Evaluation and Treatment of Acute Ischemic Stroke
January 15, 2023
AUTHORS
Samuel S. Bruce, MD, MA, Vascular Neurology Fellow, Weill Cornell Medical College, New York
Alan Z. Segal, MD, Associate Professor of Clinical Neurology, Weill Cornell Medical College, New York
PEER REVIEWER
Matthew E. Fink, MD, Louis and Gertrude Feil Professor and Chairman, Department of Neurology, Weill Cornell Medical College, Neurologist-in-Chief, New York Presbyterian Hospital/Weill Cornell Medical Center, New York, NY
EXECUTIVE SUMMARY
- Failure to report promptly to the emergency department leads to the loss of 1.9 million neurons per minute.
- After assuring hemodynamic stability and adequate airway, the next emphasis in the assessment of an acute stroke patient should shift to a focused stroke evaluation, with an emphasis on determining eligibility for intravenous thrombolysis or endovascular thrombectomy.
- Imaging studies can be used to assess the extent of threatened cerebral tissue that is amenable to reperfusion therapy.
- Intravenous thrombolysis with alteplase remains the mainstay of acute stroke treatment for patients presenting within 4.5 hours of symptom onset.
- Endovascular thrombectomy is an effective treatment for properly selected patients presenting within 24 hours of symptom onset.
Time is brain. For every minute of prolonged ischemia without treatment, 1.9 million neurons are lost.1 The faster definitive stroke treatment is administered following the onset of ischemia, the better the outcomes.2,3 Unfortunately, the majority of patients with acute ischemic stroke do not arrive to the hospital soon enough to receive emergency stroke treatment.4
Two U.S. Food and Drug Administration (FDA)-approved therapies are available for the treatment of acute ischemic stroke patients — thrombolytic treatment with intravenous tissue plasminogen activator (IV-tPA) and endovascular treatment with mechanical thrombectomy — both that reduce medium-term disability at three months without an increase in mortality.3,5-8 Both treatments are recommended by the American Heart Association (AHA) and American Stroke Association (ASA) to be administered within discrete, narrow time windows, given the diminishing net clinical benefit that occurs with the progression of time.2,3,9,10
Standard treatment windows for IV thrombolysis and endovascular thrombectomy are 4.5 and six hours from stroke symptom onset, respectively. However, imaging-based patient selection has allowed the extension of these treatments to patients outside of the standard windows, including IV thrombolysis in patients with unknown symptom onset time and endovascular thrombectomy in patients presenting six to 24 hours from symptom onset.
The primary goal of acute stroke evaluation is to rapidly identify the most appropriate candidates for IV thrombolysis and endovascular thrombectomy. The primary goal of acute stroke systems of care is to minimize barriers to quick and safe treatment to eligible patients.
Prehospital Considerations for Acute Stroke Care
Improving Prehospital Delays to Presentation
Prehospital issues are responsible for the largest portion of delay to acute stroke treatment.11,12 Failure to promptly report to the emergency department (ED) when experiencing stroke symptoms is the result of multiple factors, including a general lack of awareness and inability to identify symptoms of stroke, and self-presentation to the ED without activating the 911 emergency system.13
One useful tool for patient education is the FAST acronym, which instructs patients to identify acute onset of facial asymmetry (F), arm weakness (A), or speech disturbance (S) as indications for emergent medical attention, since time (T) is a factor. The symptoms emphasized in the FAST acronym have high sensitivity and specificity for stroke and are identical to those included in the Cincinnati Prehospital Stroke Scale, a three-item questionnaire used by emergency medical professionals to aid in the rapid identification of stroke, which also has been shown to be reliable when used by the lay population.14
Public education efforts to spread awareness of the FAST acronym through mass media have been associated with sustained improvement in prehospital delays.15 However, the FAST acronym has limitations: It does not include all possible stroke symptoms, sociodemographic disparities persist in its effectiveness, and it may be suboptimal in non-English speaking populations.16,17
Stroke 112, an educational campaign that references the universal emergency phone number in Taiwan, was developed to translate the importance of recognizing facial asymmetry (1: uneven face), arm weakness (1: uneven arm), and speech disturbance (2: incoherent lips) to non-English speaking populations.18 While study of the lay public found that the Stroke 112 approach was easier to remember than the FAST acronym, the effect of this difference on prehospital delays has yet to be elucidated.18
Prehospital Thrombolysis
A novel method of expediting acute stroke treatment is prehospital thrombolysis. Mobile stroke units (MSUs), which are ambulances equipped with computed tomography (CT) capabilities, portable laboratory testing, and in-person or telemedicine access to a vascular neurologist and radiologist, allow for efficient clinical evaluation and treatment with thrombolysis for appropriately selected patients prior to hospital arrival.
The PHANTOM-MS study, a clinical trial conducted in Germany in which stroke dispatches were randomized to transportation with MSU or conventional ambulance, demonstrated a reduction in onset to treatment times without a significant increase in adverse events.19 Two additional randomized clinical trials, BEST-MSU conducted in the United States and B_PROUD conducted in Germany, demonstrated a reduction in disability at three months with the use of MSU, as well as reduced treatment times.20,21
Moreover, recent observational data have suggested that the reduction in time to treatment associated with adoption of MSUs may be greater for patients receiving thrombectomy than thrombolysis.22 Although many hospitals have adopted the use of MSUs, there is uncertainty regarding their cost effectiveness, since they come at considerable expense in terms of equipment, staffing, and enacting of new practice protocols.23
Systems of Care for Coordinated Stroke Treatment
Like any time-based therapy that requires coordination among multiple physician specialties, an appropriately organized system of care is necessary to provide timely and evidence-based therapeutic interventions to the largest number of patients.24,25 Many considerations, such as patient or clinical characteristics, geographic restraints, and limitations in staffing or infrastructure, may affect where stroke patients ultimately receive their care. The advent of comprehensive stroke centers (CSCs) capable of providing endovascular stroke interventions has improved outcomes and allowed access to treatment for those patients with the most severe form of stroke caused by large vessel occlusions (LVOs).26
However, not all stroke patients would equally benefit from treatment in a CSC, and therefore allocation of resources is an important factor when deciding the destination for transport by emergency medical services. It has been suggested that patients with less severe strokes or those with longer travel times needed to reach a CSC be treated initially in a primary stroke center or acute stroke-ready hospital.9,27 If a stroke patient with a suspected LVO is being treated within a hospital that cannot provide endovascular therapy, this patient should be transferred immediately to a hospital with such capabilities after the decision whether to administer IV thrombolysis has been made.9
Given the close relationship between time to treatment and outcomes in thrombolysis and thrombectomy, protocols and pathways should be derived and implemented with the goal of reducing time to treatment as much as possible without sacrificing safety.3,5 With experience and the invention of novel methods aimed at expediting the evaluation of acute stroke patients, in-hospital delays to thrombolysis have improved over time and the frequency of tPA administration has increased.12,28
Simple interventions that reduce door-to-needle times can be instituted on a hospital level, such as hospital pre-notification by emergency medical services, rapid triage, notification of a stroke team with a single call or page, expedited acquisition and interpretation of brain imaging, premixing of tPA prior to CT acquisition, storage of tPA within the ED, and prompt review of feedback from performance data.29,30
Such interventions were proposed in a national campaign, Target: Stroke, spearheaded by the AHA, which promoted and monitored its effects. Widespread implementation of these interventions led to improved thrombolysis treatment times, lower mortality, lower rates of symptomatic intracerebral hemorrhage (sICH), and improved outcomes.29-31
The AHA recently has initiated a new phase of the Target: Stroke campaign with the goal of reducing time to thrombectomy, in addition to further reduction of thrombolysis treatment times.30 Still, considerable disparities persist in access to thrombolysis and thrombectomy, and more standardization of systems of care is needed to ensure these treatments are available to all who need them.32,33
Acute Evaluation of Patients with Ischemic Stroke
A patient presenting to the ED with symptoms of acute stroke should prompt the activation of a stroke team, which often involves a combination of practitioners and technicians from emergency medicine, radiology, nursing, laboratory, and pharmacy.9 See Table 1 for a list of the various duties performed by these specialists to streamline the stroke evaluation. It is imperative that the treating team obtain the time that the patient was observed last known normal, since acute stroke treatments can only be applied within narrow, discrete time windows.
Table 1. ED Diagnostic Evaluation for Acute Ischemic Stroke |
Emergency Medical Services
Emergency Department
Nursing
Stroke Team
Radiology
Laboratory
Pharmacy
ED: emergency department; LKN: last known normal; tPA: tissue plasminogen activator; IV: intravenous line; ECG: electrocardiogram; NIHSS: National Institutes of Health Stroke Scale; CT: computed tomography; CTA: computed tomography angiography; CTP: computed tomography perfusion *Stroke laboratory studies standardly include basic metabolic profile, complete blood count, coagulation studies (PT/INR and aPTT), and markers of cardiac ischemia.
|
Initial management should focus on patient stabilization, as in any emergency situation. The prompt placement of two peripheral IVs, preferably large-bore and in an antecubital location, is important to allow for the concurrent rapid administration of alteplase and acquisition of CT angiography in cases of suspected LVO.
Hypertension is common in the acute phase of stroke as a compensatory mechanism to maintain cerebral perfusion of the ischemic penumbra. Therefore, blood pressure should be carefully monitored and gently regulated if necessary. National guidelines recommend the lowering of blood pressure in hypertensive patients below 185/110 mmHg prior to tPA administration, given the increased risk of hemorrhagic conversion and other adverse events in patients with uncontrolled hypertension.9,34 Nevertheless, care should be taken to avoid hypotension, since aggressive blood pressure reduction similarly can lead to worse outcomes.34
A fingerstick glucose measurement is important since severe hypoglycemia occasionally can mimic stroke syndromes and hyperglycemia is associated with worse outcomes in acute ischemic stroke.9
After a patient is confirmed to be hemodynamically stable and protecting his or her airway, the emphasis should shift to a focused stroke evaluation, with an emphasis on determining eligibility for IV thrombolysis or endovascular thrombectomy.
Simultaneous with the initial nursing care and patient stabilization, the stroke team will perform a brief, targeted history and neurological exam. This evaluation, often performed by emergency physicians in primary stroke centers and stroke-ready hospitals or neurologists in CSCs, aims to quickly diagnose a clinical stroke and identify a recognized stroke syndrome attributable to a known vascular territory.
This neurological examination should assess the National Institutes of Health Stroke Scale (NIHSS), a 42-point validated scale that can quantify stroke severity, with higher scores referring to more severe stroke symptoms.35 (See Table 2.) The NIHSS evaluates 11 neurological domains in a focused fashion, including consciousness, language, visuospatial perception, cranial nerves, strength, sensation, and coordination.
Table 2. National Institutes of Health Stroke Scale |
||
1A |
Level of consciousness |
0 - Alert 1 - Drowsy 2 - Obtunded 3 - Coma/unresponsive |
1B |
Orientation questions a. Patient’s age b. Current month |
0 - Answers both correctly 1 - Answers one correctly 2 - Answers neither correctly |
1C |
Response to commands a. Close/open eyes b. Close/open fist |
0 - Performs both tasks correctly 1 - Performs one task correctly 2 - Performs neither |
2 |
Gaze |
0 - Normal horizontal movements 1 - Partial gaze palsy 2 - Complete gaze palsy |
3 |
Visual fields |
0 - No visual field defect 1 - Partial hemianopia 2 - Complete hemianopia 3 - Bilateral hemianopia |
4 |
Facial movement |
0 - Normal facial movement 1 - Minor facial weakness 2 - Partial facial weakness 3 - Complete unilateral palsy |
5 |
Motor function (arm) a. Left b. Right |
0 - No drift 1 - Drift before 10 seconds 2 - Hits bed before 10 seconds 3 - No effort against gravity 4 - No movement |
6 |
Motor function (leg) a. Left b. Right |
0 - No drift 1 - Drift before 5 seconds 2 - Hits bed before 5 seconds 3 - No effort against gravity 4 - No movement |
7 |
Limb ataxia |
0 - No ataxia 1 - Ataxia in 1 limb 2 - Ataxia in 2 limbs |
8 |
Sensation |
0 - No sensory loss 1 - Mild sensory loss 2 - Severe sensory loss |
9 |
Language |
0 - Normal language 1 - Mild aphasia 2 - Severe aphasia 3 - Mute or global aphasia |
10 |
Speech articulation |
0 - Normal 1 - Mild dysarthria 2 - Severe dysarthria |
11 |
Extinction/inattention |
0 - Absent 1 - Mild (abnormal in 1 sensory modality) 2 - Severe (abnormal in 2 sensory modalities) |
Nursing staff also should obtain blood for basic laboratory evaluation, including a complete blood count and coagulation studies. Other laboratory testing is not required prior to tPA administration unless the patient has medical history or recent medication use that could implicate a reason to suspect a bleeding diathesis.9 Retrospective data have demonstrated that the rate of unsuspected contraindications to thrombolysis based on previously unknown thrombocytopenia or coagulopathy is very low (0.4%).36
Point-of-care laboratory testing in the ED can significantly reduce delays to thrombolysis and remove the safety concerns related to tPA administration when the values of platelets and coagulation studies are unknown.37
An emergent non-contrast CT of the head should be promptly obtained to rule out intracranial hemorrhage, which should be excluded prior to administering tPA. The head CT occasionally may also help to rule in ischemia in the early phases of stroke, by demonstrating an intra-arterial thrombus (i.e., vessel hyperdensity), loss of the insular ribbon, or obscuration of other areas of gray-white matter differentiation.
Prior to mixing tPA, the patient’s medical history should be carefully considered to evaluate whether he or she meets any of the clinical contraindications for IV thrombolysis. (See Table 3.) Such contraindications focus on reasons a given patient may be expected to be at an increased bleeding risk, such as a large completed infarction, evidence of current or previous intracranial hemorrhage, recent bleeding, recent surgery, recent trauma, or the presence of a bleeding diathesis.
Table 3. Criteria for the Use of IV-tPA in Acute Ischemic Stroke |
Inclusion Criteria
Absolute Exclusion Criteria
|
Relative Exclusion Criteriab
Relative Contraindications to Extend the Treatment Window to 4.5 hoursc
|
a Intravenous (IV) thrombolysis may be administered if these vital sign disturbances can be corrected within an appropriate time window. b Depending on the clinical circumstances, with careful consideration of the risks and benefits, patients may receive IV thrombolysis despite one or more of these relative contraindications. c These relative contraindications are based on the ECASS III trial exclusionary criteria. Depending on the clinical circumstances, with careful consideration of the risks and benefits, patients may receive IV thrombolysis despite one or more of these relative contraindications during an extended time window. IV-tPA = intravenous tissue plasminogen activator; CT = computed tomography; aPTT = activated partial thromboplastin time; INR = international normalized ratio; PT = partial thromboplastin time; ECT = ecarin clotting time; TT = thrombin time; NIHSS = National Institutes of Health Stroke Scale |
If a patient is suspected to have an LVO based on the severity of the stroke syndrome (NIHSS score is > 10 or cortical signs such as aphasia, neglect, or visual loss), then an emergent CT angiogram (CTA) should be obtained to confirm the intracranial occlusion, assess quality of collateralization, and evaluate for the presence of comorbid extracranial carotid stenosis that may require concurrent intervention.
For patients presenting six to 24 hours from symptom onset, CTA should be accompanied by a CT perfusion (CTP) scan of the head, when available, to evaluate for a large mismatch between ischemic penumbra (i.e., tissue at risk) and infarct core (i.e., established stroke).38 (See Figure 1.) However, vessel and/or perfusion imaging should be performed after or concurrently with tPA administration for appropriate candidates, so as not to delay treatment.
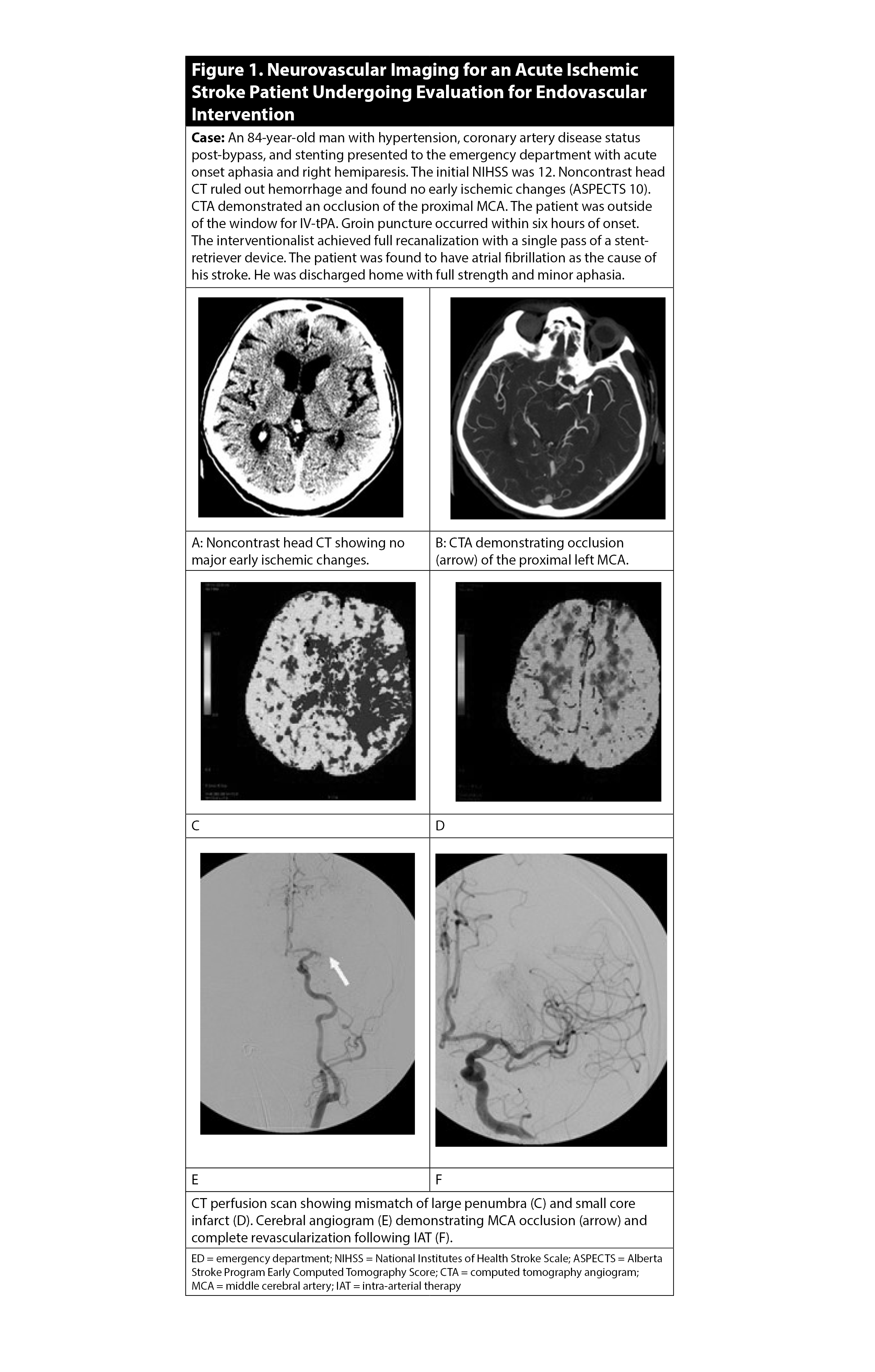
Figure 1. Neurovascular Imaging for an Acute Ischemic Stroke Patient Undergoing Evaluation for Endovascular Intervention |
Confirmation of a proximal large vessel occlusion on vessel imaging should prompt a discussion with an endovascular team at a CSC regarding eligibility for mechanical thrombectomy. Many factors may influence the ultimate decision to attempt thrombectomy, including the location of the occlusion, comorbid occlusions or stenoses elsewhere in the cervical or intracranial vasculature, premorbid functional status, and extent of early ischemia.
Often, the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) is used to standardly quantify the amount of early ischemic changes in the middle cerebral artery (MCA) territory present on a non-contrast head CT.39 The ASPECTS scale is a 10-point, validated scoring system, where an initial score of 10 indicates a normal CT scan, and one point is subtracted for each abnormal area within 10 pre-specified regions of the cortex and deep subcortical structures. Higher ASPECTS scores correlate to smaller core infarcts and thus larger presumed ischemic penumbras.
Many of the recent endovascular trials used a cutoff of 6 or better on the ASPECTS scale as part of the inclusionary criteria for trial selection, as these patients are thought to have the largest areas of salvageable brain tissue.40-42 Thus, patients with ASPECTS < 6 traditionally have been excluded from endovascular thrombectomy, although recent data suggest that endovascular therapy may still be beneficial in these patients.43
Regarding premorbid functional status, patients with pre-stroke disability were excluded from endovascular trials, and observational studies have suggested that the benefit of endovascular therapy in this population is uncertain.38,40-42,44-47
Intravenous Thrombolysis in Acute Ischemic Stroke
IV thrombolysis is the mainstay of acute stroke treatment for patients presenting within 4.5 hours of symptom onset. In the United States, IV-tPA (also known as alteplase) is FDA-approved to be administered within three hours of stroke onset, although AHA/ASA guidelines recommend its use up to 4.5 hours in certain patient populations.9,48 Within these treatment windows, IV-tPA improves the functional recovery from stroke at three or six months without an increase in mortality, despite an increased risk of sICH.3,49
Landmark IV-tPA Clinical Trials
The efficacy of IV-tPA in acute ischemic stroke was established in a series of landmark randomized clinical trials. The first of these, a 1995 American trial known as the National Institutes of Neurological Disease and Stroke (NINDS) IV tPA Study, demonstrated the effectiveness of IV-tPA in patients with acute ischemic stroke presenting within three hours of symptom onset.49 A significantly higher proportion of patients who received IV-tPA achieved complete recovery or minimal neurological deficit at three months post-treatment than patients who received placebo. Overall mortality was not significantly different between groups, despite an increase in the observed rate of sICH in the treatment group (6.4%) compared to placebo (0.6%). Treatment benefit was observed despite heterogeneity in stroke mechanisms.
A European trial published in 2008, the European Cooperative Acute Stroke Study (ECASS) III, demonstrated the effectiveness of IV-tPA in certain patients presenting between three and 4.5 hours.50 Similar to the NINDS trial, ECASS III found that patients treated with IV-tPA had a significantly higher proportion of minimal or no disability at three months post-treatment and no increase in mortality, despite a higher rate of sICH (2.4%) than that of the placebo group (0.3%). Notably, the definition of sICH used in ECASS III was more conservative than that of the NINDS trial; if the definition of the latter had been applied to patients in ECASS III, the sICH rate in the IV-tPA arm would have been 7.9%.
It also must be noted that ECASS III excluded several additional patient populations at higher risk of hemorrhagic conversion and other adverse events, such as patients older than 80 years of age, patients with both prior stroke and diabetes mellitus, patients with any recent oral anticoagulant use, and patients with very severe strokes (NIHSS score > 25).
The Third International Stroke Trial (IST-3), published in 2012, attempted to further evaluate the efficacy of alteplase in those patients excluded or not included in large numbers in the initial IV-tPA trials.51 Particularly, 53% of the patients included in the trial were older than 80 years of age, and the investigators studied the treatment of patients presenting up to six hours from symptom onset. There were no differences in the primary outcome of functional independence (determined by the Oxford Handicap Scale) between the IV-tPA and placebo arms at six months post-treatment.
Pooled analyses of these landmark trials allowed for a fuller assessment of risks and benefits associated with IV-tPA and confirmed that the net clinical benefit of tPA dampens with time, further demonstrating the concept that “time is brain.”3,52 As time unfolds, the chances of avoiding disability diminish and the risk of adverse events increases. Thus, no significant net benefit of alteplase therapy for acute ischemic stroke has been demonstrated after 4.5 hours.
Pooled analyses of these trials also confirmed the benefits of tPA within three hours in certain patient populations that were not included in large numbers in the NINDS trial, such as patients older than 80 years of age or patients with very severe strokes (NIHSS score > 25).
Unfortunately, these meta-analyses still lacked enough data to clarify whether IV-tPA was safe and beneficial in the three to 4.5 hour window for patients meeting ECASS III exclusion criteria (i.e., age > 80 years, NIHSS score > 25, diabetes and prior stroke, any anticoagulant use), but observational data have suggested that IV-tPA is not associated with an increased risk of sICH in the three to 4.5 hour window for patients older than 80 years of age or patients with diabetes and prior stroke.53-56
Minor Stroke or Non-Disabling Symptoms
Subgroup analyses from early thrombolysis trials did not consistently show a significant benefit of tPA in patients with an NIHSS score < 5 or symptoms deemed to not interfere with activities of daily living or return to work.3,57-59 The PRISMS trial, an American study published in 2018, examined this subpopulation directly.60 Patients presenting within three hours of stroke onset with an NIHSS score < 5 and deficits not otherwise deemed to be disabling were randomized to IV-tPA or aspirin. There was no significant difference between the tPA and aspirin groups in proportion of patients with minimal or no disability at three months, nor was there any difference in mortality. The tPA group had a 3.2% rate of sICH compared to 0% in the aspirin group.
As a result of this study, AHA/ASA guidelines do not recommend IV-tPA for patients presenting within three hours or three to 4.5 hours of symptom onset with non-disabling stroke symptoms and an NIHSS score < 5 who would otherwise be eligible.9
Wake-Up Stroke and Extended Treatment Window
Symptom onset times can be difficult to identify with precision, particularly in the case of stroke upon awakening, which accounts for up to 28% of strokes.61 When deciding on treatment for wake-up stroke patients, it is standard to assume that stroke onset was the time the patient went to bed normal, even though stroke onset frequency is known to be highest in the early morning.62
The WAKE-UP trial, published in 2018, demonstrated the utility of rapidly obtained magnetic resonance imaging (MRI) in selected patients for thrombolysis presenting with unknown symptom onset time.63 Patients were eligible for inclusion in the WAKE-UP trial if they had an unknown symptom onset time and an MRI showing an ischemic lesion with diffusion weighted imaging (DWI) hyperintensity and no fluid attenuated inversion recovery (FLAIR) hyperintensity, which suggested that the stroke likely occurred within 4.5 hours. Similar to prior thrombolysis trials, IV-tPA was associated with a significantly higher likelihood of minimal or no disability at three months with no increase in mortality despite a higher rate of sICH (8.0% by NINDS criteria vs. 4.9% in placebo group).
AHA/ASA guidelines recommend administration of tPA in patients with unknown symptom onset time but recognition of symptoms < 4.5 hours prior to treatment, and DWI hyperintensity without T2/FLAIR hyperintensity on MRI.9
IV Tenecteplase
Since its adoption in acute stroke treatment, numerous downsides of tPA have become apparent, including a short half-life with rapid plasma clearance and cumbersome administration, which requires both a bolus and hour-long infusion.64 Tenecteplase, a genetically engineered, mutant form tPA with a longer half-life given as a single bolus, was developed, in part, to mitigate these shortcomings.
A recently published Canadian study, called the AcT trial, demonstrated noninferiority of tenecteplase to alteplase in acute ischemic stroke presenting within 4.5 hours.65 In the AcT trial, there were similar rates of minimal or no disability at three months, mortality, and sICH between the alteplase and tenecteplase arms. Although IV tenecteplase is not yet FDA approved, many hospitals have incorporated it into acute stroke protocols given its ease of use and noninferiority to tPA.
Endovascular Thrombectomy in Acute Ischemic Stroke
One-third to one-half of acute ischemic strokes are caused by occlusion of a major intracranial artery, which typically presents with a more severe stroke syndrome and is independently associated with poorer outcomes.66,67 Unfortunately, the effect of IV-tPA diminishes with increasing clot burden, and proximal artery occlusions often are refractory to IV thrombolysis.68 Endovascular thrombectomy has emerged as the most impactful treatment option for appropriately selected patients with LVO.
In this procedure, an experienced specialist passes an arterial catheter to the site of the occlusion under angiographic guidance and removes the thrombus. This can be achieved via direct aspiration or with the aid of a stent retriever device, in which a flexible stent self-deploys and adheres itself to the thrombus, allowing it to be removed easily. In some cases, intra-arterial thrombolytic administration may be used, although currently this approach is mostly used as adjuvant or rescue therapy because of the overwhelming efficacy of mechanical thrombectomy.69
The AHA/ASA recommends endovascular thrombectomy in anterior circulation LVO within the standard treatment window of six hours from symptom onset, with extension to 24 hours with the aid of perfusion imaging.9 Emerging evidence suggests this treatment may be beneficial for additional specific populations, such as patients with basilar artery occlusion and patients with large volumes of completed infarction.7,8,43
Endovascular Thrombectomy for Anterior Circulation LVO
Before 2015, there was equipoise regarding the utility of endovascular thrombectomy in acute ischemic stroke. In 2013, three separate randomized trials (SYNTHESIS Expansion, IMS III, and MR RESCUE) were published demonstrating no benefit of endovascular therapy over the standard of care.70-72 However, multiple features of these trials likely explained their failure to find a benefit of endovascular treatment: earlier generation devices were used, the study protocols did not require the confirmation of an LVO prior to randomization, and there were delays to treatment.
It was in this context that five trials (MR CLEAN, EXTEND-IA, ESCAPE, SWIFT PRIME, and REVASCAT) with positive results were published within the span of six months in 2015.40-42,44,73 All five studies clearly demonstrated a clinical benefit of endovascular thrombectomy over standard medical care (including IV thrombolysis) without an increase in mortality or sICH.5
Although there were subtle differences in study inclusion and patient selection criteria between the endovascular trials, they all included patients presenting with acute occlusion of a large artery of the anterior circulation, typically the intracranial carotid artery (ICA) or proximal MCA, although a small number of patients included in MR CLEAN had anterior cerebral artery (ACA) occlusions.44
Each trial compared mechanical thrombectomy, largely with stent-retriever devices, to standard medical care, which most often included IV thrombolysis. The majority of patients in these randomized trials were treated within six hours, but some studies permitted a small number of patients to be randomized in later time windows.40,45
To select patients with a promising mismatch between core infarct and penumbra for study inclusion, REVASCAT allowed for the use of MRI, ESCAPE required patients to have good collateral flow on CTA, and SWIFT PRIME and EXTEND-IA employed CT perfusion imaging.40-42,45 MR CLEAN was the only study to enroll patients based only on whether an LVO was confirmed on CTA, without any additional criteria for exclusion of completed infarction on CT.44
The HERMES collaboration meta-analysis of pooled individual patient data from these trials showed that endovascular therapy initiated within the six-hour treatment window can be performed efficiently (median time to revascularization from symptom onset of 285 minutes) and with technical success (71% achieving good reperfusion).5 This procedural success results in a 19.5% reduction in risk of functional dependence, with a number needed to treat of 2.6 for a one-point decrease in modified Rankin scale score, a six-point ordinal score measuring level of disability, with 0 indicating complete lack of deficits and 6 indicating death.
Furthermore, endovascular care is safe, with no consistent significant difference in rates of sICH or mortality, although the ESCAPE trial did report an 8.6% significant reduction in mortality with endovascular treatment.40 Given the strong benefit of endovascular therapy observed in these trials, mechanical thrombectomy of proximal arterial occlusions of the anterior circulation is the standard of care within six hours of symptom onset.9
Two additional landmark trials published in 2018 demonstrated the effectiveness of thrombectomy for patients presenting six to 24 hours from stroke onset. These studies used perfusion imaging to determine which patients had a substantial amount of brain tissue that was ischemic, and therefore causing deficits, but not yet infarcted.
The first of the two trials, called DAWN, enrolled patients presenting six to 24 hours from stroke onset with proximal MCA or ICA occlusion and a mismatch in the severity of their clinical deficits and the volume of infarcted core, and randomized them to endovascular thrombectomy or medical management.46 A combination of age and NIHSS score were used as a proxy for the amount of ischemic tissue, and the infarct core was defined as the region of severely reduced cerebral blood flow on CTP or DWI hyperintensity on MRI. The authors found a significant benefit in functional outcome at three months with thrombectomy, with no significant increase in mortality or sICH.46
A second trial, DEFUSE 3, enrolled patients in a narrower time window of six to 16 hours, and used CTP or MRI perfusion imaging to define both infarct core (region of severely reduced blood flow) and ischemic penumbra (region of delayed perfusion).38 Patients with ischemic core volume of < 70 mL, ratio of penumbra to core > 1.8, and difference between
penumbra volume and core volume > 15 mL were randomized to endovascular thrombectomy or medical management. Similar to DAWN, DEFUSE 3 demonstrated better functional outcomes with thrombectomy, with an absolute reduction in mortality that did not reach statistical significance, and no significant increase in sICH. In subgroup analyses, they found similar results between patients who met eligibility criteria for DAWN (61.5% of DEFUSE 3 patients) vs. those who did not (38.5%).38
As a result of these trials, AHA/ASA guidelines recommend thrombectomy for patients presenting within six to 24 hours of stroke onset that meet inclusion criteria of either DAWN or DEFUSE 3.9
Endovascular Thrombectomy for Basilar Artery Occlusion
Acute basilar artery occlusion is rare, accounting for as little as 1% of ischemic strokes, but frequently is associated with poor prognosis in terms of disability and mortality.74,75 Although none of the landmark 2015 endovascular trials included posterior circulation occlusions, endovascular thrombectomy often has been performed for basilar artery occlusion because of its often devastating natural history. The most recent AHA/ASA guidelines, updated in 2019, say it is reasonable to perform in properly selected patients.9
More recently, the evidence for endovascular thrombectomy in basilar artery occlusion has been enriched by several randomized clinical trials. Two initial trials comparing endovascular thrombectomy and medical management had neutral results, but confounding factors complicated their interpretation.
The BEST trial, conducted in China and published in 2020, did not find a significant difference in the proportion of patients with moderate to no disability at three months in its primary intention-to-treat analysis.76 However, there was a high rate of crossover in this trial, with 27% of the medical therapy arm ultimately receiving mechanical thrombectomy. In secondary analyses with groups compared as treated or per randomization excluding crossovers, thrombectomy was associated with significantly higher odds of moderate to no disability and functional independence at three months.
The BASICS trial, conducted in seven European and South American countries and published in 2021, likewise did not show a significant benefit of endovascular thrombectomy over medical management in terms of functional outcome.77 But enrollment in the study was low, with randomization of only 300 of 424 eligible patients, and 98 of the 124 patients receiving treatment outside of the trial undergoing thrombectomy.
Two newly published trials from 2022, both performed across multiple centers in China, showed a clearer benefit of thrombectomy in basilar artery occlusion. The ATTENTION trial enrolled patients presenting within six hours of stroke onset, and found both a significant increase in proportion of patients with good functional outcome at three months and a reduction in mortality with endovascular thrombectomy compared to medical management.7 Another trial, BAOCHE, found similar results for treatment initiation six to 24 hours from symptom onset, although the reduction in mortality associated with endovascular treatment did not reach statistical significance.8
Endovascular Thrombectomy for Large Area of Infarction
Endovascular therapy typically has been deferred in patients with a large area of completed infarction seen on imaging because of concern for lack of salvageable brain tissue and higher risk of reperfusion hemorrhage.5,78 However, the RESCUE-Japan LIMIT trial, conducted in multiple centers throughout Japan and published in 2022, suggested possible benefit of thrombectomy in this population.43 Patients with anterior circulation LVO and large areas of infarcted tissue on CT or MRI, as defined by ASPECTS of 3 to 5, were randomized to thrombectomy or medical management. Thrombectomy was associated with increased chances of good functional outcome without an increase in mortality. Although it was associated with an absolute increase in risk of sICH, this increase did not reach statistical significance.
The generalizability of these results is unclear, in part because 87% of patients included in the trial had ASPECTS values calculated from MRI, which is not as readily or rapidly available as CT in most settings. Nevertheless, the study is cause for optimism in a patient population that otherwise carries a substantial risk of morbidity and mortality.
IV-tPA as Adjunctive Therapy in Patients Eligible for Thrombectomy
Despite low recanalization rates for IV-tPA in proximal arterial occlusions, 85% of patients in the landmark endovascular stroke trials underwent IV thrombolysis prior to endovascular therapy, and IV-tPA remains an important treatment for patients with LVO for two reasons.5,68
First, clot extraction performed within a proximal artery often results in distal embolization of thrombotic material into smaller arteries downstream of the target vessel.79 Such embolic particulate may not be easily accessed by catheterization techniques if lodged in small, distal arteries but potentially could be recanalized with the aid of ongoing intravenous fibrinolysis.
Second, stroke patients can be treated with IV-tPA as an initial therapy when access to endovascular intervention may be limited or delayed. For instance, many patients are first evaluated within a hospital that does not have endovascular capabilities before transfer upon evaluation and suspicion of an LVO. Transfer times can add significant delays to reperfusion, resulting in longer times to definitive treatment and worsened outcomes.80 Thus, IV-tPA may be used as a bridging therapy in these instances.
Although a meta-analysis of multiple randomized clinical trials did not show a clear improvement in functional outcome associated with combined thrombectomy and thrombolysis vs. thrombectomy alone, guidelines still recommend treating eligible patients with thrombolysis prior to thrombectomy when possible.81
Summary and Conclusions
“Time is brain” continues to be the guiding principle of acute stroke treatment. With each passing minute, ischemic tissue becomes further damaged, and the risk of permanent physical dependence rises.
The primary goal of acute stroke care is to salvage as much brain tissue as possible by identifying patients likely to benefit from IV thrombolysis and/or endovascular thrombectomy and delivering treatment safely and promptly. This is a multilayered process that begins with effective education of patients and the practitioners who treat them in the outpatient setting. It continues with streamlined systems at the prehospital and hospital level designed to minimize barriers to timely treatment. Finally, it culminates with efficient evaluation and decision-making at the point of hospital arrival to appropriately select patients and rapidly administer treatment.
Optimizing stroke outcomes also requires vigilance in systematically examining practice patterns, conducting impactful research, and incorporating the most up-to-date evidence. IV thrombolysis and endovascular thrombectomy traditionally have been limited to narrow time windows with restrictive clinical selection criteria, but recent high-quality clinical trials in specific populations have continued to extend these treatments to new groups of patients.
Nonetheless, despite the tremendous strides that have been made since the advent of tPA, enormous opportunities remain to further minimize treatment times, improve recanalization rates, and expand treatment eligibility to a greater number of stroke patients.
REFERENCES
- Saver JL. Time is brain–quantified. Stroke 2006;37:263-266.
- Fransen PS, Berkhemer OA, Lingsma HF, et al. Time to reperfusion and treatment effect for acute ischemic stroke: A randomized clinical trial. JAMA Neurol 2016;73:190-196.
- Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929-1935.
- Tong D, Reeves MJ, Hernandez AF, et al. Times from symptom onset to hospital arrival in the Get with the Guidelines--Stroke Program 2002 to 2009: Temporal trends and implications. Stroke 2012;43:1912-1917.
- Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723-1731.
- Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet 2022;399:249-258.
- Tao C, Nogueira RG, Zhu Y, et al. Trial of endovascular treatment of acute basilar-artery occlusion. N Engl J Med 2022;387:1361-1372.
- Jovin TG, Li C, Wu L, et al. Trial of thrombectomy 6 to 24 hours after stroke due to basilar-artery occlusion. N Engl J Med 2022;387:1373-1384.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344-e418.
- Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016;316:1279-1288.
- Mainz J, Andersen G, Valentin JB, et al. Treatment delays and chance of reperfusion therapy in patients with acute stroke: A Danish nationwide study. Cerebrovasc Dis 2022;Oct. 31:1-8.
- Evenson KR, Foraker RE, Morris DL, Rosamond WD. A comprehensive review of prehospital and in-hospital delay times in acute stroke care. Int J Stroke 2009;4:187-199.
- Mellon L, Doyle F, Williams D, et al. Patient behaviour at the time of stroke onset: A cross-sectional survey of patient response to stroke symptoms. Emerg Med J 2016;33:396-402.
- Hurwitz AS, Brice JH, Overby BA, Evenson KR. Directed use of the Cincinnati Prehospital Stroke Scale by laypersons. Prehosp Emerg Care 2005;9:292-296.
- Wolters FJ, Paul NL, Li L, et al. Sustained impact of UK FAST-test public education on response to stroke: A population-based time-series study. Int J Stroke 2015;10:1108-1114.
- Rioux B, Brissette V, Marin FF, et al. The impact of stroke public awareness campaigns differs between sociodemographic groups. Can J Neurol Sci 2022;49:231-238.
- Zhao J, Liu R. Stroke 1-2-0: A rapid response programme for stroke in China. Lancet Neurol 2017;16:27-28.
- Zhao J, Eckenhoff MF, Sun W-Z, Liu R. Stroke 112: A universal stroke awareness program to reduce language and response barriers. Stroke 2018;49:1766-1769.
- Ebinger M, Winter B, Wendt M, et al. Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: A randomized clinical trial. JAMA 2014;311:1622-1631.
- Grotta JC, Yamal J-M, Parker SA, et al. Prospective, multicenter, controlled trial of mobile stroke units. N Engl J Med 2021;385:971-981.
- Ebinger M, Siegerink B, Kunz A, et al. Association between dispatch of mobile stroke units and functional outcomes among patients with acute ischemic stroke in Berlin. JAMA 2021;325:454-466.
- Zhao H, Coote S, Easton D, et al. Melbourne mobile stroke unit and reperfusion therapy: Greater clinical impact of thrombectomy than thrombolysis. Stroke 2020;51:922-930.
- Navi BB, Audebert HJ, Alexandrov AW, et al. Mobile stroke units: Evidence, gaps, and next steps. Stroke 2022;53:2103-2113.
- Goyal M, Yu AY, Menon BK, et al. Endovascular therapy in acute ischemic stroke: Challenges and transition from trials to bedside. Stroke 2016;47:548-553.
- Daubail B, Ricolfi F, Thouant P, et al. Impact of mechanical thrombectomy on the organization of the management of acute ischemic stroke. Eur Neurol 2016;75:41-47.
- Iihara K, Nishimura K, Kada A, et al. Effects of comprehensive stroke care capabilities on in-hospital mortality of patients with ischemic and hemorrhagic stroke: J-ASPECT study. PLoS One 2014;9:e96819.
- Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020-3035.
- Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes 2013;6:543-549.
- Xian Y, Smith EE, Zhao X, et al. Strategies used by hospitals to improve speed of tissue-type plasminogen activator treatment in acute ischemic stroke. Stroke 2014;45:1387-1395.
- Xian Y, Xu H, Smith EE, et al. Achieving more rapid door-to-needle times and improved outcomes in acute ischemic stroke in a nationwide quality improvement intervention. Stroke 2022;53:1328-1338.
- Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA 2014;311:1632-1640.
- Ader J, Wu J, Fonarow GC, et al. Hospital distance, socioeconomic status, and timely treatment of ischemic stroke. Neurology 2019;93:e747-e757.
- Kamel H, Parikh NS, Chatterjee A, et al. Access to mechanical thrombectomy for ischemic stroke in the United States. Stroke 2021;52:2554-2561.
- Leonardi-Bee J, Bath PM, Phillips SJ, et al. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 2002;33:1315-1320.
- Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol 1989;46:660-662.
- Rost NS, Masrur S, Pervez MA, et al. Unsuspected coagulopathy rarely prevents IV thrombolysis in acute ischemic stroke. Neurology 2009;73:1957-1962.
- Walter S, Kostopoulos P, Haass A, et al. Point-of-care laboratory halves door-to-therapy-decision time in acute stroke. Ann Neurol 2011;69:581-586.
- Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708-718.
- Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol 2001;22:1534-1542.
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-1030.
- Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009-1018.
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-2295.
- Yoshimura S, Uchida K, Sakai N, et al. Randomized Clinical Trial of Endovascular Therapy for Acute Large Vessel Occlusion with Large Ischemic Core (RESCUE-Japan LIMIT): Rationale and study protocol. Neurol Med Chir (Tokyo) 2022;62:156-164.
- Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11-20.
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-2306.
- Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11-21.
- de Havenon A, Castonguay A, Nogueira R, et al. Prestroke disability and outcome after thrombectomy for emergent anterior circulation large vessel occlusion stroke. Neurology 2021;97:e1914-e1919.
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:581-641.
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-1587.
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317-1329.
- IST-3 collaborative group; Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012;379:2352-2363.
- Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet 2012;379:2364-2372.
- Cronin CA, Sheth KN, Zhao X, et al. Adherence to Third European Cooperative Acute Stroke Study 3- to 4.5-hour exclusions and association with outcome: Data from Get with the Guidelines-Stroke. Stroke 2014;45:2745-2749.
- Fuentes B, Martinez-Sanchez P, Alonso de Lecinana M, et al. Diabetes and previous stroke: Hazards for intravenous thrombolysis? Eur J Neurol 2012;19:587-593.
- Meretoja A, Putaala J, Tatlisumak T, et al. Off-label thrombolysis is not associated with poor outcome in patients with stroke. Stroke 2010;41:1450-1458.
- Karlinski M, Kobayashi A, Mikulik R, et al. Intravenous alteplase in ischemic stroke patients not fully adhering to the current drug license in Central and Eastern Europe. Int J Stroke 2012;7:615-622.
- Khatri P, Tayama D, Cohen G, et al. Effect of intravenous recombinant tissue-type plasminogen activator in patients with mild stroke in the Third International Stroke Trial-3: Post hoc analysis. Stroke 2015;46:2325-2327.
- National Institute of Neurological Disorders Stroke rt-PA Stroke Study Group. Recombinant tissue plasminogen activator for minor strokes: The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study experience. Ann Emerg Med 2005;46:243-252.
- Ingall TJ, O’Fallon WM, Asplund K, et al. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke 2004;35:2418-2424.
- Khatri P, Kleindorfer DO, Devlin T, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits: The PRISMS randomized clinical trial. JAMA 2018;320:156-166.
- Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology 2011;76:1662-1667.
- Marler JR, Price TR, Clark GL, et al. Morning increase in onset of ischemic stroke. Stroke 1989;20:473-476.
- Thomalla G, Simonsen CZ, Boutitie F, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018;379:611-622.
- Coutts SB, Yu AYX. Tenecteplase for acute stroke: The thrombolysis puzzle. Lancet Neurol 2022;21:496-497.
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): A pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet 2022;400:161-169.
- Smith WS, Lev MH, English JD, et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009;40:3834-3840.
- Zhu W, Churilov L, Campbell BCV, et al. Does large vessel occlusion affect clinical outcome in stroke with mild neurologic deficits after intravenous thrombolysis? J Stroke Cerebrovasc Dis 2014;23:2888-2893.
- Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: Real-world experience and a call for action. Stroke 2010;41:2254-2258.
- Zaidi SF, Castonguay AC, Jumaa MA, et al. Intraarterial thrombolysis as rescue therapy for large vessel occlusions. Stroke 2019;50:1003-1006.
- Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:904-913.
- Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013;368:893-903.
- Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914-923.
- Chen C-J, Ding D, Starke RM, et al. Endovascular vs medical management of acute ischemic stroke. Neurology 2015;85:1980-1990.
- Israeli-korn SD, Schwammenthal Y, Yonash-Kimchi T, et al. Ischemic stroke due to acute basilar artery occlusion: Proportion and outcomes. Isr Med Assoc J 2010;12:671-675.
- Schonewille WJ, Algra A, Serena J, et al. Outcome in patients with basilar artery occlusion treated conventionally. J Neurol Neurosurg Psychiatry 2005;76:1238-1241.
- Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): An open-label, randomised controlled trial. Lancet Neurol 2020;19:115-122.
- Langezaal LCM, van der Hoeven EJR, Mont’Alverne FJA, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med 2021;384:1910-1920.
- Cucchiara B, Kasner SE, Tanne D, et al. Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: Pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke 2009;40:3067-3072.
- Klinger-Gratz PP, Schroth G, Gralla J, et al. Protected stent retriever thrombectomy prevents iatrogenic emboli in new vascular territories. Neuroradiology 2015;57:1045-1054.
- Sun C-H, Connelly K, Nogueira RG, et al. ASPECTS decay during inter-facility transfer predicts patient outcomes in endovascular reperfusion for ischemic stroke: A unique assessment of dynamic physiologic change over time. J Neurointerv Surg 2015;7:22-26.
- Masoud HE, de Havenon A, Castonguay AC, et al. 2022 Brief Practice Update on Intravenous Thrombolysis Before Thrombectomy in Patients With Large Vessel Occlusion Acute Ischemic Stroke: A Statement from Society of Vascular and Interventional Neurology Guidelines and Practice Standards (GAPS) Committee. Stroke Vasc Interv Neurol 2022;2:e000276.
The primary goal of acute stroke care is to salvage as much brain tissue as possible by identifying patients likely to benefit from IV thrombolysis and/or endovascular thrombectomy and delivering treatment safely and promptly.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
