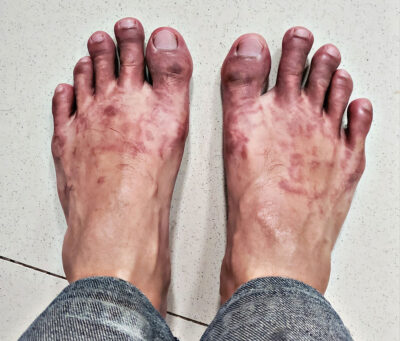
Hypothermia and Frostbite
July 15, 2024
Authors
Guhan Rammohan, MD, FACEP, Emergency Medicine Faculty, St. Luke’s Hospital, Bethlehem, PA
Joseph Skowronski, MD, Emergency Medicine Resident, St. Luke’s Hospital, Bethlehem, PA
Peer Reviewer
Sara W. Nelson, MD, MHPE, Associate Professor of Emergency Medicine, Tufts-Maine Medical Center, Portland
Executive Summary
- Hypothermia is present when the core body temperature is ≤35°C (95°F).
- Core body temperature can be actively monitored by placing a urinary catheter with a temperature probe.
- The expected physiologic responses to low temperatures can be overwhelmed by prolonged cold exposure leading to core hypothermia.
- Consider potential inciting factors when assessing the hypothermia patients: infection, myxedema coma, adrenal crisis, hypoglycemia, stroke, intoxication, and poisoning.
- Passive external rewarming techniques used in the treatment of mild hypothermia prevent further heat loss and supplement natural heat-producing mechanisms with an expected rewarming rate of 0.5°C to 2°C per hour.
- Active external rewarming techniques are used in awake, stable patients with mild to moderate hypothermia and typically raise body temperature around 2°C per hour.
- For hemodynamically stable patients with severe hypothermia, invasive internal rewarming techniques are used.
- A frostbitten extremity should be rapidly rewarmed in a water bath heated to 37°C to 39°C and rewarming is complete when the body part becomes red or purple in color and soft and pliable to the touch.
Case Introduction
A 72-year-old female is found after a neighbor performed a welfare check. As per emergency medical services (EMS), the neighbor had not seen the patient in a few days and was concerned for her well-being since she lives alone. He knocked on her door. After no response, he looked through the window and saw her slumped next to a chair in the front room. He called 911 immediately, and EMS responded quickly. The patient was found to be poorly responsive, mumbling nonsensical words, and unable to provide any history. She was noted to be covered in urine and feces. She was protecting her airway, so no advanced airway was established. Intravenous (IV) access was obtained with an 18-gauge catheter in her left antecubital fossa. Initial vital signs were temperature (T) 32°C tympanic, heart rate (HR) 55 beats per minute and regular, respiratory rate (RR) 10 breaths per minute, blood pressure (BP) 105/65 mmHg, and oxygen saturation (SpO2) 94%.
Point-of-care glucose was 45 mg/dL. An infusion of 100 mL of dextrose 10% in water (D10W) was given. EMS also initiated 1 L of normal saline. She was packaged onto a stretcher, covered in blankets, and transported to the nearest emergency department (ED).
On arrival to the ED, repeat pioint-of-care glucose is 120 mg/dL. She is placed on the monitor, and repeat vital signs are obtained: T 31.5°C rectal, HR 52 beats per minute and regular, RR 10 breaths per minute, BP 100/60 mmHg, and SpO2 94%. The patient is unable to provide any history secondary to her poor mental status. On physical exam, the patient is an ill-appearing, elderly woman with her eyes closed. She does not open her eyes to voice but does when squeezing her fingertips. She occasionally moans but does not speak sensible words. She withdraws to pain in all extremities. There are no signs of head trauma. Her neck is supple. Her pupils are 3 mm bilaterally and sluggishly reactive. Upon opening her mouth, her tongue is dry. Auscultation of the heart demonstrates a normal S1/S2 with no murmurs. Her lungs are clear to auscultation bilaterally. Her abdomen is soft and not distended. Her clothing, which is swaroaked with urine and feces, is removed. She is rolled for a full body skin and extremity exam, which shows no wounds, lesions, or rashes. Her skin feels cold and dry.
The ED initiates a workup including a complete blood count (CBC), comprehensive metabolic panel (CMP), urinalysis, thyroid function test (TSH with free T4), creatine kinase (CK), blood cultures, lactic acid, activated partial thromboplastin time (aPTT), prothrombin time/international normalized ratio (PT/INR), electrocardiogram (ECG), computed tomography (CT) head, and portable chest radiograph.
What Is Hypothermia?
Hypothermia is a medical emergency that requires immediate attention and correction. Hypothermia typically is defined as a core temperature ≤ 35°C (95°F).1-3 Hypothermia has been further classified by two widely used systems, the Swiss system and the Wilderness Medical Society classification, which suggest the expected physiologic changes and physical exam findings in addition to guiding the recommended interventions to restore a patient to normothermia.1-3 (See Tables 1 and 2.)
Table 1. Comparison of Two Common Classification Systems |
|||
Swiss Classification |
Temperature |
Wilderness Medical Society Classification |
Temperature |
Stage 1 |
32-35°C, 90-95°F |
Mild |
32-35°C, 90-95°F |
Stage 2 |
28-32°C, 82-90°F |
Moderate |
28-32°C, 82-90°F |
Stage 3 |
24-28°C, 75-82°F |
Severe |
<28°C, <82°F |
Stage 4 |
13.7-24°C, 57-75°F |
||
Stage 5 |
< 13.7°C, < 57°F |
||
Note that there are the same temperature ranges between stages 1 and 2 of the Swiss classification and the mild and moderate Wilderness Medical Society classifications, respectively.2,3 |
|||
Table 2. Common Physical Exam Findings and Physiological Changes in the Classes of Hypothermia as per the Wilderness Medical Society Classification1,3 |
||
Mild Hypothermia |
Moderate Hypothermia |
Severe Hypothermia |
|
|
|
ECG: electrocardiogram; EEG: electroencephalogram |
||
Obtaining a Core Temperature
When evaluating a patient for hypothermia, it is imperative to obtain a core temperature. Although a pulmonary artery catheter is the gold standard, the best alternative is an esophageal probe in the lower one-third of the esophagus.3,4 In the ED for patients without an advanced airway, the recommended routes are rectal, bladder, or epitympanic. Although rectal and bladder measurements are relatively easy to obtain, they can lag behind true core temperature in severe hypothermia.4
Core temperature can be actively monitored after placing a bladder Foley with a temperature probe; this also provides the additional benefits of urinalysis, accurate monitoring of urine output, and relief of possible urinary retention. Epitympanic thermometers use a soft probe that should abut the tympanic membrane to measure carotid artery temperature. These should not be confused with the more common tympanic thermometers.
Oral, axillary, tympanic, and temporal thermometers poorly correlate with true core temperature as their readings are easily confounded by the environment or improper measurement technique; therefore, these should be avoided if possible.4-6 It is reasonable to start with a screening temperature via the oral, tympanic, or temporal route, but when a patient is suspected to be hypothermic, a core temperature should be obtained to accurately guide treatment.
Epidemiology of Hypothermia
Hypothermia is a disease that has caused morbidity and mortality throughout human history. About 700 to 1,500 hypothermia-related fatalities are reported in the United States each year.7 The condition most frequently affects adults between the ages of 30 and 49 years, with occurrence 10 times greater in men than women. Homeless patients, cold weather adventurers, and those with malfunctioning heating systems at their homes, in addition to those with dementia or mental illness, are at high risk for environmental exposure.8 Infants and older adults are at higher risk because of inadequate muscle tone and less efficient rewarming mechanisms. Compared to patients with other chief concerns presenting to the ED, patients with hypothermia were more likely to be older or uninsured.9
Hypothermia leading to cardiac arrest is quite rare, with the largest centers rarely treating more than 20 patients annually and the largest studies only including a few hundred patients.7 ED patients with hypothermia were more likely to need critical care admission compared to other chief complaints.9 The lowest recorded core temperature from which a patient with accidental hypothermia was successfully resuscitated was 13.7°C; this patient had a good physical and mental recovery.10
Quick recognition and intervention for hypothermia is vital, as delayed recognition is linked to poor outcomes in numerous situations, including critical illness, infection, burns, trauma, and surgery.11-14 Additionally, hypothermia contributes to postoperative wound infection, adverse myocardial events, increased blood loss through coagulopathy, decreased metabolism of sedative drugs, and overall increased mortality.11-14
Physiologic Response to Hypothermia
The hypothalamus — the center of thermoregulation of the body — is responsible for setting the body’s thermic set point and supervising temperature homeostasis. It receives temperature information from peripheral and central thermoreceptors of the skin, viscera, and spinal cord and, accordingly, activates heat regulation mechanisms to adjust the temperature.15 There is some developing evidence that body temperature is not managed solely by a specific hypothalamic set point, but rather a collective temperature average of countless independent thermoeffector loops of afferent and efferent neurons.4,16
Hypothermia generally occurs when the body releases more heat than it generates or absorbs, whether from environmental exposure or deficiencies in the body’s innate mechanisms for regulating body temperature and generating heat. Body heat is lost to the environment via four mechanisms: radiation, conduction, convection, and evaporation.1,17 Radiation is heat transfer through electromagnetic waves, such as heat loss from an exposed head or scalp to the colder environment. Conduction occurs through direct contact with colder substances, and heat losses are considerable during immersion injuries. Convection occurs with the movement of fluids, whether gas or liquid, and heat losses are substantial in windy conditions. Evaporation occurs when heat is lost through the process of sweat or other surface liquids turning to gas from body heat.
When the hypothalamus senses too low of a temperature, it responds by activating cellular cascades and signaling to raise the body temperature. Some of the body’s responses include arteriolar and arteriovenous vasoconstriction; piloerection; skeletal muscle shivering; increasing metabolic rate via adrenal and thyroid glands; and increasing activity via movement, huddling, and eating.1,15,18,19 Unfortunately, these processes can become overwhelmed with prolonged cold exposure or acquired deficits in these pathways, which leads to core hypothermia.
Causes of Hypothermia
When a patient presents with hypothermia, it usually is from impaired thermoregulation, decreased heat production, or increased heat loss.7 Hypothermia can be multifactorial, and it is important to consider medical causes in addition to environmental exposures. Hypothermia can be induced by medications or drugs such as ethanol, opioids, tricyclic antidepressant overdose, beta-blocker overdose, clonidine overdose, inhaled anesthesia, carbon monoxide (CO) poisoning, or lithium toxicity.1,20,21
Other major inciting factors may be systemic illnesses that alter temperature regulation such as infection, myxedema coma, adrenal crisis, hypoglycemia, strokes, intoxication, poisoning, trauma/blood loss, burns, vitamin deficiencies such as thiamine, and states of shock.1,20,21 Iatrogenic hypothermia may be induced after massive transfusion or major surgery.1,20,21
Treatment for Hypothermia
The mainstay of treatment for hypothermia is supportive care, rewarming, and focused treatment targeting the cause of the hypothermia. Prehospital and initial management of the hypothermic patient requires support of the airway, breathing, and circulation. Special care must be taken in moving the hypothermic patient, as rough handling can precipitate arrhythmias due to the high myocardial irritability. Endotracheal intubation may be considered as an early intervention in those patients with altered mental status or a decreased cough reflex, as this can help clear secretions produced by cold-induced bronchorrhea.
Although most hypothermic patients should be brought to a hospital for rewarming, there is indication for termination in the field if there are other fatal injuries such as decapitation, open head injury with loss of brain matter, truncal transection, incineration, or a chest wall so stiff compressions are not possible.22
To obtain an accurate temperature and guide rewarming, a core temperature should be obtained, ideally rectal, bladder, esophageal, or epitympanic. Immediately check a point-of-care glucose on all patients and administer IV glucose if below 70 mg/dL.
Detection of a pulse in patients with hypothermia can be challenging. It is recommended that pulse checks are done for a full minute and from a central pulse, specifically for patients who are severely hypothermic (< 28°C).3 Helpful adjuncts for accurately assessing for a pulse include Doppler sonography and bedside transthoracic echocardiography. End-tidal CO2 monitoring can be helpful as its presence can correlate with the presence of vital signs and physiologic function.7
If there is no pulse after one minute, cardiopulmonary resuscitation (CPR) should be commenced.3 If indicated, defibrillation can be attempted at < 30°C, but if unsuccessful, it is recommended to wait until core temperature has reached 30°C to try further defibrillation or antiarrhythmic medications, as repeat attempts are unlikely to be successful.3,21
Passive and active rewarming techniques should commence immediately depending on the initial temperature. It is essential to warm the thorax before the extremities to avoid a worsening core hypothermia that can develop when cold, acidic blood returns to the heart that can cause hemodynamic instability through hypotension and arrhythmia.23,24
If the patient appears clinically hypovolemic, warmed IV fluids should be infused using an in-line warming device. If this device is unavailable, there have been reports of microwaving fluids for around two minutes on high setting then shaking to evenly distribute the fluid temperature to obtain an adequate warmed temperature (< 40°C).25 Warmed IV fluids are not effective to raise body temperature but will prevent iatrogenic hypothermia, which can occur when room temperature fluids are infused.
Examine the patient for evidence of frostbite, especially in cases of severe hypothermia, and treat accordingly. A screening ECG is helpful to evaluate for cardiac conduction abnormalities and can suggest possible electrolyte abnormalities. The characteristic J wave (Osborn) wave may be seen in precordial leads. This ECG finding is a distortion of the beginning of ventricular repolarization seen as an elevation of the J point. (See Figure 1.)
Figure 1. ECG with Osborn Waves |
A positive deflection at the junction of the QRS-complex and the ST-segment, best seen in leads II, III, and V2-V5 |
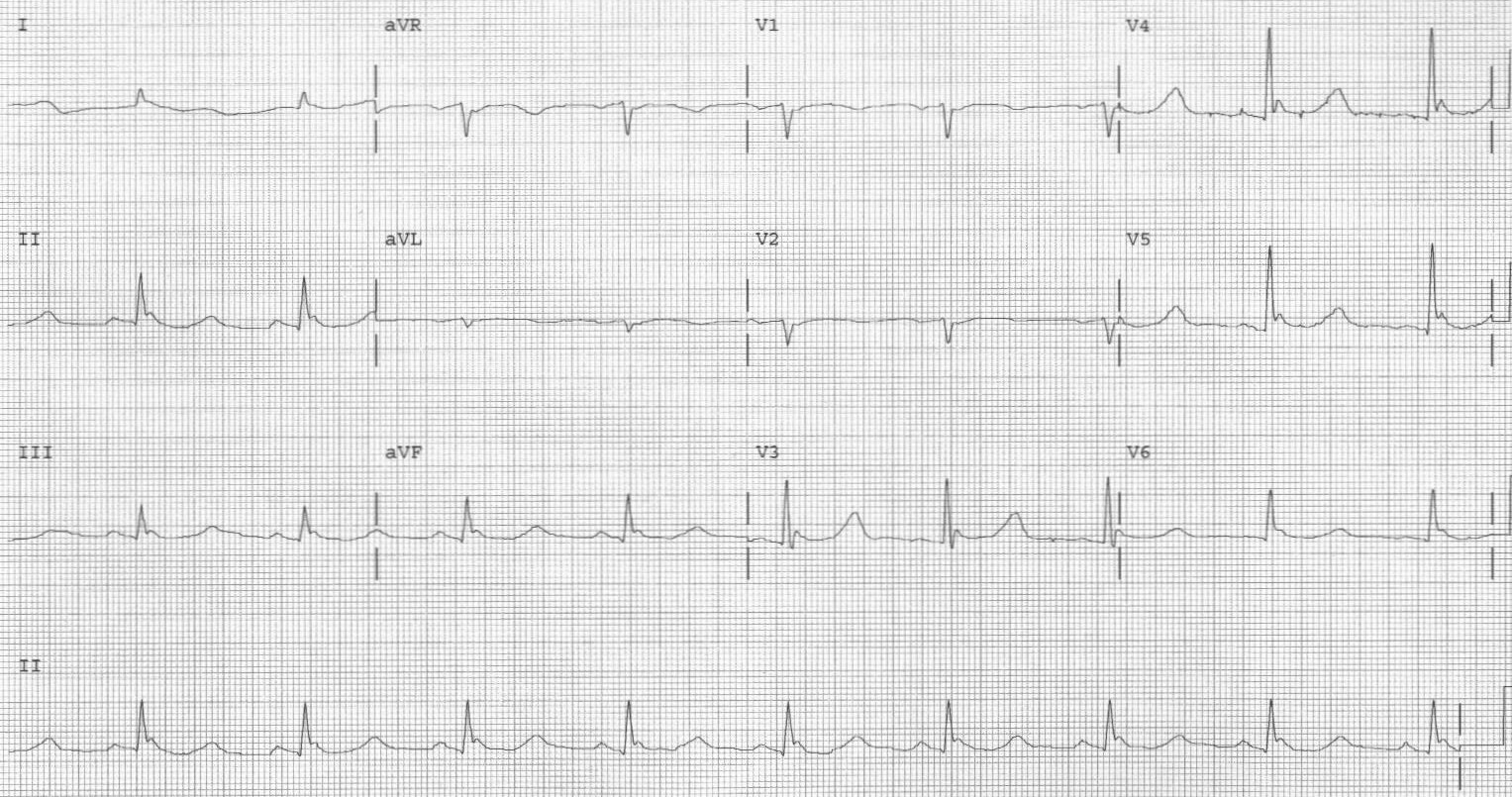 |
Image courtesy of J. Stephan Stapczynski, MD |
Guided by the patient’s condition, medical history, and physical examination, obtain pertinent laboratory tests and imaging modalities. A basic laboratory workup should include at least a CBC and CMP. Other possible tests to consider include blood cultures, urinalysis, cortisol, TSH with free T4, coagulation studies, fibrinogen, lactic acid, lipase, and CK. Obtain imaging studies as clinically indicated.
To officially declare a hypothermic patient dead, the patient should be warmed and CPR provided. While there is not a specific threshold temperature as per guidelines such as the Wilderness Medical Society, a review article from Brown et al recommends a minimum of 32°C.20 If there is no evidence of cardiac activity on bedside ultrasound after rewarming, this is an indication to terminate resuscitation efforts if no other reversible causes are identified. Hyperkalemia over 12 mmol/L, specifically over 14 mmol/L, has been associated with incredibly poor outcomes and is a reason to terminate resuscitation even before rewarming.3,26,27
Rewarming Strategies
Passive external rewarming techniques can be used for mild hypothermia. Remove all cold and/or wet clothing and place the patient into dry clothing or blankets. The patient’s own shivering reflex is helpful to generate significant internal heat. These passive techniques prevent further heat loss and supplement the body’s natural heat-producing mechanisms, and the expected rewarming rate is 0.5°C to 2°C per hour.28 Should the patient not meet this target, active rewarming strategies should commence.28 Always consider medical causes of hypothermia besides environmental exposure.
When passive modalities are insufficient, start active external rewarming strategies. In most ED encounters, this will be done in parallel with passive rewarming. Techniques include warmed blankets, raising the temperature setting in the room, forced air conduction systems such as the Bair Hugger, and heating pads such as the Arctic Sun. Infants should be placed in a radiant heater device. Monitor for signs of early skin changes that could indicate developing burns in patients on radiant heaters. Active external rewarming should raise body temperature around 2°C per hour.20 There is no evidence that one active external rewarming technique is better than any other.
When active external rewarming is unsuccessful, or the patient is severely hypothermic, invasive rewarming techniques should be commenced. Rewarming via the lungs with high-flow nasal cannula or positive airway pressure set at the highest temperature settings is not particularly effective at raising core temperature but is helpful to prevent heat loss from alveolar gas exchange. If the patient is intubated, the ventilator circuit can be set at maximum temperature settings.
For hemodynamically stable patients, an endovascular temperature control catheter (Quattro, Icy, and Cool Line, Zoll Medical Corporation) can be used if available. This device is placed into a large vein, usually the femoral vein, and actively warms blood by circulating temperature-controlled water through the catheter.29 However, an observational retrospective study found that the endovascular temperature control catheter did not produce increased rates of rewarming or reduce mortality when compared to other methods of active rewarming.30 There is a heat exchange device placed into the esophagus that has shown success in active rewarming although it is unfortunately not widely available.31
For patients with hemodynamic instability, those unresponsive to pressors and inotropes, and severe hypothermia (< 28°C), extracorporeal life support (ECLS) — such as venous-arterial extracorporeal membrane oxygenation (VA ECMO), venous-venous extracorporeal membrane oxygenation (VV ECMO), or cardiopulmonary bypass (CPB) — is indicated to reliably and efficiently raise body temperature through an external warming circuit.3,32
If these options are available, it would be prudent to involve the appropriate ECLS team immediately while also performing passive and active rewarming.3 VA ECMO is preferred over VV ECMO given the likely cardiac instability of these patients. These techniques have shown to raise body temperature 3°C to 6°C per hour. ECLS requires systemic anticoagulation to prevent thromboembolism with the potential of increasing the already high bleeding risk from hypothermia.20
Hemodialysis also is an option if ECMO is not available. Blood can be exchanged through the external heating element to raise the temperature about 3°C per hour.33
If ECLS is not available, lavage techniques can be considered. Lavage historically has been performed via the thorax, peritoneum, gastrointestinal tract, or bladder and should be performed with warmed (< 40°C) isotonic saline. Thoracic lavage is ideally performed in the right thorax, as the left thorax is closer to the irritable heart and can trigger arrhythmias.34,35 Bilateral pleural lavage should be avoided in the perfusing patient but can be considered for a patient in cardiac arrest.34,35
A single or double chest tube setup can be used to bathe the pleura, but a double tube setup is more efficient. Ideally, two large-bore thoracostomy tubes are placed to allow infusion and drainage of saline. Historically, the bore is 36-40 Fr, but a smaller sized tube should allow adequate flow rates.34,35 One tube should be placed superior and anterior with the other tube placed inferior and posterior in the pleural cavity.34,35 Between 200 mL to 300 mL warmed saline should be infused at a time through the anterior tube and then drained posteriorly.34,35
Peritoneal irrigation for rewarming is similar to performing a diagnostic peritoneal lavage. Lastly, bladder irrigation can be used with a Foley catheter. Generally, this is less effective than pleural or peritoneal irrigation because of the bladder’s small volume and longer irrigation cycle turnover; therefore, it is not recommended. Gastric and colonic lavage should be avoided because of aspiration risk and the likelihood to cause electrolyte abnormalities, further predisposing to arrhythmia.
Complications from Hypothermia and Rewarming
The presence of hypothermia in addition to the eventual return to normothermia for patients who survive unfortunately are not without their own comorbid sequelae. Hypothermic patients are prone to cold-induced diuresis and acute kidney injury; therefore, frequent assessment of volume status is key. Cold exposure to the external body can lead to rhabdomyolysis and subsequent hyperkalemia and frostbite. The prompt awareness and treatment of frostbite can salvage limbs.
Internal hypothermia can lead to pulmonary edema, ataxia, pancreatitis, and cardiac arrhythmias.24,36 During the rewarming process, complications include stress cardiomyopathy, systemic inflammatory response, significant electrolyte shifts, rhabdomyolysis, cardiac arrhythmias, rewarming vasoplegic shock, infection, coagulopathy, and alterations in glucose homeostasis.24,36
Frostbite Introduction
For patients with environmental exposures, especially freezing ambient temperatures, cold water, snow, and ice, frostbite injuries are of significant concern. Patients who are outside, such as cold weather recreationalists, outdoor workers, the homeless, those stranded outdoors in cold weather, and those with inoperable home heating, are at high risk of experiencing frostbite.
Alcohol, other substance use, dementia, and mental illness can alter decision-making for patients already at risk for environmental hypothermia and increase their risk for frostbite due to increased exposure. Patients with medical comorbidities, such as peripheral vascular disease, diabetes, and neuropathy, are at increased susceptibility to frostbite.37 However, most cases of frostbite are seen in adults between 30 and 49 years of age due to increased cold exposure and risk-taking behavior.37,38
Frostbite is defined as tissue damage caused by heat loss sufficient to cause ice crystal formation in superficial or deep tissues.39 Factors that contribute to frostbite include the exposing temperature, wind chill factor, length of exposure, and what area of the body is exposed.40 Due to their peripheral anatomical position and more exposed nature, the fingers, toes, nose, ears, and cheeks are most vulnerable to developing frostbite. Cases of buttock and perineum frostbite have been reported from patients sitting on cold metal seats, and penile frostbite has been observed in joggers and skiers.37 Corneal frostbite has been reported in people exposed to strong, freezing winds.41 However, the hands and feet account for 90% of frostbite injuries.37
Frostbite Pathophysiology
When the entire body is exposed to a cold environment, the body’s physiological response is peripheral vasoconstriction to shunt blood away from the extremities to the vital organs of the core.42,43 This combination of external cold exposure and vasoconstriction leads to localized hypothermia, usually of the distal or most exposed parts of the body.42,43 As the local temperature drops, ice crystals will begin to form around 0°C (32°F).39,42,43
As this freezing occurs, the tissues and vessels begin to expand, causing vascular permeability, fluid shifts, and continued freezing.42,43 A cascade of decreased blood flow and increased permeability of warmed, oxygenated blood leads to an expanding area of frostbite.42,43 Decreasing temperatures cause hypercoagulability and localized thrombosis, further increasing ischemia.42,43 Localized cold exposure may generate a paradoxical vasodilation to increase blood flow to the cold area.39,44 However, eventually vasoconstriction will predominate.39,42,43
The increased permeability may lead to blister formation as fluid translocates to the superficial skin layers.42,43 As the frozen area extends deeper into the tissues, vascular damage can occur, leading to impaired blood flow, and nerve damage can occur, leading to desensitization.42,43
Defining Frostbite
Frostnip involves severe vasoconstriction of the skin with frost on the surface but without deeper skin involvement; it fully resolves after rewarming.39 Frostnip can be a precursor to frostbite but is not always present or a requirement.39
Frostbite traditionally has been categorized into four tiers of injury similar to the classification scheme for thermal burn injuries.39 First-degree presents with numbness and erythema, and a white or yellow firm; slightly raised plaque develops in the area of injury.39 No gross infarction occurs, and there may be some epidermal sloughing.39 Second-degree shows evidence of superficial skin vesiculation.39 A clear or milky fluid is present in the blisters with surrounding erythema and edema.39 Third-degree causes deeper hemorrhagic blisters with injury extension into the deeper vascular tissues of the dermis. 39 (See Figure 2.) Lastly, fourth degree extends fully through the dermis and involves muscle and possibly bone.39
Figure 2. Left Foot Third-Degree Frostbite |
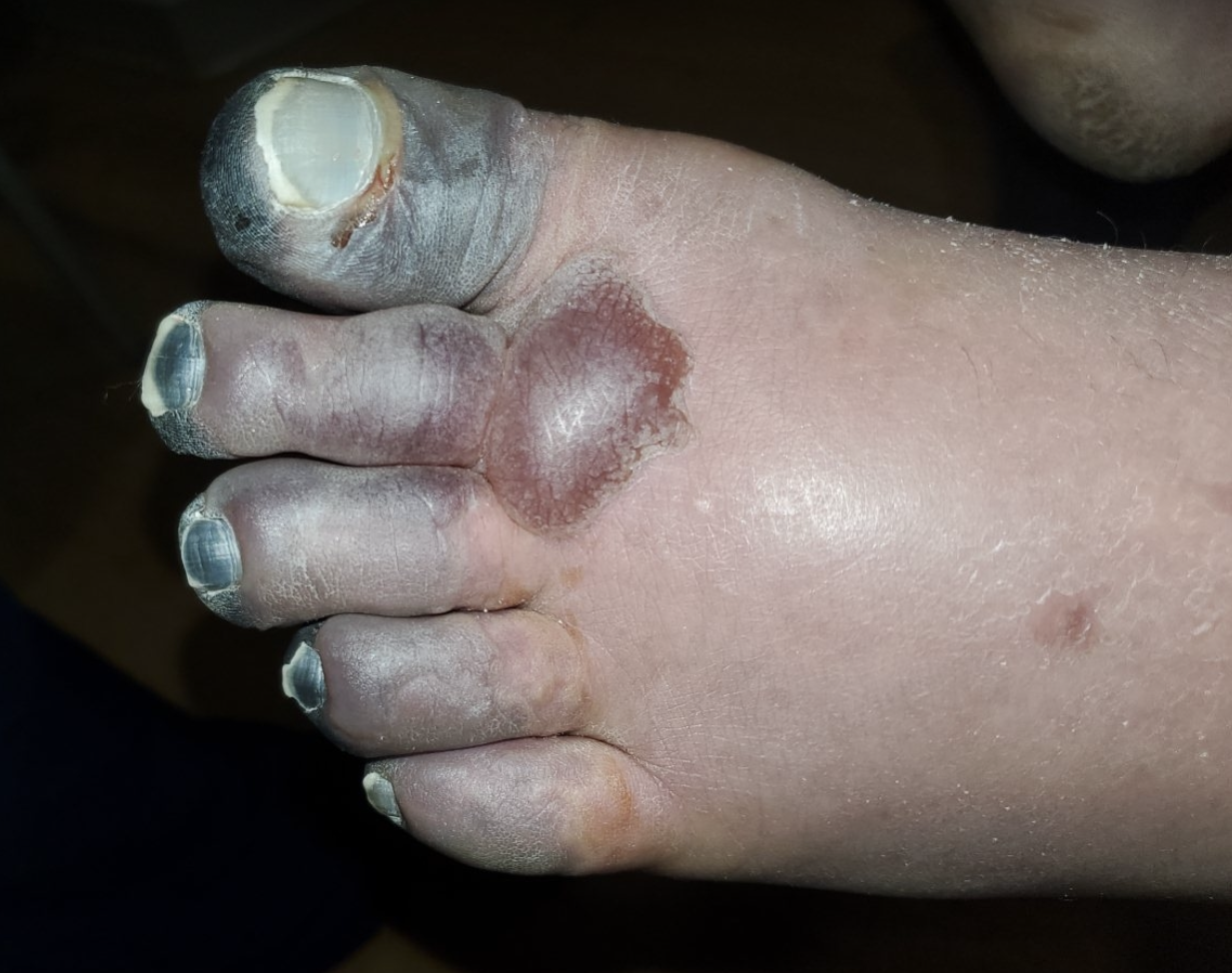 |
CheckDO, CC BY-SA 4.0 https://creativecommons.org/li..., via Wikimedia Commons |
A more preferred classification of frostbite is the Cauchy method. (See Figure 3.)This classification is preferred because it measures the anatomical extent of frostbite for the distal extremities after rewarming. The grading scale is from 1 to 4 and can provide an expected clinical prognosis.45 Grade 1 is defined by no cyanosis of the extremity and predicts no amputation or sequela.45 Grade 2 involves cyanosis of the distal phalanx and predicts only soft tissue amputation and fingernail or toenail sequela.45 Grade 3 is characterized by intermediate and proximal phalangeal cyanosis and predicts bone amputation of the digit with functional sequela.45 Lastly, grade 4 involves cyanosis over the carpal or tarsal bones and predicts limb amputation with functional sequela.45
Figure 3. Cauchy Classification with Physical Exam Findings and Prognosis |
||||||
Extent of Cyanosis After Rewarming |
Bullae After Rewarming |
Bone Scan Findings at Day 2 |
Hand |
Foot |
Prognosis |
|
Grade 1 |
Absent |
Absent |
N/A |
 |  | No amputation risk |
Grade 2 |
Only on distal phalanx |
Clear bullae |
Hypofixation of radiotracer uptake area |
 |  | Amputation risk |
Grade 3 |
Proximal phalanx involvement |
Hemorrhagic bullae on digits |
Absence of radiotracer uptake area on the digit |
 |  | Amputation risk |
Grade 4 |
Carpal/tarsal involvement |
Hemorrhagic bullae over carpal/tarsal |
Absence of radiotracer uptake area on the carpal/tarsal |
 |  | Amputation risk |
Frostbite grading (grades 1-4) is assigned through the presence and extent of cyanosis and bullae as well as bone scan findings. Grade 1 is the least severe form and typically displays no cyanosis and portends no amputation risk to patients. Grade 2 often displays cyanosis on distal phalanx and patients have less than 1% amputation risk. For grade 3, cyanosis at proximal phalanx suggests an amputation risk of 23% to 83%. Grade 4 frostbite is the most severe form of frostbite injury with carpal/tarsal involvement and a 99% amputation risk. Adapted from: emottawablog.com/wp-content/uploads/2023/08/Classification-of-frostbite-2-595x643.pngFree Open Access Medical Education content by EMOttawa is under a Creative Commons Attribution-NonCommercial ShareAlike 4.0 International License |
||||||
This classification may be helpful in expectant amputation risk for a patient with grade 3 and 4 more predictive of the need for amputation and systemic involvement including risk of subsequent infection.45 Recognition of early frostbite may be difficult in patients with darker skin tones.
Management of Frostbite
Unfortunately, there is a paucity of high-quality evidence to guide frostbite management, so multiple guidelines that have been developed provide mostly suggested recommendations based on expert opinion.42
Initial management of frostbite, which in most cases occur in the prehospital setting and is done by EMS personnel, is to remove the patient from the cold environment into a warm environment. Remove all cold or wet clothing and place the patient into dry clothing. All jewelry should be removed in anticipation of possible swelling and to prevent subsequent tourniquet formation.37,39 When outside the hospital, field rewarming should commence only if the frozen part can be kept thawed and warm until the patient arrives to the hospital for definitive care as refreezing can cause more tissue damage.39
Once the patient reaches the hospital, initial care consists of treating life-threatening conditions, including trauma and hypothermia; rapid rewarming; wound care; efforts to enhance tissue viability; and prevention of complications.
Rapid rewarming with a water bath heated to 37°C to 39°C should commence once the patient has reached a warmer environment.37 As the injured body part begins to warm, the water bath subsequently will cool. Ideally, the water bath is an actively warmed system. However, if there is no active heating component and to avoid the water bath becoming too cool, a larger volume bath is preferred or another water bath can be warmed while the other is warming the body part.
Rewarming is complete when the body part becomes red or purple in color and soft and pliable to touch. Adding an antiseptic solution to the water bath has not been shown to reduce the risk of future infection, but there does not appear to be any harm.39
Rewarming is incredibly painful, so pain medications including nonsteroidal anti-inflammatory drugs (NSAIDs), which theoretically help reduce local inflammation during the rewarming process, and opioid medications should be provided judiciously. For areas not amenable to a warming bath, the patient should rewarm in a warm room and can place their own warmed hands to the area or cover the area with a heated blanket.
After rewarming, wound care techniques can commence. Pat dry the affected area with a dry towel and apply aloe vera ointment, which shows some evidence to improve outcomes by reducing the inflammatory cascade, and then cover with a bulky, loosely wrapped, dry dressing.39,42,46
If blisters are present, there is not convincing evidence whether they should be aspirated or left alone. Some authors suggest aspirating blisters can decrease localized inflammation while others have concerns for increased risk of subsequent infection.8,37 However, hemorrhagic blisters should not be aspirated.
Antibiotics are not indicated routinely but can be considered for comorbid conditions.39 Update tetanus vaccination if needed. Depending on where the frostbite is located, early consultation with an appropriate surgical specialist with experience managing frostbite is recommended, especially if the patient ultimately will need surgical debridement.
For those patients with small areas of frostbite or superficial frostbite, a nonadherent gauze can be placed as the first dressing layer and then a loose bulky dressing can be placed. If involving the digits, it is recommended to insert pledgets between digits to prevent tissue maceration. These patients should be given follow-up with a surgical specialist.
The role of imaging in acute evaluation of frostbite is limited. Plain radiographs are useful only to screen for trauma-related fractures. CT angiography and bone scans have been used historically to determine candidates for thrombolytic therapy; however, this practice has been questioned because imaging can delay the warm ischemia time, and thus is not recommended.47
After involving the appropriate specialist, deeper injuries may be investigated using angiography or technetium-99 bone scans to determine the depth of involvement, provide prognosis, and guide amputation if necessary, but usually only in the subsequent days or weeks after the injury.37,48 Bone scan and magnetic resonance imaging are considered the gold standard tests to assess tissue viability. Plain radiographs, if obtained as part of a routine workup for other causes in the ED, may show bony destruction or damage to growth plates in children.49
If there is not a reported history of a thaw and refreeze cycle, infusions with tissue plasminogen activator (tPA) or iloprost can be considered to enhance tissue viability. Although the studies in support of tPA and iloprost are small in enrollment population, they do show significant decreases in amputation rates.42,50,51 For patients with Cauchy grade 3 and 4 frostbite, IV thrombolytic therapy can be administered to decrease the chance of future amputation.52,53
As with consideration of systemic thrombolytic therapy for any condition, evaluate the patient for any contraindications. The typical IV dosing of tPA is 0.15 mg/kg bolus followed by 0.15 mg/kg/hour for six hours for a maximum administration of 100 mg.54 If intra-arterial administration is an option, a recommended regimen is a 2 mg to 4 mg bolus followed by 1 mg/hour for 24-48 hours along with heparin at a rate targeted to twice the control PTT value.37 An alternative to IV heparin is 1 mg/kg enoxaparin twice daily for 14 days.54
If IV iloprost is available, it can be considered instead of tPA, as it has shown to significantly reduce the rate of amputation. Current guidelines recommend IV iloprost for Cauchy grade 2-4 frostbite, especially considering its notable safety profile.39,42 The recommended infusion of IV iloprost is 0.5 ng/kg/min to 2 ng/kg/min increased every 30 minutes by 0.5 ng/kg/min until the patient develops unacceptable or intolerable side effects such as headache or hypotension, then reduce the rate by 0.5 ng/kg/min and remain at that rate. The infusion should go for six hours per day for five to eight days at the highest tolerated rate.37,55
Iloprost (Aurlumyn) was approved by the Food and Drug Administration to treat frostbite in the United States in February 2024, and may not be widely accessible to use for frostbite treatment. The decision between infusing tPA or iloprost should involve the appropriate specialist consultants and pharmacy team, the capabilities of the hospital, and the patient’s risk factors for adverse events from administration. Regardless of the medication decision, a delayed time to administration has been shown to increase risk of amputation.56
Long-Term Sequela of Frostbite
The most feared complication of frostbite is amputation leading to significant functional morbidity. Fortunately, frostbite is not a disease of mortality, but it does suggest global hypothermia, which should be evaluated as hypothermia is a sign of impending mortality. Admission should be considered on a case-by-case basis and may require transfer to a burn center.
Superficial frostbite can likely be discharged home with follow-up. Deeper frostbite is more likely to succumb to complications such as amputation, compartment syndrome, and infection. Fasciotomy should be performed if there is clinical evidence of compartment syndrome, and compartment pressures can be verified with an intra-compartmental pressure monitor if needed.
It can take up to three months for final demarcation of tissue necrosis and eventual development of dry gangrene, so amputation can be delayed assuming there are no other emergent concerns present. However, if the patient shows signs of local infection or systemic sepsis, amputation should be performed immediately.39
Chronic nerve damage is common with frostbite and neuropathy may be amenable to medications commonly used for other neuropathies. Some patients develop hypersensitivity and others hyposensitivity in the frostbitten area. Chronic arthritis of the affected hands and feet joints is common. Early changes seen are flexion contractures of the proximal interphalangeal joints that may improve in the subsequent months or unfortunately become permanent.57
Patients who experience frostbite should avoid cold exposure for six months after minor frostbite and 12 months after significant frostbite, as they are at an increased risk of developing frostbite again due to impaired cold perception.58
Conclusion
Hypothermia often is an easy diagnosis to make, but it is a condition that must be considered in the setting of altered mental status, medical illness, trauma, and toxic ingestions. Treatment strategies depend on the severity of the hypothermia. Mild hypothermia can be treated with passive rewarming, while those with severe hypothermia should be treated with more active core rewarming techniques. Patients who are unstable or presenting with cardiac arrest will require more advanced management, including invasive rewarming techniques, vasoactive medications, CPR, and possibly ECLS.
Frostbite is a common problem during winter months and is associated with high morbidity. Any part of the body can be affected, with fingers, toes, ears, and nose being the most common areas. Management includes first addressing life-threatening issues, such as trauma and hypothermia. The goal of the initial care is to rapidly rewarm the affected areas so as to provide the best chance for tissue viability and to prevent complications, such as infection, compartment syndrome, and amputation. Good wound care, possible infusions of thrombolytics or iloprost, and early specialist involvement have all also been shown to improve morbidity secondary to frostbite.
Case Conclusion
The patient has an additional 18-gauge catheter placed in the right forearm, and another 1 L of warmed normal saline is given. After blood cultures and urine are obtained, 1 g of ceftriaxone is empirically started for possible infection. After 30 minutes of warmed blankets and IV fluids, her rectal temperature is 31°C. A glucose recheck is 115 mg/dL. A Foley with a temperature probe is placed for continuous temperature monitoring and a more active rewarming technique is employed with a convective temperature blanket (Bair Hugger). The patient’s ECG shows sinus bradycardia with J waves noted.
The patient’s metabolic panel is significant for a potassium of 5 mmol/L, bicarbonate of 17 mmol/L, Cr of 1.8 mg/dL, and a glucose of 125 mg/dL. Her CBC shows an elevated white blood count (WBC) of 15,000/uL. Her TSH and T4 are within normal limits. Her lactate is elevated at 3.5 mmol/L, and her CK is elevated at 1,800 U/L. The patient’s urinalysis is positive for large leukocytes and nitrites with innumerable WBC and bacteria. CT head showed no intracranial abnormalities, and chest radiograph showed no infiltrates.
Two hours after the initiation of treatments, she has repeat vital signs with a bladder temperature of 33°C, HR 62 beats per minute and regular, RR 12 breaths per minute, BP 120/75 mmHg, and SpO2 95%. Her mentation starts to improve, but she still is speaking nonsensical words. The patient is admitted for hypothermia secondary to presumed sepsis from a urinary tract infection.
References
- McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician 2004;70:2325-2332.
- Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol 2003;4:99-103.
- Dow J, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society Clinical Practice Guidelines for the Out-of-Hospital Evaluation and Treatment of Accidental Hypothermia: 2019 Update. Wilderness Environ Med 2019;30(4S):S47-S69.
- Hymczak H, Golab A, Mendrala K, et al. Core temperature measurement-principles of correct measurement, problems, and complications. Int J Environ Res Public Health 2021;18.
- Erickson RS, Kirklin SK. Comparison of ear-based, bladder, oral, and axillary methods for core temperature measurement. Crit Care Med 1993;21:1528-1534.
- Lefrant JY, Muller L, de La Coussaye JE, et al. Temperature measurement in intensive care patients: Comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med 2003;29:414-418.
- Paal P, Pasquier M, Darocha T, et al. Accidental hypothermia: 2021 update. Int J Environ Res Public Health 2022;19.
- Biem J, Koehncke N, Classen D, Dosman J. Out of the cold: Management of hypothermia and frostbite. CMAJ 2003;168:305-311.
- Baumgartner EA, Belson M, Rubin C, Patel M. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med 2008;19:233-237.
- Gilbert M, Busund R, Skagseth A, et al. Resuscitation from accidental hypothermia of 13.7 degrees C with circulatory arrest. Lancet 2000;355:375-376.
- van Veelen MJ, Brodmann Maeder M. Hypothermia in trauma. Int J Environ Res Public Health 2021;18.
- Kurz A. Thermal care in the perioperative period. Best Pract Res Clin Anaesthesiol 2008;22:39-62.
- Driver J, Fielding A, Mullhi R, et al. Temperature management of adult burn patients in intensive care: Findings from a retrospective cohort study in a tertiary centre in the United Kingdom. Anaesthesiol Intensive Ther 2022;54:226-233.
- Drewry A, Mohr NM. Temperature management in the ICU. Crit Care Med 2022;50:1138-1147.
- Osilla EV, Marsidi JL, Shumway KR, Sharma S. Physiology, Temperature Regulation. StatPearls. StatPearls Publishing; 2024.
- Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol 2018;156:3-43.
- Hanania NA, Zimmerman JL. Accidental hypothermia. Crit Care Clin 1999;15:235-249.
- Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ 2015;39:139-148.
- Paal P, Brugger H, Strapazzon G. Chapter 33. Accidental Hypothermia. In: Handbook of Clinical Neurology. Elsevier; 2018;157:547-563.
- Brown DJ, Brugger H, Boyd J, Paal P. Accidental hypothermia. N Engl J Med 2012;367:1930-1938.
- Rathjen NA, Shahbodaghi SD, Brown JA. Hypothermia and cold weather injuries. Am Fam Physician 2019;100:680-686.
- Paal P, Milani M, Brown D, et al. Termination of cardiopulmonary resuscitation in mountain rescue. High Alt Med Biol 2012;13:200-208.
- Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am 1992;10:311-327.
- Danzl DF, Pozos RS. Accidental hypothermia. N Engl J Med 1994;331:1756-1760.
- Lindhoff GA, Mac GPJH. An assessment of the thermal safety of microwave warming of crystalloid fluids. Anaesthesia 2000;55:251-254.
- Hilmo J, Naesheim T, Gilbert M. “Nobody is dead until warm and dead”: Prolonged resuscitation is warranted in arrested hypothermic victims also in remote areas—a retrospective study from northern Norway. Resuscitation 2014;85:1204-1211.
- Schaller MD, Fischer AP, Perret CH. Hyperkalemia. A prognostic factor during acute severe hypothermia. JAMA 1990;264:1842-1845.
- Delaney KA, Vassallo SU, Larkin GL, Goldfrank LR. Rewarming rates in urban patients with hypothermia: Prediction of underlying infection. Acad Emerg Med 2006;13:913-921.
- Laniewicz M, Lyn-Kew K, Silbergleit R. Rapid endovascular warming for profound hypothermia. Ann Emerg Med 2008;51:160-163.
- Klein LR, Huelster J, Adil U, et al. Endovascular rewarming in the emergency department for moderate to severe accidental hypothermia. Am J Emerg Med 2017;35:1624-1629.
- Primozic KK, Svensek F, Markota A, Sinkovic A. Rewarming after severe accidental hypothermia using the esophageal heat transfer device: A case report. Ther Hypothermia Temp Manag 2018;8:62-64.
- Mazur P, Kosinski S, Podsiadlo P, et al. Extracorporeal membrane oxygenation for accidental deep hypothermia-current challenges and future perspectives. Ann Cardiothorac Surg 2019;8:137-142.
- Rahman S, Rubinstein S, Singh J, et al. Early use of hemodialysis for active rewarming in severe hypothermia: A case report and review of literature. Ren Fail 2012;34:784-788.
- Plaisier BR. Thoracic lavage in accidental hypothermia with cardiac arrest—report of a case and review of the literature. Resuscitation 2005;66:99-104.
- Kjaergaard B, Bach P. Warming of patients with accidental hypothermia using warm water pleural lavage. Resuscitation 2006;68:203-207.
- Tveita T, Sieck GC. Physiological impact of hypothermia: The good, the bad, and the ugly. Physiology (Bethesda) 2022;37:69-87.
- Handford C, Buxton P, Russell K, et al. Frostbite: A practical approach to hospital management. Extrem Physiol Med 2014;3:7.
- Reamy BV. Frostbite: Review and current concepts. J Am Board Fam Pract 1998;11:34-40.
- McIntosh SE, Freer L, Grissom CK, et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Frostbite: 2024 Update. Wilderness Environ Med 2024;35:183-197.
- Regli IB, Strapazzon G, Falla M, et al. Long-term sequelae of frostbite-a scoping review. Int J Environ Res Public Health 2021;18.
- Long WB 3rd, Edlich RF, Winters KL, Britt LD. Cold injuries. J Long Term Eff Med Implants 2005;15:67-78.
- Lorentzen AK, Davis C, Penninga L. Interventions for frostbite injuries. Cochrane Database Syst Rev 2020;12:CD012980.
- McMahon JA, Howe A. Cold weather issues in sideline and event management. Curr Sports Med Rep 2012;11:135-141.
- Cheung SS. Responses of the hands and feet to cold exposure. Temperature (Austin) 2015;2:105-120.
- Cauchy E, Chetaille E, Marchand V, Marsigny B. Retrospective study of 70 cases of severe frostbite lesions: A proposed new classification scheme. Wilderness Environ Med 2001;12:248-255.
- McCauley RL, Hing DN, Robson MC, Heggers JP. Frostbite injuries: A rational approach based on the pathophysiology.
J Trauma 1983;23:143-147. - Wibbenmeyer L, Lacey AM, Endorf FW, et al. American Burn Association Clinical Practice Guidelines on the Treatment of Severe Frostbite. J Burn Care Res 2024;45:541-556.
- Gross EA, Moore JC. Using thrombolytics in frostbite injury. J Emerg Trauma Shock 2012;5:267-271.
- Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: Pathogenesis and treatment. J Trauma 2000;48:171-178.
- Jones LM, Coffey RA, Natwa MP, Bailey JK. The use of intravenous tPA for the treatment of severe frostbite. Burns 2017;43:1088-1096.
- Lee J, Higgins M. What interventional radiologists need to know about managing severe frostbite: A meta-analysis of thrombolytic therapy. AJR Am J Roentgenol 2020;214:930-937.
- Sheridan RL, Goldstein MA, Stoddard FJ Jr., Walker TG. Case records of the Massachusetts General Hospital. Case 41-2009. A 16-year-old boy with hypothermia and frostbite. N Engl J Med 2009;361:2654-2662.
- Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma 2005;59:1350-1354; discussion 1354-1355.
- Hickey S, Whitson A, Jones L, et al. Guidelines for Thrombolytic Therapy for Frostbite. J Burn Care Res 2020;41:176-183.
- Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med 2011;364:189-190.
- Nygaard RM, Lacey AM, Lemere A, et al. Time matters in severe frostbite: Assessment of limb/digit salvage on the individual patient level. J Burn Care Res 2017;38:53-59.
- Welch GS, Gormly PJ, Lamb DW. Frostbite of the hands. Hand 1974;6:33-39.
- Ervasti O, Hassi J, Rintamaki H, et al. Sequelae of moderate finger frostbite as assessed by subjective sensations, clinical signs, and thermophysiological responses. Int J Circumpolar Health 2000;59:137-145.
Hypothermia is a medical emergency that requires immediate attention and correction. For patients with environmental exposures, especially freezing ambient temperatures, cold water, snow, and ice, frostbite injuries are of significant concern.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
