Hypertensive Disorders of Pregnancy: More than Hypertension and Proteinuria
November 1, 2022
AUTHORS
Daniel J. Brown, MD, FACEP, Department of Emergency Medicine Wright State University, Dayton, OH; Department of Emergency Medicine, University of Cincinnati, OH
Kasey W. Rawlins, MD, Department of Emergency Medicine Wright State University, Dayton, OH
PEER REVIEWER
Larissa I. Velez, MD, Associate Dean for Graduate Medical Education, Professor and Vice Chair for Education, Michael P. Wainscott Professorship in Emergency Medicine, Department of Emergency Medicine, UT Southwestern Medical Center, Dallas, TX
EXECUTIVE SUMMARY
- Hypertensive disorders in pregnant patients include chronic hypertension, gestational hypertension, preeclampsia, and eclampsia. Because blood pressure decreases early in pregnancy, the finding of new hypertension should be concerning.
- Most patients who develop hypertension during pregnancy can be managed as an outpatient; however, the emergency department physician should ensure obstetric follow-up.
- Preeclampsia traditionally has been diagnosed by the presence of proteinuria, hypertension, and edema. However, proteinuria is not always present, and edema is not diagnostic of preeclampsia. While patients who are obese, diabetic, or chronically hypertensive are at greater risk, about half of patients with preeclampsia will have no risk factors.
- Eclampsia may or may not be preceded by preeclampsia. Seizures are treated with magnesium. Patients may become ecalmptic six weeks (and rarely even longer) postpartum.
Pregnant patients are a high-risk population, where small physiologic changes may indicate severe disease and result in increased morbidity and mortality for mother and fetus.
This article explains the current diagnostic criteria for hypertensive disorders of pregnancy and how they are interrelated. It also describes evidence-based interventions for emergency providers, who must know how to diagnose and treat these conditions, and when it is safe for discharge, as well as to arrange outpatient follow-up.
Introduction
Hypertensive disorders of pregnancy are one of the leading causes of maternal and perinatal mortality worldwide.1,2,3 Symptoms, presentations, and disease severity vary greatly, and acute recognition and management by the emergency provider often are required. Hypertensive disorders of pregnancy represent a spectrum of disease states that includes gestational hypertension, preeclampsia, HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome, and eclampsia. According to the American College of Obstetricians and Gynecologists (ACOG), there are four distinct categories of hypertensive states in pregnant patients.4 These include chronic hypertension, gestational hypertension, preeclampsia, and chronic hypertension with superimposed eclampsia. Hypertension is defined as a systolic blood pressure (SBP) of 140 mmHg or greater or a diastolic blood pressure (DBP) of 90 mmHg on two occasions at a minimum of four hours apart.5 In the emergency department, each of these conditions other than mild chronic hypertension requires consultation with OB/GYN specialists. Following are important definitions:
Chronic hypertension is defined as elevated blood pressure present prior to pregnancy or before 20 weeks of gestation. Chronic hypertension does not preclude patients from developing preeclampsia during their pregnancies, and it actually increases the risk of superimposed preeclampsia. When a patient with chronic hypertension develops proteinuria or evidence of end-organ damage, it is considered chronic hypertension with superimposed preeclampsia.
Gestational hypertension is defined by an SBP ≥ 140 mmHg or a DBP ≥ 90 mmHg in a woman with a previously normal range blood pressure, found on two occasions at a minimum of four hours apart, after 20 weeks of gestation. “Severe features” is added to gestational hypertension if SBP reaches 160 mmHg or greater or DBP reaches 110 mmHg or greater. Blood pressure levels usually return to a normal range postpartum.6
Preeclampsia has been defined classically as gestational hypertension in combination with proteinuria, but other diagnostic criteria now exist. (See Table 1.) Preeclampsia may be diagnosed in the absence of proteinuria. The official diagnostic criteria now include gestational hypertension (new-onset SBP ≥ 140 mmHg/DBP ≥ 90 mmHg on two occasions, four hours apart) in addition to proteinuria or the presence of end-organ damage. Proteinuria can be determined by three methods: 300 mg or more of protein on a 24-hour urine collection (gold standard), a protein to creatinine ratio of 0.3 mg/dL, or a urine dipstick result of 2+ protein. End-organ damage includes thrombocytopenia (platelet count less than 100,000 × 109/L); abnormal liver function (two times the upper limit of normal of aspartate aminotransferase [AST] or alanine transaminase [ALT] or severe persistent right upper quadrant or epigastric pain unresponsive to medications); renal dysfunction (serum creatinine levels > 1.1 mg/dL or two times normal creatinine level in the absence of other renal pathology); pulmonary edema; or neurological abnormalities (new-onset headache unresponsive to medications and not explained by other diagnoses or visual disturbances).6 When present, these signs of end-organ damage with or without proteinuria also are referred to as preeclampsia with severe features or gestational hypertension with severe features.6
Table 1. Diagnostic Criteria for Preeclampsia |
Blood Pressure Systolic or diastolic
|
Plus any of the following:
|
Based on American College of Obstetricians and Gynecologists (ACOG)6 guidelines unless otherwise noted. DIC: disseminated intravascular coagulation; AST: aspartate aminotransferase; ALT: alanine transaminase * Canada’s Society of Obstetricians and Gynecologists (SOGC) recommends repeated measurements at least 15 minutes apart13 α The United Kingdom’s National Institute for Health and Care Excellence (NICE)14 recommends avoiding first morning urine to quantify proteinuria. Alternative albumin/creatinine ratio ≥ 8 mg/mmol is diagnostic. Platelet count < 150 x 109/L is concerning. β Society of Obstetric Medicine Australia and New Zealand (SOMANZ)15 guidelines |
Hemolysis, elevated liver enzymes, and low platelet count, or HELLP syndrome, has multiple definitions, but the diagnostic criterion accepted by ACOG is the combination of a lactate dehydrogenase (LDH) level of above 600 IU/L, AST and ALT of 2 × the upper limit of normal, and thrombocytopenia < 100,000 × 109/L.7 HELLP syndrome is thought to be a more severe form of preeclampsia, rather than a separate disease entity. Interestingly, it may occur in the absence of either prerecorded hypertension or proteinuria in up to 15% of patients.8,9
Eclampsia is considered the most severe manifestation of the hypertensive disorders of pregnancy and is defined as new-onset seizure without other identifiable cause. Although traditionally thought to be a linear disease process, in which a patient progresses from gestational hypertension to preeclampsia to the development of severe features and finally eclampsia, the general consensus now is that this is not the case. Instead, any hypertensive disorder can manifest in convulsions regardless of prior disease severity. Additionally, studies have shown that eclamptic seizures may occur in isolation in patients, without any signs or symptoms of prior disease.10,11,12
Epidemiology
Throughout the world, an estimated 2% to 12% of all pregnancies are complicated by hypertensive disorders. Between 3% and 5% of all pregnancies present with preeclampsia, and 0.016% to 0.1% with eclampsia in developed countries.16,17 An estimated 70,000 maternal deaths and 500,000 fetal deaths worldwide are attributed to preeclampsia.18 As such, it is one of the leading causes of prematurity worldwide and in the United States. Infants also are at an increased risk for intrauterine growth restriction and low birth weight.19 Globally, hypertensive disorders of pregnancy are the second leading cause of maternal death.20 Women with preeclampsia are at risk for cardiovascular disease, such as stroke and acute myocardial infarction, later in life.6 There is evidence that the incidence of preeclampsia has been on the rise over the past few decades.21,22 This may be attributed to parallel increases in the prevalence of associated characteristics, such as advanced maternal age, obesity, and diabetes, which increase the risk of hypertensive disorders of pregnancy.
An estimated one of every nine pregnancies in the United States is affected by a hypertensive disorder. Hypertensive disorders of pregnancy may present six weeks, and in rare cases even several months, postpartum. As such, emergency department (ED) staff must carry a high index of suspicion when interacting with patients in the postpartum period and consult obstetric specialists when caring for affected mothers.
Etiology
Several risk factors for the development of preeclampsia have been identified in the literature. (See Table 2.) The most significant risk factors include prior preeclampsia, chronic hypertension, pre-gestational diabetes mellitus, multiple gestation, body mass index (BMI) > 30 (pre-pregnancy), and antiphospholipid syndrome. Other moderate risk factors include advanced maternal age (> 35 years), nulliparity, systemic lupus erythematosus, chronic kidney disease, history of stillbirth, BMI > 25 (pre-pregnancy), prior placental abruption, assisted reproductive technology, and family history of preeclampsia.23 Other studies have listed polycystic ovarian syndrome, sleep disordered breathing, thrombophilia, and hydatidiform mole as clinical risk factors.24,25,26 Furthermore, there is ongoing research that suggests there is a genetic component to preeclampsia.27,28 Despite this, the majority of preeclampsia cases occur in individuals who are previously healthy and without risk factors.6
Table 2. Preeclampsia Risk Factors in Order of Greatest Risk |
|
Pathophysiology
The leading theory is that preeclampsia is secondary to abnormal implantation and immune maladaptation. Dysfunctional placental implantation is thought to occur early in the first trimester. This is led in part by the insufficient fetal cytotrophoblast invasion of the spiral arteries and myometrium, which in turn leads to incomplete spiral artery remodeling.29,30 The result is narrow vessels that impede blood flow to the placenta, which cause reduced perfusion and ultimately lead to placental ischemia. This hypoperfusion is thought to prompt the release of antiangiogenic factors into the maternal circulation by the placenta. These factors lead to a cascade of systemic inflammatory response and endothelial dysfunction, which triggers many of the clinical features seen in preeclampsia, including hypertension, edema, and proteinuria. The generalized inflammation also leads to widespread vasoconstriction, reduced intravascular volume, and activation of the coagulation cascade, resulting in platelet aggregation and consumption.19,31 In the kidneys, endothelial cell swelling, basement membrane disruption, and microangiopathy occur in a pathologic state known as glomerular endotheliosis.32 This leads to the nonspecific leakage of protein into the urine. This microvascular damage and ischemic-reperfusion injury with associated immune response also leads to liver dysfunction, as seen in HELLP syndrome.33,34
The underlying pathophysiology of eclampsia is not well understood. The most widely accepted hypothesis is a malfunction of the blood brain barrier because of the systemic inflammation and endothelial dysfunction described earlier. This creates increased permeability of fluids and plasma protein into the brain parenchyma.35 Another proposed mechanism is the dysfunction of the autoregulatory system of the brain in the setting of elevated blood pressure, similar to hypertensive encephalopathy.36,37 A combination of decreased cerebral vascular resistance and hydrostatic pressure lead to cerebral edema, inflammation of the neurons, and neural damage.
Disease Course
Only 10% to 50% of women with gestational hypertension will progress to preeclampsia during the course of their pregnancy.38,39 Furthermore, only about 2% of women with preeclampsia ultimately developed eclampsia, in the absence of treatment.40,41 Although most eclampsia seizures are preceded by signs of cerebral irritation (headache, visual disturbances, etc.), some patients will convulse suddenly and without warning.42 In fact, some patients with eclampsia do not even have hypertension or proteinuria prior to their seizure.43,44 Preeclampsia diagnostically has signs of end-organ damage compared to gestational hypertension;45 however, the notion that gestational hypertension is significantly less worrisome than preeclampsia is false.44
Roughly 85% of patients with a hypertensive disorder of pregnancy present at 34 weeks of gestation or later, often during labor. Another 10% will present before 34 weeks and around 5% present in the postpartum period.46 Eclamptic convulsions also can occur any time during the antepartum, intrapartum, and postpartum period. In fact, eclampsia is more common in postpartum women than preeclampsia, with an estimated 20% to 40% of seizures occurring in the postpartum period. Almost half of all eclamptic seizures occur more than 48 hours after delivery, with a median of five days postpartum, often after the patient has been discharged from the hospital.47,48,49
Long-Term Sequelae
Women with hypertensive disorders of pregnancy are at risk for long-term complications. Studies have shown an increased risk of stroke (hazard ratio [HR], 2.27; 95% confidence interval [CI], 1.37-3.76), coronary artery disease (1.89; 1.26-2.82), cardiac arrhythmias (1.62; 1.28-2.05), chronic kidney disease (2.41; 1.54-3.78), and multimorbidity (1.25; 1.15-1.35) in those with hypertensive disease of pregnancy.50 Stuart et al found similar results when following 57,974 participants free of cardiovascular disease prior to their first pregnancy. After a median of 34 years of follow-up since the first pregnancy, women with hypertensive disease of pregnancy had a 63% increased rate of cardiovascular disease compared with normotensive first pregnancies. Among this cohort, the average age at the time of first birth of all participants was 27-28 years, and statistically significant cardiovascular disease was noted at age 40-49 years for those who had hypertensive disease of pregnancy.51
Studies show that the offspring also are affected, with long-term development of cardiovascular complications. A growing body of literature suggests that offspring of pregnant women with hypertensive disease of pregnancy develop a vascular phenotype that predisposes them to hypertension, even at an early age. This phenotype manifests as increased carotid intima-media thickness.52 Multiple studies from Finland have shown that children born to mothers with severe preeclampsia have a 1.5-times relative risk of developing hypertension. A meta-analysis that compiled data from more than 45,000 offspring of pre-eclamptic mothers revealed that even from an early age, children can have a 2.39 mmHg higher systolic and 1.35 mmHg higher diastolic blood pressure. If tracked into adulthood, this difference would increase these individuals’ risk of ischemic heart disease and stroke.52 Figure 1 summarizes the effects of preeclampsia to the fetus and offspring.52
As discussed earlier, dementia is a well-studied long-term complication in women with a history of preeclampsia and other hypertensive disorders of pregnancy. One study with a cohort of 1,178,005 women showed that preeclamptic patients had a three-times higher risk of developing vascular dementia as compared to pregnant women who never developed any hypertensive disorders of pregnancy. The association was particularly strong for late-onset vascular dementia, and less for early-onset vascular dementia, Alzheimer’s dementia, or other types of dementia.53
Figure 1. Effects of Preeclampsia on the Fetus and Offspring |
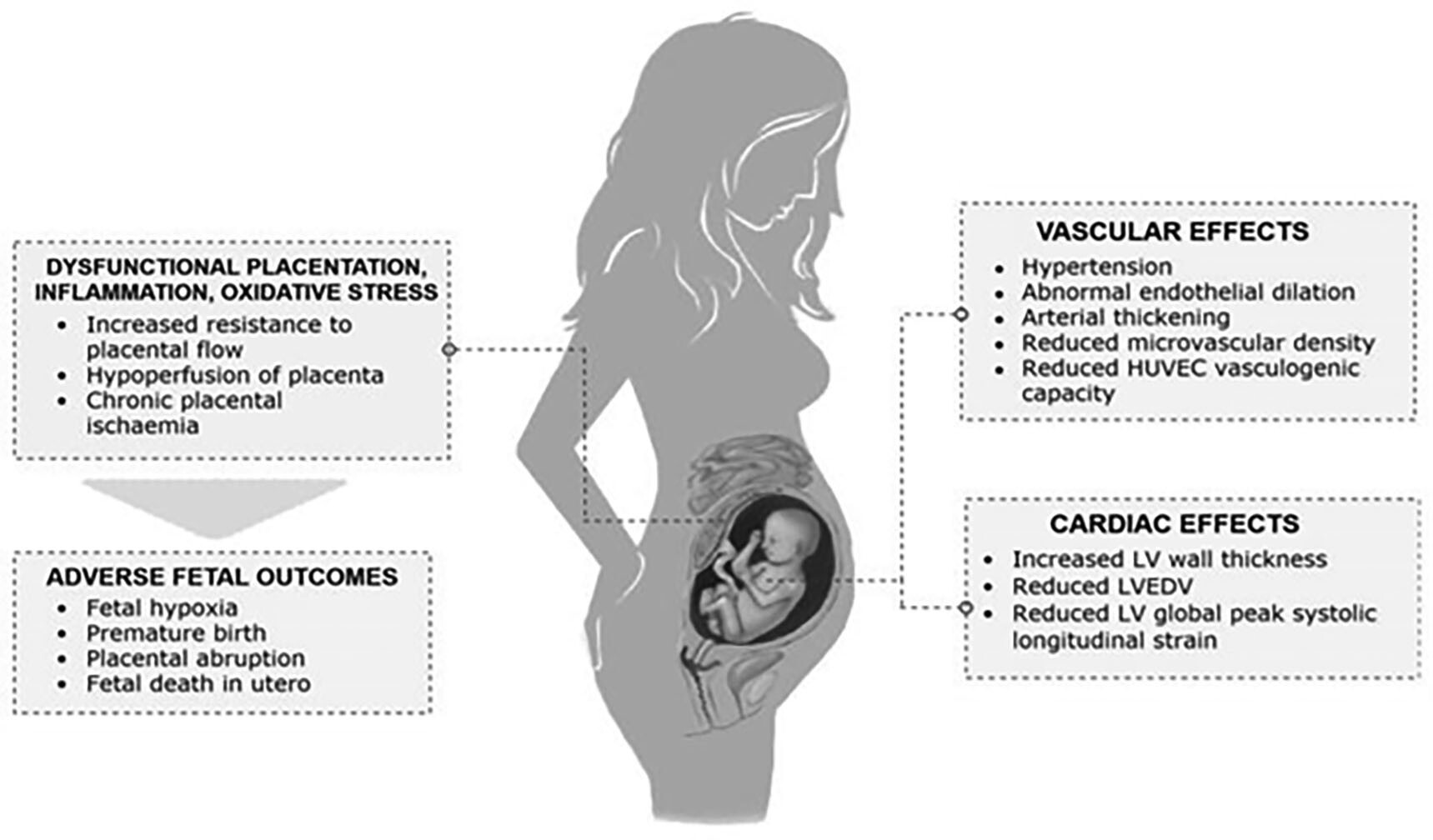 |
HUVEC: human umbilical vein endothelial cells; LV: left ventricle; LVEDV: left ventricular end-diastolic volume Source: Fox R, Kitt J, Leeson P, et al. Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med 2019;8:1625. https://creativecommons.org/licenses/by/4.0/ |
Clinical Presentation
The clinical presentation of a patient with a hypertensive disorder of pregnancy is extremely varied. Most often, patients present to the ED for a completely unrelated reason and are found to have elevated blood pressure or protein in their urine. One in five women have primary headache disorders, and those with migraines have a four-times increased risk of developing preeclampsia.54 ACOG defines a preeclampsia-related headache as one that is “new-onset” and “unresponsive to acetaminophen and not accounted for by alternative diagnoses or visual symptoms.” Patients may endorse visual disturbances, such as blurry vision, scotomas, or photophobia. Altered mental status may be seen in patients, with confusion, altered behavior, or agitation. Hyperreflexia is another possible sign of neurologic dysfunction. The aforementioned symptoms are commonly referred to as signs of “cerebral irritation” and are very concerning because they precede seizures in 75% to 80% of eclamptic women.49,55 Seizures of eclampsia can be focal but most often are generalized tonic-clonic convulsions followed by a post-ictal state.35
Right upper quadrant/epigastric areas secondary to liver involvement may be seen, but abdominal pain also may be generalized. Abdominal pain may be accompanied by nausea and vomiting. Petechiae or bleeding from the gums or nose may be signs of thrombocytopenia. Lastly, dyspnea, orthopnea, cough, or chest pain can be seen in the setting of pulmonary edema. Unfortunately, the most common symptoms of headache, visual disturbances, and epigastric pain are not reliable enough alone to accurately predict nor exclude imminent eclampsia. Headache is the most sensitive symptom (57%), but it captures only about half of the women who go on to develop eclampsia and has the lowest specificity (83%).56 Other symptoms may include peripheral edema, rapid weight gain, or facial edema. These are common in all pregnancies, but when they are rapid or more pronounced, they may warrant further investigation for the disease.
Examination
When evaluating a patient with concern for a hypertensive disorder of pregnancy, precise blood pressure measurement is vital. Because pregnancy brings several physiologic changes to the body and the vasculature, inaccuracies may result from devices that are not validated in pregnant women. Therefore, specialty guidelines focus on using a device that is validated for pregnant women and for those with preeclampsia.57,58 In the ED, however, providers generally will use whatever automated vital signs device is on hand. Factors that may result in inaccurate measurements include inappropriately sized cuffs, inaccurate placement of the cuff, a full bladder, talking or crossing legs during measurement, recent activity in the past five minutes, ingestion of caffeine or nicotine within the past 30 minutes, or sitting in an incorrect position.59 Any combination of these factors may result in a falsely elevated reading and should be controlled for as much as possible when measuring blood pressure on any pregnant patient in the ED. (See Table 3.)
Table 3. Inaccuracies in Blood Pressure Measurement |
|
Type of Measurement Inaccuracy |
False Measurement |
|
High |
|
Low |
The majority of blood pressure readings in the ED now are performed with automated devices that use the oscillometric method to read blood pressure. These are more prone to error and require periodic recalibration. Confirmation by auscultation method may be performed when there is concern for an inaccurate reading, such as large body habitus or suspicious symptoms.57 The use of invasive blood pressure monitoring is helpful in the critically ill patient to provide real-time assessments of changes in hemodynamics.57
Although the official diagnosis of hypertension in pregnancy is a blood pressure measurement of ≥ 140/90 mmHg on two separate occasions at least four hours apart, this often is not feasible nor is it practical in the ED. In a critically ill patient, perform serial blood pressure readings in a matter of minutes rather than hours to facilitate prompt diagnosis and timely administration of antihypertensives.
Another important step is determining whether an elevated blood pressure in a pregnant patient is a new finding or an already established diagnosis. As discussed, patients with chronic hypertension still can develop superimposed preeclampsia. Therefore, screen all pregnant patients with preexisting (chronic) hypertension for proteinuria or evidence of end-organ damage. Lastly, in patients presenting prior to 20 weeks with elevated BP or other signs or symptoms of preeclampsia, investigate alternative diagnoses, including, but not limited to, molar pregnancy, drug use, renal disease, autoimmune disease, thrombotic thrombocytopenic purpura, and hemolytic–uremic syndrome.
The examination of the hypertensive pregnant patient after 20 weeks of gestation should be centered not only on their chief complaint, but also on the evaluation for evidence of end-organ damage. Examination of the abdomen may reveal right upper quadrant tenderness to palpation. Respiratory distress and rales might be found on cardiopulmonary exam secondary to pulmonary edema. Although common in any pregnancy and not diagnostic for preeclampsia, lower extremity edema is evident in many patients with preeclampsia. When edema is present with preeclampsia, it usually is more sudden in onset and more pronounced. The neurologic examination may demonstrate decreased visual acuity, visual field deficits, changes in mental status, and hyperreflexia. The fundoscopic examination may reveal abnormalities, including hypertensive retinopathy or serous retinal detachment.60 Generalized tonic-clonic seizures are eclamptic in nature in any antepartum
(> 20 weeks) or postpartum woman until proven otherwise.61
Diagnostic Studies
In pregnant or postpartum women with concerning risk factors, obtain a complete blood count (CBC), particularly to evaluate the platelet count. A platelet count less than 100,000 × 109/L is used as part of the diagnosis in both preeclampsia and HELLP syndrome. A serum chemistry is used to evaluate for electrolyte abnormalities and for kidney function. Liver function tests may demonstrate elevated AST and/or ALT levels, with two times the upper limit of normal being used for evidence of end-organ damage. Draw a lactate dehydrogenase (LDH) level if the patient’s liver enzymes are abnormal. An LDH value above 600 IU/L is another diagnostic criterion of HELLP syndrome.
Assess for proteinuria in all women being evaluated for hypertensive disorders of pregnancy. In the ED, obtain a random urine sample and order a routine urinalysis. A dipstick reading of 2+ protein on a midstream clean catch is acceptable for diagnosis. However, a single urine protein to creatinine ratio of ≥ 0.3 is more accurate and preferred to a dipstick reading.6 Order urine creatinine and urine protein if these tests are available with a reasonable turnaround time. Further testing, such as electrocardiogram (ECG) and troponins, may be ordered for pregnant patients with hypertensive disorders of pregnancy and symptoms of chest pain or dyspnea to assess for cardiac end-organ damage.
Ultrasonography is useful in assessing fetal well-being. In the setting of right upper quadrant (RUQ) pain or tenderness, an RUQ ultrasound may be used in the evaluation of alternative diagnoses. Chest X-ray or lung ultrasound may show findings of pulmonary edema, such as cephalization, interstitial or alveolar edema, pulmonary venous congestion, pleural effusions, and cardiomegaly. The hemodynamic changes that can accompany preeclampsia often result in changes to the cardiac function and structure. These changes can be visualized with echocardiography and include increased vascular resistance, left ventricular hypertrophy, diastolic dysfunction, and even reduced stroke volume.62,63 Point-of-care ultrasound (POCUS) may be used to assess fluid status, identify pulmonary edema, measure optic nerve sheath diameter, and recognize diastolic dysfunction by performing echocardiography as described earlier. Computed tomography and magnetic resonance imaging (MRI) of the brain have been found to demonstrate cerebral edema and ischemic changes in the posterior hemispheres similar to that of posterior reversible encephalopathy syndrome (PRES).64 Routine neuroimaging is not recommended for every eclamptic patient, and instead should be considered only in those with focal neurologic deficits, prolonged coma, or recurrent convulsions.35
Prevention
An intervention to completely reduce the risk of progression has yet to be found. Currently, aspirin is the only agent that is recommended as a potential method of lowering the risk of preeclampsia. The proposed mechanism of action is through the inhibition of the cyclooxygenase-1 enzyme, which ultimately reduces the amount of thromboxane and downregulates platelet aggregation.65 Evidence of aspirin’s efficacy has been debated for years, but more recently the Aspirin for Evidence-Based Preeclampsia Prevention (ASPRE) trial found that of women who were high risk for preeclampsia and took 150 mg aspirin daily starting between 11 and 14 weeks of gestation, only 1.6% ultimately developed preterm preeclampsia as opposed to 4.3% in the placebo group.66 Furthermore, a recent meta-analysis found evidence to suggest that the initiation of aspirin before 16 weeks of gestation reduced the risk of development of preeclampsia, severe preeclampsia, and fetal growth restriction.67 Based on these findings, both the ACOG and the U.S. Preventive Services Task Force (USPSTF) currently recommend low-dose (81 mg) aspirin daily starting between 12 and 28 weeks of gestation in women with risk factors for preeclampsia.6,68
Management
Since the presentation and severity may vary greatly, having a high index of suspicion is crucial to identifying patients at risk of serious morbidity and mortality. In the hypertensive pregnant patient presenting to the ED, the initial focus should be on stabilization and the ABCs (airway, breathing, circulation). For a stable and well-appearing patient with gestational hypertension, identify any associated complications or severe features of their disease. Prior to 37 weeks of gestation and in the absence of severe features, close monitoring as an outpatient has been deemed appropriate by ACOG. Coordination of care should be discussed with obstetrics, since these patients require extremely close follow-up for both maternal and fetal monitoring.
Several studies have compared outcomes between tight blood pressure control and loose control in non-proteinuric patients with mild to moderate gestational hypertension (DBP < 109 mmHg) and found no difference in pregnancy loss, overall maternal complications, or need for high-level neonatal care.69 A systematic review published in 2018 confirmed that there appears to be no difference in progression to preeclampsia or risk of fetal/neonatal death by treating women with mild to moderate hypertension. However, there was evidence to suggest that tighter blood pressure control reduces the progression to severe hypertension by nearly half.70 The ACOG currently recommends initiation of antihypertensive medication once patients reach SBP or DBP > 160/110 mmHg that is found to be persistent, or lasting ≥ 15 minutes.6
Several oral antihypertensive medications are available for use in this patient population. No single agent has been deemed superior to the others in the literature. Possible medications include labetalol, nifedipine, methyldopa, hydrochlorothiazide (HCTZ), and hydralazine. The recommended dose for labetalol is 200 mg/d to 2,400 mg/d in two to three divided doses. For nifedipine, it is 30 mg to 120 mg once daily. Methyldopa is 0.5 g/d to 3 g/d in two to three divided doses. HCTZ is dosed as 12.5 mg/d to 25 mg/d. Lastly, hydralazine is not recommended as monotherapy, but as adjunct therapy, and is given two to four times a day at a dose of 50 mg to 200 mg.61,71,72 All agents listed previously have been assigned to Pregnancy Category C (other than methyldopa, which is Category B), which means risk cannot be ruled out either because of a lack of human studies or because of animal studies showing potential risk to the fetus, but potential benefit of treatment likely outweighs risks.73 Because of these risks, new antihypertensive therapies should be initiated only in consultation with obstetricians.
When severe features are present (see Table 4) or when the patient is unstable and urgent blood pressure control is warranted, there are a number of other options available. The three most commonly used medications include intravenous (IV) labetalol, IV hydralazine, and oral immediate-release nifedipine. Both IV options are preferred, but immediate-release nifedipine is useful when a patient does not have IV access. Any combination of these three agents may be used in an escalating stepwise manner. Labetalol may be given initially as 10 mg to 20 mg IV over two minutes, followed by additional doses of 20 mg to 80 mg every 10-30 minutes. For hydralazine, the initial dose is 5 mg over one to two minutes with a repeat of 5 mg to 10 mg IV every 20-40 minutes. Finally, nifedipine is suggested to be administered at a starting dose of 10 mg to 20 mg orally (PO) with an additional dose optional at 20 minutes, followed by 10 mg to 20 mg PO every two to six hours. Maximum doses of these medications are 300 mg of labetalol, 20 mg of hydralazine, and 180 mg nifedipine. Alternatively, labetalol may be infused at a continuous rate of />1 mg/min to 2 mg/min IV or hydralazine at a rate of 0.5 mg/hr to 10 mg/hr IV.5 These agents should be titrated to maintain a blood pressure goal of < 160 mmHg systolic or < 110 mmHg diastolic. Similar to outpatient maintenance therapy, there is no evidence to suggest one agent is superior to the others.74 Figure 2 represents a useful algorithm for urgent blood pressure reduction in the preeclamptic patient.61
Table 4. Treatment of Preeclampsia with Severe Features |
|
Severe Features |
Treatment |
|
If systolic or diastolic blood pressure
PLUS
|
AST: aspartate transaminase; ALT: alanine transaminase; IV: intravenous; PO: orally * Check for magnesium toxicity: loss of deep tendon reflexes and respiratory depression. Treat with 10% calcium gluconate 10 mL to 30 mL over 3-5 minutes. Note: This is a simple summary. Please refer to the text for more details. |
|
Figure 2. Medications for Acute Blood Pressure Management for Severe Preeclampsia/Eclampsia |
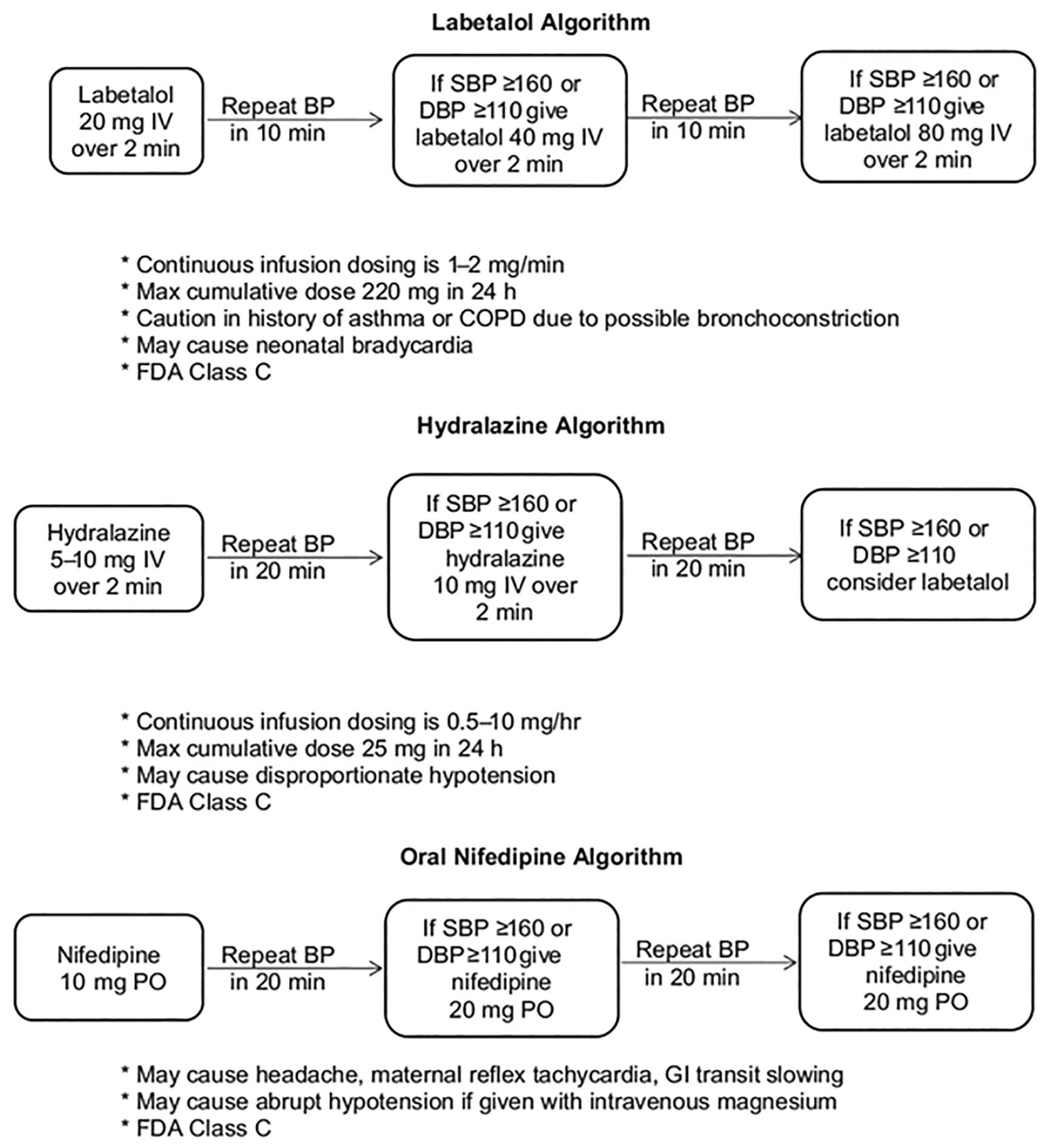 |
Reprinted with permission from: Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am 2019;37:301-316. |
Seizure prophylaxis and control is another major aspect of the management of this patient population in the ED. The mainstay of this treatment is magnesium, which reduces seizure rate by half with treatment.75 Currently, only women presenting with preeclampsia with severe features or eclampsia are recommended to undergo treatment with magnesium. ACOG currently does not recommend routine administration in those with only gestational hypertension or preeclampsia without severe features because of the lack of definitive benefit and a high number needed to treat (129).6 The recommended dosing in the United States typically is a loading dose of 4 g to 6 g IV over 15-30 minutes with a maintenance dose of 1 g/hr to 2 g/hr. Additionally, boluses of 2 g may be administered for any eclamptic seizures that occur after the loading dose. If IV access cannot be obtained, intramuscular (IM) magnesium can be administered with a loading dose of 10 g (5 g IM in each buttock) followed by additional 5 g every four hours, which may be mixed with local anesthetic to help with pain at the injection site. Typically, magnesium is administered for up to 24 hours after delivery.
Monitor all pregnant patients receiving magnesium treatment by routinely checking patellar tendons and observing the respiratory rate. The side effects of magnesium are thought to be related to the smooth muscle relaxation. Loss of deep tendon reflexes occurs first and is believed to be around a serum magnesium level of 8.5 mg/dL to 12.0 mg/dL. This is followed by respiratory depression at 12 mg/dL to 16 mg/dL and cardiac arrest at 30 mg/dL.76 Additionally, consider magnesium is excreted almost exclusively through the urine. Monitor urine output while a patient is receiving a magnesium infusion. If a patient has evidence of mild renal insufficiency (serum creatinine of 1.0 mg/dL to 1.5 mg/dL) or oliguria (urine output < 30 mL/hr for over four hours), ACOG recommends a normal loading dose of 4 g to 6 g with a maintenance dose of only 1 g/hr. If a patient develops severe magnesium toxicity, the treatment is with 10% calcium gluconate at a dose of 15 mL to 30 mL administered intravenously over two to five minutes. A lower dose of 10 mL may be used for less severe toxicity. Intravenous furosemide also may be used to increase the excretion of magnesium in the urine.6 If there is impending respiratory failure, endotracheal intubation and mechanical ventilation can be considered.
Basic management principles of actively seizing patients also should be employed for the eclamptic patient in the ED. The patient should be placed in the lateral decubitus position to avoid aspiration. Suction should be used to clear the airway and oxygen administered for hypoxia. Bedrails should be raised with padding applied to prevent maternal injury. If the patient has already received the loading dose and is seizing, an additional 2 g bolus of magnesium may be given over three to five minutes. If the patient continues to seize after this bolus, 4 mg IV lorazepam is recommended.35 Alternative diagnoses should be considered in patients with focal neurologic deficits, those prior to 20 weeks of gestation, those with normal blood pressures or lack of proteinuria, or those with prolonged loss of consciousness.35 Consider and treat concerns for hypoglycemia, hyponatremia, or toxic ingestions that may lead to seizures.
Disposition
It is extremely important to involve obstetrics or maternal-fetal medicine early in the decision-making for these patients. Patients with gestational hypertension or preeclampsia without severe features likely can be discharged with close outpatient monitoring. Even so, a conversation with obstetrics should occur to ensure appropriate follow-up.77 For patients with preeclampsia with severe features or HELLP syndrome, guidelines recommend delivery at 34 weeks of gestation or beyond, because of the high risk of complications. Between 28 and 34 weeks of gestation, the decision is less clear, but expectant management in these patients may be associated with improved neonatal outcomes.78 Any patient who presents to the ED and is found to have severe features will require admission to the nearest labor and delivery unit. Stabilize and admit all eclamptic patients as well, since the only definitive treatment for eclampsia is delivery.10
Practice Gaps
Eclampsia has not been found to be a progression from severe preeclampsia. However, because eclamptic seizures occur in a subset of preeclamptic patients, it is important to continue to screen and treat this population.10
Many emergency providers may continue to rely on proteinuria for the screening and diagnosis of preeclampsia. However obstetric guidelines regarding diagnosis were updated in 2013 and now include other criteria related to end-organ damage. (See Table 1.) When a pregnant woman presents to the ED with elevated blood pressure, screen for the severe features listed in Table 4. Consult obstetrics immediately regarding admission, blood pressure treatment, and seizure prophylaxis in hypertensive pregnant women with severe features, regardless of gestational age. Women with gestational age < 34 weeks are at very high risk and will need further obstetric inpatient treatment prior to delivery.4,6
Headache is a common presenting complaint in pregnant women. Any new-onset headache unresponsive to medication and not accounted for by alternative diagnoses or any visual disturbance meets diagnostic criteria for preeclampsia with severe features regardless of any evidence of proteinuria.6 In coordination with obstetrics, these patients require admission and further treatment.
Emergency providers may start newly diagnosed pregnant patients on prenatal vitamins for preventive health. ED physicians can consider preventive treatment for patients at high risk for developing preeclampsia, including daily treatment with low-dose aspirin in consultation with obstetrics.68 Additionally, consider providing education in discharge instructions regarding lifestyle modification recommendations to help those women who are obese, have chronic hypertension, and have diabetes, since all of these conditions place women at risk for developing preeclampsia.79
Summary
Hypertensive disorders of pregnancy are common and lead to significant maternal and perinatal morbidity and mortality. Screen all pregnant patients in the ED and have a low threshold to consult obstetric specialists for any pregnant patient with elevated blood pressure. Consider prescribing daily low-dose aspirin for patients at risk of developing hypertensive disorders of pregnancy, starting in the second trimester. In consultation with OB, treat those with severe blood pressure (systolic or diastolic > 160/110 mmHg) with oral antihypertensive medications as outpatients unless they have severe features.
Evaluate all hypertensive pregnant patients for preeclampsia with severe features. The diagnosis of preeclampsia includes the presence of proteinuria, but that finding is not required to make the diagnosis. New-onset headache unrelieved with medications and not explained by alternative diagnoses, in the setting of hypertension in pregnancy at least 20 weeks of gestation, is among the diagnostic criteria for preeclampsia with severe features. Patients with severe features require immediate OB consultation, antihypertensive medication treatment, seizure prophylaxis with magnesium sulfate, and admission.
Eclampsia is a true emergency and requires immediate consultation with obstetrics and emergent transfer to labor and delivery. Immediately start an IV magnesium sulfate infusion as well as IV lorazepam for any eclamptic seizures that occur after the loading dose of magnesium. Treat concurrent hypertension in eclampsia with a goal blood pressure < 160/110 mmHg. Rapid treatment initiated in the ED and urgent delivery by OB provide the optimal care to reduce morbidity and mortality.
References
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: A WHO systematic analysis. Lancet Glob Health 2014;2:e323-e333.
- Creanga AA, Berg CJ, Ko JY, et al. Maternal mortality and morbidity in the United States: Where are we now? J Womens Health (Larchmt) 2014;23:3-9.
- Hao J, Hassen D, Hao Q, et al. Maternal and infant health care costs related to preeclampsia. Obstet Gynecol 2019;134:1227-1233.
- [No authors listed]. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122-1131.
- [No authors listed]. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000;183:S1-S22.
- [No authors listed]. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol 2020;135:1492-1495.
- Audibert F, Friedman SA, Frangieh AY, Sibai BM. Clinical utility of strict diagnostic criteria for the HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. Am J Obstet Gynecol 1996;175:460-464.
- Reubinoff BE, Schenker JG. HELLP syndrome—a syndrome of hemolysis, elevated liver enzymes and low platelet count—complicating preeclampsia-eclampsia. Int J Gynaecol Obstet 1991;36:95-102.
- Martin JN Jr, Rinehart BK, May WL, et al. The spectrum of severe preeclampsia: Comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol 1999;180(6 Pt 1):1373-1384.
- Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: Toward a new paradigm. Am J Obstet Gynecol 2000;182:1389-1396.
- Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ 1994;309:1395-1400.
- Sibai BM, Abdella TN, Spinnato JA, Anderson GD. Eclampsia. V. The incidence of non-preventable eclampsia. Am J Obstet Gynecol 1986;154:581-586.
- Butalia S, Audibert F, Côté AM, et al. Hypertension Canada’s 2018 Guidelines for the Management of Hypertension in Pregnancy. Can J Cardiol 2018;34:526-531.
- Webster K, Fishburn S, Maresh M, et al; Guideline Committee. Diagnosis and management of hypertension in pregnancy: Summary of updated NICE guidance. BMJ 2019;366:l5119.
- Lowe SA, Bowyer L, Lust K, et al. The SOMANZ Guidelines for the Management of Hypertensive Disorders of Pregnancy 2014. Aust N Z J Obstet Gynaecol 2015;55:11-16.
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 2010;376:631-644.
- Liu S, Joseph KS, Liston RM, et al; Maternal Health Study Group of the Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol 2011;118:987-994.
- Rana S, Lemoine E, Granger JP, Anath Karumanchi S. Preeclampsia: Pathophysiology, challenges, and perspectives. Circ Res 2019;124:1094-1112.
- Teng H, Wang Y, Han B, et al. Gestational systolic blood pressure trajectories and risk of adverse maternal and perinatal outcomes in Chinese women. BMC Pregnancy Childbirth 2021;21:155.
- Kassebaum NJ, Barber RM, Bhutta ZA, et al; GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1775-1812.
- Wang W, Xie X, Yuan T, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021;21:364.
- Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens 2008;21:521-526.
- Bartsch E, Medcalf KE, Park AL, et al. High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016;353:i1753.
- Yu HF, Chen HS, Rao DP, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4863.
- Soto-Wright V, Bernstein M, Goldstein DP, Berkowitz RS. The changing clinical presentation of complete molar pregnancy. Obstet Gynecol 1995;86:775-779.
- Facco FL, Parker CB, Reddy UM, et al. Association between sleep-disordered breathing and hypertensive disorders of pregnancy and gestational diabetes mellitus. Obstet Gynecol 2017;129:31-41.
- McGinnis R, Steinthorsdottir V, Williams NO, et al. Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nat Genet 2017;49:1255-1260.
- Kikas T, Inno R, Ratnik K, et al. C-allele of rs4769613 near FLT1 represents a high-confidence placental risk factor for preeclampsia. Hypertension 2020;76:884-891.
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019;366:l2381.
- Nirupama R, Divyashree S, Janhavi P, et al. Preeclampsia: Pathophysiology and management. J Gynecol Obstet Hum Reprod 2021;50:101975.
- Garovic VD, Dechend R, Easterling T, et al; American Heart Association Council on Hypertension; Council on the Kidney in Cardiovascular Disease, Kidney in Heart Disease Science Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; and Stroke Council. Hypertension in pregnancy: Diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the American Heart Association. Hypertension 2022;79:e21-e41.
- Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol 2007;18:2281-2284.
- Stojanovska V, Zenclussen AC. Innate and adaptive immune responses in HELLP syndrome. Front Immunol 2020;11:667.
- Alese MO, Moodley J, Naicker T. Preeclampsia and HELLP syndrome, the role of the liver. J Matern Fetal Neonatal Med 2021;34:117-123.
- Fishel Bartal M, Sibai BM. Eclampsia in the 21st century. Am J Obstet Gynecol 2022;226:S1237-S1253.
- van Veen TR, Panerai RB, Haeri S, et al. Cerebral autoregulation in normal pregnancy and preeclampsia. Obstet Gynecol 2013;122:1064-1069.
- Jones-Muhammad M, Warrington JP. Cerebral blood flow regulation in pregnancy, hypertension, and hypertensive disorders of pregnancy. Brain Sci 2019;9:224.
- Barton JR, O’Brien JM, Bergauer NK, et al. Mild gestational hypertension remote from term: Progression and outcome. Am J Obstet Gynecol 2001;184:979-983.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998;105:1177-1184.
- Sibai BM, Sarinoglu C, Mercer BM. Eclampsia. VII. Pregnancy outcome after eclampsia and long-term prognosis. Am J Obstet Gynecol 1992;166(6 Pt 1):1757-1761; discussion 1761-1763.
- Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: Recurrence risk and long-term prognosis. Am J Obstet Gynecol 1991;165(5 Pt 1):1408-1412.
- Cooray SD, Edmonds SM, Tong S, et al. Characterization of symptoms immediately preceding eclampsia. Obstet Gynecol 2011;118:995-999.
- Noraihan MN, Sharda P, Jammal AB. Report of 50 cases of eclampsia. J Obstet Gynaecol Res 2005;31:302-309.
- Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: Toward a new paradigm. Am J Obstet Gynecol 2000;182:1389-1396.
- Homer CS, Brown MA, Mangos G, Davis GK. Non-proteinuric pre-eclampsia: A novel risk indicator in women with gestational hypertension. J Hypertens 2008;26:295-302.
- Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med 1992;326:927-932.
- Cairns AE, Pealing L, Duffy JMN, et al. Postpartum management of hypertensive disorders of pregnancy: A systematic review. BMJ Open 2017;7:e018696.
- Yancey LM, Withers E, Bakes K, Abbott J. Postpartum preeclampsia: Emergency department presentation and management. J Emerg Med 2011;40:380-384.
- Berhan Y, Berhan A. Should magnesium sulfate be administered to women with mild pre-eclampsia? A systematic review of published reports on eclampsia. J Obstet Gynaecol Res 2015;41:831-842.
- Garovic VD, White WM, Vaughan L, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol 2020;75:2323-2334.
- Stuart JJ, Tanz LJ, Rimm EB, et al. Cardiovascular risk factors mediate the long-term maternal risk associated with hypertensive disorders of pregnancy. J Am Coll Cardiol 2022;79:1901-1913.
- Fox R, Kitt J, Leeson P, et al. Preeclampsia: Risk factors, diagnosis, management, and the cardiovascular impact on the offspring. J Clin Med 2019;8:1625.
- Basit S, Wohlfahrt J, Boyd HA. Pre-eclampsia and risk of dementia later in life: Nationwide cohort study. BMJ 2018;363:k4109.
- Digre KB. Headaches during pregnancy. Clin Obstet Gynecol 2013;56:317-329.
- Cooray SD, Edmonds SM, Tong S, et al. Characterization of symptoms immediately preceding eclampsia. Obstet Gynecol 2011;118:995-999.
- Hastie R, Brownfoot FC, Cluver CA, et al. Predictive value of the signs and symptoms preceding eclampsia: A systematic review. Obstet Gynecol 2019;134:677-684.
- Bello NA, Woolley JJ, Cleary KL, et al. Accuracy of blood pressure measurement devices in pregnancy: A systematic review of validation studies. Hypertension 2018;71:326-335.
- Hurrell A, Webster L, Chappell LC, Shennan AH. The assessment of blood pressure in pregnant women: Pitfalls and novel approaches. Am J Obstet Gynecol 2022;226:S804-S818.
- Khedagi AM, Bello NA. Hypertensive disorders of pregnancy. Cardiol Clin 2021;39:77-90.
- Abu Samra K. The eye and visual system in the preeclampsia/eclampsia syndrome: What to expect? Saudi J Ophthalmol 2013;27:51-53.
- Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am 2019;37:301-316.
- Castleman JS, Ganapathy R, Taki F, et al. Echocardiographic structure and function in hypertensive disorders of pregnancy: A systematic review. Circ Cardiovasc Imaging 2016;9:e004888.
- Gyselaers W. Hemodynamic pathways of gestational hypertension and preeclampsia. Am J Obstet Gynecol 2022;226:S988-S1005.
- McDermott M, Miller EC, Rundek T, et al. Preeclampsia: Association with posterior reversible encephalopathy syndrome and stroke. Stroke 2018;49:524-530.
- Rolnik DL, Nicolaides KH, Poon LC. Prevention of preeclampsia with aspirin. Am J Obstet Gynecol 2022;226:S1108-S1119.
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017;377:613-622.
- Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am J Obstet Gynecol 2018;218:287-293.e1.
- US Preventive Services Task Force; Davidson KW, Barry MJ, Mangione CM, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US Preventive Services Task Force recommendation statement. JAMA 2021;326:1186-1191.
- Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med 2015;372:407-417.
- Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev 2018;10:CD002252.
- Leavitt K, Običan S, Yankowitz J. Treatment and prevention of hypertensive disorders during pregnancy. Clin Perinatol 2019;46:173-185.
- ElFarra J, Bean C, Martin JN Jr. Management of hypertensive crisis for the obstetrician/gynecologist. Obstet Gynecol Clin North Am 2016;43:623-637.
- Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension 2008;51:960-969.
- Duley L, Meher S, Jones L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev 2013;2013:CD001449.
- Altman D, Carroli G, Duley L, et al; Magpie Trial Collaboration Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet 2002;359:1877-1890.
- Lu JF, Nightingale CH. Magnesium sulfate in eclampsia and pre-eclampsia: Pharmacokinetic principles. Clin Pharmacokinet 2000;38:305-314.
- Koopmans CM, Bijlenga D, Groen H, et al; HYPITAT study group. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks’ gestation (HYPITAT): A multicentre, open-label randomised controlled trial. Lancet 2009;374:979-988.
- Odendaal HJ, Pattinson RC, Bam R, et al. Aggressive or expectant management for patients with severe preeclampsia between 28-34 weeks’ gestation: A randomized controlled trial. Obstet Gynecol 1990;76:1070-1075.
- Sole KB, Staff AC, Laine K. Maternal diseases and risk of hypertensive disorders of pregnancy across gestational age groups. Pregnancy Hypertens 2021;25:25-33.
This article explains the current diagnostic criteria for hypertensive disorders of pregnancy and how they are interrelated. It also describes evidence-based interventions for emergency providers, who must know how to diagnose and treat these conditions, and when it is safe for discharge, as well as to arrange outpatient follow-up.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
