the eye, then place fluorescein in the eye and check if dye comes out of the laceration; if this occurs, it suggests canalicular involvement.
Treatment and Disposition
If there are any features of complicated laceration, then referral to an ophthalmologist for repair is warranted. Because lacerations should be repaired as soon as possible, transfer the patient if necessary. The following is a summary of the features that warrant repair by an ophthalmologist. If providers are uncomfortable, they should not attempt to repair these injuries, even if none of these high-risk features are met:
- deep lacerations (anything deeper than the epidermis should be referred to ophthalmology — orbicularis oculi, tarsal cartilage, subcutaneous fat, or through-and-through lacerations);
- involvement or potential involvement of the lacrimal system (medial third of the eye or fluorescein seen coming from the wound);
- involvement of the lid margin (which, if improperly repaired, can lead to lid deformity and chronic eye irritation);
- ptosis on exam (which suggests severing of the levator palpebrae muscle); and
- inability to close the eye (which suggests severing of the orbicularis oculi muscle).
When referring to an ophthalmologist, have the patient place a pad of gauze soaked in cold saline over the eye while they await consultation. This can decrease lid swelling and improve their ultimate repair.12
Assuming there is a simple laceration that does not require ophthalmology consultation, begin by achieving anesthesia with a regional block. The supraorbital and infraorbital nerve blocks provide anesthesia without causing tissue distortion. To perform the supraorbital block, have the patient look straight ahead and inject local anesthetic (lidocaine or bupivacaine) into the area directly above the pupil in line with the eyebrow. This will numb the medial aspect of the upper eyelid. To perform the infraorbital block, feel for the infraorbital foramen, which will feel like a small divot in the upper cheek. Pull the upper lip back and inject local anesthetic toward this notch. Once the patient is numb, use a 6-0 or 7-0 absorbable suture (fast or chromic gut is best).13 If the laceration is close to the lid margin, tie the suture ends of the ties closest to the margin underneath the knots of the ties farther away from the margin. This prevents the ties from twisting into the eye and causing a corneal abrasion.13 Put the least amount of vertical tension on the knots as possible. Vertical tension can cause lid retraction.14 Refer to ophthalmology after the repair to ensure proper healing.
Lastly, as with any laceration, ensure the patient’s tetanus immunity is up to date.
Subconjunctival Hemorrhage
Anatomy and Pathophysiology
The conjunctiva is the most superficial layer of the eye over the sclera and inner eyelids. It is a thin, translucent mucosal membrane that keeps the eye moist. There are fragile blood vessels within the conjunctiva that are prone to rupture, and when these vessels bleed, blood becomes trapped between the sclera and conjunctiva. Bleeding can happen with blunt or penetrating trauma, with increased blood pressure of the head and neck, or spontaneously. Sources of increased head and neck blood pressure include coughing, sneezing, vomiting, strangulation, and Valsalva (such as from heavy lifting or straining with bowel movements). Many cases happen without any clear trigger.
Presentation and Evaluation
Subconjunctival bleeding will not affect the patient’s vision. Large subconjunctival hemorrhage may cause mild discomfort, but it generally is painless. Physical exam will show a well-demarcated area of bright red blood overlying the sclera that does not cross the limbus or iris. Because these are largely asymptomatic, a patient with spontaneous subconjunctival hemorrhage often will notice the bleeding incidentally when they look in the mirror or when told by someone else. When a child presents with traumatic subconjunctival hemorrhage, the emergency provider needs to evaluate for globe rupture, particularly if it is a large hemorrhage with chemosis (bulging of the conjunctiva). These patients likely will report eye pain and may report foreign body sensation. In these cases, clinicians need to evaluate for foreign body, corneal abrasion, and globe rupture. Traumatic subconjunctival hemorrhage with any concerning features for globe rupture warrants emergent ophthalmologic evaluation. Also, consider nonaccidental trauma in the differential for young children.
Treatment and Disposition
No further treatment or follow up is required for isolated traumatic subconjunctival hemorrhage. Counsel the patient that the bleeding can take two weeks to resolve and may change color as the blood breaks down. Consultation with ophthalmology is required if there is a concern for concomitant globe rupture. Consider consultation with a child abuse specialist if there is any suspicion for abuse.
Corneal Abrasion
Anatomy and Pathophysiology
The cornea is a superficial layer of epithelium that lies over the central part of the anterior globe. It is superficial to the anterior chamber and connects to the sclera at the limbus. The cornea protects the part of the eye that allows light to pass through to the lens. Because of its role in protecting vision, the cornea is extremely sensitive. In fact, it is the most densely innervated tissue in the human body.15 Any injury to it is extremely sensitive. A corneal abrasion occurs when there is a partial-thickness tear into the corneal epithelium. The cornea heals very quickly when abraded, typically within 48 to 72 hours.16 In rare cases, corneal abrasions can lead to serious infections, such as corneal ulcers (ulcerative keratitis) and bacterial endophthalmitis. Antibiotic prophylaxis to prevent these outcomes is warranted.
Etiology and Epidemiology
Corneal abrasions can occur through a variety of mechanisms: improper contact lens use, fingernails (particularly during contact lens application or infants with untrimmed fingernails), sand or other tiny objects on a windy day, projectiles during sanding or grinding work, eyelash curlers, bungee or elastic cords, and airbag deployment, to name just a few. Dry eyes are especially sensitive to abrasion.
Presentation and Evaluation
Patients with corneal abrasion will present with tearing, photophobia, a foreign body sensation, and intense pain after eye trauma. Nonverbal children may present with fussiness, a closed eye, and eye irritation as the only complaints. Vision typically is not affected unless the abrasion is located over the pupil. If there is no history of eye trauma, then providers should be suspicious for other pathologies, such as keratitis or conjunctivitis. Intense pain can be delayed, so be sure to ask about injuries that may have occurred in the preceding days.
When there is suspicion for corneal abrasion, begin by examining for signs of globe rupture (e.g., hyphema, pupillary distortion, tenting of the iris, and protrusion of pigmented material from the sclera or cornea). If hard signs of globe rupture are visible, then stop and consult ophthalmology. If there are no signs concerning for globe rupture, then invert the lids to look for trapped foreign bodies. Finally, perform fluorescein staining. Pay special attention to how the patient feels after anesthetic drops are given during the fluorescein exam. Because corneal abrasions are superficial injuries, relief of pain with anesthetic eyedrops is virtually diagnostic of corneal abrasion. Abrasions can be linear or involve a wide swath of tissue. Multiple linear abrasions near one another should raise suspicion for a retained foreign body under the eyelid. If linear abrasions are present, evert the eyelids to check for foreign body and consider irrigation to wash out occult foreign body.
Treatment and Disposition
Cases of uncomplicated corneal abrasion — in which globe rupture and foreign body have been ruled out — are treated with pain control and antibiotic prophylaxis. Pain management is best achieved with systemic nonsteroidal anti-inflammatory drugs (NSAIDs). Antibiotic ointment (discussed later) also can help with pain control because the ointment coats the abraded tissue. Topical anesthetics are controversial. A recent randomized controlled trial found that 24 hours of home tetracaine drops controlled pain without leading to increased negative outcomes.17 However, some ophthalmologists fear the overuse of anesthetic drops as well as the possible increased risk of corneal ulcer from patients not protecting an insensate eye and advise against this practice.18 Patients with refractory pain can undergo a trial of cycloplegics (such as cyclopentolate or homatropine) to treat underlying iris spasm. Antibiotics are prescribed to prevent ulcerative keratitis and endophthalmitis. Patients who use contact lenses need coverage for Pseudomonas (usually calling for tobramycin or ciprofloxacin) and should not wear their contact lens until they follow up with ophthalmology. Those who do not use contact lenses can be treated with erythromycin. In both cases, use ointment to help soothe the eye. While patching used to be standard, data show that there is no increased rate of healing, and it is no longer recommended.19,20
Any complicating features, such as concern for globe rupture, keratitis, retained foreign body, or uncertain diagnosis, should prompt evaluation by an ophthalmologist as soon as possible.
Chemical Injuries
Anatomy and Pathophysiology
The eyes are a particularly dangerous area for exposure to harmful chemicals. Many critically important structures are millimeters from the surface, and the ocular epithelium is a thin, soft, permeable layer that offers little protection. While any chemical can cause damage, the ones that most commonly cause serious injury are caustic substances — those that are highly acidic or basic. Caustic chemicals cause damage to cellular proteins that leads to tissue necrosis, which results in long-term fibrotic scarring of the eye that can permanently impair vision. Alkali injuries usually are worse because they penetrate deeper into the tissue.21,22 The extent of visual impairment typically depends on how much of the cornea is involved.
Etiology and Epidemiology
Exposure to chemicals often occurs in the home or occupational setting. Children are exposed to dangerous substances in the form of home products related to cleaning, automotive work, lawn care, crafts (such as paint or glue), or plumbing. Recent data show that children 1-2 years of age have the highest risk of ocular caustic injury of any age group.23 Liquid products may be splashed or sprayed onto the eyes. Solid products, such as chemical granules, have the potential to be lodged and stuck in the ocular fornices.
Presentation and Evaluation
As soon as an ocular chemical exposure in a patient becomes evident, begin irrigation immediately, even prior to a full exam, pH testing, or diagnostic tests.24,25 Caustic substances can cause permanent vision loss in as little as 10 minutes after exposure, so every minute counts.26 Proper ED irrigation of ocular chemical exposure has been shown to correlate with improved long-term outcomes.27 Place the patient on a flat surface with either towels or a catch basin beneath them. Any clean, large-volume fluid is appropriate.28 Typically, intravenous (IV) fluid boluses or saline bottles for wound irrigation are used. Using IV tubing connected to either an external ocular irrigation cap, a Morgan lens, or just the end of IV tubing, irrigate the eye for 10-15 minutes prior to examining it. Young children likely will require restraint using a blanket wrap, multiple holders, and may even require anxiolysis. Anesthetic eye drops can be used for comfort, but irrigation should not be delayed to obtain anesthesia.29
After the first 10-15 minutes of irrigation, allow two to three minutes for the irrigating solution to flow out of the eye before checking pH. When checking pH, use narrow-range strips (which go from pH 6 to 8), rather than the usual urine strips that have a much broader range. These strips are more accurate for the desired ocular range. The goal eye pH is 7.0 to 7.2. Continue irrigating until the pH in this range, which can take several liters depending on the amount of exposure. When pH has normalized, stop irrigating, but continue checking pH for 30-60 minutes afterward to ensure that no rebound pH change occurs, which is worrisome for retained granules of chemical substance. For noncaustic substances in which pH will be normal from the beginning, 10-15 minutes is sufficient.30
After irrigation, a full eye exam is warranted, including visual acuity, visual fields, pupillary response, and fluorescein staining. The exam commonly shows tearing and redness and may show corneal clouding. Concomitant injuries to evaluate for include thermal burns (if the substance was hot), globe rupture (particularly if the substance was under pressure), retained foreign body (for solid, granular chemicals), and abrasion.
Treatment and Disposition
After the initial irrigation and exam, emergent consultation with ophthalmology is warranted in any case with concerning features by history, physical exam, or pH. Mild cases with no concerning features by the type of chemical involved, physical exam, or pH may not require emergent ophthalmologic consultation, but patients should have outpatient follow-up with ophthalmology in two to three days. If there is unfamiliarity with the type of chemical to which the child was exposed, or if there is concern for systemic absorption, then refer to the material safety data sheet and consider consultation with toxicology.
Ocular Foreign Body
Anatomy and Pathophysiology
Ocular foreign bodies come in two broad groups: extraocular and intraocular. Extraocular foreign bodies are stuck to the conjunctiva and may get trapped under the eyelid. This type of foreign body can lead to corneal abrasion and, often, parallel abrasions from being repeatedly dragged under the eyelid. Intraocular foreign bodies have pierced the globe and are lodged within any of the globe’s inner structures (the anterior chamber, posterior chamber, lens, or iris). Ocular foreign bodies can lead to corneal ulcers and endophthalmitis if not treated with prompt removal and prophylactic antibiotics.
Etiology and Epidemiology
Foreign body injuries most often occur from activities that involve small, high-velocity projectiles. Classic examples include hammering (particularly metal on metal), sawing, grinding, or sanding. Ocular trauma is made less common by using protective eyewear.
Presentation and Evaluation
Patients will present with a painful, red, tearing eye after exposure to projectiles or other small objects. They likely will report a sensation of foreign body. Visual acuity may be impaired if the object is overlying the cornea. Just like corneal abrasions, the pain from an extraocular foreign body will be relieved with topical anesthesia.
Always perform a thorough exam, with special attention to inverting the eyelids to sweep for foreign bodies. Significant subconjunctival hemorrhage (particularly complete opacification of the sclera with blood) in the setting of a projectile exposure is concerning for globe rupture. Regardless, if there is subconjunctival hemorrhage, always perform a fluorescein stain to examine for globe rupture. Fluorescein staining of an area with globe rupture will show a positive Seidel sign, in which aqueous humor pours out of a defect and looks like a dark waterfall through the bright fluorescein stain.
Imaging should be obtained if the suspicion for intraocular foreign body is moderate or high. X-ray misses up to 60% of foreign bodies, so they usually are not obtained.30 If there are no obvious signs of globe rupture, then point-of-care ultrasound should be the first-line imaging modality because it is radiation-free, quick, and can reveal if foreign bodies are mobile or fixed.31 Ocular ultrasound shows objects as hyperechoic signals within an anechoic fluid and may show the twinkle artifact.32 If the point-of-care ultrasound is negative, noncontrast CT of the orbit should be done because it is more sensitive.33 Of note, point-of-care ultrasound should not be done if there are clinical signs of globe rupture because of the concern that increased pressure can worsen the protrusion of globe contents.
Treatment and Disposition
Removal of ocular foreign bodies always is indicated to prevent corneal ulceration and endophthalmitis. Extraocular foreign bodies can be removed by ED providers. To do so, first anesthetize both eyes (numbing the contralateral eye helps to diminish reflexive blinking). Next, irrigate the affected eye with the same setup described in the chemical injury section. This alone may be enough to dislodge and wash away superficial objects. If irrigation does not remove the object, then gently roll a cotton-tipped swab back and forth over the object.
Finally, if both these methods are unsuccessful, attempt to remove the object with a needle in a calm, cooperative child. To do this, sit the patient comfortably at the slit lamp. Find the object through the viewfinder. Using a 25- or 27-gauge needle with the bevel pointing away from the cornea, come in from the side with the needle nearly parallel to the eye (to decrease the risk of perforation). Use gentle movements to attempt to dislodge the object. Once finished, repeat the fluorescein exam to detect any potential iatrogenic globe rupture. In a child who is unable to cooperate, obtain ophthalmology consultation.
Any signs of globe rupture or intraocular foreign body warrant emergent referral to ophthalmology. If there is a rust ring or any residue left by an extraocular object, then refer to ophthalmology within 24 hours for removal.34 If urgent follow-up with ophthalmology is not possible, then removal of a rust ring by the emergency physician also is possible in a calm, cooperative child. To do this, follow the same numbing and positional setup described for needle removal of foreign bodies, but use a corneal burr to brush away the rust ring until it is no longer visible. Also test postintervention fluorescein for iatrogenic globe rupture.
As with any puncture wound, treat all foreign bodies by updating tetanus.35 Prophylactic antibiotics are warranted in both extraocular and intraocular foreign bodies, with Pseudomonas coverage for those who use contact lenses.36
Traumatic Iritis
Anatomy and Pathophysiology
The iris is a muscular ring of tissue that lies between the anterior chamber and the lens. The iris is connected to the ciliary body at its outward edges, which is connected to the choroid, the middle part of the globe’s outer tissue. The iris contracts and relaxes to allow the pupil to dilate and constrict. Any type of blunt trauma can cause contusion of the iris that can induce inflammation, called traumatic iritis.
Etiology and Epidemiology
Children with traumatic iritis will present with monocular eye pain that usually presents one to two days after a blunt trauma. There are two classic characteristics of the pain: It does not improve with topical anesthetics, and it worsens with miosis so shining a light in either eye, or performing an accommodation test, causes pain in the affected eye. Patients also may report floaters or blurry vision. The physical exam may show tearing, anisocoria, a sluggish pupillary response, and perilimbal injection that worsens near the iris. A slit lamp exam may show flare and hypopyon. A full eye exam should be performed, including visual acuity, slit lamp, and tonometry. Common concomitant injuries to rule out are abrasions, hyphema, and retinal detachment.
Treatment and Disposition
Patients with traumatic iritis who have no other confirmed or suspected eye injuries can be discharged with outpatient ophthalmologic follow-up. Using sunglasses can help with the pain of miosis. They can take NSAIDs for analgesia, and if those are insufficient to manage pain in the ED, patients can be given topical cycloplegics, such as scopolamine, cyclopentolate, and homatropine. A potential complication of traumatic iritis is synechiae (malformations and adhesions of the iris) that can impact vision, so outpatient follow-up should be emphasized at discharge.
Hyphema
Anatomy and Pathophysiology
Hyphema is bleeding into the anterior chamber of the eye. The anterior chamber is the part of the eye that is behind the cornea and in front of the iris. It is full of clear fluid that allows light to pass through to the lens. Blood is thought to come from the iris and ciliary body. The most serious short-term complication of hyphema is elevated intraocular pressure caused by the blockage of the chamber’s draining system with clotted blood. This can happen to any child but is especially common in children with sickle cell disease.37 The increased intraocular pressure can threaten vision. Those who have had hyphema can have rebleeding in the days afterward, but this seems to be uncommon in the pediatric population.38 In the long term, hyphema can lead to corneal staining and synechiae of the iris.39
Etiology and Epidemiology
Hyphema can be spontaneous or result from trauma. Traumatic sources include both penetrating and blunt trauma, although hyphema caused by penetrating trauma should be managed as a globe rupture. Hyphema from blunt trauma in the pediatric population is commonly caused by fists, sports injuries, or projectiles.37
Presentation and Evaluation
Patients will present with eye pain after trauma. On visual inspection, hyphema will appear as blood in front of the iris. Examining the patient upright helps to detect blood because it causes blood to settle in a layer. In a supine patient (such as during a trauma), blood can settle in a thinner layer over the iris and be less obvious. Recent bleeding will appear as bright red, but as time passes, blood oxidizes and appears black. Hyphema can present on a spectrum from microhyphema, in which blood is only detected floating in the chamber on slit lamp, all the way to 100% occlusion of the anterior chamber visible on basic inspection. As in all types of ocular trauma, a thorough eye exam is needed to assess for potential concomitant injuries. This includes a fluorescein stain, baseline intraocular pressure, and a slit lamp exam.
Treatment and Disposition
Because hyphema is a vision-threatening injury, emergent consultation with an ophthalmologist is necessary. When hyphema is detected on exam, some initial interventions can be performed as ophthalmology consultation is initiated. First, make sure that the head of the bed is elevated to 45 degrees. Having the patient upright is thought to help prevent total blockage of the draining system because it causes the blood to sink and frees up at least the top part of the eye to drain chamber fluid. Second, do not give NSAIDs, which might worsen active bleeding. Third, consider if the patient might have a coagulopathy that needs to be corrected. Coagulopathy is uncommon in the pediatric population, but if a child has a bleeding disorder, such as hemophilia, von Willebrand disease, or thrombocytopenia, then they may require intervention to help prevent worsening of their bleed.
There are medical interventions for hyphema that ophthalmologists may recommend. These include tranexamic acid and aminocaproic acid to stop bleeding, cycloplegics and miotics to increase drainage, and steroids. A recent Cochrane review found no benefit to medical interventions for traumatic hyphema, but because of the theoretical benefit and low risk of these interventions, they may be worthwhile to try.40 However, they should be done only with consultation by ophthalmology.
Many patients with small hyphemas (less than one-third of the anterior chamber) with no complications can be discharged after consultation with ophthalmology. Any complicating feature, such as elevated intraocular pressure, coagulopathy, or sickle cell disease, should warrant admission to measure trending intraocular pressure, administer medical interventions at the direction of ophthalmology, and prepare for the potential need for operative intervention (e.g., washout of the anterior chamber) if conservative management fails. Normal intraocular pressure is 10 mmHg to 21 mmHg. If a patient has traumatic hyphema and an intraocular pressure greater than 21 mmHg, this is concerning for developing glaucoma, particularly if the value is significantly above 21 mmHg. While awaiting ophthalmology, patients with elevated intraocular pressure should be started on topical beta-blockers, such as timolol; topical alpha-agonists, such as brimonidine; and oral acetazolamide to decrease their intraocular pressure.
Lens Dislocation
Anatomy and Pathophysiology
The lens is a tough capsule that focuses light on the retina. It is posterior to the iris in the pupillary space. The lens is held in place by suspensory ligaments, called zonular fibers, that extend radially outward from the lens. When these ligaments are disrupted, the lens can become dislodged. If it is only partially dislodged but still visible within the pupil, it is subluxed. If it is fully separated from the pupillary space, it is dislocated. The lens can dislocate posteriorly into the vitreous humor or anteriorly into the anterior chamber.
Both subluxation and dislocation can cause trauma to the outer capsule of the lens. When the capsule is traumatized, the lens can develop traumatic cataracts. Anterior dislocation can cause acute angle closure glaucoma because of direct occlusion of the draining system in the anterior chamber.
Etiology and Epidemiology
Traumatic lens dislocation usually occurs from blunt trauma, not penetration. Compression of the globe in the anterior-posterior aspect leads to compensatory expansion in the radial direction. The radial expansion stretches the zonular fibers, which can cause them to tear. Lens dislocation is more common in children who have connective tissue disorders, such as Marfan syndrome, Ehlers-Danlos syndrome, and homocystinuria. It also is more common in people who have had prior ophthalmologic surgeries, particularly cataract surgery, although this is uncommon in the pediatric population.
Presentation and Evaluation
Children with traumatic lens dislocations will present with monocular diplopia and/or loss of monocular visual acuity after blunt trauma. Lens dislocation by itself is painless (as is known from patients who have spontaneous lens dislocation), but there may be pain from concomitant soft tissue injuries related to the blunt trauma. They will have exquisite globe pain if the dislocated lens has caused angle closure glaucoma.
If lens dislocation is suspected, a full eye exam is warranted, including slit lamp, tonometry, and fluorescein (if pain is present). In patients with subluxation, the lens will be visible as a crescent-shaped object that is visible across the pupil when performing the red-light reflex. In patients with anterior dislocation, the lens will be visible as a translucent capsule in front of the iris. In those with posterior dislocation, there may be no apparent visual evidence of lens dislocation because the lens has gone further back into the vitreous chamber.
Point-of-care ultrasound of the globe can help to evaluate for dislocation. Using the linear probe in the ocular setting, the lens is visible as an anechoic oval. It should appear posterior to the iris in the midline of the eye in both sagittal and axial views. Any disturbance of this confirms lens dislocation. Note that pupillary dilation can make dislocation easier to see, but there is a risk that dilation can cause a subluxation to become an anterior dislocation, which may cause glaucoma. Thus, dilation should take place only at the discretion of an ophthalmologist.
Injuries that are associated with lens subluxation and dislocation are hyphema, vitreous hemorrhage, retinal detachment, and globe rupture. Close attention needs to be paid to exclude these concomitant injuries.
Treatment and Disposition
A patient with lens subluxation or dislocation is safe for discharge with urgent outpatient ophthalmologic follow-up if they have normal intraocular pressure and no other concomitant eye injury. If lens subluxation is present with elevated intraocular pressure, then emergent ophthalmologic evaluation is warranted.
Retinal Detachment
Anatomy and Pathophysiology
The retina is the innermost layer of the posterior wall of the globe. The retina lies between the vitreous membrane, which is deep to the retina and contains the vitreous fluid of the posterior chamber, and the choroid membrane, which is the middle layer of the globe. The retina is a layer of nerves that detects and transmits light.
Blunt trauma can cause the vitreous fluid of the posterior chamber to suddenly shift. This movement can cause shearing forces that pull on the vitreous membrane. When the vitreous membrane is pulled, the retina can be torn off the wall of the globe because of its attachment to the vitreous membrane. This process is called rhegmatogenous detachment. There are other mechanisms by which the retina can be torn, but this is the usual mechanism of traumatic retinal detachment.41 When a part of the retina is torn off by the vitreous membrane, the fluid of the posterior chamber can flow through the hole to the potential space between the retina and choroid. This causes the retina to detach further.
When the retina is detached, it can no longer transmit light. Thus, patients will report a curtainlike area of vision loss where the retina is detached. Tugging on the retina can induce flashes of light called photopsia. Patients also will frequently report flashes of light and floaters followed by an area of vision loss. If the macula (the area of highest concentration of retinal neurons) is involved in the detachment, then the patient will report significant loss of visual acuity.
Etiology and Epidemiology
In children, trauma is the most common cause of retinal detachment.42 Any type of blunt trauma can cause it. Retinal detachment is more common in young males, with peak incidence of 9 to 12 years of age.40
Presentation and Evaluation
Children with traumatic retinal detachment will present with monocular floaters, flashes of light, curtainlike vision loss, or (if the macula is involved) significant loss of visual acuity. Retinal detachment typically is painless, but patients may have other soft tissue injury from trauma that will cause pain. They physical exam will show a normal external eye, extraocular movements, pupillary response, tonometry, and slit lamp exam. Fundoscopy will show an area of the retina that is wrinkled, opaque, and gray.
When retinal detachment is suspected, the emergency physician should perform point-of-care ultrasound of the eye to help confirm the diagnosis. A 2019 meta-analysis showed that point-of-care ultrasound performed by emergency physicians has both a high sensitivity and specificity for retinal detachment, both greater than 90%.43 Using a linear probe on ocular settings, ultrasound will show retinal detachment as a hyperechoic stripe that floats freely in the posterior chamber. Retinal detachment can appear similar to vitreous detachment. The retina will be thicker than the vitreous membrane and will not be detached from the optic nerve (whereas the vitreous membrane can be detached from the optic nerve).44
Serious injuries that can occur with retinal detachment and need to be ruled out are globe rupture and orbital fracture.
Treatment and Disposition
Untreated retinal detachment can lead to permanent visual impairment if not corrected urgently. Consultation with ophthalmology prior to discharge always is recommended. Most cases of retinal detachment will either be admitted for surgical correction or have urgent outpatient correction scheduled prior to discharge.45 Ophthalmologists will correct it by one of several ways, including photocoagulation, vitrectomy, and scleral buckling.
While awaiting ophthalmologic disposition, it is recommended to have the patient lie in a position that causes the detached portion of the retina to be dependent. It is thought that this positioning may help prevent worsening of the detachment. Because the mechanism of detachment usually is a rhegmatogenous tear in the retina through which vitreous fluid is flowing and expanding the detachment, it is advised that the affected eye be manipulated as little as possible to avoid pushing more fluid through the hole.
Globe Rupture
Anatomy and Pathophysiology
The term globe rupture means any injury with a full-thickness defect to the outer layer of the eye. As a brief review, the outer layer is made of the cornea in the anterior-central part of the eye. Around the cornea, the outer layer is the sclera, which is covered by the conjunctiva. Deep to the sclera is the choroid, which contains blood vessels. The retina is the innermost layer of the posterior eye.
Etiology and Epidemiology
Globe rupture can result from penetrating or blunt trauma. Penetrating trauma typically affects the anterior surface of the eye because of the protection of the posterior part by the orbit, but posterior penetrating trauma is possible if a projectile passes through the bony orbit or from major orbital fractures that lead to jagged orbital bone. Blunt trauma can cause rupture by compressing the eye and leading to hydraulic rupture of the sclera at its weakest points (the limbus and insertion points of the extraocular muscles).46 Common mechanisms include hammering, sawing, mowing, and grinding without eye protection.47 In children, glass, fireworks, and assault/abuse are the most common causes.
Presentation and Evaluation
Patients will present after blunt or penetrating trauma. They may report eye pain that may feel superficial with a foreign body sensation or feel deep and boring. Depending on the degree of the rupture, vision may or may not be affected. Like all the injuries discussed in this article, globe rupture frequently co-occurs with other types of ocular trauma. Be especially suspicious for globe rupture in patients with full-thickness eyelid lacerations, displaced orbital fractures, blowout orbital fractures, or traumatic hyphema.
The physical exam for globe rupture entails visual inspection of the eye, slit-lamp exam, and fluorescein staining. Signs concerning for globe rupture are:
- distortion of the pupil and iris (“teardrop-shaped” pupil);
- hyphema;
- pigmented tissue emanating from the sclera (protrusion of the choroid);
- significant subconjunctival hemorrhage (particularly if it encompasses all of the sclera);
- hemorrhagic chemosis.
CT of the orbit can show signs of rupture, but it is only moderately sensitive and should not be relied on to rule out rupture.48
Treatment and Disposition
Any of the mentioned physical exam findings in a child with ocular trauma warrants immediately stopping the evaluation, covering the eye with a protective shield, and contacting ophthalmology for emergent evaluation. Manipulation of the eye can increase the hydraulic pressure of the vitreous and aqueous humor. Pressure on a ruptured globe can worsen the injury by increasing the amount of fluid drainage and soft tissue protrusion from the defect. Do not test the intraocular pressure or perform point-of-care ultrasound on an eye that has obvious signs of rupture.
While awaiting consultation by ophthalmology, limit pressure to the eye. Elevate the head of the bed to 45 degrees and prescribe antiemetics to prevent vomiting, which is another potential source of increased intraocular pressure. Ensure the patient’s tetanus status is up to date. Endophthalmitis occurs in about 7% of cases of globe rupture, so broad-spectrum antibiotics with vancomycin and a third-generation cephalosporin are indicated.49
Retrobulbar Hematoma
Anatomy and Pathophysiology
The eye and surrounding soft tissues are contained in a cone of fascia within the orbital fossa. In the anterior aspect, this fascial cone is contained by the tarsal plates of the eyelids, which are anchored at the lateral eyelids by the canthal ligaments.50 Blunt trauma can lead to bleeding behind the globe of the eye, which accumulates into what is called a retrobulbar hematoma. Bleeding is most commonly from the infraorbital artery.51
Because the eye is contained by tough layers of fascia, bleeding can cause acute orbital compartment syndrome (AOCS). Like other types of compartment syndrome, the increased pressure of AOCS can lead to impaired perfusion and ischemia. In the case of the eye, ischemia can lead to permanent vision loss if not promptly treated.44 Even as little as 120 minutes of AOCS can cause permanent monocular blindness in a child.52
Etiology and Epidemiology
Any type of blunt trauma can cause retrobulbar hematoma. Falls, motor vehicle accidents, and assaults are the most common causes.53 It is more common in children with bleeding problems, such as hemophilia, von Willebrand disease, and patients on anticoagulation medications.
Presentation and Evaluation
Patients with retrobulbar hematoma will present after blunt facial trauma. They likely will complain of orbital pain and may report diplopia and monocular vision loss. The physical exam will show periorbital ecchymosis, dysconjugate gaze, afferent pupillary defect, and exophthalmos (most noticeable from the superior aspect).54 Because AOCS leads to vision loss after as little as 120 minutes, evaluate the intraocular pressure as soon as suspicion for retrobulbar hematoma arises from the physical exam and history. If intraocular pressure exceeds 40 mmHg, emergent lateral canthotomy is warranted. If the pressure is below that level, further evaluation with imaging should be ordered. CT orbit is the preferred imaging modality. Ocular point-of-care ultrasound can help rule out concomitant lens dislocation, retinal detachment, and vitreous hemorrhage, but ruling out retrobulbar hematoma is difficult because retrobulbar blood can appear isoechoic to retrobulbar fat.55,56
Treatment and Disposition
As mentioned, patients with AOCS warrant immediate lateral canthotomy to preserve vision. To perform lateral canthotomy, anesthetize the lateral canthus with 1% to 2% lidocaine. Clamp the lateral canthus with a straight Kelly clamp horizontally pointing toward the ear for about one minute to limit blood flow. Use scissors to cut the clamped tissue. Retract the inferior eyelid down and use fingers or an instrument to feel for the inferior ligament, which will feel like a tough band. Cut the inferior ligament with scissors and recheck pressure. If it is still above 40 mmHg then cut the superior ligament in the same way.34 Emergent consultation with ophthalmology is warranted in all cases of retrobulbar hematoma, even if patients do not have AOCS during assessment. While awaiting ophthalmologic evaluation, limit further increases in the patient’s intraocular pressure. Elevate the head of bead, provided the patient does not have concomitant injury that precludes this, and treat nausea to limit vomiting. Ophthalmology may recommend treatment with agents, such as mannitol, acetazolamide, or steroids, to decrease intraocular pressure. Patients typically will require admission to monitor serial pressures, but ultimate disposition is at the guidance of ophthalmology.
Summary
A summary of the pathologies discussed in this review, their associated findings, and steps for emergent management can be found in Table 1.
Table 1. Summary of Pediatric Ocular Trauma Pathologies |
||
| Pathology | Findings | Emergent Management |
Orbital fractures |
|
|
Eyelid laceration |
|
|
Subconjunctival hemorrhage |
|
|
Corneal abrasion |
|
|
Caustic injury |
|
|
Foreign body |
|
|
Hyphema |
|
|
Lens dislocation |
|
|
Traumatic iritis |
|
|
Retinal detachment |
|
|
Globe rupture |
|
|
Retrobulbar hematoma |
|
|
CT: computed tomography; POCUS: point-of-care ultrasound; IV: intravenous |
||
References
To view the references, visit bit.ly/3iwHWiR.
Pediatric ocular injuries are predominantly minor but may be devastating. The emergency provider must understand the anatomy and injuries that may result in significant damage and a timely critical approach to preserve the child’s vision.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
