Hyponatremia: Evaluation and Management in the Emergency Department
February 15, 2023
Related Articles
AUTHORS
Christie Lech, MD, MHPE, Assistant Professor of Clinical Emergency Medicine, Weill Cornell Medical College,
New York, NY
Lauren Kaplan, MD, Emergency Medicine Resident, NewYork-Presbyterian, New York, NY
PEER REVIEWER
Catherine A. Marco, MD, FACEP, Professor, Department of Emergency Medicine, Penn State Health – Milton S. Hershey Medical Center, Penn State College of Medicine
EXECUTIVE SUMMARY
- Hyponatremia, defined as a serum sodium concentration < 135 mEq/L, is a common condition in the emergency department.
- Hyponatremia can be categorized according to serum sodium concentration as mild (130 mEq/L to 135 mEq/L), moderate (125 mEq/L to 129 mEq/L), and severe (< 125 mEq/L).
- Hyponatremia can occur acutely (< 24 hours) or be chronic (> 48 hours).
- The most common symptoms of hyponatremia are nausea, headache, and malaise.
- Nonspecific symptoms include gait instability, cognitive impairment, and vertigo.
- Severe symptoms include seizures, confusion, somnolence, coma, and cardiovascular instability.
- Hypotonic hyponatremia is the most common form of hyponatremia seen in the emergency department.
- Hypotonic hyponatremia is further subdivided according to volume status.
- Treatment of hyponatremia is guided by symptoms, volume status, and acuity.
- Acute severe symptomatic (seizures, impaired consciousness) hyponatremia is treated with an infusion of hypertonic saline (3%) 100 mL to 150 mL over 20 minutes.
- Hypovolemic hyponatremia is treated with normal saline infusion.
- Euvolemic hyponatremia is treated with fluid restriction.
- Hypervolemic hyponatremia is treated with sodium and fluid restriction and a loop diuretic if necessary.
Definition
Hyponatremia, defined as serum sodium concentration < 135 mEq/L, is one of the most common electrolyte derangements among adult patients presenting to the emergency department (ED).1-4 Hyponatremia can be categorized in several ways: severity, occurrence, symptoms, and volume status. Severity typically is classified by the serum sodium concentration: mild (130 mEq/L to 135 mEq/L), moderate (125 mEq/L to 129 mEq/L), and severe (< 125 mEq/L).2,4-6 A 12-year study from two tertiary hospital EDs found that 10% of the patients had mild hyponatremia (126 mEq/L to 134 mEq/L) and 1% had profound hyponatremia (≤ 125 mEq/L).4
Acute hyponatremia develops within less than 48 hours, and chronic hyponatremia is when the condition is present for greater than 48 hours.7 The occurrence of symptoms is strongly linked to the rapidity of hyponatremia development; acute hyponatremia is more likely to produce clinical symptoms than chronic hyponatremia.2
Hyponatremia also is classified according to the patient’s volume status: hypovolemic, euvolemic, or hypervolemic.7 This approach often is used to guide therapy, but as noted later, assessment of volume status is imperfect and sometimes inaccurate.8-10
Hyponatremia has significant implications for patient morbidity and mortality, as well as healthcare costs. The presence of hyponatremia increases patients’ mean hospital length of stay, increases the probability of readmission, and increases the risk of in-hospital patient death.3,11-13 More specifically, studies have found that mortality can be as high as 10% for inpatients with severe hyponatremia.1,11 In cases of severe hyponatremia when a too rapid correction of the sodium occurs, observed in up to 14% of patients, serious neurologic complications may result.1
When discussing hyponatremia, is it important to be aware of pseudohyponatremia. Pseudohyponatremia is caused by endogenous solutes, which lead to laboratory artifact, and occurs in cases of hyperproteinemia (e.g., macroglobulinemia, multiple myeloma) and hyperlipidemia.2
Epidemiology
Various factors are associated with an increased risk of developing hyponatremia. Hyponatremia is most common among the elderly patient populations. This is thought to be because of age-related changes in physiology, comorbid medical conditions, and decreased ability to cope with physical and environmental stressors, among other factors.2,4
Prior studies also have suggested that female sex, presence of chronic heart or kidney disease, medication use (e.g., thiazides, serotonin reuptake inhibitors, drugs that cause renin-angiotensin system blockade), syndrome of inappropriate antidiuretic hormone secretion (SIADH), and certain genetic factors are risk factors for hyponatremia.2,14
Rates of hyponatremia have been cited as up to 30% for patients with acute-on-chronic kidney disease, as well as 21% in patients with chronic kidney disease and 22% in those with acute kidney injury.2,15 These findings may be the result of these conditions’ effect on patients’ ability to dilute or concentrate urine, as well as the higher prevalence of diuretic use in patients with chronic kidney disease.15
Prior studies also have suggested a relationship between outdoor temperature and relative humidity on the incidence of hyponatremia.2,4 A positive correlation was found between higher outdoor temperatures and the incidence of profound hyponatremia, while mild hyponatremia was not associated with outdoor temperature.4 The relationship between outdoor temperature and incidence of hyponatremia is thought to be due to sweating and the greater ingestion of hypotonic fluids relative to hypertonic fluid loss.2
Etiology
Classification by Serum Osmolality
Normal serum osmolality is between 280 mOsm/kg H2O and 285 mOsm/kg H2O. The occurrence of hyponatremia with normal serum osmolality is termed isotonic hyponatremia or pseudohyponatremia, and as noted before, is due to the presence of excess serum proteins or lipids.
Hyponatremia with an increased serum osmolality (> 285 mOsm/kg H2O) is termed hypertonic hyponatremia, and usually is due to hyperglycemia, but it also can be seen after use of mannitol, sorbitol, or radiocontrast media.
Hyponatremia with a decreased serum osmolality (< 280 mOsm/kg H2O) is termed hypotonic hyponatremia and is the most common cause of hyponatremia. Thus, the following discussion will focus on this form of hyponatremia.
Hypotonic hyponatremia most commonly is caused by water retention.16 Water retention can occur if mechanisms to produce dilute urine are impaired or if the kidneys’ excretory capacity for water is overwhelmed by excessive water intake (e.g., use of electrolyte-free irrigation solutions, psychogenic polydipsia, etc.).16
Classification by Volume Status
Hypotonic hyponatremia can be classified further according to volume status as hypovolemic, euvolemic, and hypervolemic. As noted, clinical assessment of volume status is inaccurate, and these categories are guidelines, not absolute distinctions.
Hypovolemic hypotonic hyponatremia can be due to extrarenal sodium loss or renal sodium loss. Examples of extrarenal sodium loss include burns, vomiting, diarrhea, severe hemorrhage, and third spacing as may occur with ileus, pancreatitis, peritonitis, and rhabdomyolysis.16 Hypovolemic hypotonic hyponatremia resulting from renal sodium loss can be caused by osmotic diuresis, diuretic use, mineralocorticoid deficiency, salt-wasting nephropathy, and cerebral salt wasting.16
Euvolemic hypotonic hyponatremia typically is caused by SIADH, which can be secondary to medications (nonsteroidal anti-inflammatory drugs [NSAIDs], anticonvulsants, antipsychotics, etc.), malignancy (lymphoma, small cell lung cancer, gastrointestinal and oropharyngeal cancer), stress (nausea, postoperative state, pain, endurance exercise), pulmonary disease (cystic fibrosis, acute respiratory distress syndrome, chronic obstructive pulmonary disease [COPD], etc.), nutritional solute deficiency, and endocrine disorders, among other causes.16 Hypothyroidism is a rare cause of hyponatremia in cases with marked thyroid hypofunction.
Excess water ingestion beyond the kidneys’ ability to excrete is termed primary polydipsia and can produce euvolemic hypotonic hyponatremia.17 Psychogenic polydipsia is one form of primary polydipsia, and it typically is seen in patients with psychiatric disease, with hyponatremia being a severe complication of the disease.17 It is hypothesized that in psychogenic polydipsia there is impaired functioning of the cholinergic and dopaminergic systems, which leads to dysfunction of the hippocampus and thirst centers.17 This dysregulation leads to the behavior of constant drinking.17
Dipsogenic polydipsia or compulsive water drinking as a conscious effort for health can produce euvolemic hypotonic hypernatremia. Excess ingestion of low solute fluids also can cause euvolemic hypotonic hyponatremia, classically associated with a period of binge drinking along with a poor diet, termed “beer potomania.”18
A cause of chronic euvolemic hypotonic hyponatremia is a reset osmostat.19 A reset osmostat can develop from a variety of conditions, including chronic psychogenic polydipsia, as chronic hyponatremia alters the hypothalamic-pituitary axis that regulates thirst. These patients adapt to a lower serum osmolality by adjusting their ability to concentrate or dilute urine at a lower serum sodium level than normal.
The final category of hyponatremia is hypervolemic hypotonic hyponatremia. Causes of hypervolemic hypotonic hyponatremia include renal failure, hepatic cirrhosis, nephrotic syndrome, and congestive heart failure.16
Alternative Classification
More recently, research has suggested moving away from traditional approaches to hyponatremia where hyponatremia is classified according to volume status, since assessments of a patient’s volume status often are flawed and inaccurate. Although the following discussion partially delves into laboratory evaluation and treatment, it will be discussed here with the goal of describing it as an alternative approach to hyponatremia classification.
Researchers have examined the relationship between hyponatremia and hypouricemia as a method to distinguish between SIADH and other causes of hyponatremia.20 More specifically, the FEurate (the filtered urate load excreted in the urine or fractional excretion of urate) described as a percentage can be used to differentiate between causes of hyponatremia.20 (See Table 1.) Urate is filtered in the glomerulus and transported down the proximal tubule, with net reabsorption of 89% to 96% of urate in that segment. This leaves 4% to 11% of urate remaining in the proximal tubule. Since urate is not transported in the distal nephron, this percentage is the total urate load expected to be excreted in the urine.20
Table 1. Characterization of Hyponatremia According to FEurate |
||
FEurate % = (urine urate/serum urate)/(urine creatinine/serum creatinine) x 100 = (urine urate x serum creatinine)/(urine creatinine x serum urate) x 100 |
||
FEurate < 4% (low) |
FEurate 4% to 11% (normal) |
FEurate > 11% (high) |
|
|
|
SIADH: syndrome of inappropriate antidiuretic hormone secretion |
||
In cases where the FEurate is less than 4%, causes of hyponatremia include prerenal azotemia (i.e., from renal and hepatic dysfunction and congestive heart failure) and Addison’s disease.20 When FEurate is between 4% and 11%, causes of hyponatremia include etiologies such as psychogenic polydipsia or reset osmostat. When FEurate levels are greater than 11%, it is recommended that providers correct hyponatremia, and if the FEurate decreases to less than 11%, hyponatremia can be due to SIADH or use of thiazide diuretics. If FEurate is persistently greater than 11% after treatment, it is thought that hyponatremia is related to renal salt wasting.20
In addition to evaluating the FEurate to classify hyponatremia, studies have suggested the use of an isotonic infusion challenge as a way to distinguish whether a patient’s hyponatremia is secondary to SIADH, a process further described in the following section, or other etiologies. With an isotonic fluid challenge, patients with SIADH will not demonstrate correction of hyponatremia and will not dilute their urine.20 While this approach may be diagnostic, it is not routinely part of standard of practice. The use of intravenous fluids in patients with hyponatremia should be done with caution, since fluids may cause further electrolyte derangements that can affect patient morbidity and mortality.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
SIADH is one of the most common causes euvolemic hyponatremia. It involves the non-osmotic and non-volume-based release of antidiuretic hormone (ADH) by either the pituitary gland or ectopic production, leading to excess free water retention and inappropriate concentration of urine.2,14 SIADH may be primary or secondary. In primary SIADH, there is dysfunction of the central nervous system mediation of plasma osmolality and/or thirst mechanisms.
Secondary SIADH involves pituitary-independent causes of increased ADH. Increased levels of ADH can result from a myriad of acute and chronic medical conditions, as well as from medications. These aforementioned medical conditions include, but are not limited to, malignancy (lung, gastrointestinal, genitourinary, mediastinal tumors), asthma and COPD, pulmonary infections (e.g., lung abscess, tuberculosis, aspergillosis), cerebrovascular accidents (CVAs), head trauma, and acquired immunodeficiency syndrome (AIDS), which can lead to elevations in ADH.7 SIADH also can be caused by medication side effects, stress (i.e., postoperative state, prolonged exercise), and endocrine disorders (adrenal disease, thyroid disease), among other causes.16
For emergency physicians, it is essential to identify patients with potential SIADH to reduce the risk of further decline in serum sodium due to hypertonic fluid administration.2
Medications
Diuretics induce mild hypovolemia as well as stimulate release of ADH.2 More specifically, thiazide diuretics directly impair the kidneys’ ability to dilute urine, increasing free water resorption and increasing the risk for hyponatremia in a dose-dependent manner.2,21 Among the thiazide diuretics, chlorthalidone is associated with the highest risk of hyponatremia, and hydrochlorothiazide is associated with the lowest risk. Hyponatremia is common in patients taking thiazide diuretics and was present in 22% of patients in one study.21
Medications, such as NSAIDs, antipsychotics, amiodarone, antidepressants, opioids, proton-pump inhibitors, and anticonvulsants, also can cause secondary SIADH.7
Drugs of Abuse
3,4-methylenedioxymethamphetamine (MDMA) is a recreational drug that has effects on epinephrine, serotonin, and dopamine, increasing their presence in the synaptic cleft and inhibiting their reuptake.22 Serotonergic effects can affect body temperature regulation, causing hypernatremia and polydipsia.22 There are two theories regarding the etiology of hyponatremia with MDMA use: increased secretion of ADH and water intoxication; and polydipsia due to hyperpyrexia.22
Volume Depletion
Causes of volume depletion, such as hemorrhage or gastrointestinal or renal losses, can cause elevations in ADH levels, since they result in a loss of sodium and intravascular volume.2 Sweat is hypotonic compared to plasma, so sodium loss from sweating would not be expected to cause hyponatremia by itself. If excess sweating leads to volume depletion, and especially with excess hypotonic fluid ingestion, subsequent hyponatremia can occur.2 Severe diarrhea causes sodium loss through stool. Excessive vomiting triggers renal sodium loss along with bicarbonate in the urine to counteract the resultant metabolic alkalosis.2
On the opposite end of the spectrum, excess free water intake relative to sodium intake (e.g., beer drinkers’ potomania, psychogenic polydipsia, tea-and-toast diet) also can lead to hyponatremia.2
Acute and Chronic Comorbid Conditions
Renal disease can induce renal salt wasting.2 Cerebral salt wasting also can be a consequence of intracranial pathology, trauma, or surgery, and this occurs through mechanisms that are incompletely understood.2
Reduced effective circulating volume triggers non-osmotic ADH release and retention of free water; conversely, activation of the renin-angiotensin-aldosterone system (RAAS) leads to sodium retention, while angiotensin II leads to an increased sense of thirst.2 Hyponatremia is induced when increased thirst and ADH action outweigh sodium retention.2
Cirrhosis induces splanchnic vasodilation, which similarly leads to decreased effective circulating volume, triggering RAAS activation and ADH release. Nephrotic syndrome leads to decreased effective circulating volume through decreased oncotic pressure due to protein loss, which causes activation of RAAS and ADH release.2
Patients with hyponatremia are at increased risk for mortality and poor outcome, but it is not clear whether it is due to the hyponatremia alone or a reflection of the severity of multiple underlying diseases.12 One study of elderly patients admitted with hyponatremia is typical of such individuals: 71% had hypertension, 34.4% had coronary artery disease, and 33.5% had diabetes mellitus.11 Risk factors for patient mortality included malignancy, cirrhosis, dialysis treatment, increased Charlson Comorbidity Index (CCI, a predictor of in-hospital mortality based on underlying medical comorbidities), elevated urea and C-reactive protein (CRP), and use of antineoplastic drugs.11
Hyponatremia is prevalent among patients with acute COPD exacerbations (in up to one out of five patients).23,24 While one recent study did not find any significant association between hyponatremia and adverse outcome or mortality in patients with an acute COPD exacerbation,23 another study found it to be an independent predictor of a return ED visit within one year after initial ED discharge.24
Hyponatremia is the most common electrolyte disorder complicating community-acquired pneumonia (CAP) in emergency patients and is an independent predictor of prolonged hospital stay.25 In addition, moderate to severe hyponatremia at ED presentation independently predicts mortality in patients with sepsis.26 After the start of the COVID-19 pandemic, one recent study found hyponatremia to be a predictive marker of adverse outcome and higher 30-day mortality in patients with COVID-19 infection.27
Pathophysiology
The Edelman equation describes the serum sodium level. The simplified Edelman equation is:
[Na+] = (Na+ exchangeable + K+ exchangeable)/Total Body Water (TBW)
The serum sodium concentration is the ratio of the total exchangeable sodium and total body water. Because osmotically active sodium and potassium are exchangeable, they both are used in the equation to determine the serum sodium.2 Potassium is the main intracellular solute, while sodium is the main extracellular solute. As water moves freely across the cell wall, an equal solute concentration is maintained both intracellularly and extracellularly.2
Based upon the principles of this equation, hyponatremia develops if there is a loss of sodium/potassium, a gain of free water, or a combination of both. Notably, when treating hyponatremia, simultaneous correction of hypokalemia can lead to a higher correction rate of sodium.2
There also are multiple causes of non-hypotonic hyponatremia. This includes the presence of either “effective” or “ineffective” osmoles that increase serum osmolality, as well as the presence of endogenous solutes that cause pseudohyponatremia.2 “Effective” (i.e., osmotically active) osmoles that increase the serum osmolality include glucose, mannitol, and glycine.2 These osmoles cause shifts of free water from the intracellular space to the extracellular space, leading to dilutional hyponatremia.2
In patients with hyponatremia, two formulas have been proposed to correct for hyperglycemia.
• Katz proposed a correction factor in 1973 based on theoretical calculations of a hypothetical 70-kg patient.29 His calculation yielded a decrease in serum sodium (in mEq/L) for every 0.016 increase in serum glucose (in mg/dL). The Katz correction formula typically is displayed as:
Corrected sodium = Measured Na + [(glucose level - 100) × 0.016]
• Hillier et al proposed a different correction factor in 1999 based on a study of induced hyperglycemia in six healthy individuals with actual measurement of serum sodium levels.30 They found a decrease in serum sodium (in mEq/L) for every 0.024 increase in serum glucose (in mg/dL). The Hillier correction formula typically is displayed as:
Corrected sodium = Measured Na + [(glucose level - 100) × 0.024]
Stated differently, the serum sodium level decreases by 1.6 mEq/L or 2.4 mEq/L for each 100 mg/dL increase in serum glucose.28,29 The accuracy of either formula is unproven, and either one appears acceptable for clinical use.
With regard to TBW, changes in thirst, fluid consumption, satiation, and ADH are essential for regulation of body water.2 When plasma osmolality rises over 285 mOsm/kg H2O, ADH is secreted. At higher plasma osmolality levels, thirst develops, leading to fluid ingestion and subsequent reabsorption of free water by the kidneys through the actions of ADH.2 ADH also is triggered by stretch-sensitive baroreceptors; small reductions in blood pressure, therefore, lead to increased ADH. Notably, baroregulation can override osmoregulation in cases of more severe hypovolemia.2
Clinical Features
Severity of symptoms depends on the rapidity with which hyponatremia develops. Symptoms may range from mild symptoms, such as fatigue, to life-threatening cerebral edema.2 Some of the most frequently encountered symptoms of hyponatremia are nausea, headache, and malaise.2,6,7 Signs of hyponatremia also can be nonspecific and include instability with falls, weakness, syncope, cognitive dysfunction, and vertigo.2,6,7,21
In cases of chronic hyponatremia (i.e., duration > 48 hours) patients generally are asymptomatic or with milder symptoms, such as changes in gait or attention.7 In more severe cases of hyponatremia, symptoms include altered mental status (lethargy, somnolence, coma) as well as seizures.2,6,7
The brain can compensate, to an extent, for hypoosmolar environments by losing up to 20% of their intracellular solutes. However, rapidly developing and profound hyponatremia can lead to cellular swelling and life-threatening edema.2 Stated differently, hyponatremia can cause intracranial hypertension.30 In chronic hyponatremia, although cells can adapt to the hypo-osmolality, the loss of intracellular solutes still can lead to neurologic deficits.2
Diagnostic Studies
The work-up of hyponatremia in the ED should involve determination of chronicity (if possible) and etiology of hyponatremia, including a thorough medication review.2 Determination of volume status, as described earlier, is one method of differentiating etiologies of hyponatremia.
The clinical approach to detect hypovolemia involves assessment of tachycardia, orthostatic hypotension, dry oral mucosa, absence of axillary moisture, a decrease in skin turgor on the subclavicular chest or forearm, and delayed capillary refill time. The sensitivity of individual findings varies from 20% to 70% and is dependent on the duration of the illness.
The clinical approach to detect hypovolemia involves assessment for peripheral edema, pulmonary rales, and elevated jugular venous pressure. The sensitivity for individual findings varies from 50% to 90%. By default, if the patient does not have enough features to be judged either hypovolemic or hypervolemic, they are assumed to be euvolemic.
The use point-of-care ultrasound (POCUS) to assess volume status is more promising than the clinical criteria noted earlier, especially in acute and critically ill patients.31 A description of this technique is beyond the scope of this article.
Laboratory Studies
Blood glucose levels will help clinicians exclude hyperglycemia-induced hyponatremia.2 Electrolyte derangements, particularly hypokalemia, also are commonly found in conjunction with hyponatremia, and an electrolyte panel should be obtained.2 Serum osmolality, creatinine to evaluate renal function, urea nitrogen to aid in determination of volume status, and thyroid stimulating hormone (TSH) levels should be obtained. Acquisition of random serum cortisol levels and/or adrenocorticotropic hormone (ACTH) stimulation testing will help providers to evaluate adrenal insufficiency in patients with euvolemic hyponatremia.
Notably, SIADH is a diagnosis of exclusion. Adrenal insufficiency, among others, should be ruled out prior to provision of a diagnosis of SIADH. Patients with adrenal insufficiency can present with signs of dehydration along with hyponatremia and hyperkalemia.32 One study found that up to 14.2% of patients presenting with euvolemic hyponatremia had undiagnosed adrenal insufficiency secondary to exogenous steroid use or previously undiagnosed hypopituitarism.33 Thus, it is imperative that a thorough endocrinopathy work-up be pursued in these hyponatremia patients when indicated.
The collection of urine samples prior to any treatment interventions (i.e., intravenous fluids, medications) to evaluate for urine chemistry, osmolality, and sodium concentration will help in diagnosis and treatment of hypotonic hyponatremia.2 (See Table 2.) Urine sodium levels can help providers determine if the hyponatremia is renal or non-renal in origin.
Table 2. Assessment of Hypotonic Hyponatremia Using Urine Sodium and Osmolality |
|||
Volume Status |
Urine Sodium |
Urine Osmolality |
Conditions |
Hypovolemia |
> 20 mEq/L |
Renal loss for diuretics or adrenal insufficiency |
|
< 20 mEq/L |
External renal loss, such as vomiting or diarrhea |
||
Euvolemia |
> 20 mEq/L |
> 100 mOsm/kg H2O |
SIADH, stress, drug use, adrenal insufficiency, hypothyroidism |
< 100 mOsm/kg H2O |
Primary polydipsia, beer potomania |
||
Variable |
Reset osmostat |
||
Hypervolemia |
> 20 mEq/L |
Renal failure |
|
< 20 mEq/L |
Heart failure, cirrhosis, nephrotic syndrome |
||
SIADH: syndrome of inappropriate antidiuretic hormone secretion |
|||
In cases of hypervolemic hyponatremia secondary to non-renal causes, such as cirrhosis or congestive heart failure, urine sodium levels are less than 20 mEq/L secondary to renal hypoperfusion.32 In renal causes of hypervolemic hyponatremia or in cases of SIADH, patients typically have urine sodium levels of greater than 20 mEq/L, since their kidneys do not retain sodium.32
For those patients with euvolemic hyponatremia, urine sodium concentration usually is greater than 20 mEq/L, since free water retention leads to volume expansion.32
In cases of hypovolemic hyponatremia secondary non-renal causes, urine sodium levels typically are less than 20 mEq/L, as the kidneys try to retain solutes.32 For patients with hypovolemic hyponatremia secondary to renal causes, urine sodium levels are greater than 20 mEq/L, as sodium is not retained by the kidneys.32
Imaging
Since hyponatremia can lead to intracranial hypertension, prior studies have suggested that the use POCUS measurements of the optic nerve sheath diameter could guide hyponatremia management, since increases in intracranial pressure increases the optic nerve sheath diameter.30
If concern exists for central pontine myelinolysis (CPD) after rapid correction of hyponatremia, in addition to medical management of the serum overcorrection, imaging can be helpful to distinguish CPD from other etiologies of neurological symptoms. In these cases, assuming the patient has been stabilized, magnetic resonance imaging (MRI) of the brain is the preferred imaging study. The classic finding of CPD is the “trident sign,” a trident-shaped pontine lesion with sparing of the descending corticospinal tract.34 While the pons typically is most susceptible to injury from CPD, the basal ganglia, thalamus, hippocampus, and periventricular white matter also may be affected.34
Management
Treatment should be guided by the presence of symptoms, volume status, and focus on distinguishing between acute and chronic hyponatremia.2 (See Table 3 and Figure 1.)
Patients with severe symptoms from hyponatremia, as manifested by seizures, altered mental status (confusion, somnolence, coma), or cardiorespiratory instability, typically evolve over less than 24 hours and have serum sodium levels less than 120 mEq/L. They are at risk for irreversible neurologic injury, respiratory arrest, and brainstem herniation. Such patients should be treated with 100 mL to 150 mL of 3% (hypertonic) saline intravenously over 20 minutes. The sodium level should then be re-checked 20 minutes after the infusion is completed.
Table 3. Treatment of Acute Hypotonic Hyponatremia |
||
Category |
Characteristics |
Initial Treatment |
Severe symptomatic |
Seizures, confusion, somnolence, coma, cardiorespiratory instability, serum sodium typically < 120 mEq/L |
Hypertonic (3%) saline 100 mL to 150 mL IV over 20 minutes, recheck serum sodium 20 minutes after infusion complete, repeat if still symptomatic and serum sodium increase is < 5 mEq/L |
Hypovolemic |
GI or renal fluid loss, mineralocorticoid deficiency |
Normal saline 20 mL/kg IV over 24 hours |
Euvolemic |
SIADH, polydipsia |
Fluid restriction; salt and protein not restricted |
Hypervolemic |
Heart failure, cirrhosis, renal injury |
Sodium and fluid restriction, loop diuretics |
IV: intravenous; GI: gastrointestinal; SIADH: syndrome of inappropriate antidiuretic hormone secretion |
||
Figure 1. Treatment of Hyponatremia |
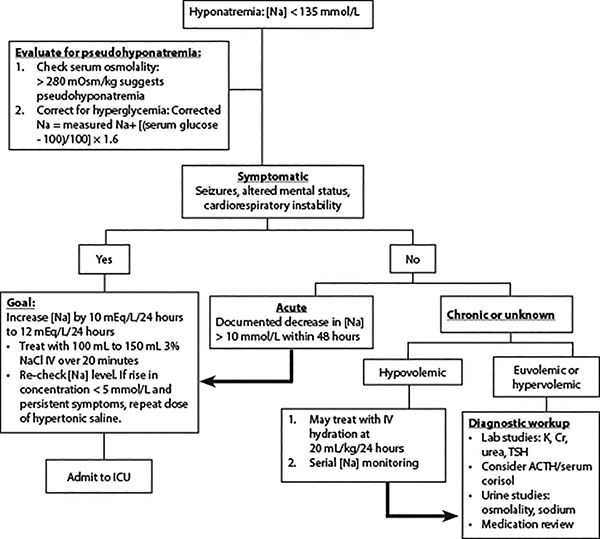 |
Na: sodium; NACl: sodium chloride; IV: intravenous; ICU: intensive care unit; K: potassium; Cr: creatinine; TSH: thyroid stimulating hormone; ACTH: adrenocorticotropic hormone |
If the patient still is symptomatic and the rise in sodium concentration is < 5 mEq/L, repeat the dose of the hypertonic saline. An increase of 5 mEq/L serum sodium has been found to reduce intracranial pressure by about 50%, with subsequent improvement in neurological symptoms.1
If the symptoms have improved with the initial bolus of 3% saline, the treatment approach shifts to gradual correction of serum sodium at a rate no faster than 0.5 mEq/L/hour.1 The serum sodium correction limit should be within a range of 10 mEq/L to 12 mEq/L in 24 hours and 18 mEq/L in 48 hours.1
An observational study evaluating treatment, specifically a 150 mL bolus of 3% saline (per European guidelines), compared to conventional treatment with normal saline, found that the sodium increase was more consistent with the use of hypertonic saline, but the overcorrection (exceeding 10 mEq/L to 12 mEq/L in 24 hours) rate also was high.35 American guidelines recommend a 100 mL bolus of 3% saline for up to three doses, while European guidelines stipulate a minimum of two boluses of 150 mL 3% saline.
If the patient has experienced an acute fall in serum sodium > 10 mEq/L within 48 hours, even if asymptomatic, treat similarly to patients with symptomatic hyponatremia. When treating hyponatremia, hypokalemia also should be treated aggressively.32
Studies have compared the bolus method vs. continuous infusion of hypertonic saline demonstrating mixed results regarding the ability to achieve the targeted increase in serum sodium without causing overcorrection.35 Despite the aforementioned definitions regarding serum sodium correction, there is heterogeneity of overcorrection definitions, and, thus, prevalence of sodium serum overcorrection varies depending on patient cohort.5 One systematic review found 14 different methodologies that were used to determine overcorrection of hyponatremia.5
An important pitfall to note is that symptoms caused by hypovolemia may be misinterpreted as severely symptomatic hyponatremia. Providers should be thoughtful regarding bolus volume and regularly re-evaluate treatment to avoid overcorrection.35
Hypovolemic hyponatremic patients without severe symptoms should be treated with intravenous normal saline at a rate of 20 mL/kg over 24 hours.2
If patients are hypervolemic, the initial treatment is with sodium and fluid restriction. If fluid restriction is inadequate, loop diuretics, such as furosemide, can be used.32 In cases of patients with euvolemic hyponatremia secondary to SIADH, treatment consists of fluid restriction and addressing the underlying cause to definitively treat this condition.32
If patients with hyponatremia have high output of dilute urine, desmopressin can be administered to prevent further urine electrolyte-free water loss.36 In addition, for patients at elevated risk of serum sodium overcorrection, providers may prophylactically give patients desmopressin, 1 mcg to 2 mcg intravenously or subcutaneously every six hours to similarly prevent urine electrolyte-free water loss.36
Regarding treatment of hyponatremia, it is important for providers to remember that hyponatremia in and of itself can not only lead to significant patient morbidity and mortality, but it also has predictive value of poor patient outcomes. As one report stated, hyponatremia likely signifies the need for greater clinical attention with regard to a patient’s evaluation; fixating on correcting sodium homeostasis may be incorrectly emphasizing the “signifier, not the signified.”37
Thus, hyponatremia can be a useful biomarker to identify higher-risk patients who could benefit from more frequent monitoring, multidisciplinary input, and more intensive follow-up and is more a reflection of higher-risk populations vs. directly contributing to pathology.37
Special Populations
Geriatric Population
Prior studies have reported that hyponatremia is found in approximately 18% of community-living elderly patients and almost 20% of elderly patients presenting to the ED.6 Increased age is associated with decreased ability to adapt to disease and environmental-related stressors, which, in turn, influences sodium and water balance.2,4 Elderly individuals also have decreased total body water, altered thirst perception, decreased renal function, and decreased renal sodium-conserving ability.4 Increasing age leads to decreased appetite and reduced solute and protein intake, which affects water excretion, and, thus, the risk of hyponatremia.4
In addition, in elderly patients, hyponatremia also may be secondary to physiological factors, such as cognitive impairment, decreased physical conditioning, polypharmacy, and comorbid medical conditions.4,6 In the elderly, the development of hyponatremia during hospitalization significantly increases the length of hospitalization irrespective of the etiology.13
While mild chronic hyponatremia typically is asymptomatic, older patients may carry a higher risk of impairment in cognitive function and gait changes, as well as an increased risk of injurious falls.6 In addition, patients with mild chronic hyponatremia are more likely to develop osteoporosis, which can subsequently place them at increased risk for fracture.6,7 Hyponatremia also may result from decreased bone mineral density secondary to activation of osteoclasts.7
Pediatric Population
As with elderly patients, hyponatremia is similarly the most common electrolyte disturbance among pediatric patients, particularly in children younger than 4 years of age.2,38 Children with underlying medical conditions are at increased risk of moderate and severe hyponatremia. This has been demonstrated in various studies, finding the presence of hyponatremia to be associated with increased risk of hospitalization, increased pediatric intensive care unit (PICU) stays, and death.2,38
Studies have demonstrated that asymptomatic hyponatremia is common in healthy infants presenting with dehydration in the setting of gastroenteritis, moderate to severe bronchiolitis, and pyelonephritis.14 Inappropriate use of hypotonic solutions, such as bottled drinks or diluted milk, also can contribute to dilutional hyponatremia in children.38
Iatrogenic hyponatremia can develop when using hypotonic fluids. One recent study found that severe hyponatremia developed in one out of every 998 acutely ill children receiving moderately hypotonic (60 mmol/L to 80 mmol/L of sodium) intravenous fluid therapy.39 Despite guideline recommendations from the American Academy of Pediatrics published in 2018 recommending the use of isotonic maintenance intravenous fluids, which include appropriate potassium chloride and dextrose supplementation, physicians continue to use hypotonic fluids (e.g., D5-0.45% normal saline) in pediatric populations.
The application of clinical practice improvement strategies, such as institutional discussions based on literature review, education on updated guidelines, electronic medical record changes prompting the use of isotonic intravenous fluids, and group practice review with individual physician audit and feedback, all have been shown to effectively alter practice patterns to align with national recommendations.40
Pitfalls
Rapid correction of hyponatremia can result in central pontine myelinolysis (CPM) or osmotic demyelination syndrome (ODS), which is associated with high morbidity and mortality.1,5,41,42 Symptoms of these syndromes include hypotension, dysphagia, dysarthria, and flaccid paralysis.32 Identified patient characteristics that increase the risk for osmotic demyelination syndrome include a history of malnutrition, cirrhosis, the presence of hypokalemia, and profoundly low initial serum sodium level.5,32 Of note, cases of osmotic demyelination syndrome are rare in patients with serum sodium levels > 116 mEq/L.42
Risk factors for overcorrection of serum sodium include symptomatic hyponatremia at presentation and use of 3% saline for the treatment of the patient’s hyponatremia.1 Successful treatment of hyponatremia and, thus, mitigation of risk of CPM and ODS is contingent upon targeting the underlying mechanism and understanding the pathophysiology of the disease process.2
In addition, the rate of correction of serum sodium is a modifiable risk factor for ODS.5 Overcorrection of serum sodium can be defined as a rate of greater than 10 mmol/L to 12 mmol/L over 24 hours or 18 mmol/L per 48 hours.5 For those at elevated risk for ODS, a correction rate of 8 mmol/L over 24 hours is recommended.5
If sodium overcorrection occurs leading to ODS, fluids containing sodium should be held and D5W should be administered.32
Disposition
Patients with neurological signs and symptoms (i.e., altered mental status, and/or patients who required the administration of hypertonic saline fluids) should be admitted to a critical care unit for close monitoring.32 More stable patients who have had appropriate increases in their sodium with treatment are safe for admission to floor beds with telemetry monitoring.32 While provider practice and literature recommendations may differ, patients generally are safe for discharge when their serum sodium levels reach 130 mEq/L, assuming the patient is reliable and has close outpatient follow-up.32
Summary
Hyponatremia is one of the most common electrolyte derangements among adults presenting to the ED and is associated with significant morbidity and mortality. A variety of factors and disease processes can contribute to the development of hyponatremia, varying in both chronicity and in subsequent symptomatology. Understanding the varied etiologies of hyponatremia is essential for the emergency physician to appropriately manage this electrolyte disorder, ensuring appropriate treatment and disposition in a common but potentially dangerous disease process.
REFERENCES
- Sumi H, Imai N, Shibagaki Y. Incidence and risk factors of overcorrection in patients presenting with severe hyponatremia to the emergency department. Clin Exp Nephrol 2022;26:1086-1091.
- Lindner G, Schwarz C, Haidinger M, Ravioli S. Hyponatremia in the emergency department. Am J Emerg Med 2022;60:1-8.
- Tazmini K, Nymo SH, Louch WE, et al. Electrolyte imbalances in an unselected population in an emergency department: A retrospective cohort study. PLoS One 2019;14:e0215673.
- Sailer CO, Winzeler B, Nigro N, et al. Influence of outdoor temperature and relative humidity on incidence and etiology of hyponatremia. J Clin Endocrinol Metab 2019;104:1304-1312.
- Woodfine JD, van Walraven C. Criteria for hyponatremic overcorrection: Systematic review and cohort study of emergently ill patients. J Gen Intern Med 2020;35:315-321.
- Boyer S, Gayot C, Bimou C, et al. Prevalence of mild hyponatremia and its association with falls in older adults admitted to an emergency geriatric medicine unit (the MUPA unit). BMC Geriatr 2019;19:265.
- Rocha AFB, Sá MVBO, Elihimas Junior UF. Hyponatremia in elderly patients with fragility fractures of the proximal femur: A cross-sectional study. J Bras Nefrol 2019;41:518-525.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination. Is this patient hypovolemic? JAMA 1999;281:1022.
- Kalantari K, Chang JN, Ronco C, Rosner MH. Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int 2013;83:1017.
- Maurer C, Wagner JY, Schmid RM, Saugel B. Assessment of volume status and fluid responsiveness in the emergency department: A systematic approach. Med Klin Intensivmed Notfmed 2017;112:326.
- Bozkurt Babuş S, Köse A, Erdoğan S, et al. Risk factors and mortality in elderly patients with severe hyponatremia admitted to the emergency department. Ir J Med Sci 2022; Apr 14. doi: 10.1007/s11845-022-02989-w. [Online ahead of print].
- Falchi AG, Mascolo C, Sepe V, et al. Hyponatremia as a predictor of outcome and mortality: Results from a second-level urban emergency department population. Ir J Med Sci 2022; Feb 20. doi: 10.1007/s11845-022-02953-8. [Online ahead of print].
- Baser S, Yılmaz CN, Gemcioglu E. Do the etiology of hyponatremia and serum sodium levels affect the length of hospital stay in geriatric patients with hyponatremia? J Med Biochem 2022;41:40-46.
- Mazzoni MB, Milani GP, Bernardi S, et al. Hyponatremia in infants with community-acquired infections on hospital admission. PLoS One 2019;14:e0219299.
- Woitok BK, Funk GC, Walter P, et al. Dysnatremias in emergency patients with acute kidney injury: A cross-sectional analysis. Am J Emerg Med 2020;38:2602-2606.
- Burst V. Etiology and epidemiology of hyponatremia. Front Horm Res 2019;52:24-35.
- Kotagiri R, Kutti Sridharan G. Primary polydipsia. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan. Updated July 25, 2022.
- Lodhi MU, Saleem TS, Kuzel AR, et al. “Beer potomania” – a syndrome of severe hyponatremia with unique pathophysiology: Case studies and literature review. Cureus 2017;9:e2000.
- Kamel HM, Upadhyay A, Borkan SC. Intractable hyponatremia complicated by a reset osmotat: A case report. J Med Case Rep 2023;17:13.
- Maesaka JK, Imbriano LJ, Grant C, Miyawaki N. New approach to hyponatremia: High prevalence of cerebral/renal salt wasting, identification of natriuretic protein that causes salt wasting. J Clin Med 2022;11:7445.
- Ravioli S, Bahmad S, Funk GC, et al. Risk of electrolyte disorders, syncope, and falls in patients taking thiazide diuretics: Results of a cross-sectional study. Am J Med 2021;134:1148-1154.
- Elkattawy S, Mowafy A, Younes I, et al. Methylenedioxymethamphetamine (MDMA)-induced hyponatremia: Case report and literature review. Cureus 2021;13:e15223.
- Lindner G, Herschmann S, Funk GC, et al. Sodium and potassium disorders in patients with COPD exacerbation presenting to the emergency department. BMC Emerg Med 2022;22:49.
- Tokgöz Akyıl F, Tural Önür S, Abalı H, et al. Hyponatremia is an independent predictor of emergency department revisits in acute exacerbation of COPD. Clin Respir J 2021;15:1063-1072.
- Ravioli S, Gygli R, Funk GC, et al. Prevalence and impact on outcome of sodium and potassium disorders in patients with community-acquired pneumonia: A retrospective analysis. Eur J Intern Med 2021;85:63-67.
- Castello LM, Gavelli F, Baldrighi M, et al. Hypernatremia and moderate-to-severe hyponatremia are independent predictors of mortality in septic patients at emergency department presentation: A sub-group analysis of the need-speed trial. Eur J Intern Med 2021;83:21-27.
- Atila C, Sailer CO, Bassetti S, et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur J Endocrinol 2021;184:409-418.
- Katz MA. Hyperglycemia-induced hyponatremia — calculation of expected serum sodium depression. N Engl J Med 1973;289:843.
- Hillier TA, Abbott RD, Barrett EJ. Hyponatremia: Evaluating the correction factor for hyperglycemia. Am J Med 1999;106:399.
- Demir TA, Yılmaz F, Sönmez BM, et al. Association of optic nerve sheath diameter measurement with hyponatremia in emergency department. Am J Emerg Med 2019;37:1876-1879.
- Pourmand A, Pyle M, Yamane D, et al. The utility of point-of-care ultrasound in the assessment of volume status in acute and critically ill patients. World J Emerg Med 2019;10:232.
- Pfennig CL, Slovis CM. Electrolyte disorders: Hyponatremia. In: Walls RM, Hockberger R, Gausche-Hill M, et al, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 10th ed. Elsevier;2023.
- Kumar A, Ghosh M, Jacob JJ. Prevalence of adrenal insufficiency among patients with euvolemic hyponatremia. Endocr Connect 2021;10:1623-1631.
- Shah NR, Tavana S, Opoku A, Martin D. Toxic and metabolic leukoencephalopathies in emergency department patients: A primer for the radiologist. Emerg Radiol 2022;29:545-555.
- Chifu I, Gerstl A, Lengenfelder B, et al. Treatment of symptomatic hyponatremia with hypertonic saline: A real-life observational study. Eur J Endocrinol 2021;184:647-655.
- Seay NW, Lehrich RW, Greenberg A. Diagnosis and management of disorders of body tonicity — Hyponatremia and hypernatremia: Core curriculum 2020. Am J Kidney Dis 2020;75:272-286.
- O’Sullivan M, McCarthy KF. Sodium: Sign, signifier, or signified, of sepsis? Eur J Intern Med 2021;83:10-11.
- Yen CW, Yu MC, Lee J. Serum electrolyte abnormalities in pediatric patients presenting to an emergency department with various diseases: Age-related differences. Pediatr Neonatol 2022;63:575-581.
- Lehtiranta S, Honkila M, Kallio M, et al. Severe hospital-acquired hyponatremia in acutely ill children receiving moderately hypotonic fluids. Pediatr Nephrol 2022;37:443-448.
- Akinsola B, Cheng J, Iyer SB, Jain S. Improving isotonic maintenance intravenous fluid use in the emergency department. Pediatrics 2021;148:e2020022947.
- Lehtiranta S, Honkila M, Kallio M, et al. Risk of electrolyte disorders in acutely ill children receiving commercially available plasmalike isotonic fluids: A randomized clinical trial. JAMA Pediatr 2021;175:28-35. Erratum in: JAMA Pediatr 2021;175:212.
- Woodfine JD, Sood MM, MacMillan TE, et al. Derivation and validation of a novel risk score to predict overcorrection of severe hyponatremia: The Severe Hyponatremia Overcorrection Risk (SHOR) score. Clin J Am Soc Nephrol 2019;14:975-982.
Hyponatremia is one of the most common electrolyte derangements among adults presenting to the emergency department and is associated with significant morbidity and mortality. A variety of factors and disease processes can contribute to the development of hyponatremia, varying in both chronicity and in subsequent symptomatology. Understanding the varied etiologies of hyponatremia is essential for the emergency physician to appropriately manage this electrolyte disorder, ensuring appropriate treatment and disposition in a common but potentially dangerous disease process.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
