AUTHORS
Thomas Powell, MD, MS, FAWM, Chief Resident, Emergency Medicine, Ohio State University Wexner Medical Center, Columbus
Peter Rinne, DO, Emergency Medicine Resident, Ohio State University Wexner Medical Center, Columbus
Timothy Hoffman, MD, Emergency Medicine Resident, Ohio State University Wexner Medical Center, Columbus
Matthew Malone, MD, Clinical Assistant Professor of Emergency Medicine, Ohio State University Wexner Medical Center, Columbus
PEER REVIEWER
Mark Pittman, MD, FACEP, FAAEM, FAWM, Clinical Associate Professor, Emergency Medicine, University of South Carolina School of Medicine, Greenville
EXECUTIVE SUMMARY
- The primary recommendation of acclimatization to altitude is through the slow, deliberate increase in sleeping elevation by 500 m per day above 3,000 m. Further, an additional day of rest for every 1,000 m increase during these 500 m ascents has been demonstrated to reduce the incidence of altitude illness. A second preventive measure is the use of inhaled oxygen.
- Acute mountain sickness (AMS) manifests with the generally accepted constellation of symptoms suggested by the Lake Louise Criteria: a headache plus one of the following symptoms: insomnia, anorexia, nausea, vomiting, and dizziness.
- High altitude cerebral edema (HACE) is clinically defined as AMS progressing to ataxia or altered mental status. Immediate descent is the treatment of choice. If immediate descent is not possible, dexamethasone can be given as 8 mg initially and then 4 mg every six hours, given intravenous/intramuscular or orally, with a plan for descent as soon as possible. Acetazolamide 250 mg two times per day also can be used as treatment while awaiting descent. Oxygen, if on hand, also should be given. Fabric hand- or machine-pumped altitude chambers, colloquially known as “Gamow bags” after their original manufacturer, allow for a rescue party to “descend” the patient until definitive transport and are proven life-saving interventions if available for patient treatment.
- High altitude pulmonary edema (HAPE) can manifest as a dry cough that quickly becomes wet early in the disease course, and it can be mistaken for a benign upper respiratory tract infection, a reaction to the dry, cold air of altitude, or a side effect of the dusty environments found above the tree line. However, this dry cough progresses quickly to become productive, with frothy, pink sputum. Additional initial symptoms can include decreased exercise performance, prolonged exercise recovery time, dyspnea on exertion, and localized rales. The treatment of HAPE is immediate descent.
- If lower oxygen partial pressures at elevation were not enough, the body has to spend a significant amount of energy maintaining a core body temperature within the margin of about 2°C. For every 1,000 m someone ascends in elevation, the ambient temperature drops by 9.8°C. Humans must balance heat production and heat loss to stay within this margin.
Some patients love to challenge themselves, seeking new locations and activities, pushing themselves to perform in extreme environments. In this issue, the authors explore the physiology of altitude and the various illnesses encountered by people working and playing in the higher areas of the earth!
— Ann M. Dietrich, MD, Editor
Introduction
High on mountain slopes and plateaus reside harsh environments that have tested the limits of human endurance and tenacity. Because of their unique location high in the earth’s atmosphere, human activity in these regions is challenged by cold, high winds, frequent storms, and, most significantly, a hypobaric atmosphere that encourages the development of hypoxia. Despite these dangers, humans flock to these locales in ever-greater numbers for activities such as mountain climbing, alpine skiing, eco-tourism, scientific endeavors, and collection of natural resources.
At approximately 5,500 m above sea level, the earth’s atmosphere contains approximately half as many gaseous molecules as it does at sea level. Since the atmosphere contains only 21% oxygen, the human body must adapt to a lower availability of oxygen to maintain the oxygen-hungry tissues of the brain and other organs. Upon exposure to these oxygen-deprived environments, even the healthiest, most vigorous individuals will begin to experience various manifestations of low blood oxygen concentration at a pressure less than sea level, a phenomenon known as hypobaric hypoxia. This physiological state results in dangerous mental and physical symptoms that pose a challenge to even experienced personnel encountering this environmental hazard. Individuals who are exposed to the relatively gradual loss of atmospheric pressure, most commonly mountain climbers and skiers, experience manifestations of hypobaric hypoxia known as acute mountain sickness (AMS). AMS presents as a range of symptoms and in its most advanced stages is fatal.
High-altitude environments also are home to cold temperatures, even on the equator, because of the steadily decreasing temperature of the atmosphere as altitude is gained and harsh upper-level winds that encourage convective cooling. Personnel exposed to these temperatures and winds are at significant risk of hypothermia, frostbite, and other cold-related injuries. This article will review the physiology of altitude and the various illnesses encountered by people working and playing in the higher areas of the earth.
Altitude Physiology
The root of all high-altitude diseases is the issue that, because of the effect of gravity on Earth’s atmosphere, the number of gas molecules decreases with increasing altitude. This creates a hypobaric state at high altitude, with the atmosphere containing roughly half the number of molecules at 5,500 m (18,000 ft) as it does at sea level. Interestingly, because of Dalton’s law, which states that as the pressure of the gas decreases, the relative mixture of the gases remains constant, the concentration of oxygen at altitude remains the same as at sea level. A person standing on top of a mountain that is 5,500 m high still is inhaling a gas mixture composed of 21% oxygen, but the partial pressure of this oxygen is less than half of what is normally experienced at sea-level. (See Figure 1.)
Figure 1. Dalton’s Law |
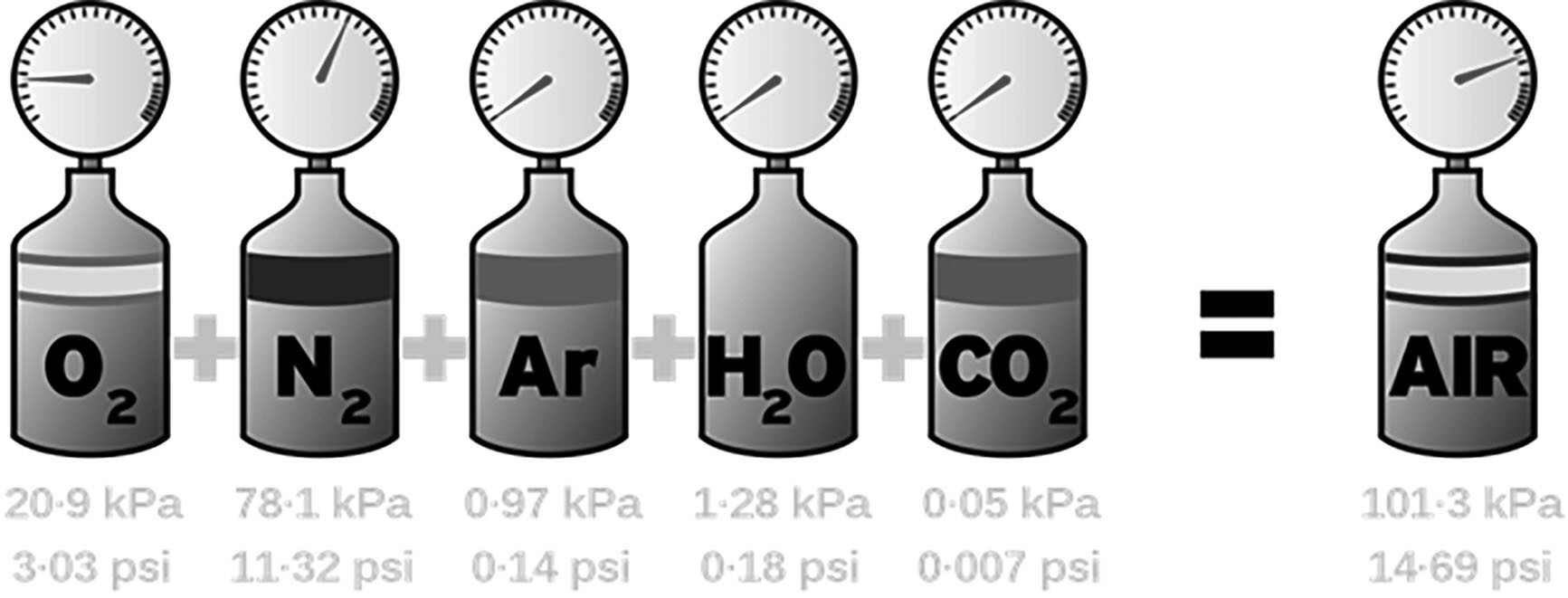 Per Dalton’s Law, the pressure of the air is equal to the sum of the partial pressures of the component gases of the air mixture. This stays constant as one ascends in altitude. |
Source: Andrew Jarvis, Wikimedia Commons. https://creativecommons.org/licenses/by-sa/4.0/deed.en |
Since body transport of free and hemoglobin-bound oxygen depends on the partial pressure of oxygen being inhaled, a lower outside partial pressure of oxygen greatly reduces the efficiency of the system. The lowered quantity of oxygen being transported in the blood creates hypoxemia, since the lungs are unable to bring in sufficient oxygen to maintain normal arterial oxygen content. Acute exposure to profound hypobaric hypoxemia, such as a rapid depressurization of an aircraft cabin, results in a very short period of useful consciousness since the body has not had a chance to adapt to the sudden lack of oxygen. If the altitude exposure is experienced gradually, as occurs when climbing a mountain, the body compensates through a variety of mechanisms, including increased minute ventilation, increased hemoglobin production, and hypoxic vasoconstriction of lung tissue.
Should the exposure last a few days or more, the body compensates at the molecular level by producing hypoxia-inducible factors (HIFs) to alter the transcription of cellular deoxyribonucleic acid (DNA).1 These factors upregulate mitochondrial metabolism, anaerobic metabolism, erythropoiesis, and angiogenesis to allow for the body to adapt to the chronic hypoxia it is experiencing. This is why the hematocrit levels of those who live and train at high altitude are higher and their athletic performance at sea level is so robust. In fact, many U.S. Olympic athletes train at approximately 2,100 m to provide a competitive advantage from these performance-enhancing changes. The body’s response to altitude can be so robust that individuals training at 2,500 m can acclimate to a similar level of performance to their previous training at sea level.2
People who live and work at high altitude for long periods of time eventually become accustomed to the hypobaric hypoxia of moderate altitude, but the human body is physiologically limited to approximately 4,500 m in its ability to adapt. Ethnic groups who have lived at high altitude for generations, such as the Tibetans, Sherpa, and Andeans, de-monstrate increased physiologic adaptations to the rigors of altitude, but even they are limited in the highest altitude to which they can adapt.1 The villages of Dho-Tarap in Nepal and La Rinconada in Peru are the highest continuously inhabited places on Earth, each with an altitude above 4,000 m. Above this level, humans can tolerate brief sojourns to higher elevations, such as Himalayan and Karakorum peaks, but they cannot tolerate continuous habitation of these altitudes.
Preventing the Effects of Altitude
Individuals traveling from lower elevations who wish to be effective at altitude can do so by following well-established guidelines from the Wilderness Medicine Society (WMS). The primary recommendation of acclimatization to altitude is through the slow, deliberate increase in sleeping elevation by 500 m per day above 3,000 m. Further, an additional day of rest for every 1,000 m increase during these 500 m ascents has been demonstrated to reduce the incidence of altitude illness.3 Interestingly, fitness at sea level does not confer any protection.2 Although the research currently is ongoing, pre-acclimation by living in tent-like habitats that simulate hypobaric-hypoxic conditions have demonstrated efficacy.3 This option may be attractive to those who have a limited amount of time to climb a certain peak, such as Everest, and are unable to do a prolonged acclimatization. These devices are expensive and can be tricky to use properly, so consultation with a professional company developing these products often is necessary.
A second preventive measure is the use of inhaled oxygen. Climbers ascending high mountains often use oxygen canisters to supplement their oxygen intake and, therefore, reduce their susceptibility to altitude illness. Since oxygen only makes up 21% of the earth’s atmosphere, the fraction of inspired oxygen, or FiO2, does not need to be very high to provide a substantial “decrease” in altitude. A good rule of thumb followed by climbers is that an increase in 1% FiO2 above 21% provides the equivalent of a 300 m decrease in altitude. Therefore, an FiO2 of 31% can “descend” the climber physiologically as much as 3,000 m.1,4 Unfortunately, oxygen bottles are heavy and can encumber climbers, making their use on-site more difficult. However, administration of inhaled oxygen is one of the mainstays of therapy for any severe altitude illness.
For high-altitude buildings, enriching the working environment with oxygen has been shown to be protective. The high-altitude telescopes of the Atacama Desert in Chile are one such project, using a working atmosphere of 27% FiO2 to “descend” the astronomers from 5,000 m to a more tolerable 3,200 m.1 Finally, some medications used for the treatment of AMS also can offer a degree of prophylactic protection against the development of mountain sickness, and these are described in the following respective sections.
Acute Mountain Sickness
Rapidly ascending from sea level to elevations above 2,500 m (8,200 ft) can create a syndrome known as AMS. Although it can manifest as a myriad of symptoms, the generally accepted constellation of symptoms, suggested by the Lake Louise Criteria, are a headache plus one of the following symptoms: insomnia, anorexia, nausea, vomiting, and dizziness.5 Often, those affected will exhibit more than two of these symptoms, resulting in a miserable experience that is not unlike influenza or another viral syndrome. The onset of AMS can be rapid, as soon as six to 12 hours upon ascent.4 It has been seen in as many as 40% of skiers at higher-altitude Colorado skiing destinations and 70% of climbers on Mt. Rainier.4 Rapid ascent to altitudes above 3,000 m by unacclimatized persons nearly guarantees its development.3
The pathophysiology of AMS is incompletely understood, but the general theory is related to a combination of inflammation and hypoxic vasodilatation of the tissues of the brain in a chronically oxygen-starved state. From animal studies and limited human studies, the general model is thought to be that brain hypoxia leads to vasogenic edema of the cerebral vasculature, which encourages loosening of the blood brain barrier, exacerbating the vasogenic edema.6 This vasogenic edema leads to increased intracranial pressure, causing the headache and lassitude seen in affected persons.6 As the edema worsens with increased altitude and/or prolonged exposure, the vasogenic edema results in cytotoxic effects at the microscopic level, which in turn worsen the edema through the activation of local inflammatory cascades and cytotoxic necrosis.
Experiments have demonstrated that the hypobaria of altitude worsens the symptoms by encouraging fluid shift from out of the plasma and into the brain, but that chronic hypoxia by itself produces the same symptoms.6 Interestingly, in experiments involving magnetic resonance imaging (MRI) machines and hypobaric chambers, it was demonstrated that the effects of both hypoxia and hypobaria resolve within an hour on return to sea level.6
AMS as an illness is both insidious and debilitating, with effects beginning to affect the mountaineer gradually as the person ascends to a higher altitude. The illness often is mistaken for dehydration or another illness, and this in turn encourages the afflicted to ignore the symptoms or treat them improperly. Although dehydration is risk factor for AMS, additional risk factors include a previous episode of AMS, rapid ascent, the intensity of the exercise at the higher altitude, and the home elevation of the person being exposed.4 Initially, patients generally will notice a dull, worsening headache, known as high altitude headache. As the individual ascends to a greater altitude without sufficient acclimatization, insomnia, anorexia, nausea, and the other symptoms described earlier begin to manifest. As the affected person continues to ascend or foregoes treatment, they risk worsening edema and, therefore, worsening illness. As AMS continues to progress, it begins to manifest as worsening neurological function in the case of high altitude cerebral edema (HACE), described in its own section later.
AMS can be prevented or lessened through slow ascent over a period of several days. Current guidelines recommend daily increases in sleeping elevation to be no more than 500 m per day.3 Prophylaxis with acetazolamide (Diamox) at a dose of 125 mg twice daily offers an excellent degree of protection as well.3 Should AMS develop, acetazolamide 250 mg orally twice daily is the mainstay of treatment.3
Acetazolamide encourages the secretion of bicarbonate into the urine, resulting in a more acidotic blood plasma, which encourages more rapid respirations. This helps to combat the relative bradypnea of altitude by encouraging more rapid respirations, particularly during deep sleep.3 Although acetazolamide is considered to be a “sulfa drug,” individuals with a sulfa allergy rarely experience allergy symptoms while taking this medication.4 Dexamethasone is even more efficacious for the treatment of AMS, since it acts directly on the vasogenic inflammatory effects of altitude. Dexamethasone is given as two doses of 4 mg over a 12-hour period either orally or intravenously/intramuscularly (IV/IM) depending on whether the patient can tolerate oral intake. Although it is a powerful drug against high altitude disease, dexamethasone use carries the risk of steroid-induced mania, which often is deleterious to others in a climbing party.
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, also have been shown to be helpful with the headache symptoms, and standard anti-emetics, such as ondansetron, also are useful for symptom management. Encouraging oral hydration to help alleviate the dehydrating effects of altitude likewise is beneficial. If the illness is severe with debilitating symptoms, oxygen administration is beneficial. If the symptoms are unbearable, even with medication, descent often is the best course of action, with symptoms lessening as the patient is lowered from the higher altitude. Unfortunately, having AMS predisposes one to repeat episodes despite good physical condition. The only true preventive measure is slow acclimatization to altitude, but prophylactic acetazolamide and/or dexamethasone has demonstrated reasonable outcomes in studies.3
It is worth mentioning that if the provider is at a high enough altitude to see these illnesses, they themselves are at risk for developing these pathologies. It is of the utmost importance that any provider practicing at high altitudes be familiar with preventive and treatment strategies for these high-altitude diseases so they do not fall victim to them and create a dangerous situation for the group they are caring for. Attitudes such as invincibility or machismo have no place at high altitudes. Provider self-care in these environs is of the utmost importance. Ascend slowly, consider chemical prophylaxis, remain hydrated, and monitor your own symptoms closely.
High Altitude Cerebral Edema
As climbers continue into high altitude, especially with untreated AMS symptoms, they begin to develop cerebral edema as their brain microvasculature continues to secrete fluid due to the patient’s chronically hypoxic state. Known as high altitude cerebral edema (HACE), this condition results in ataxia and malaise, with altered mental status developing shortly thereafter. Representing the final stage of AMS, it often presents with the full spectrum of severe AMS symptoms, such as intractable nausea and vomiting.4 This predictable disease progression defines HACE clinically as AMS in addition to ataxia or altered mental status.6 These symptoms sometimes have been confused for hypothermia, and the symptoms are similar, but evaluation of a core temperature will find the patient’s temperature as normal. In severe cases, papilledema and extensor plantar reflexes can be seen on physical exam.1
Although HACE has been reported as low as 2,100 m, it occurs more commonly above 3,000 m.4 HACE is an emergency because the patient’s declining physical and mental abilities will make them a hazard to themselves and others on the mountain. The illness progresses rapidly, with mild symptoms progressing to coma in as little as 12 hours, but 24-48 hours is more typical.4 The edema itself is caused by the same increased vascular permeability seen in AMS, but on a grander scale. If this vasogenic edema progresses unimpeded, it creates a negative feedback loop of worsening secondary edema from cytotoxic processes within the brain itself, resulting in an acute worsening of symptoms. The intracerebral pressures that develop can be extreme, with lumbar punctures in cases demonstrating cerebral pressures as high as 300 cm H2O.4 Given these high pressures, both retinal and cerebral microhemorrhages develop, exacerbating the edema and resulting in a breakdown of brainstem homeostasis and death. Immediate descent is the treatment of choice. If immediate descent is not possible, dexamethasone can be given as 8 mg initially and then 4 mg every six hours, given as IV/IM or orally (PO), with a plan for descent as soon as possible. Acetazolamide 250 mg two times per day also can be used as treatment while awaiting descent.5 Oxygen, if on hand, also should be given.
Fabric hand- or machine-pumped altitude chambers, colloquially known as “Gamow bags” after their original manufacturer, allow for a rescue party to “descend” the patient until definitive transport and are proven life-saving interventions if available for patient treatment. (See Figure 2.)
Figure 2. Portable Hyperbaric Chamber |
 A portable hyperbaric chamber, or “Gamow bag,” allows a team to “descend” a person in place while definitive rescue is organized. |
Source: Mckeeman at English Wikipedia. https://creativecommons.org/licenses/by/3.0/deed.en |
It is important to distinguish HACE from mimicking diseases that also are found in high-altitude, mountainous, exercise-intensive environs. Ataxia and altered mental status can be caused by profound dehydration, hypoglycemia, trauma, or other illness. It is important to fully assess patients who develop these symptoms to rule out these other causes. Delayed capillary refill, a history of diarrhea, or decreased skin turgor should raise one’s suspicion for dehydration as the diagnosis. Diet, water sources, and endemic illness all are sources of diarrheal illness in many mountainous areas around the world. High-altitude air also tends to be drier, which works to dehydrate individuals exerting themselves at a higher altitude. Excessive exertion, particularly in people with diabetes, also can lead to hypoglycemia. A portable glucometer can be helpful in fully assessing patient symptoms and ensuring not to mistake hypoglycemia for HACE.
Head trauma from falls or rocks is another condition to consider. Although one may think that head trauma in a climber would be obvious, motivated individuals in a climbing party who experience head trauma may be hesitant to discuss the full extent of their symptoms until they become ataxic or altered for fear of slowing the climbing party down or losing their chance to summit. A careful history for any encounters with falling rocks or falls, along with an examination of the face, scalp, and neck, should be performed.
Finally, heat sources being used in enclosed, tight areas always raise the possibility of carbon monoxide poisoning, especially if the heat sources are being used inside poorly ventilated tents or structures. The body’s ability to use the thin atmospheric oxygen already is compromised at altitude, and given carbon monoxide’s higher affinity for hemoglobin, the effects of even a small exposure to carbon monoxide will be acute. Again, a careful history and physical exam are vital to ensure proper management.
Interestingly, there are case reports of persons who were thought to be experiencing HACE but instead were discovered to have hypotonic or exercise-induced hyponatremia.7 Highly motivated climbers, especially in hot, dry areas, may find themselves overhydrating, particularly if they are mistaking early AMS signs for dehydration. Most people with hypotonic hyponatremia experience headache, then nausea with emesis, followed by altered mental status and/or ataxia, and finally seizure.7 These symptoms are very similar to HACE, but the treatment for exercise-induced hyponatremia is hypertonic saline, whereas the recommendations for HACE are to use dexamethasone and descend. Given that both processes are caused by cerebral edema, it has been proposed to use hypertonic saline for severe HACE. Unfortunately, given the relatively low number of severe HACE episodes and austere environs in which it generally occurs, the use of hypertonic saline in the treatment of HACE has not been studied and its use should only be considered as a last resort.4
Because these diseases share many of the same symptoms and susceptible populations, it is important for a healthcare provider to consider both when constructing a differential in a person at high altitude experiencing altered mental status. However, while a broad differential should be considered, the treatment of HACE and many of the other illnesses is best done at a location with definitive care at a lower altitude. Consideration of the differential and treatment attempts should not delay descent.
High Altitude Pulmonary Edema
The most dangerous form of altitude illness is high altitude pulmonary edema or HAPE.1 As the patient experiences more severe symptoms of AMS, their body develops a greater amount of vasogenic edema. Further, as lung tissue becomes more hypoxic, it undergoes vasoconstriction to limit blood going into the unventilated lung, leading to shunting. This increasing vasoconstriction increases pulmonary artery pressure, which in turn increases lung capillary pressure, encouraging third-spacing of interstitial fluid. When combined with the vasoedemic processes of altitude, this pulmonary vasoconstrictive process can generate substantial edema. A non-cardiac cause of pulmonary edema, HAPE can generate substantial edema in otherwise previously healthy individuals with outstanding cardiac function.
HAPE can manifest as a dry cough that quickly becomes wet early in the disease course, and it can be mistaken for a benign upper respiratory tract infection, a reaction to the dry, cold air of altitude, or a side effect of the dusty environments found above the tree line. However, this dry cough progresses quickly to become productive, with frothy, pink sputum. Additional initial symptoms can include decreased exercise performance, prolonged exercise recovery time, dyspnea on exertion, and localized rales. Oxygen saturations for these patients can be shockingly low, as low as 40% to 50% depending on the altitude. Testing of HAPE patients reveals elevated pulmonary artery pressures combined with profound hypoxia; mean SpO2 in HAPE patients has been found to be 56%.4 As the edema and pulmonary hypertension progress, the capillaries of the lung tissue begin to burst, causing hemoptysis. Significant capillary hemorrhage into the airway heralds the end stage of the disease, with a mortality rate as high as 50%, even for individuals who receive treatment.8
Typically seen only above 4,000 m, there are reports of HAPE occurring as low as 2,500 m.4 HAPE as a syndrome generally is seen as one of two types. The first is the classic presentation of HAPE, as described earlier, which manifests from a quick ascent to a high altitude for an individual who normally lives at low altitude or in HAPE-susceptible individuals who have not acclimated properly. The second type is reentry HAPE, which is when an individual who normally resides at a high altitude ascends after time spent at a lower altitude. Individual risk varies, and risk factors include altitude attained, rate of ascent, and time at high altitude. Additionally, having had HAPE previously is its own risk factor, with a recurrence rate of 60% in those who have had HAPE previously and who ascend to 4,500 m over two days.
Diagnosis for these patients is primarily clinical. The physical exam can reveal tachypnea, pulmonary crackles, and tachycardia, among other things. Even though the diagnosis is primarily clinical, certain imaging modalities can help confirm the diagnosis. X-ray imaging of subjects demonstrates unilateral or bilateral infiltrates, but a frank effusion is rare.1 B-lines on ultrasonography demonstrate the pulmonary edema well, and so this can be considered for point-of-care testing for patients, especially in a more austere setting. If the patient is brought to a hospital setting, there really are no laboratory tests that would be useful in distinguishing the diagnosis. However, it is important to consider that there are diagnoses that can be confused with HAPE. Pneumonia, for example, should be considered, since there is significant overlap in the symptomatology. Other considerations include pulmonary embolism, bronchitis, and reactive airway disease. These diagnoses also can coexist within a patient, so care should be taken to monitor symptoms for continued improvement. Given that, if a patient is brought to normal altitude and still struggles with oxygenation, the provider should consider broadening the differential for the patient.
The treatment of HAPE is immediate descent. The goal is to do this as soon as possible to avoid late-stage symptoms, especially hemoptysis, which could compromise the airway. If a situation occurs in which a patient is unable to descend immediately, such as if there is inclement weather, a Gamow bag can be used to alleviate symptoms as a temporizing measure prior to or during descent. (See Figure 2.) This piece of equipment serves to decrease the effective altitude of the patient by altering the pressure in the bag compared to outside the bag. However, in situations in which this technology is used, it is important to ensure that the patient will be able to tolerate the procedure. Those who experience claustrophobia may have trouble tolerating this procedure. For example, if the patient is experiencing possible HACE as well, it may not be feasible for the provider to attempt this treatment for the patient. Finally, if the patient is already at the point at which hemoptysis is occurring, the Gamow bag would be contraindicated. In addition, expiratory positive airway pressure (EPAP) has been shown to improve gas exchange in HAPE patients but has not been studied to measure clinical outcomes in HAPE patients.9
A single, nonrandomized, unblinded study has demonstrated the utility of nifedipine in HAPE treatment when oxygen or descent is not available.10 In addition, there is extensive clinical experience with nifedipine use as an adjunct to oxygen or descent. The Wilderness Medicine Society Practical Guideline recommends 30 mg of the extended-release form twice daily without a loading dose as adjunctive treatment of HAPE. It is not recommended that nifedipine be used as treatment of HAPE in situations where descent is impossible and access to supplemental oxygen or portable hyperbaric therapy cannot be arranged.3
Many people who plan to ascend to high altitude look for prophylactic methods to prevent this complication. The first and foremost way to prevent HAPE is gradual ascent, with a recommendation of an ascent rate of no more than 500 m per day. For medical prophylaxis, nifedipine 30 mg extended release every 12 hours has been demonstrated to be efficacious.3 This is reserved primarily for those individuals considered to be at high risk, such as those with a history of HAPE. The medication ideally should be started the day prior to ascent and continue to be used for five days.3 Other studies have suggested dexamethasone or PDE-5 inhibitors could be used prophylactically against the illness, but further research needs to be done for these medications.
Acetazolamide hastens acclimatization and should be effective at preventing all forms of acute altitude illness by blunting hypoxic pulmonary vasoconstriction. However, there are no data specifically supporting the role of acetazolamide in HAPE prevention. Of note, anecdotal clinical observations suggest that acetazolamide may prevent reentry HAPE in children who reside at high altitude, travel to lower elevation, and then develop HAPE upon rapid return to a higher altitude.3
Special Populations and Altitude Illness
As mountainous and other high-altitude areas continue to become more accessible, people who may not have been physically able to traverse these areas, such as pregnant women and children, now are exposed to high altitude on a more regular basis. These populations have some special considerations for the prevention and treatment of these illnesses.
Active, young women may inadvertently expose a first trimester pregnancy to altitude because they do not know they are pregnant. A retrospective survey of reproductive-age women who discovered they were pregnant after a trip to high altitude found that many women who were engaged in high altitude sporting activities were doing so in the first trimester of pregnancy without knowing they were pregnant.11 Most of these women noted no complication with this and reported a miscarriage rate consistent with the general population.11
The effect of altitude on pregnancy in later stages is not as well studied, but it is thought that pregnant women are more susceptible to AMS because of their already greater rate of respiration and physiological alkalosis. The additional weight and physiological load of pregnancy also increases the physical exertion component of activity at altitude.12
Overall, expert consensus agrees that pregnant individuals who dwell near sea level should avoid altitude, and especially strenuous activity at altitude, with the exception of brief sojourns. If they must ascend, a slow acclimatization process must be employed since they cannot take acetazolamide for AMS prophylaxis because of its potential as a teratogen. If acetazolamide has been consumed during the first trimester because of lack of knowledge of the pregnancy, reassurance should be given that the likelihood of birth defect is low.12 If altitude-illness symptoms develop in a pregnant patient, even during the first trimester, descent to a less strenuous altitude must be accomplished as soon as possible.
As areas of breathtaking beauty and winter sports found at high altitude become more and more accessible, greater numbers of lowlander children also are being exposed to altitude, often with a short duration of ascent. Just like their adult counterparts, children also develop AMS and its more dangerous forms when exposed to altitude. Case reports from Himalaya trekking seasons have demonstrated HACE in teenagers among the trekking groups.13 The presentation is typical of standard AMS, with headache, nausea, and anorexia. Just like adults, ataxia generally is the first neurological symptom. Interestingly, one child, a 12-year-old male, reported diplopia associated with his case of HACE.14
Current consensus for pediatric cases is to prevent AMS by ascending no more than 500 m in sleeping elevation each night and to administer acetazolamide at 2.5 mg/kg, with a maximum dose of 125 mg every 12 hours.14 Should HACE or HAPE develop in a child, dexamethasone at a dose of 0.15 mg/kg with a maximum dose of 8 mg, then 0.075 mg/kg up to 4 mg every six hours should be given.14 The route of administration does not affect the level of dosage. If available, portable oxygen should be given. Descent should be arranged as soon as possible.
Another special population to consider when thinking about the effects of altitude is sickle cell disease patients (SCD). These patients have an increased likelihood of doing poorly at high altitudes. The lower oxygen tensions at higher altitude promote sickling, and this can lead to a higher chance of pain crises. In fact, studies have shown that the risk of altitude is additive to the higher risk of pain crises observed in SCD patients. To combat this, patients with sickle cell disease who plan to travel to altitude need to prepare. Since these patients are functionally asplenic, they should take vaccinations corresponding to encapsulated organisms, such as the pneumococcal and meningococcal vaccines. Also, considerations should be made for the patient’s overall health. SCD patients are at a higher risk of having pulmonary hypertension, and this can lead to a higher likelihood of pulmonary complications seen at altitude.
A gradual conditioning program can be used to prepare SCD patients for trips to altitude. It should be noted that those patients with sickle cell trait are mostly asymptomatic at higher altitudes. However, their condition at altitude does predispose them to a higher risk of dehydration, sudden death, and splenic infarcts. Care should be taken when traveling with or treating these patients. Those patients should be keenly aware of possible signs or symptoms that could portend negative effects of the altitude, such as left upper quadrant pain corresponding to splenic infarct.
Finally, similar to sickle cell patients, patients with thalassemias need to be cautious when traveling at higher altitude. These patients also should be screened for possible pulmonary hypertension. These patients need to be screened for appropriate cardiac function prior to ascension to altitude, since many of them can develop underlying cardiac issues. Many thalassemia patients require iron chelation therapy, and if possible, this should be continued for patients traveling to high altitude so as not to predispose them to iron overload.
Hypothermia
If lower oxygen partial pressures at elevation were not enough, the body has to spend a significant amount of energy maintaining a core body temperature within the margin of about 2°C. For every 1,000 m someone ascends in elevation, the ambient temperature drops by 9.8°C.1,4 Humans must balance heat production and heat loss to stay within this margin. This becomes increasingly more difficult at high altitudes because the environment saps heat from the human body in several ways. The most basic way is through radiation of the body’s heat via infrared radiation to the surrounding environment. A second way, conduction, encourages heat to move down a gradient from an area of higher heat (i.e., the body) and into the area of lower heat (i.e., the ground or air). Evaporation of sweat, often insensible in the cold, the dry environment of altitude also results in evaporative heat loss, especially during exertion. Proper clothing, moisture-wicking fabrics, and minimized contact with cold surfaces all work to minimize heat losses through these three mechanisms.
Introducing wind or water into the equation allows for heat loss through convection, the most efficient mechanism of the four, which dramatically increases heat loss and is more difficult to combat. Generally known as “wind chill,” the wind whips the cold air around the skin surface, sapping body heat rapidly through convection. Figure 3 shows that even a small amount of wind dramatically decreases the time to produce frostbite in an unprotected individual.
Figure 3. Wind Chill Chart |
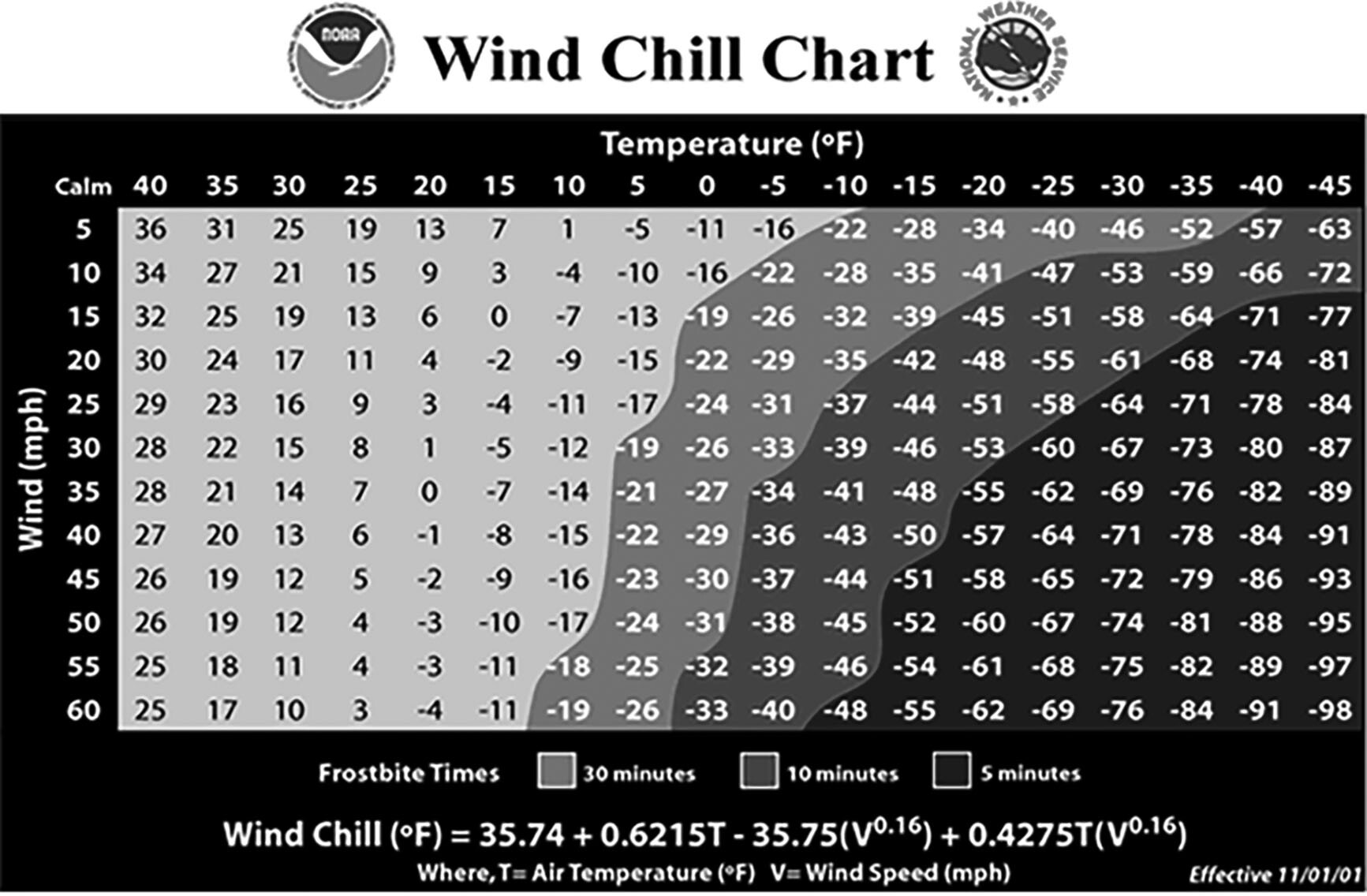 This chart demonstrates the power of even a modest amount of wind in cold environments. Patients exposed to a windy, cold environment will develop hypothermia rapidly through convection cooling without proper equipment. |
Source: National Weather Service, National Oceanic and Atmospheric Administration |
As these four mechanisms of heat transfer reduce the body’s ability to maintain homeostasis, a physiologic response to hypothermia begins to be evident at around 35°C, where the Swiss Hypothermia Classification System (HT) begins. The HT system provides a scale at which various predictable symptoms begin and end relative to core body temperature. These are found at 35°C to 32°C for mild (HT-1), 32°C to 28°C for moderate (HT-2), 28°C to 24°C for severe (HT-3), and < 24°C represents profound hypothermia (HT-4/5).13 Since individuals can have somewhat varying responses to hypothermia, there are limitations to these classification systems, but they can serve as a good rule of thumb in the field where physical exam is critical and testing is limited. The gross physical exam findings of the four stages of hypothermia are described in Table 1.
Table 1. Physical Exam Findings | ||
Classification | Presentation | Temperature |
Normal |
| > 95°F (35°C) |
Mild |
| 95°F to 90°F |
Moderate |
| 90°F to 82°F |
Severe |
| < 82°F (28°C) |
Adapted from: Section of Community Health and EMS. Cold Injuries Guidelines. Alaska Multilevel 2003 Version. | ||
The impact of hypothermia on the body’s physiology occurs gradually at first and then cascades into more severe illness as core temperature falls. As tissues begin to cool into the mild range, their metabolism decreases, which leads to inhibition of neurologic function and signaling via lateral spinothalamic tracts to the hypothalamus.15 Initially, during mild hypothermia, oxygen consumption by the cardiovascular system increases due to the nervous system response to decreased core body temperature resulting in shivering, which increases metabolism, ventilation, and cardiac output. Peripheral vasoconstriction likewise occurs, shunting more blood volume to the body’s core in an attempt to minimize heat loss in the extremities. This increase in core volume causes the kidneys to begin to excrete more urine, a phenomenon known as cold exposure diuresis.16 This can be dehydrating to affected individuals, especially if they are taking acetazolamide as an altitude illness prophylactic, placing them at risk for continued physiological decline.
If these first countermeasures are ineffective and temperature continues to drop into the moderate range, the nervous system begins to lose capacity, resulting in slurred speech and impaired judgment, which often leads to poor decision making and can exacerbate the situation. Moderate hypothermia affects the cardiac tissue by generating a linear decrease in pulse rate, which becomes significantly bradycardic as the body cools below 28°C. For every 8°C drop in body temperature, there is a 50% decrease in CO2 production and there are alterations of pH, hemoglobin-bound oxygen, and serum electrolytes, resulting in the myocardium being more irritable.4,11 This alters electrical conduction at the tissue and cellular level, leading to the development of atrial fibrillation and other arrhythmias. Electrocardiogram (ECG) findings in moderate hypothermia begin with initial prolongation of the PR interval, followed by subsequent widening of the QRS then QTC segments. As core temperature continues to drop into late moderate or profound hypothermia, pathognomonic J or Osborn waves are seen on ECG. Respiration rate also is slowed in severe hypothermia. The patient may be breathing as little as five to 10 times per minute.
When core body temperature drops into deep severe or profound hypothermia, induced ventricular fibrillation and asystole are more likely to occur. These life-threatening arrhythmias occur due to worsening metabolic derangements lowering the transmembrane resting potential.
Once profound hypothermia is achieved, a ventilation perfusion mismatch occurs due to lung atelectasis, with subsequent decreased minute ventilation, and increased secretions and decreased ciliary motility leading to noncardiogenic pulmonary edema. Although many hypothermic patients often are found in urban settings close to definitive care, cold, windy, and/or wet wilderness conditions that are conducive to the development of hypothermia can greatly delay hospital care of hypothermic patients and, therefore, familiarity with prehospital field management is necessary.
The WMS has published clinical practice guidelines and consensus statements around the prehospital management of hypothermia. The WMS guideline outlines an algorithmic approach to the evaluation of a potentially hypothermic patient, beginning with a severely hypothermic patient without a pulse or other signs of life. After ensuring scene safety for the rescuers to prevent additional casualties, this guideline recommends initiation of advanced cardiac life support (ACLS) if there are no signs of life and no pulse. Notably, this pulse check should take 60 seconds to confirm if a pulse is present, not the normal ACLS time frame of 10 seconds. Unless the patient has evidence of lethal injury, a chest wall that is too stiff for cardiopulmonary resuscitation (CPR), has been buried in an avalanche for greater than 35 minutes, or has a large quantity of snow found in the patient’s airway, the WMS clinical practice guideline recommends to begin ACLS interventions.
Chest compressions should be performed via standard rate and depth, but ventilatory rate should be performed at half normal (~6 BPM) because of the patient’s low metabolic demand. If ventricular fibrillation is present on the first rhythm check, it should be defibrillated at 360 J once; no further shocks should be delivered until the patient is warmed to 30° C. A severely hypothermic patient should not be pronounced deceased until they have been rewarmed to a temperature of 32° C. For patients with hypothermia, this profound external rewarming can be started, but it is far more effective to warm the patient using heated high-flow nasal cannula, thoracic or peritoneal lavage, or if the capability is available, extracorporeal membrane oxygenation (ECMO) cannulation provides the most rapid rewarming as well as excellent tissue perfusion as part of the patient’s resuscitation. For patients in moderate hypothermia, prompt external rewarming is necessary but should be performed with some precautions, especially in the field environment where medical resources are limited.
Rewarming patients in moderate hypothermia encourages vasodilation in the peripheral vasculature, where the warm blood begins to perfuse the cooler extremities, causing the core body temperature to begin dropping again. This is most important when the patient is on the threshold of moderate and severe hypothermia where cardiovascular collapse due to contact with the now colder blood may occur. This can be prevented by warming the core as opposed to the peripheral tissues initially in patients with moderate to severe hypothermia. For these patients being removed from watery or submerged environments, another phenomenon known as circumsaccate collapse can occur during and just after removal from water. This is caused by decrease of the hydrostatic pressure from the water previously surrounding the victim’s body, allowing blood to return to the cold dependent areas and cooling the heart upon its return to the core, not unlike core temperature after-drop. Keeping the patient horizontal and warming the core after rescue can mitigate this phenomenon. Additionally, the patient should avoid major physical efforts, which can encourage premature peripheral perfusion and precipitate after-drop. Ensure that all hypothermia casualties are placed into a dry environment that prevents further heat loss. External heat sources should be applied to the axilla, chest, and back. If using commercial chemical heat packs, although effective when applied to the axilla and groin, care should be taken to avoid pressure injury or burn, especially in patients with no or a low state of consciousness.
The ideal rate of active rewarming is 1°C to 2°C/hour. In-hospital treatment of hypothermia is dependent on the stage the patient is in on arrival and which interventions have been performed. For mild hypothermia patients, dry clothes, warmed oral fluids/blankets, and physical activity may be all that is necessary. Patients in moderate or early severe hypothermia will benefit from warmed intravenous (IV) fluids and heated high-flow nasal canula in addition to the interventions described earlier. If patients are hypotensive on arrival, do not administer vasoactive medication, since hypothermia creates a basal vasoconstriction and additional vasopressors would not be beneficial to increase blood pressure at the expense of a greater risk of arrhythmia. For severe hypothermia patients, lavage generally is indicated or ECMO, if available, for rapid, even rewarming.
Cold Tissue Injuries
A discussion of illnesses found at high altitude would not be complete without a review of cold-induced pathologies, since high altitude generally exacerbates these illnesses. Further, the high-altitude environment generally is much colder than lower altitudes because of the decreasing temperature of the atmosphere due to a phenomenon known as the dry adiabatic lapse rate. This rate is due to the cooling effect air has as it expands and flows from the warm ground to the cooler atmosphere, resulting in an average temperature loss of 10°C for every kilometer of altitude gained. Cold-induced tissue injuries are placed into one of two categories: prolonged exposure to low temperatures in humid environments that still are above freezing, and tissue freezing injuries. The former includes injuries such as chilblains or pernio, trench foot, and immersion foot. Tissue-freezing injuries, colloquially known as frost nip and frostbite, recently have been reclassified into a depth of tissue damage scheme similar to thermal burns, obsoleting the older system of classifying the damage by physical signs alone. These injuries tend to occur more intensely at high altitude as well because of the extreme weather that often is found at high elevations and the increased metabolic demands of altitude. For any provider examining a suspected case of cold tissue injury, examining the casualty for other pathologies of low temperatures and altitude, such as AMS and its various manifestations and hypothermia, is important to consider.
Non-freezing cold tissue injuries are caused by the inflammatory effects of poor circulation and/or excessive tissue moisture. Although not frostbite, they can be severe enough to mimic frostbite and also can create local tissue destruction with long-term debilitating effects. Chilblains, or pernio, is an inflammatory condition caused by prolonged exposure to cold but not freezing conditions. This chronic cold exposure reduces peripheral circulation to the tissues resulting in a metabolic supply and demand mismatch. The subsequent inflammation from this long-term mismatch creates localized erythema and tissue damage. Treatment of this condition involves gradual rewarming of the affected tissues with warm air and removal of the patient from the cool environment. Although generally reversible, the injury can result in long-term cold sensitivity.4 (See Figure 4.)
More severe is trench foot and its warmer weather form, immersion foot. Although often associated with grueling military operations, hence its name, trench foot develops anytime the feet are exposed to chronic cold and wet conditions. (See Figure 5.) Exposure to chronic cold and wet conditions triggers an inflammatory condition within the foot that produces edema and subsequent capillary destruction, which results in impaired perfusion and peripheral nerve injury.17 The peripheral nerve injury produces the pain and eventual numbness of the foot. Long-term injury will reduce the arterial diameter of the vessels found in the distal foot, resulting in profound ischemia and gangrene.17 The loss of sensation also can lead to injuries that produce infection and gangrene, not unlike diabetic foot ulcers. Even mild trench foot can have long-term effects; nerve conduction studies in armed service members with previous mild trench foot demonstrate reduced intraepidermal nerve fibers.17 Immersion foot occurs through a similar process where feet are exposed to chronic wet conditions, creating localized inflammation and capillary destruction.
Figure 4. Pernio from Chronic Inflammation |
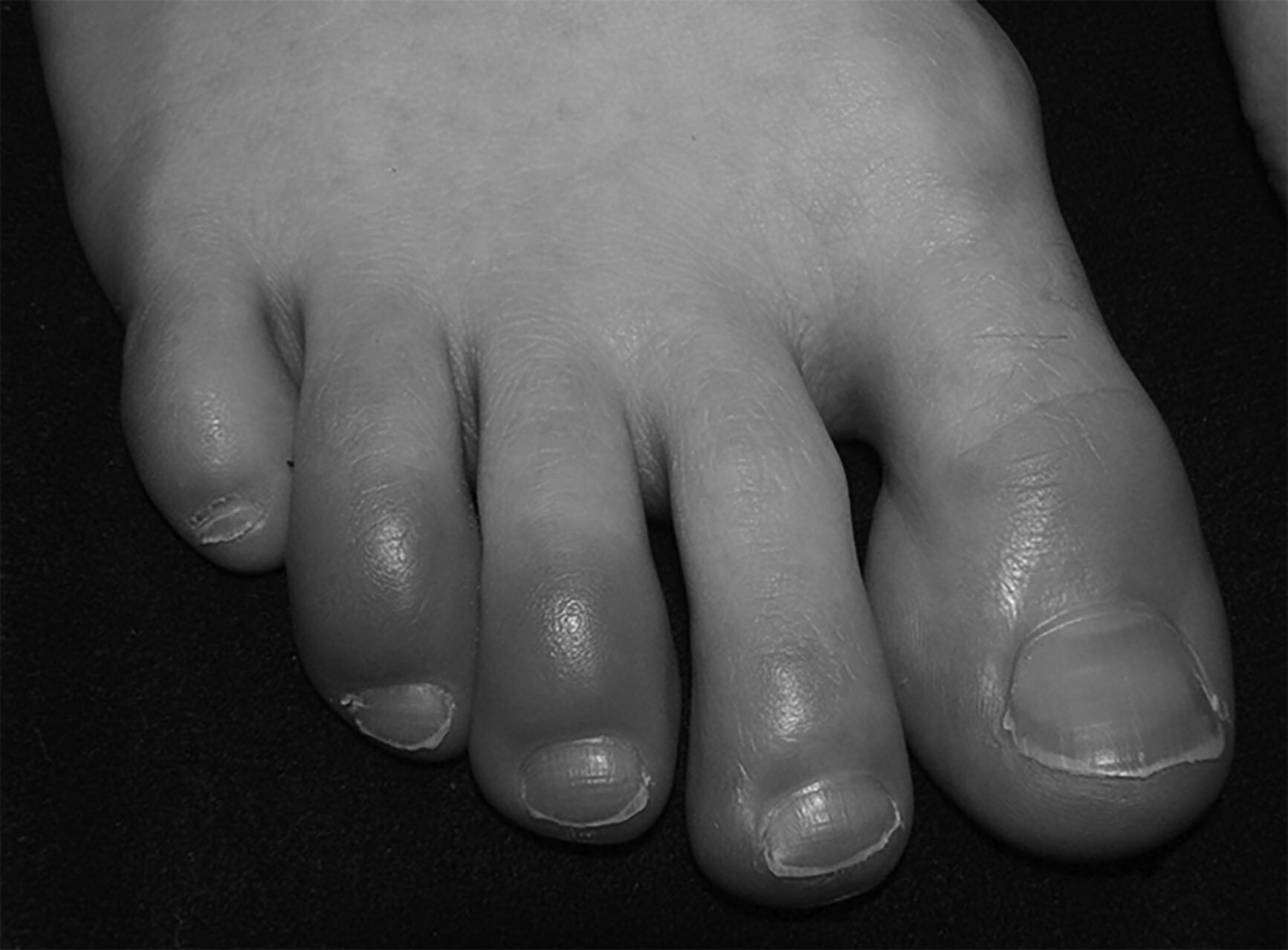 These toes are not frostbitten, since they have not been exposed to freezing temperatures. Instead, these digits have been affected by chronic inflammation leading to pernio. |
Source: Wikimedia Commons. [public domain] |
Figure 5. Trench Foot |
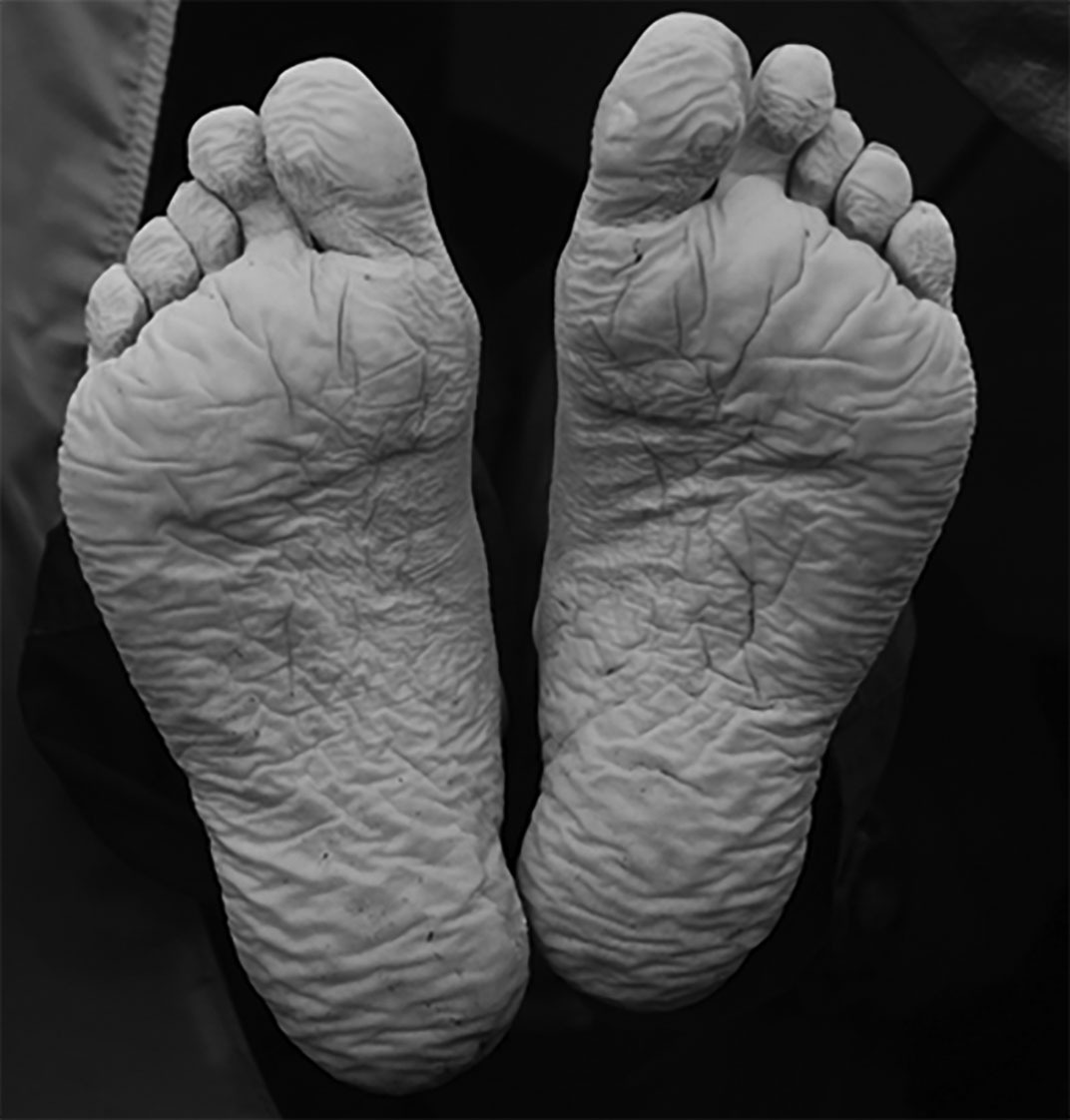 These feet have a wrinkled, inflamed look from early trench foot. Continued exposure will result in tissue ischemia and loss. |
Source: Mehmet Karatay, Wikimedia Commons. https://creativecommons.org/licenses/by-sa/3.0/deed.en |
Treatment of trench foot is largely preventive, with individuals encouraged to keep their feet warm, always wearing warm, dry socks/footwear. Should it develop, removal of the patient from the offending conditions and replacing their footwear should allow for improvement of their symptoms. Prostaglandin administration to improve peripheral blood flow has been demonstrated to improve paresthesia in trench foot, but this has not been well studied.17 Because of the impaired nerve conduction of feet with this injury, careful observation for signs of tinea or cellulitis must be performed since the loss of normal sensation removes typical symptoms of these diseases. Cellulitis in particular can lead to necrotizing infections that will result in preventable tissue destruction.17 Should the illness progress to the point where the ischemia has resulted in gangrene, surgical debridement often is the only remaining option. Immersion foot should be treated in the same way, with a heavy focus on prevention and supportive care with close observation for signs of secondary infection.
Frostbite, the most widely known cold tissue injury, is universally dreaded because of the potentially disfiguring and debilitating effects of the illness. The mere mention of the name elicits visions of necrotic digits, feet, and noses. Treatment options used to be limited, but new research is discovering effective treatment modalities that have improved outcomes in even severe frostbite. For frostbite to develop, the tissue must be exposed to temperatures below freezing. Planning for the windchill factor, previously mentioned in the discussion of hypothermia, also is of critical importance in preventing the development of frostbite injury. As noted in Figure 3, increasing wind speeds result in faster frostbite times and in more intense frostbites. Even a moderate wind will greatly decrease the time required to produce a frostbite injury in cold air temperatures.
Proper clothing and equipment is absolutely essential if working or recreating in below-freezing temperatures, especially for long periods of time or conditions that produce intense wind chills. The body parts most often at risk for frostbite are structures away from the core, such as the distal extremities, penis, ears, and nose, because of the body’s reflexive peripheral vasoconstriction when exposed to cold temperatures. Covering these body parts adequately is crucial in the prevention of frostbite.
Previously, frostbite had been thought of in four distinct degrees, with an increasing number representing a more significant injury. First-degree frostbite causes numbness and erythema. A white or yellow, firm, and slightly raised plaque develops in the area of injury. No gross tissue infarction occurs. Second-degree frostbite injury causes superficial skin vesiculation; a clear or milky fluid is present in the blisters, surrounded by erythema and edema. Third-degree frostbite causes deeper hemorrhagic blisters, indicating that the injury has extended into the reticular dermis and beneath the dermal vascular. Fourth-degree frostbite extends completely through the dermis and involves the comparatively avascular subcutaneous tissues, with necrosis extending into muscle and bone. To better classify frostbite injuries, pre-hospital and in-hospital grading scales based on the depth of tissue damage have been adopted. The prehospital scale is calculated after rewarming and divided into either superficial or deep. Superficial corresponds with first- and second-degree injuries and is associated with minimal or no anticipated tissue loss. Deep corresponds with third- and fourth-degree injuries, and there is anticipated tissue loss. Once the patient has been transported to more definitive care, more advanced classification can be determined.
Field treatment of frostbite is performed by gradually rewarming the tissue in water at a temperature of 40° C. This methodology is greatly preferred to rewarming with an ambient heat source, such as a stove, since it ensures universal heating of the tissue at a low enough temperature to prevent additional damage.16 If there is a chance that frostbite can recur after the rewarming, the rewarming should be delayed until definitive treatment is obtained. Rewarming of the frozen tissue will be excruciatingly painful, and use of opioid analgesics or peripheral nerve blocks is highly recommended.16
Since frozen tissue often contains clotted blood resulting from the inflammatory response to freezing, this furthers the ischemia and prevents reperfusion of the tissue after warming. It has been demonstrated that the use of targeted thrombolytic therapy into the blood vessels has allowed for much greater tissue rescue than previous therapies.18
A further advanced therapy for the treatment of frostbite includes synthetic prostacyclins, such as iloprost. Synthetic prostacyclins encourage vasodilation and improve circulation to the damaged tissues. Studies have shown that their use greatly improves tissue recovery even in severe frostbite, in some cases salvaging what otherwise would have been amputated tissue.
The current guidelines recommend using iloprost within 24 hours.19,20 Small studies have demonstrated using iloprost in combination with tissue plasminogen activator (tPA) is very effective and its use in frostbite injuries within 24 hours is recommended.17 A recent case series demonstrated that infusing iloprost at a rate of 2 ng/kg/min was highly effective in salvaging frostbite injury at 72 hours after injury.21 This treatment still is experimental, and more studies are needed before adopting it into general practice.
Conclusion
Around the world, majestic mountains and the stories of those who have climbed them previously inspire many to sojourn into high-altitude environments. Although often thrilling and beautiful, the deleterious effect of high altitude on the human body and the challenges of inclement weather can produce dangerous illnesses that have crippled and killed even the most experienced alpinists. Most humans have evolved for a life at or near sea level, meaning that for most of us, a gradual, cautious ascent into the thin air of altitude is the safest and most responsible option. This caution is especially important for providers who may find themselves providing medical care for others at high altitude and must be of sound mental and physical health to do so. Although many of these illnesses lack formal randomized controlled trials, there exists a fairly large body of expert consensus, case reports, and guidelines the experience of which has been summarized here. As greater numbers of travelers, adventure seekers, and scientists make their way into the higher, less traveled regions of the world, altitude illnesses and cold weather injuries will need to be considered and cared for. Should a provider encounter any of these travelers or experience these illnesses themselves, these guidelines will serve as an excellent resource for diagnosing and treating these injuries.
REFERENCES
- West JB, Schoene RB, Luks AM, Milledge JS. High Altitude Medicine and Physiology. 5th ed. Chapman and Hall Medical; 2012.
- Campbell AD, McIntosh SE, Nyberg A, et al. Risk stratification for athletes and adventurers in high-altitude environments: Recommendations for preparticipation evaluation. Clin J Sport Med 2015;25:404-411. Erratum in: Clin J Sport Med 2015;25:553.
- Luks AM, Auerbach PS, Freer L, et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Acute Altitude Illness: 2019 Update. Wilderness Environ Med 2019;30:S3-S18.
- Auerbach PS. Wilderness Medicine. 6th ed. Elsevier/Mosby; 2012.
- Small E, Phillips C, Marvel J, Lipman G. Older age as a predictive risk factor for acute mountain sickness. Am J Med 2022;135:386-392.e1.
- Hackett PH. The cerebral etiology of high-altitude cerebral edema and acute mountain sickness. Wilderness Environ Med 1999;10:97-109.
- Spano SJ, Reagle Z, Evans T. Symptomatic hypotonic hyponatremia presenting at high altitude. Wilderness Environ Med 2014;25:69-74.
- Jensen JD, Vincent AL. High Altitude Pulmonary Edema. In: StatPearls [Internet]. StatPearls Publishing. Updated July 20, 2021.
- Schoene RB, Roach RC, Hackett PH, et al. High altitude pulmonary edema and exercise at 4,400 meters on Mount McKinley. Effect of expiratory positive airway pressure. Chest 1985;87:330-333.
- Oelz O, Maggiorini M, Ritter M, et al. Nifedipine for high altitude pulmonary oedema. Lancet 1989;2:1241-1244.
- Keyes LE, Hackett PH, Luks AM. Outdoor activity and high altitude exposure during pregnancy: A survey of 459 pregnancies. Wilderness Environ Med 2016;27:227-235.
- Jean D, Moore LG. Travel to high altitude during pregnancy: Frequently asked questions and recommendations for clinicians. High Alt Med Biol 2012;13:73-81.
- Pasquier M, Carron PN, Rodrigues A, et al. An evaluation of the Swiss staging model for hypothermia using hospital cases and case reports from the literature. Scand J Trauma Resusc Emerg Med 2019;27:60.
- Church BJ, Basnyat B, Mattingly B, Zafren K. Pediatric high altitude cerebral edema in the Nepal Himalayas. Wilderness Environ Med 2019;30:306-309.
- Kanna B, Wani S. Giant J wave on 12-lead electrocardiogram in hypothermia. Ann Noninvasive Electrocardiol 2003;8:262-265.
- Granberg PO. Human physiology under cold exposure. Arctic Med Res 1991;50(Suppl 6):23.
- Mistry K, Ondhia C, Levell NJ. A review of trench foot: A disease of the past in the present. Clin Exp Dermatol 2020;45:10-14.
- Giesbrecht GG. The respiratory system in a cold environment. Aviat Space Environ Med 1995;66:890.
- McIntosh SE, Freer L, Grissom CK, et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Frostbite: 2019 Update. Wilderness Environ Med 2019;30:S19-S32.
- Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med 2011;364:189-190.
- Pandey P, Vadlamudi R, Pradhan R, et al. Case report: Severe frostbite in extreme altitude climbers-The Kathmandu iloprost experience. Wilderness Environ Med 2018;29:366-374.
