Evaluation and Management of Neck Trauma
March 1, 2024
Related Articles
AUTHORS
Eileen Chu, MS, MD, Resident, Department of Emergency Medicine, University of Maryland Medical Center, Baltimore
R. Gentry Wilkerson, MD, Associate Professor, Department of Emergency Medicine, Director of Clinical Research, Assistant Residency Program Director, University of Maryland School of Medicine, Baltimore
PEER REVIEWER
Howard A. Werman, MD, Professor Emeritus of Emergency Medicine, The Ohio State University, Columbus
EXECUTIVE SUMMARY
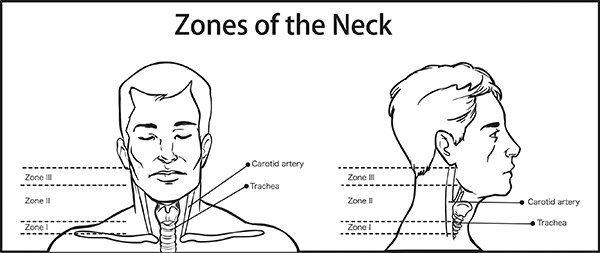
- Zone I extends from the clavicles and sternum to the level of the cricoid cartilage. Major vessels within zone I include the proximal portion of the carotid arteries, the jugular-subclavian junctions, and the left innominate vein. Zone II is the largest zone and the most commonly injured. It extends from the superior aspect of the cricoid cartilage to the level of the angle of the mandible. Major vessels within zone II include the internal and external jugular veins, the common carotid arteries and their bifurcation into the internal and external carotid arteries, and the vertebral arteries. The trachea and esophagus are aerodigestive structures that extend through both zones.
- Zone III extends from the angle of the mandible to the base of the skull. The vessels within zone III include the carotid and vertebral arteries and the internal jugular veins. Zone III also contains cranial nerve IX (glossopharyngeal), cranial nerve X (vagus), cranial nerve XI (accessory), and cranial nerve XII (hypoglossal).
- Improvements in radiological techniques and widespread screening protocols for trauma patients have led to increased detection, with reported rates of blunt cerebrovascular injury (BCVI) of 0.9% to 2.7% in blunt trauma. Failure to diagnose and appropriately treat BCVI can lead to 80% morbidity and 40% mortality. Strokes are a major complication concern when it comes to blunt neck trauma and BCVI.
- Multidetector neck computed tomography angiography (CTA) is the first-line imaging study of choice for penetrating or blunt neck trauma patients who do not require immediate operative exploration.
- Four percent to 24% of patients who only exhibited soft signs of injury along with a positive CTA required intervention. A CTA study also can help determine if an endovascular repair may be more appropriate than open surgery.
- Patients presenting with hard signs or hemodynamic instability, regardless of zone, may proceed directly to the operating room. Asymptomatic patients could be observed with serial exams, while the rest of the patients with suspected injury were managed based on the location of the injury zone.
- Medical therapy with antiplatelet agents or anticoagulation to prevent thrombosis is the first-line treatment for BCVI.
The neck is a complex region that may have injuries that range from minor to life-threatening. An understanding of the anatomy and potential injuries is essential to optimize patient care and outcomes.
— Ann M. Dietrich, MD, Editor
Introduction
The neck is a small but complex region that contains many important vascular, aerodigestive, and neurological structures, which, anteriorly, do not have substantial overlying tissue providing protection from trauma. Subsequently, neck trauma management can be especially challenging to the emergency clinician since injuries to this area and its underlying structures can range from minor to life-threatening. Trauma to the neck may be less clinically apparent and missed, or in the setting of polytrauma, may be considered a lower priority. Depending on the type of neck injury, such as penetrating vs. blunt, there may be different implications with respect to diagnostic and therapeutic management. Evaluations of the neck should be prompt, systematic, and thorough as the presentation has the potential for significant morbidity and even mortality. This review will be limited to injuries to the anterior neck and will not discuss cervical spine or spinal cord trauma.
Anatomy
Understanding the normal anatomical layout of the neck and its internal structures helps guide the evaluation and management. The outermost layer of the neck is the skin, and any interruption of the skin should raise the suspicion for a penetrating neck injury. Anteriorly, the platysma is a thin muscle that lies below the subcutaneous fascia and extends from the lower mandible down to the deltoid and pectoris muscles. Violation of the platysma has been used to define the presence of a penetrating neck injury.1 Posteriorly, beneath the skin of the neck are the bones of the spine and thick muscles that provide some degree of protection.
The neck was arbitrarily divided into three zones by Monson et al in 1969.2 This was further refined by Roon and Christenson in 1979.3 Traditionally, the evaluation of penetrating neck injuries was based on the zone of the location of the injury. The most caudal zone is zone I, and zone III is the most cephalad. (See Figure 1.) Zone I extends from the clavicles and sternum to the level of the cricoid cartilage. Major vessels within zone I include the proximal portion of the carotid arteries, the jugular-subclavian junctions, and the left innominate vein. Zone II is the largest zone and the most commonly injured. It extends from the superior aspect of the cricoid cartilage to the level of the angle of the mandible. Major vessels within zone II include the internal and external jugular veins, the common carotid arteries and their bifurcation into the internal and external carotid arteries, and the vertebral arteries. The trachea and esophagus are aerodigestive structures that extend through both zones I and II. Lastly, zone III extends from the angle of the mandible to the base of the skull. The vessels within zone III include the carotid and vertebral arteries and the internal jugular veins. Zone III also contains cranial nerve IX (glossopharyngeal), cranial nerve X (vagus), cranial nerve XI (accessory), and cranial nerve XII (hypoglossal).4 The contents located in each zone are summarized in Table 1.
Figure 1. Zones of the Neck |
 |
Source: Getty Images |
Table 1. Summary of Anatomic Neck Zones and Structural Contents |
|||||
Zone |
Zone Boundaries |
Vascular Structures |
Aerodigestive Structures |
Neurologic Structures |
Miscellaneous Structures |
Zone I (caudal) |
|
|
|
|
|
Zone II (middle) |
|
|
|
|
|
Zone III (cephalad) |
|
|
|
|
|
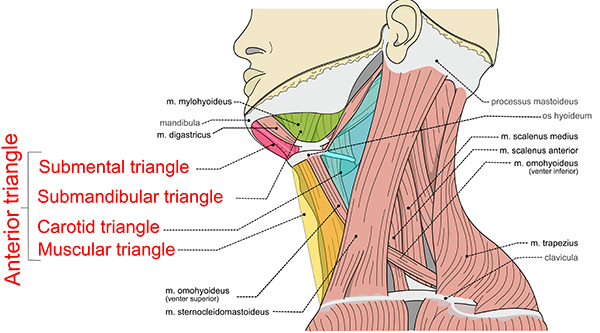
The anatomy of the neck also can be separated into anterior and posterior triangles, each of which is further subdivided into additional triangles. (See Figure 2.) The anterior triangle is bounded superiorly by the mandible, medially by the midline of neck, and laterally by the sternocleidomastoid muscle. Within the anterior triangle lie more of the neck’s vital structures. It is where the common carotid artery diverges, and it also contains numerous cranial nerves, including the facial, glossopharyngeal, vagus, accessory, and hypoglossal nerves. Additionally, it houses the trachea, larynx, pharynx, and esophagus. The posterior neck is surrounded by the sternocleidomastoid muscle anteriorly, trapezius posteriorly, and the clavicle inferiorly. Within the posterior neck lie the subclavian artery and external jugular vein, which empties into the subclavian vein along with the transverse cervical vein. It also contains the thoracic duct, spinal column, accessory nerve, phrenic nerve, and brachial plexus.5
Figure 2. Triangles of the Neck |
 |
Source: Olek Remesz. https://commons.wikimedia.org/... |
Epidemiology and Etiology
In the final reports from the National Trauma Data Bank in 2016, neck trauma accounted for approximately 2.3% of adult and 2.2% of pediatric trauma cases. For patients with neck trauma and an Abbreviated Injury Score (AIS) ≥ 3, the case fatality rate was 17.4% for adults and 14.5% for pediatric patients, which was the highest of all body regions for adults and second highest for pediatrics.6,7 There is seasonal variation, with neck injuries occurring more frequently in the spring and summer months.8 Injuries to the neck can be secondary to penetrating trauma or blunt trauma, including strangulation and near-hanging. Patients with penetrating trauma are more likely to be younger and male as compared to those with blunt neck injury.9
Penetrating Neck Trauma
Penetrating neck trauma is defined as any neck injury that causes violation of the platysma muscle. Penetrating neck trauma represents up to 10% of all adult traumas, with mortality rates reaching 10% to 15%.1,10 Common causes of penetrating neck trauma include stab wounds, gunshot wounds (GSWs), self-injury, and motor vehicle collisions. Stabbings often cause less damage because of the lower velocity mechanism when compared to GSWs, which typically are higher energy.11 GSWs can be classified further as low- or high-energy injuries (e.g., handguns and shotguns vs. military grade weaponry) and have features of both penetrating and blunt trauma. As the bullet passes through tissue, it creates a permanent cavity. Additionally, the kinetic injury of the bullet creates a shock wave known as the secondary cavity that results in stretching and snapping back of surrounding tissue, similar to blunt force injury. Injury occurs when the forces exerted exceed the elasticity of the tissue.12
One of the major concerns with penetrating neck trauma is the possibility of vascular injury. More than half of the deaths in patients with penetrating neck trauma are the result of severe hemorrhage from vascular injury.13 Vascular injury may result in extravasation of blood, dissection, partial or complete occlusion, development of pseudoaneurysm, and arteriovenous fistula formation. Arterial injury occurs in approximately 25% of penetrating neck trauma. In cases of arterial injury, the carotid artery is involved in almost 80% of cases and the vertebral artery in approximately 43% of cases. Aerodigestive injury is the second most common injury in penetrating neck trauma, occurring in 23% to 30% of patients.14 The trachea, followed by the larynx, is the most commonly injured part of the airway.15
Blunt Neck Trauma
While less common than penetrating neck trauma, it is estimated that blunt neck trauma constitutes 5% of all neck injuries. Injury mechanisms can include motor vehicle collisions (most common), strangulation, near-hanging, or direct blows.16 Despite these injuries having the potential for significant morbidity and mortality, they often may have an asymptomatic clinical presentation. Any blunt trauma to the neck can result in severe injury. Direct or shearing forces to vessels of the neck can lead to blunt cerebrovascular injury (BCVI). Historically, BCVI was thought to occur rarely, with an incidence of approximately 0.1% in hospitalized trauma patients.17 Improvements in radiological techniques and widespread screening protocols for trauma patients have led to increased detection, with reported rates of BCVI of 0.9% to 2.7% in blunt trauma.18,19 In contrast to penetrating neck trauma where the common carotid artery is involved most commonly, blunt neck trauma most commonly involves the carotid bifurcation or the internal carotid artery.20 Failure to diagnose and appropriately treat BCVI can lead to 80% morbidity and 40% mortality.21 Strokes are a major complication concern when it comes to blunt neck trauma and BCVI. In a single-center study of 147 patients with BCVI, the overall incidence of stroke was 12.2%. In patients not treated with anticoagulation or antiplatelet agents, the incidence of stroke was 25.8%, whereas for patients who were treated, the incidence was 3.9%.22
Clinical Features
Patients presenting with neck trauma, whether isolated or associated with multiple traumatic injuries, should be evaluated with a standardized ABCDE (airway, breathing, circulation, disability, and exposure/environment) assessment.
Hard and Soft Signs of Neck Trauma
Once the primary survey has been completed, the secondary survey should include a thorough evaluation of the neck with special attention focused on detection of hard and soft signs of injury. The physical examination must be thorough, including inspection, palpation, and auscultation since many patients with neck injuries will be asymptomatic initially. Having the patient breathe, speak, and swallow will aid in the evaluation for these signs. Deeper structural injuries should be suspected if platysma violation is identified. Subcutaneous emphysema, tracheal tenderness or deviation, and difficulty speaking or swallowing may be evidence of aerodigestive tract injury. The carotid arteries should be auscultated to assess for the presence of bruits, although this can be challenging in a busy trauma bay. A concerning sign for vascular injury is the presence of an expanding hematoma. The posterior neck also should be evaluated. Tenderness over the cervical spine should increase suspicion for a bony injury. A neurologic examination should be performed to assess for central or peripheral nervous system injuries. The presence of a hard sign generally warrants proceeding to the operating room (OR) for exploration, although recently there has been some controversy about this approach. The hard and soft signs are summarized in Table 2.
Table 2. Hard and Soft Signs of Neck Trauma |
||
Injury Type |
Hard Signs |
Soft Signs |
Vascular injury |
|
|
Aerodigestive tract injury |
|
|
* Some sources may include dysphonia or subcutaneous emphysema as a hard sign indication rather than soft sign. Adapted from: Paladino L, Baron BJ, Shan G, Sinert R. Computed tomography angiography for aerodigestive injuries in penetrating neck trauma: A systematic review. Acad Emerg Med 2021;28:1160-1172. |
||
Blunt Cerebrovascular Injury Signs
Patients who present with blunt neck trauma at risk for BCVI can have a wide spectrum of presentations from entirely asymptomatic to having devastating neurologic involvement. A retrospective analysis of the Japan Trauma Data Bank found that patients with BCVI had differences in presenting signs and symptoms based on the vessel injured. The initial Glasgow Coma Scale (GCS) score for patients with carotid injuries was 7 (interquartile range [IQR], 3-14). For those with vertebral artery injuries the presenting GCS was 14 (IQR, 9-15). The median Injury Severity Score was 26 (IQR, 17-35) for those with carotid injuries and 20 (IQR, 16-28) for those with vertebral injuries.24 Patients may develop symptoms within 10 to 72 hours after injury, and sometimes the first symptoms of a BCVI are symptoms of a stroke. Signs and symptoms of BCVI may include arterial hemorrhage from neck/nose/mouth, cervical bruit, expanding cervical hematoma, focal neurological deficit, neurologic deficit with normal head computed tomography (CT) findings, and stroke on secondary CT scan.25 The cervical seat belt sign is an abrasion or other soft tissue injury of the neck and upper torso area that results from trauma during a motor vehicle collision. Two older retrospective studies have failed to demonstrate that the cervical seat belt sign is independently associated with BCVI.26,27 However, both studies were done on patients who were evaluated prior to the implementation of protocols with more liberal indications for BCVI evaluation.
Diagnostic Considerations
Radiography
Plain film radiographs of the chest and neck can be obtained as adjuncts to the primary survey. One benefit of plain film radiographs is the ease by which they can be obtained as a portable study while the patient still is in the clinical area. Chest radiography can be helpful in the evaluation of hemothorax, pneumothorax, rib fractures, or pneumomediastinum. Patients with zone I injuries may have a higher likelihood of lung parenchyma injury because of the presence of the apices of the lung extending into that area. Neck radiography also can be considered in the setting of penetrating neck trauma, especially if secondary to a ballistic mechanism to assess for retained foreign bodies. Including a lateral neck view may help assess for subcutaneous air and identify laryngotracheal injury to guide airway management.28 With the wide accessibility of CT scan, plain films of the neck are obtained less frequently now.
Computed Tomography Angiography
Multidetector neck CT angiography (CTA) is the first-line imaging study of choice for penetrating or blunt neck trauma patients who do not require immediate operative exploration. Penetrating injury from a high-energy mechanism can produce a serious internal injury despite having what appears to be an innocuous entry wound.15 Obtaining a CTA of the neck can be helpful in determining the trajectory of a missile and ultimately guiding the decision for operative vs. nonoperative management.29 For penetrating trauma, CTA is a noninvasive diagnostic tool that has both a high sensitivity and specificity. In stable patients who had either hard or soft signs of injury, CTA has a sensitivity of 94% to 100% and a specificity of 96% to 100%.30 Compared to esophagogastroduodenoscopy (EGD), esophagram, and bronchoscopy, CTA was found also to have 100% sensitivity and 100% negative predictive value for aerodigestive injuries. However, the specificity and positive predictive value were poor.31 The importance of accurately diagnosing these injuries is demonstrated by the fact that 4% to 24% of patients who only exhibited soft signs of injury along with a positive CTA required intervention.10 A CTA study also can help determine if an endovascular repair may be more appropriate than open surgery.
As CTA technology improved, its use started to replace the use of digital subtraction angiography (DSA) as the screening modality for diagnosing BCVI. The benefit of using CTA rather than DSA is that CTA is less resource intensive, most trauma patients already are having CT imaging performed, and most hospitals have the capability to obtain these studies. In 2009, the Western Trauma Association (WTA) recommended that 16-slice CTA was the screening test of choice.32 The following year, the Eastern Association for the Surgery of Trauma (EAST) guidelines stated that angiography remained the gold standard test but with Level III evidence to support its use, 8-slice or greater CTA may be considered as a screening modality.33 Missed lesions on CTA may be due to mistiming of the contrast bolus, which can result in suboptimal visualization, and the presence of streak-like artifact from retained metallic fragments, which can obscure the vessels.20
In 2011, a study performed at Elvis Presley Memorial Trauma Center in Memphis found that 32-slice CTA had a sensitivity of only 51% and, thus, concluded that it was inadequate as a screening tool.34 In 2014, using data from the same trauma center, researchers found that 64-slice CTA had a sensitivity of 68% and that 62% of false-negative studies missed only low-risk, grade I injuries, suggesting that the technology had reached a point to be used as a screening tool.35 Because of the continued high rate of false-positive CTA studies (45%) found in subsequent data collected, that study group continued to recommend DSA for confirmation of injury to avoid unnecessary anticoagulation.36
Which patients should undergo screening for BCVI remains controversial. Routine imaging with a CTA of the neck may increase the yield for identifying a BCVI that could be missed if only BCVI screening guideline risk criteria were used.37,38 (See Table 3.) However, this could lead to an increase in false-positive studies. To date there has been only one study that assessed the test characteristics of 64-slice CTA technology for the diagnosis of BCVI.35 Further research is warranted in the use of advanced CTA technology to help guide which patients require screening.
Table 3. Expanded Denver Criteria for Blunt Cerebrovascular Injury |
|
Signs/Symptoms |
BCVI Risk Factors |
|
|
BCVI: blunt cerebrovascular injury; TIA: transient ischemic attack; CT: computed tomography; MRI: magnetic resonance imaging; GCS: Glasgow Coma Scale; MS: mental status Used with permission from: Geddes AE, Burlew CC, Wagenaar AE, et al. Expanded screening criteria for blunt cerebrovascular injury: A bigger impact than anticipated. Am J Surg 2016;212:1167-1174. |
|
Digital Subtraction Angiography
DSA is a fluoroscopy exam performed by interventional radiologists and involves acquisition of a noncontrast image followed by contrast-enhanced images. The noncontrast mask images are digitally subtracted from the contrast-enhanced images. This technique removes static nonenhancing vascular and nonvascular structures. DSA has been considered the gold standard for evaluating patients for BCVI and still has a diagnostic accuracy that is superior to CTA.39 As mentioned earlier, CTA has largely replaced DSA as the choice for screening; however, DSA frequently is used as a confirmatory study.36
Laboratory Evaluation
Laboratory testing in patients with neck trauma is similar to that for any other trauma patient. Most laboratory testing in the trauma patient is done by a protocol developed and implemented at the clinician’s institution. These studies should include a complete blood count (CBC) with differential to monitor for drops in hemoglobin or hematocrit due to ongoing blood loss. A complete metabolic panel will help monitor for electrolyte abnormalities, assess kidney function, and provide insight into the acid-base status of the patient. Laboratory testing of the coagulation profile, including prothrombin time (PT), international normalized ratio (INR), and partial thromboplastin time (PTT) with fibrinogen levels, is useful in evaluating a patient on anticoagulation and those at risk of developing disseminated intravascular coagulation. A type and screen is indicated in case the patient requires a transfusion of blood products. A pregnancy test (urine or serum human chorionic gonadotropin [hCG]) always should be performed on females of childbearing age because pregnancy status may alter management. A pregnant patient with potential for abdominal or pelvic trauma should have their Rh status determined because all Rh (-) mothers with abdominal trauma are at risk for alloimmunization resulting from fetomaternal hemorrhage (FMH). Thromboelastography (TEG) assays also can be obtained, if available, to help with precise coagulopathy management and reduce the use of blood products.40
Management
In the absence of needing immediate resuscitation, approaching a neck trauma patient should be systematic and follow Advanced Trauma Life Support (ATLS) guidelines. Providers should follow the ABCDE approach to evaluating and treating trauma patients: airway, breathing, circulation, disability, and exposure/environment.41 This should be followed regardless of whether the injury was due to blunt or penetrating mechanism. Airway and circulation should be prioritized, with a particular focus on active bleeding. There should be an emphasis on identification of shock and hemorrhage control during the initial assessment because massive hemorrhage can be more deadly than poor airway management.28 However, during the primary survey it still is important to note airway patency and the presence of any signs of aerodigestive injury, such as tracheal deviation or air bubbling from a wound, since it could affect future airway management.
In conjunction with the primary survey, other team members should obtain venous access by placing large bore intravenous catheters, place the patient on a cardiac monitor with pulse oximetry, and provide supplemental oxygen, if needed. During the secondary survey the neck should be examined for obvious signs of platysma violation and for the presence of any hard or soft signs to establish the need for immediate operative intervention. In cases of possible wound contamination, empiric antimicrobial coverage can be considered.
Hemorrhage Control and Resuscitation in Penetrating Neck Trauma
Probing a wound is discouraged because it could dislodge a clot, worsen bleeding, or extend an injury. Direct external compression typically is appropriate and successful in controlling bleeding. However, when the source of bleeding is deeper in the neck, direct compression may not be feasible or effective, and it may require tight packing of the wound with hemostatic dressings. Another alternative is to place a Foley catheter into the wound, which may tamponade the bleeding vessel once the balloon has been inflated.13,42 Foley catheter balloon tamponade has a fairly good rate of success in achieving control. A Foley catheter (18 or 20 French) can be directly inserted into the wound toward the presumptive source of bleeding, and the balloon inflated with saline or sterile water until the bleeding ceases. Additional Foley catheters may be inserted as needed for continued bleeding around the site. Once bleeding has been controlled, the wound then can be sutured closed to prevent slippage of the Foley catheter.43,44
The current paradigm of damage control resuscitation in trauma balances the restriction of fluid administration until bleeding has been controlled with the need to prevent shock state. For patients with traumatic hemorrhagic shock, early resuscitation with whole blood, if available, is preferred. An alternative to whole blood is the use of a fixed-ratio strategy of 1:1:1 packed red blood cells to plasma to platelets.45 Studies have suggested that whole blood transfusion may have mildly improved resuscitation outcomes.46 Excessive bleeding may be due to anticoagulant use and may require reversal in presentations with life-threatening bleeds or need for immediate operative intervention. Reversal strategies will differ based on the patient’s home anticoagulation (vitamin K antagonist, direct thrombin inhibitor, factor Xa inhibitor) since this will affect the choice of reversal agent. However, the use of a nonspecific reversal agent, such as prothrombin complex concentrate, may be considered instead since it is likely to be more readily available in an emergent situation. The administration of tranexamic acid (TXA), which inhibits the conversion of plasminogen to plasmin and ultimately preserves formed clots, has been shown to reduce death due to bleeding. TXA may be considered if the patient is within a three-hour window since the injury occurred.47 For further hemorrhage resuscitation, allowing for permissive hypotension with a mean arterial pressure range of 50 mmHg to 60 mmHg is ideal.48
Airway Management
Airway is the first step in the ABCDE mnemonic used in ATLS training.41 Securing the airway in a neck trauma patient can be difficult for reasons ranging from blood in the airway to oropharyngeal and tracheal injuries that distort the normal anatomy. Approximately 11% of patients with penetrating neck injuries may need immediate airway management.49 The cervical trachea is the part of the airway most commonly injured in penetrating neck traumas.15 However, both blunt and penetrating traumas can cause tracheal or laryngeal injury. It is critical for providers to continue to monitor the airway frequently since neck injuries may progress rapidly, resulting in the loss of the airway patency despite an initially unremarkable exam. For example, ongoing swelling, tracheal disruption, or a rapidly expanding hematoma could lead to displacement or airway obstruction.
Concern for airway compromise, such as with the development of inspiratory stridor, should warrant establishing a definitive airway to prevent further decompensation.50 The airway plan should include multiple suction set-ups, various sized endotracheal tubes, rescue airway devices, and back-up modalities, including a surgical airway kit. Emergency clinicians also should consider the most appropriate approach to secure the airway in the context of the patient’s injury and the involvement of the surgical team if a surgical airway is considered. For complicated airways, it is recommended that the most experienced provider perform the intubation, if possible.
Pre-oxygenation or re-oxygenation after a failed attempt with bag valve mask ventilation should be limited, since the positive pressure administered can worsen subcutaneous emphysema and distort airway anatomy.14,15 It also is recommended that supraglottic devices should be avoided in cases of laryngeal trauma since these devices do not pass distal to the site of possible injury to the airway allowing for continued air leak.15,51
If the anatomic structures of the neck appear to be preserved, rapid sequence intubation still may be the safest and most effective approach to pursue.11,14,50 Higher rates of first-pass success have been found when video laryngoscopy (VL) is used rather than direct laryngoscopy (DL).52 VL also has been shown to be superior to DL in patients identified with predicted or anatomically difficult airways.53 However, DL still may be preferred if there are VL visualization difficulties secondary to hemorrhage obscuring the camera. The use of a bougie also may increase first-pass success rates during intubation of difficult airways.54 The use of cricoid pressure is not recommended because of the risk of further worsening tracheal injury.15
In cases of distorted oropharyngeal or neck anatomy, awake fiberoptic intubation is another technique that may avoid worsening the area of injury. However, this technique can be limited due to blood in the airway, available equipment, operator experience, and the patient’s ability to cooperate or tolerate the procedure.14,55 In cases of neck trauma with distorted anatomy, a second clinician, if available, should be at the bedside prepared to convert to a surgical airway in case of a failed airway.
Emergent tracheotomy or cricothyrotomy may be performed if indicated in severe cases or if prior attempts to obtain a definitive airway have been unsuccessful. A cricothyrotomy may be preferable in emergent situations since it takes less time to perform compared to a tracheotomy. Although penetrating trauma resulting in tracheal dissection is rare, it should prompt immediate airway management and further surgical airway with tracheotomy below the level of injury.15 Tracheotomy should be followed by operative repair. In patients with known laryngeal fractures or tracheal trauma with an unstable airway, an awake tracheotomy would be ideal if the situation allows. It is possible that patients with a visible airway dissection can be intubated directly through the larynx or trachea in these scenarios. With this, however, there may be increased risk of further damaging the larynx by creating a false passage or causing complete airway obstruction, which is why direct visualization typically is preferred.15,56
Use of a Cervical Collar
Preventing cervical spine injury by placing a cervical collar is a common practice in the prehospital setting. However, for penetrating neck injuries, there is a question of its benefit. There is a very low incidence of an unstable cervical spinal injury in penetrating neck injuries, including GSWs.13,57 Subsequently, unless there is a high suspicion for spinal cord injury or there is a neurologic deficit present, a cervical collar is not recommended.13,14 A cervical collar may affect visualization of a penetrating wound or hinder control of bleeding with subsequent increased risk of death and an increase in the risk of elevated intracranial pressure in patients with head injury.58,59
Zones vs. No-Zones Approach
The most appropriate management of penetrating neck trauma remains an ongoing debate. Whether a zones-based or no-zones-based approach is used likely will continue to vary from institution to institution until further research has been performed. The most recent guidelines by the WTA in 2013 use anatomic zones to help guide management for operative exploration in patients with penetrating injuries.60 The use of the zones-based approach assumed that the location of the external wound closely correlated with the location of internal injuries. Patients presenting with hard signs or hemodynamic instability, regardless of zone, may proceed directly to the operating room. Asymptomatic patients could be observed with serial exams, while the rest of the patients with suspected injury were managed based on the location of the injury zone. Patients with zone I and zone III injuries often underwent additional workup with endoscopy or angiography due to more difficult operative management in these zones. Vertebral artery injuries, for example, can be more difficult to gain surgical control of bleeding and may benefit from endovascular repair instead. Traditional teaching led to mandatory surgical exploration for zone II injuries, even if clinically stable, because of the easier accessibility to this area of the neck.14 However, mandatory zone II exploration led to high negative exploration rates in up to 56% in patients with stable injuries.11,30 Additionally, exploration in these stable patients resulted in longer hospitalization and increased risk of post-operative complications.11 Mandatory zone II exploration also leads to higher expenses and resource use.
While the anatomic zones of the neck have guided the approach to penetrating neck trauma for decades, management has continued to evolve and become less rigid. There has been growing controversy regarding the zones, leading to the more selective no-zone approach being favored instead, given the negative exploration rates and accuracy of CTA.29 The EAST guidelines for penetrating neck trauma from 2008 state that mandatory exploration of zone II injuries vs. a selective approach are equally justified and safe.33 Since the WTA and EAST guidelines were published, additional studies have demonstrated that external wounds may not necessarily correlate with internal wound injury, even with hard signs or aerodigestive injury.61,62 Wounds, especially ballistic, can travel through more than just one zone. In addition, there is growing support that demonstrates the zone of injury is not an independent predictive factor for internal injuries.10 Operating by the zone approach increases the risk of missing occult injuries secondary to the uncertainty of trajectory. Therefore, the no-zone approach is not based on the location of injury but on the neck as a single entity instead. The assessment is largely based on hemodynamic stability, physical exam, signs, and symptoms. This allows for further routine evaluation with CTA to identify or rule out injuries, such as tracheal or esophageal injuries, irrespective of zone. With this approach, only patients with a positive finding on CTA will proceed to the operating room (OR), and this has reduced missed injury and exploration rates.29,30,63 For patients with hard signs of injury or hemodynamic instability, evidence still suggests a benefit in exploring this group even with a more selective approach.10,11,14 Patients with soft signs or no symptoms at all with a negative workup that includes a negative CTA can be observed rather than explored.30
Current Approach to Blunt Neck Trauma
The EAST guidelines published in 2020 recommend screening patients at risk for BCVI using screening tools that have been developed to identify those at higher risk for injury.64 Biffl et al published a landmark study in 1999 whereby patients were selected to undergo angiography based on a set of anatomic and mechanistic variables.65 Of the 249 patients included in the study, 85 (34%) were found to have an injury. There were 209 patients included who were asymptomatic. Of these, 57 (27%) were found to have injury. Additional research has led to the development of the Expanded Denver Criteria for BCVI.66 (See Table 3.) In an external validation study, use of the expanded criteria was found to be associated with a 16% increase in the detection of BCVI.67 Since then, additional studies have shown increased detection of BCVI with the addition of more criteria, such as any suspected cervical spine and any high-energy deceleration trauma with impact on the chest, abdomen, or pelvis.16,68
While angiography is the gold standard for diagnosis of BCVI, CTA has become the first choice for screening.69 As the use of screening criteria alone has continued to miss a substantial number of BCVIs, some have advocated for a relaxed screening approach with CTA.70 A 48-hour repeat CTA typically is recommended for asymptomatic patients with initial imaging indeterminate for BCVI.71,72 Follow-up CTA imaging within seven to 10 days after in patients medically managed with a grade I to III BCVI injury typically is recommended. If the injury is completely healed at that time, antithrombotic treatment can be discontinued; if not, treatment should be continued for three to six months. Higher-grade lesions are unlikely to have healed significantly within this timeframe, and repeat imaging for these lesions may be a poor use of time and resources.20
BCVI grading classification of carotid or vertebral artery lesions helps stratify therapeutic management and risk of future stroke. The various types of vascular injury include minimal luminal irregularity or dissection (grade I); dissection or intramural hematoma, intramural thrombus, or raised intimal flap (grade II); pseudoaneurysm (grade III); occlusion (grade IV); and transection with active extravasation (grade V).73 High-risk BCVIs should prompt vascular surgery consultation if available.
It was previously thought that BCVI treatment should include endovascular stenting; however, this is no longer routinely recommended for all patients because of increased costs, potential risks of stent thrombosis, and no additional reduction in stroke risk.62,74 Instead, medical therapy with antiplatelet agents or anticoagulation to prevent thrombosis is the first-line treatment for BCVI. The EAST guidelines recommend starting antithrombotic treatment for all patients diagnosed with BCVI to reduce the risk of both stroke and mortality.64 Heparin may be preferred in the acute setting because of its reversibility; however, studies have not shown evidence of benefit of one treatment strategy over any other.75 Heparin is started at 15 units/kg/hour titrated to a PTT of 40 to 50 seconds and a loading dose is not needed. If antiplatelet therapy is preferred, aspirin 325 mg daily or clopidogrel 75 mg daily typically is started. Treatment should be initiated as soon as possible but with risks weighed in the context of any contraindications, such as the patient’s other injuries. For patients with a high risk of bleeding, antiplatelet agents were considered an acceptable alternative to anticoagulation.76 Antithrombotic treatment in patients with BCVI and concomitant traumatic brain injury (TBI) has been shown to be safe and effective.77
Surgically accessible grade II-IV injuries may undergo operative repair or be treated with antithrombotic therapy. Grade V injuries require definitive treatment and should undergo either endovascular or operative repair.
Consults
Except for very apparent superficial injuries, immediate trauma or surgical team notification should be obtained for all cases of penetrating neck trauma to potentially assist with a surgical airway or need for emergent operative intervention. Based on institutional protocols, services such as anesthesia or otolaryngology may be consulted for other airway interventions. Patients with suspected or known vascular injuries also would benefit from early vascular surgery consultation to provide control with definitive management, such as embolization, ligation, or revascularization.
Disposition
Patients who exhibit hard signs or are hemodynamically unstable should be taken to the OR for definitive treatment. Ideally, these patients, as well as patients with soft signs, should be admitted or transferred to a tertiary trauma center given the significant morbidity and mortality associated with neck trauma. Patients at risk for significant injury also should be transferred. Transferring patients from a nontertiary (Level III or IV) trauma center to even a Level II trauma center can have some survival benefit since these centers still have resources that can optimize patient care.78 Patients who may be asymptomatic can be admitted for observation, serial examinations, and repeat imaging if indicated.
Summary
While neck trauma is not particularly common, it poses a significant risk of morbidity and mortality given the vital structures contained in the neck and the wide range of presentations. The increased potential for morbidity and mortality necessitates a systematic approach to evaluation and management to optimize patient outcomes. The emergency clinician should understand the potential structural injuries and be able to anticipate the need for airway management, hemorrhage control, diagnostic intervention, and multidisciplinary care.
References
- Chandrananth ML, Zhang A, Voutier CR, et al. ‘No zone’ approach to the management of stable penetrating neck injuries: A systematic review. ANZ J Surg 2021;91:1083-1090.
- Monson DO, Saletta JD, Freeark RJ. Carotid vertebral trauma. J Trauma 1969;9:987-999.
- Roon AJ, Christensen N. Evaluation and treatment of penetrating cervical injuries. J Trauma 1979;19:391-397.
- Anand T, Tang A, Joseph B. Penetrating neck trauma: A review. Curr Trauma Rep 2019;5:12-18.
- Xu H, Jazayeri L, Matros E, Henderson PW. Anatomy, exposure, and preparation of recipient vessels in microsurgical head and neck reconstruction. J Reconstr Microsurg 2021;37:97-110.
- Chang MC, ed. National Trauma Data Bank 2016 Annual Report. American College of Surgeons Committee on Trauma Leadership; 2016. https://www.facs.org/media/ez1hpdcu/ntdb-annual-report-2016.pdf
- Chang MC, ed. National Trauma Data Bank 2016 Annual Pediatric Report. American College of Surgeons Committee on Trauma Leadership; 2016. https://www.facs.org/media/d3ufvsmy/ntdb-pediatric-annual-report-2016.pdf
- Sethi RKV, Kozin ED, Fagenholz PJ, et al. Epidemiological survey of head and neck injuries and trauma in the United States. Otolaryngol Head Neck Surg 2014;151:776-784.
- Dodds N, Hollis S, Islam M, Thompson J. Anatomical injury patterns, demographics and outcomes data for blunt and penetrating neck injuries on the Trauma Audit and Research Network database. Bull R Coll Surg Engl 2023;105:70-75.
- Ko JW, Gong SC, Kim MJ, et al. The efficacy of the “no zone” approach for the assessment of traumatic neck injury: A case-control study. Ann Surg Treat Res 2020;99:352-361.
- Hamilton JM, Chan TG, Moore CE. Penetrating head and neck trauma: A narrative review of evidence-based evaluation and treatment protocols. Otolaryngol Clin North Am 2023;56:1013-1025.
- Ditkofsky N, Nair JR, Frank Y, et al. Understanding ballistic injuries. Radiol Clin North Am 2023;61:119-128.
- Jenkins LN, Rezende-Neto JB. Current management of penetrating traumatic cervical vascular injuries. Curr Surg Rep 2020;8:15.
- Nowicki J, Stew B, Ooi E. Penetrating neck injuries: A guide to evaluation and management. Ann R Coll Surg Engl 2018;100:6-11.
- Duggan LV, Doyle LN, Zunder JS, Hanna M. Blunt and penetrating airway trauma. Emerg Med Clin N Am 2023;41:e1-e15.
- Farhat-Sabet A, Lauerman M, Chavez A, et al. Blunt cerebrovascular injury screening criteria should include motor vehicle crash characteristics. Am Surg 2021;87:390-395.
- Davis JW, Holbrook TL, Hoyt DB, et al. Blunt carotid artery dissection: Incidence, associated injuries, screening, and treatment. J Trauma 1990;30:1514-1517.
- Mutze S, Rademacher G, Matthes G, et al. Blunt cerebrovascular injury in patients with blunt multiple trauma: Diagnostic accuracy of duplex Doppler US and early CT angiography. Radiology 2005;237:884-892.
- Tso MK, Lee MM, Ball CG, et al. Clinical utility of a screening protocol for blunt cerebrovascular injury using computed tomography angiography. J Neurosurg 2017;126:1033-1041.
- George E, Khandelwal A, Potter C, et al. Blunt traumatic vascular injuries of the head and neck in the ED. Emerg Radiol 2019;26:75-85.
- Alfanek Z, Herzog A, Taylor N, et al. Evaluating the routine use of head computed tomography angiography in blunt cerebrovascular trauma. J Surg Res 2022;269:129-133.
- Stein DM, Boswell S, Sliker CW, et al. Blunt cerebrovascular injuries: Does treatment always matter? J Trauma 2009;66:132-144.
- Paladino L, Baron BJ, Shan G, Sinert R. Computed tomography angiography for aerodigestive injuries in penetrating neck trauma: A systematic review. Acad Emerg Med 2021;28:1160-1172.
- Shibata J, Okada Y, Osawa I, et al. Trauma mechanisms and patterns of blunt cervical vascular injury: A descriptive study using a nationwide trauma registry. Am J Emerg Med 2023;71:117-122.
- Burlew CC, Biffl WL, Moore EE, et al. Blunt cerebrovascular injuries: Redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg 2012;72:330-337.
- Rozycki GS, Tremblay L, Feliciano DV, et al. A prospective study for the detection of vascular injury in adult and pediatric patients with cervicothoracic seat belt signs. J Trauma 2002;52:618-624.
- DiPerna CA, Rowe VL, Terramani TT, et al. Clinical importance of the “seat belt sign” in blunt trauma to the neck. Am Surg 2002;68:441-445.
- Burgess CA, Dale OT, Almeyda R, Corbridge RJ. An evidence based review of the assessment and management of penetrating neck trauma. Clin Otolaryngol 2012;37:44-52.
- Shiroff AM, Gale SC, Martin ND, et al. Penetrating neck trauma: A review of management strategies and discussion of the ‘no zone’ approach. Am Surg 2013;79:23-29.
- Ibraheem K, Wong S, Smith A, et al. Computed tomography angiography in the “no-zone” approach era for penetrating neck trauma: A systematic review. J Trauma Acute Care Surg 2020;89:1233-1238.
- Adra A, Brigode W, Bokhari F. An evaluation of diagnostic tests for aerodigestive injuries in penetrating neck trauma. Am Surg 2023;89:6353-6355.
- Biffl WL, Cothren CC, Moore EE, et al. Western Trauma Association critical decisions in trauma: Screening for and treatment of blunt cerebrovascular injuries. J Trauma 2009;67:1150-1153.
- Bromberg WJ, Collier BC, Diebel LN, et al. Blunt cerebrovascular injury practice management guidelines: The Eastern Association for the Surgery of Trauma. J Trauma 2010;68:471-477.
- DiCocco JM, Emmett KP, Fabian TC, et al. Blunt cerebrovascular injury screening with 32-channel multidetector computed tomography: More slices still don’t cut it. Ann Surg 2011;253:444-450.
- Paulus EM, Fabian TC, Savage SA, et al. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: More slices finally cut it. J Trauma Acute Care Surg 2014;76:279-285.
- Shahan CP, Magnotti LJ, Stickley SM, et al. A safe and effective management strategy for blunt cerebrovascular injury: Avoiding unnecessary anticoagulation and eliminating stroke. J Trauma Acute Care Surg 2016;80:915-922.
- Bruns BR, Tesoriero R, Kufera J, et al. Blunt cerebrovascular injury screening guidelines: What are we willing to miss? J Trauma Acute Care Surg 2014;76:691-695.
- Harper PR, Jacobson LE, Sheff Z, et al. Routine CTA screening identifies blunt cerebrovascular injuries missed by clinical risk factors. Trauma Surg Acute Care Open 2022;7:e000924.
- Kik CC, Slooff WM, Moayeri N, et al. Diagnostic accuracy of computed tomography angiography (CTA) for diagnosing blunt cerebrovascular injury in trauma patients: A systematic review and meta-analysis. Eur Radiol 2022;32:2727-2738.
- Meizoso JP, Barrett CD, Moore EE, Moore HB. Advances in the management of coagulopathy in trauma: The role of viscoelastic hemostatic assays across all phases of trauma care. Semin Thromb Hemost 2022;48:796-807.
- American College of Surgeons, Committee on Trauma. Advanced Trauma Life Support Student Course Manual. 10th ed. American College of Surgeons; 2018.
- Boulton AJ, Lewis CT, Naumann DN, Midwinter MJ. Prehospital haemostatic dressings for trauma: A systematic review. Emerg Med J 2018;35:449.
- Jose A, Arya S, Nagori SA, Thukral H. Management of life-threatening hemorrhage from maxillofacial firearm injuries using Foley catheter balloon tamponade. Craniomaxillofac Trauma Reconstr 2019;12:301-304.
- Kong V, Ko J, Cheung C, et al. Foley catheter balloon tamponade for actively bleeding wounds following penetrating neck injury is an effective technique for controlling non-compressible junctional external haemorrhage. World J Surg 2022;46:1067-1075.
- Woolley T, Thompson P, Kirkman E, et al. Trauma Hemostasis and Oxygenation Research Network position paper on the role of hypotensive resuscitation as part of remote damage control resuscitation. J Trauma Acute Care Surg 2018;84:S3-S13.
- McCoy CC, Brenner M, Duchesne J, et al. Back to the future: Whole blood resuscitation of the severely injured trauma patient. Shock 2021;56:9-15.
- CRASH-2 trial collaborators; Shakur H, Roberts I, Bautista R, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010;376:23-32.
- Tran A, Yates J, Lau A, et al. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: A systematic review and meta-analysis of randomized controlled trials. J Trauma Acute Care Surg 2018;84:802-808.
- Mandavia DP, Qualls S, Rokos I. Emergency airway management in penetrating neck injury. Ann Emerg Med 2000;35:221-225.
- Jain U, McCunn M, Smith CE, Pittet JF. Management of the traumatized airway. Anesthesiology 2016;124:199-206.
- Oliveira J, Maia N, Gonçalves J, Almeida V. Airway management for penetrating neck trauma: A case report. Cureus 2023;15:e33441.
- Michailidou M, O’Keeffe T, Mosier JM, et al. A comparison of video laryngoscopy to direct laryngoscopy for the emergency intubation of trauma patients. World J Surg 2015;39:782-788.
- Ruderman BT, Mali M, Kaji AH, et al. Direct vs video laryngoscopy for difficult airway patients in the emergency department: A National Emergency Airway Registry Study. West J Emerg Med 2022;23:706-715.
- Driver BE, Prekker ME, Klein LR, et al. Effect of use of a bougie vs endotracheal tube and stylet on first-attempt intubation success among patients with difficult airways undergoing emergency intubation: A randomized clinical trial. JAMA 2018;319:2179.
- Evans C, Chaplin T, Zelt D. Management of major vascular injuries neck, extremities, and other things that bleed. Emerg Med Clin North Am 2018;36:181-202.
- Lee WT, Eliashar R, Eliachar I. Acute external laryngotracheal trauma: Diagnosis and management. Ear Nose Throat J 2006;85:179-184.
- Lustenberger T, Talving P, Lam L, et al. Unstable cervical spine fracture after penetrating neck injury: A rare entity in an analysis of 1,069 patients. J Trauma 2011;70:870-872.
- Vanderlan WB, Tew BE, McSwain NE Jr. Increased risk of death with cervical spine immobilisation in penetrating cervical trauma. Injury 2009;40:880-883.
- Núñez-Patiño RA, Rubiano AM, Godoy DA. Impact of cervical collars on intracranial pressure values in traumatic brain injury: A systematic review and meta-analysis of prospective studies. Neurocrit Care 2020;32:469-477.
- Sperry JL, Moore EE, Coimbra R, et al. Western Trauma Association critical decisions in trauma: Penentrating neck trauma. J Trauma Acute Care Surg 2013;75:936-940.
- Low GMI, Inaba K, Chouliaras K, et al. The use of the anatomic ‘zones’ of the neck in the assessment of penetrating neck injury. Am Surg 2014;80:970-974.
- Madsen AS, Nair V, Loots E, Kong V. Penetrating pharyngoesophageal injuries. Curr Trauma Rep 2019;5:26-34.
- Prichayudh S, Choadrachata-anun J, Sriussadaporn S, et al. Selective management of penetrating neck injuries using “no zone” approach. Injury 2015;46:1720-1725.
- Kim DY, Biffl W, Bokhari F, et al. Evaluation and management of blunt cerebrovascular injury: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2020;88:875-887.
- Biffl WL, Moore EE, Offner PJ, et al. Optimizing screening for blunt cerebrovascular injuries. Am J Surg 1999;178:517-521.
- Geddes AE, Burlew CC, Wagenaar AE, et al. Expanded screening criteria for blunt cerebrovascular injury: A bigger impact than anticipated. Am J Surg 2016;212:1167-1174.
- Grigorian A, Kabutey NK, Schubl S, et al. Blunt cerebrovascular injury incidence, stroke-rate, and mortality with the expanded Denver criteria. Surgery 2018;164:494-499.
- Bensch FV, Varjonen EA, Pyhältö TT, Koskinen SK. Augmenting Denver criteria yields increased BCVI detection, with screening showing markedly increased risk for subsequent ischemic stroke. Emerg Radiol 2019;26:365-372.
- Filiberto DM, Kerwin AJ. Blunt cerebrovascular injury. Curr Surg Rep 2023;11:81-85.
- Schmidt JC, Huang DD, Fleming AM, et al. Missed blunt cerebrovascular injuries using current screening criteria — The time for liberalized screening is now. Injury 2023;54:1342-1348.
- Crawford JD, Allan KM, Patel KU, et al. The natural history of indeterminate blunt verebrovascular injury. JAMA Surg 2015;150:841-847.
- Vogt K, Kaminsky M, Joos E, Ball CG; Evidence Based Reviews in Surgery (EBRS) Group. Universal screening for blunt cerebrovascular injury: A critical appraisal. Evidence-based reviews in surgery. J Trauma Acute Care Surg 2021;91:e142-e145.
- Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: Implications of a new grading scale. J Trauma 1999;47:845.
- Shahan CP, Sharpe JP, Stickley SM, et al. The changing role of endovascular stenting for blunt cerebrovascular injuries. J Trauma Acute Care Surg 2018;84:308-311.
- Cothren CC, Biffl WL, Moore EE, et al. Treatment for blunt cerebrovascular injuries: Equivalence of anticoagulation and antiplatelet agents. Arch Surg 2009;144:685-690.
- Russo RM, Davidson AJ, Alam HB, et al. Blunt cerebrovascular injuries: Outcomes from the American Association for the Surgery of Trauma PROspective Observational Vascular Injury Treatment (PROOVIT) multicenter registry. J Trauma Acute Care Surg 2021;90:987-995.
- Callcut RA, Hanseman DJ, Solan PD, et al. Early treatment of blunt cerebrovascular injury with concomitant hemorrhagic neurologic injury is safe and effective. J Trauma Acute Care Surg 2012;72:338-346.
- Garwe T, Cowan LD, Neas B, et al. Survival benefit of transfer to tertiary trauma centers for major trauma patients initially presenting to nontertiary trauma centers. Acad Emerg Med 2010;17:1223-1232.
The neck is a complex region that may have injuries that range from minor to life-threatening. An understanding of the anatomy and potential injuries is essential to optimize patient care and outcomes.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
