Epistaxis: Evaluation and Management in Patients Taking Antiplatelet Drugs
March 15, 2024
AUTHOR
Shayne Gue, MD, MSMEd, FACEP, FAAEM, Director of Education, Emergency Medicine Residency Program; Program Director, Medical Education Fellowship Program, University of Central Florida/HCA Florida Healthcare GME (Greater Orlando/Osceola); Assistant Professor of Emergency Medicine, University of Central Florida College of Medicine, Orlando
PEER REVIEWER
Steven Winograd, MD, FACEP, Attending Emergency Physician, Trinity, Samaritan Hospital, Troy, New York
EXECUTIVE SUMMARY
- Epistaxis has many causes, but it often is difficult to ascribe a specific cause to the bleeding episode.
- Patients on antiplatelet therapy have an increased risk of epistaxis.
- Patients taking antiplatelet therapy often have multiple comorbidities and are more likely to have complex nosebleeds requiring hospitalization and invasive interventions.
- Basic approaches to controlling nosebleeds should be followed regardless of the complexity of the patient.
- Nasal cautery and topical tranexamic acid are more effective conservative therapies than nasal packing when first-line measures fail.
- In more severe bleeds, posterior nasal packing and/or surgical management may be needed.
- Antiplatelet therapy should not be stopped unless the specialist/consultant says otherwise.
- Patients should be educated on self-treating nosebleeds and how to prevent them.
Overview
Epistaxis (nosebleed) is rarely fatal but very common, with about 60% of the general population experiencing at least one episode within their lifetime.1,2 Up to 10% of these episodes necessitate further evaluation and management by a healthcare professional, often in the emergency department (ED), accounting for approximately one in 200 ED visits.1-5
Although the complaint of epistaxis often is perceived as less severe when compared to other ED complaints, it still may pose a challenge requiring expertise in its acute management. As pertains to this article, between 2% and 5% of patients taking antiplatelet agents will experience epistaxis requiring medical attention.
Anatomy
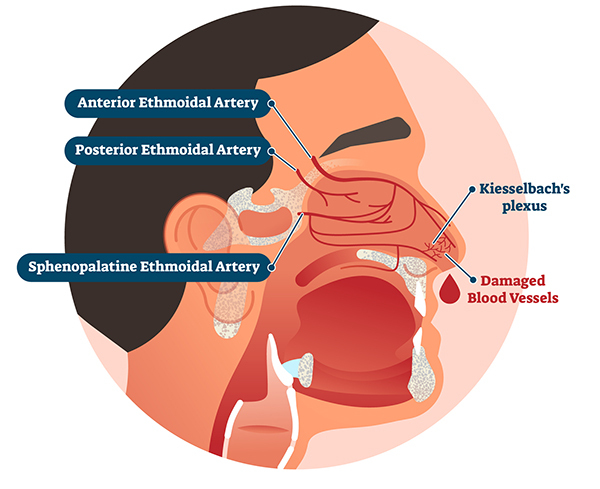
Nosebleeds typically originate from disrupted blood vessels within the nasal mucosa.2 The vascular supply to the nasal cavity arises from branches of the external and internal carotid arteries.6 The external carotid artery supplies the sphenopalatine artery, greater palatine artery, and septal branch of the superior labial artery. The internal carotid artery supplies the ophthalmic artery, from which the anterior and posterior ethmoidal arteries derive.6
An anastomosed vascular network of these five arteries forms Kiesselbach’s plexus, which lies on the anterior septum of the nasal cavity. (See Figure 1.) This plexus is responsible for the origin of 90% of epistaxis cases called “anterior bleeding.”2,4,7 Fortunately, bleeding from Kiesselbach’s plexus usually is amenable to external compression to slow or stop the bleeding.
Figure 1. Epistaxis |
 |
Source: Getty Images |
Posterior bleeding usually originates from the sphenopalatine and/or greater palatine arteries.8 Bleeding from these arteries usually is more severe and difficult to control compared to anterior bleeding because of the posterior anatomic location that is not amenable to external compression.
Etiology
Many conditions can precipitate epistaxis. They often are divided into local, systemic, and idiopathic causes, including trauma (internal or external to the nasal cavity), allergic rhinitis, infectious rhinosinusitis, bleeding disorders, and neoplasms.9,10 (See Table 1.) Low humidity, as seen with high altitudes or during the winter months, can dry out the nasal mucosa, promoting epistaxis.10,11 Despite these known associations, the majority of cases of spontaneous epistaxis are not noted to have a direct or documented cause.3 Additionally, episodes of epistaxis may be recurrent. A single episode of epistaxis increases the risk of recurrent or subsequent severe epistaxis.12
Table 1. Etiology of Epistaxis |
|
Local |
Systemic |
Trauma
|
Coagulopathies
|
Infectious/Inflammatory
|
Medications
|
Neoplasm
|
Vascular
|
Chemical/Irritants
|
Granulomatous Disorders
|
Medications
|
Other
|
Environmental
|
|
Anatomic
|
|
Adapted from: Jagmias L, Daniel M. Management of epistaxis in the emergency department. Emerg Med Rep 2006;27:240. |
|
Epistaxis is more common in males, with an increased frequency in the first two decades of life (likely due to increased exposure to trauma) and then again later in life, mostly seen in older adult patients with underlying comorbidities.2,8,10,13,14 After adjusting for comorbidities, along with antiplatelet and anticoagulation therapy, the authors of one study noted there was an association between epistaxis and cardiovascular disease, implying that epistaxis may be a result of atherosclerotic vascular disease.15 Interestingly, older adult patients of higher socioeconomic status were more likely to seek out care in the ED compared to patients of lower socioeconomic status.16
As the risk for cardiovascular disease increases with age, older adult patients are more likely to be taking antiplatelet therapy, which increases their risk of developing epistaxis.8,11,16,17 There are three main categories of antiplatelet agents: irreversible inhibitors of cyclooxygenase-1 (aspirin/triflusal), selective irreversible P2Y12 inhibitors (clopidogrel/prasugrel/ticlopidine), and reversible P2Y12 blockers (ticagrelor/cangrelor).18
Aspirin is absorbed swiftly in the gastrointestinal (GI) system, with peak plasma levels at 30 to 40 minutes after ingestion of non-enteric-coated preparations. Inhibition of platelet function is evident at one hour and lasts the lifespan of the platelet. The plasma half-life of aspirin is 15 to 20 minutes, but because of the irreversible inhibition of exposed platelets to aspirin, the pharmacologic effect on platelet function lasts until circulating platelets are replaced by bone marrow production, approximately 10% to 12% per day. Aspirin metabolites do not interfere with transfused or newly formed platelets.19
Drugs like clopidogrel or prasugrel are prodrugs, metabolized by the cytochrome P450 pathway into active metabolites that affect circulating and transfused platelets.19 These metabolites irreversibly bind to P2Y12 adenosine diphosphate receptors, reducing platelet activation and aggregation for approximately seven days, since this is the period required to create new platelets.20,21
Drugs like ticagrelor or cangrelor also have active metabolites but reversibly inhibit the P2Y12 adenosine diphosphate receptors. Therefore, recovery of platelet function is dependent on the half-life of the drug. Ticagrelor has a half-life of seven hours for the parent drug and nine hours for the active metabolite. Cangrelor has the shortest half-life of the commonly used antiplatelet agents (only three to six minutes) and, therefore, is only used intravenously in coronary interventions.
Even with these wide-ranging differences in metabolism, a recent comparison of old and relatively new antiplatelets found no difference in the risk of developing epistaxis.22 Patients taking antiplatelet agents are more likely to have frequent, recurrent, or severe episodes of epistaxis compared to patients taking anticoagulants (warfarin, rivaroxaban, or apixaban).4,17,19,23 Even regular monotherapy with aspirin has been linked to recurrent and more severe episodes of epistaxis, regardless of dosage.24-26
Patients who are post percutaneous coronary intervention usually require antiplatelet therapy to prevent severe recurrent thrombotic events. These patients are commonly started on dual antiplatelet therapy (DAPT).27 Most bleeding complications for these patients are more likely to occur within the first 30 days of starting DAPT.27,28 The incidence of epistaxis in DAPT patients is up to 17.6%, second only to GI bleeds. Overall, DAPT patients are twice as likely to develop major bleeding when compared to patients taking mono-antiplatelet therapy.4,27,29 Other patients may be placed on single-agent or combination therapy for conditions like atrial fibrillation, pulmonary embolism, and cardiac stenting. These patients are at higher risk for spontaneous epistaxis and recurrent nosebleeds overall.5,11,26,30,31
Another rare but very complicated subset of patients are those with left ventricular assist devices (LVADs). At baseline, these patients have higher rates of bleeding episodes, thought to be due to the development of acquired von Willebrand disease along with the need for concomitant anticoagulation.32 Unsurprisingly, epistaxis is a common complication in these patients, with even simple bleeding episodes often requiring numerous interventions in the ED and possibly requiring admission for specialist consultation and more invasive management.33 Management for patients requires a delicate balance of bleeding control while maintaining adequate anticoagulation for proper machine functioning.
The global coronavirus pandemic led to the emergence of another concerning population: those patients who have contracted COVID-19 and may be on antiplatelet/anticoagulation therapy for thrombotic comorbidities. One observational case series of 104 patients admitted for COVID-19 pneumonia and respiratory failure found that 30 patients developed spontaneous epistaxis.34 All 30 were receiving low-molecular-weight heparin, 10 patients were on aspirin, and three patients were receiving clopidogrel for either thromboprophylaxis or therapy. All patients with epistaxis were considered to have non-allergic atrophic (crusty) rhinitis from the use of non-humidified oxygen. In 20 patients, direct nasal compression and topical vasoconstrictor agents resolved the bleeding; the other 10 patients required the use of cautery or nasal packing.
These special populations are at an increased risk of treatment failure and may require admission, specialist consultation, and prolonged hospital stays due to antiplatelet therapy for their chronic and comorbid conditions.14,26 With this in mind, the emergency physician should be prepared with multiple approaches to the evaluation and management of such patients in the ED.
Diagnosis
As with many emergency complaints, the initial approach to patients with epistaxis should be an evaluation of hemodynamic and respiratory status: assessing mental status, peripheral perfusion (pulse rate, blood pressure, peripheral pulses, capillary refill), and ventilation (respiratory rate, pulse oximetry). Then, estimate the severity of bleeding by noting the nature of bleeding from the nares and in the posterior pharynx. For active bleeding, apply compressive pressure to the anterior nose, which can be accomplished with a nasal epistaxis clip or with direct compression by the patient’s own fingers over the lower third of the nose. When confident that the patient is stable, proceed with an examination of the nasal cavity, starting with the region of Kiesselbach’s plexus, the vestibule, the septum, and finally the turbinates to identify the source of bleeding.35
Identifying the source of bleeding is an important step in treatment.23 The severity of bleeding can be estimated using the duration of the current bleeding episode, history of previous episodes, and concerning signs and symptoms (e.g., altered mental status, dizziness, syncope).4 If the source of bleeding cannot be determined, then suspicion for a posterior bleed should be high.35
As with any patients with potentially infectious diseases, such as COVID-19, it is imperative that the treating physician remember to don appropriate personal protective equipment (PPE), including mask, goggles or face shield, gloves, and gown, to avoid being exposed to potential blood, droplets, and aerosolized infectious particles.7,36,37
Treatment
Conservative Measures
For the most part, initial treatment for patients who present with epistaxis while on antiplatelet therapy should be no different than for patients without such therapy.4 After completing the primary survey, the next step is to have the patient be seated upright in bed, ensuring that airway, breathing, and circulation remain intact with stable vitals.7,38 A stepwise approach to the management of epistaxis is displayed in Figure 2.
Figure 2. Stepwise Approach to the Management of Epistaxis in the Emergency Department |
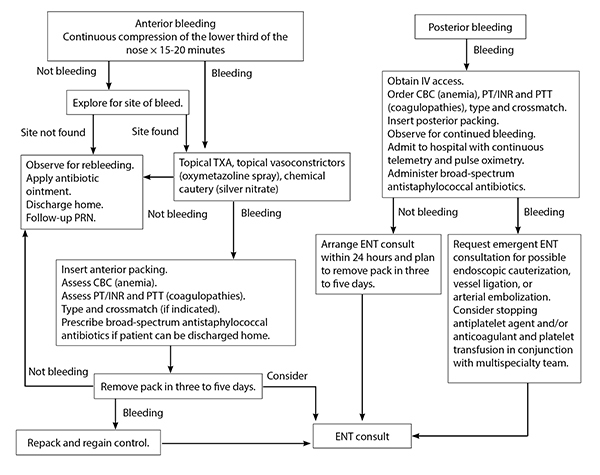 |
IV = intravenous; CBC = complete blood count; PT = prothrombin time; PTT = partial thromboplastin time; PRN = as needed; ENT = ear, nose, and throat/otolaryngologist; INR = international normalized ratio; TXA = tranexamic acid Adapted from: Jagmias L, Daniel M. Management of epistaxis in the emergency department. Emerg Med Rep 2006;27:243. |
The patient then should be instructed to compress the nose over the nasal ala for at least 15-20 continuous minutes, leaning forward with the head extended in an effort to avoid swallowing blood that may go down the nasopharynx.7,38-41 This direct compression can be accomplished using the patient’s own fingers or by using nasal clips or two tongue depressors taped together. After at least 15 minutes, the physician may examine the nasal cavity to identify the source of bleeding. A topical vasoconstrictor (such as oxymetazoline spray) can be administered in the affected nare if there is only mild oozing. However, if bleeding persists, it will be necessary to escalate to more invasive measures.7,39 Throughout the process, the patient should be re-evaluated frequently to ensure stability as well as the patient’s comfort.37 Of note, many clinicians are hesitant to use topical vasoconstrictors, particularly in patients with hypertension or coronary artery disease (CAD) because of the potential for systemic effects. Multiple studies have supported its safe and efficacious use in these populations.9,42
A helpful practice for patients with active bleeding is to have appropriate equipment and topical vasoconstrictors and analgesics at the bedside, including suction. In addition it is helpful for the patient to blow their nose to remove the larger clots that can obscure the bleeding site. (See Tables 2 and 3.)
Table 2. Suggested ENT Tray Contents |
|
Adapted from: Jagmias L, Daniel M. Management of epistaxis in the emergency department. Emerg Med Rep 2006;27:242. |
Table 3. Topical Vasoconstrictors |
|
Source: Jagmias L, Daniel M. Management of epistaxis in the emergency department. Emerg Med Rep 2006;27:242. |
Tranexamic Acid
Tranexamic acid (TXA) is an antifibrinolytic agent that enhances clot stability and is commonly used as an adjunct to reduce blood loss in hemorrhagic states.21 Two major benefits of TXA are that it can be applied topically to the bleeding site and it is economical, priced at approximately $5.70 per gram.43 Consider the use of topical TXA when initial compression therapy has failed.38,44 If effective, its use avoids the discomfort of nasal packing (both for the provider and the patient) and the required follow-up for assessment and removal of the packing.44
Topical TXA is superior to oxymetazoline spray therapy alone for both control of bleeding and the rate of treatment failure.45 When compared to phenylephrine-lidocaine anterior nasal packing for patients on antiplatelet agents, topical TXA had significantly decreased times for bleeding control and ED length of stay, along with reduced rates of epistaxis recurrence and discharge times from the ED.1,39,43,46,47 Similar results were noted when comparing TXA and anterior nasal packing with 2% lidocaine, along with an associated increase in patient satisfaction.47 No increase in adverse effects (headache, lightheadedness, nausea, vomiting, or treatment intolerance) was seen with topical TXA treatment.43,48
Although the majority of studies show benefits with topical TXA, a randomized controlled clinical trial in the United Kingdom found that topical TXA applied in the bleeding nostril on a cotton wool dental roll is no more effective than placebo in reducing the need for anterior nasal packing, hospitalization, or blood transfusion.49 Perhaps an important difference in this study from the others was that 65% of the patients were taking an oral anticoagulant medication. On the contrary, another recent study showed that TXA reduced the need for anterior nasal packing (number needed to treat of 8).50
Although it is not commonly used for epistaxis, oral TXA had no clinically significant difference in controlling bleeding or preventing recurrence of epistaxis when compared to topical TXA.51
Cautery
Treatment with either chemical (silver nitrate) or electrical (bipolar forceps) cautery is another appropriate step after conservative therapy has failed.3,52 To better visualize the nasal cavity, rhinoscopy can be used to locate the source of the bleeding and facilitate the use of cautery.4
The majority of patients taking antiplatelet therapy who had been treated with chemical and/or electrical cautery had decreased rates of failure and decreased rates of recurrence when compared to anterior nasal packing.5,11,26,52 Also, patients successfully treated with nasal cautery do not require a return visit for removal of the anterior nasal packing.26 Notably, cautery is largely dependent on and limited by the provider’s ability to directly visualize the origin of bleeding.11,51
Comparable to other conservative management options, chemical cautery with silver nitrate may provide temporary cessation of bleeding. However, it has had less success in LVAD patients who were noted more often to have recurrent epistaxis requiring further, and more invasive, interventions.33
Nasal Packing
Although nasal packing historically has been considered the next step after failed noninvasive measures, there is newer evidence that this technique should not be the “go-to” next treatment option because it is associated with increased ED re-visits and recurrent, prolonged hospital stays.40,43,52 However, if the decision is made to pursue nasal packing, it is suggested that a resorbable packing be used for patients taking antiplatelet/anticoagulants or those with inherent bleeding disorders.3,39 For patients with LVADs, though, either absorbable or nonabsorbable nasal packing (regardless of the brand used) helps control acute bleeding as well as reduces recurrent episodes, which supports the use of anterior nasal packing as first-line therapy in this special population.33
A variety of commercially available materials can be used for anterior nasal packing. Classically, anterior nasal packing was done using petrolatum ribbon gauze (0.5 × 72 inches), covered with an antibiotic ointment, gently layered like an accordion to pack the anterior nasal cavity using Bayonet forceps.53 This nonabsorbable gauze will require removal in two to three days.
Another nonabsorbable packing material is compressed polyvinyl acetate that expands up to six times in diameter as it absorbs moisture after placement in the nose.54,55 The two most common products that use polyvinyl acetate are Merocel nasal packing and Rhino Rocket nasal packing. Both products are available in different widths and lengths. The polyvinyl acetate nasal tampon in the Rhino Rocket product is inside a plastic plunger to facilitate placement.
Another popular product for anterior nasal packing is the Rapid Rhino, which is comprised of a longitudinal inflatable balloon covered with an outside coating of carboxymethylcellulose that encourages platelet aggregation.53 The device is soaked for 30 seconds in water to ease insertion. The balloon is slowly inflated with air until the pilot cuff feels firm. This inflatable feature reduces pain on insertion, and deflation prior to removal helps to maintain the freshly formed clot. Nasal tampons, either inflatable or noninflatable, typically are removed after two to four days.
One disadvantage of nonabsorbable nasal packing is that, during removal, the blood clot at the bleeding site can be disturbed, provoking recurrent bleeding. To combat this, resorbable materials such as Surgicel (a regenerated cellulose requiring water to help form a clot-like structure), Gelfoam (porcine skin gelatin), or Floseal (a gelatin-thrombin mold) can be applied directly to the bleeding site and do not necessitate removal.54,55
There is no consistent difference in bleeding control using either absorbable or nonabsorbable packing; however, there is some evidence to support combining different materials, such as Merocel and Surgicel, with improved bleeding cessation.54,55
Traditional methods of packing with these materials often lead to considerable pain, which can be further complicated by rebleeding, infection, alar necrosis, septal perforation, and toxic shock syndrome.37,54,55 Because of this risk, all nasal tampons should be coated with an anti-staphylococcal ointment prior to placement to prevent toxic shock syndrome.53
Prophylactic oral antibiotics also frequently are prescribed, but there is no evidence to support that this practice reduces the risk of infection.37,53
The only contraindications to nasal packing are in patients with (or with concern for) basilar skull fractures, major or extensive nasal bone and/or other facial fractures, or if the patient is unstable and requires airway protection with mechanical ventilation or other hemodynamic support.38 If the issue is instability, the primary objective is to secure the airway and stabilize the patient first. Then the epistaxis can be addressed (given that it is not the cause of instability).
Because anterior epistaxis is far more common than posterior bleeding, anterior packing may address the majority of nosebleed patients who present to an ED. Posterior epistaxis often is associated with severe bleeding and, therefore, requires posterior packing with hospital admission and consultation with an otolaryngologist (ENT).35 With this in mind, it is important to review the following steps for the process of posterior nasal packing to aid in the emergent treatment of these more severe cases.
If the decision is made to perform posterior packing, one can use a balloon catheter (such as a Foley) or a red rubber catheter along with a gauze pack. If using the Foley catheter method, the catheter is guided down through the nasal cavity to the oropharynx, as one would normally place a nasogastric tube. The balloon should be inflated with about 8-10 mL of water and then carefully pulled back until it rests in the posterior choana.
If using a gauze pack for control of posterior epistaxis, a red rubber catheter is advanced through the nasal cavity and into the back of the oropharynx, where it can be pulled out through the mouth by grasping with ring forceps. A gauze pack then is attached to the end of the red rubber catheter and thereafter retracted into the mouth and the choana. In each instance, the catheter should be secured outside the nostril to preserve traction, providing gentle pressure to tamponade the source of posterior bleeding. It is important to remember that posterior packing can be quite uncomfortable for patients and comes with an increased risk of dislodgement, infection, and vasovagal reflex which necessitates hospital admission with continuous telemetry monitoring.35
Surgery/Embolization
For ongoing or recurring nosebleeds, nasal endoscopy and/or surgical management may be required to visualize the source of bleeding.39 If the patient has had their nasal cavity packed but the packing becomes soaked with blood quickly, or if the patient continues to complain of blood trickling down the throat, then definitive care will be needed via urgent ENT consultation.37
The threshold for consulting an ENT specialist should be decreased for patients on antiplatelet therapy; this is because nasal packing commonly fails, with up to 37.5% of patients eventually requiring admission and subsequent surgical management.4,8,46 Sometimes, even with definitive surgical management by an ENT specialist, patients taking P2Y12 inhibitors have an increased risk of recurrent epistaxis post-embolization and a decreased success rate compared to those who are not taking antiplatelet medications.6
Should Antiplatelet Therapy Be Stopped?
As previously discussed, patients with significant thrombotic risk factors necessitate antiplatelet therapy to prevent complications. The question then becomes “Should antiplatelet therapy be temporarily suspended in the setting of acute epistaxis?” The guidelines from multiple specialty organizations recommend that patients who have undergone percutaneous coronary intervention with coronary stenting should be treated with antithrombotic therapy (aspirin or other antiplatelets) immediately and long term, and that pausing therapy for whatever reason proportionally increases the risk of worsening cardiac and thrombotic events.17,19,20,28
Even if antiplatelet therapy is paused during an acute bleed, it may be of little value, as multiple antiplatelet agents are irreversible and may take up to five to seven days for hemostasis to normalize.7,20,25 A systematic review found only one study that compared epistaxis outcomes in patients who stopped vs. those who continued antiplatelet therapy.21 There was no significant difference in incidences of re-bleeding, re-packing, need for blood transfusion, surgical interventions, or re-admission.56
Even surgical candidates undergoing transnasal endoscopy while on antiplatelet therapy had no difference in rates of nosebleeds compared to those who were not receiving antiplatelet therapy.57 With this in mind, patients whose antiplatelet/anticoagulant therapy was interrupted still experienced repeated episodes of epistaxis, alluding to the idea that another underlying issue may be at play.33
If there is a question of whether to pause or continue antiplatelet therapy in an acute bleeding episode, that decision should be made on a patient-dependent basis, focusing on the degree of bleeding (the largest indicator of morbidity and mortality) and the ramifications of discontinuing therapy.28 For example, because of the complexity of their condition requiring anticoagulation, patients with LVADs require healthcare professionals from multiple specialties to aid in the treatment of epistaxis, since some may require interruption of their anticoagulation while others may need to have it continued.33
Even in less medically complex patients, those with cases of severe bleeding while on antiplatelet therapy should have their prescribing provider or specialist notified to help guide therapy.4 In cases of uncontrolled massive bleeding (or potential antiplatelet/anticoagulation therapy overdose), working in conjunction with the patient’s primary provider or specialist to modify the anticoagulation or antiplatelet regimen may be required.7 Platelet transfusion may be of use in a minority of cases, although overall, there has been no proven benefit.19,21,25
Preventive Measures
When patients are discharged from the ED, they should be educated on ways to prevent nosebleeds, such as using humidifiers, saline nasal moisturizers, or even antiseptic nasal cream during the winter months and avoiding nose picking.4,7 Patients also should be educated and sent home with written instructions on how to self-manage epistaxis in case they experience a minor repeat bleeding episode.4
Conclusion
Epistaxis is the one of the most common ENT complaints in patients presenting to the ED. It mostly affects older adult males who may have multiple comorbidities requiring them to take antiplatelet therapy. This places patients at risk for worsening and recurrent bleeding with increased treatment failure rates.
The protocol (see Figure 2), as previously described, should begin with conservative treatment, including compression therapy and oxymetazoline spray, and move on to topical TXA or nasal cautery. If either of these management options fails, either anterior or posterior nasal packing should be pursued in an attempt to stop the bleeding, but understand that this approach has many complications and risks. If needed, the patient should be admitted for further evaluation and management by an ENT specialist.
Regardless of the patient’s condition, antiplatelet therapy should not be interrupted, especially in high-risk populations, such as patients with an LVAD. Instead, their specialist should be consulted to help guide therapy. Finally, patients discharged from the ED after their epistaxis has been controlled should be instructed on first aid treatment and prevention of recurrent nosebleeds.
Acknowledgment
The author would like to acknowledge the contributions of Dr. Elizabeth Janevski in research and writing.
REFERENCES
- Cai J, Ribkoff J, Olson S, et al. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur J Haematol 2020;104:79-87.
- Tabassom A, Dahlstrom JJ. Epistaxis. In: StatPearls [Internet]. Sept. 12, 2022. StatPearls Publishing; 2024 Jan–. PMID: 28613768.
- Tunkel DE, Anne S, Payne SC, et al. Clinical practice guideline: Nosebleed (epistaxis). Otolaryngol Head Neck Surg 2020;162(Suppl 1):S1-S38.
- Smith J, Hanson J, Chowdhury R, Bungard TJ. Community-based management of epistaxis: Who bloody knows? Can Pharm J (Ott) 2019;152:164-176.
- Gomes P, Salvador P, Lombo C, et al. Role of age and anticoagulants in recurrent idiopathic epistaxis. Acta Otorrinolaringol Esp (Engl Ed) 2020;71:160-165.
- Robinson AE, McAuliffe W, Phillips TJ, et al. Embolization for the treatment of intractable epistaxis: 12 month outcomes in a two centre case series. Br J Radiol 2017;90:20170472.
- Beck R, Sorge M, Schneider A, Dietz A. Current approaches to epistaxis treatment in primary and secondary care. Dtsch Arztebl Int 2018;115:12-22.
- Marin E, Watelet JB, Gevaert P, Van Zele T. Severe spontaneous epistaxis: Retrospective study in a tertiary ENT centre. Eur Arch Otorhinolaryngol 2019;276:1693-1699.
- Seikaly H. Epistaxis. N Engl J Med 2021;384:944-951.
- Samuel EA, Kingsly S, Kumar C, et al. A clinical study of etiopathogenesis of epistaxis. Int J Surg Sci 2021;5:6-10.
- Carey B, Sheahan P. Aetiological profile and treatment outcomes of epistaxis at a major teaching hospital: A review of 721 cases. Ir J Med Sci 2018;187:761-766.
- André N, Klopp-Dutote N, Biet-Hornstein A, et al. Cardiovascular risk and severity factors in patients admitted to hospital for spontaneous epistaxis. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135:119-122.
- Sampigethaya S, Cherian E, Pratap D, Mani I. A clinical study of epistaxis. Int J Otorhinolaryngol Head Neck Surg 2018;4:555-558.
- Kallenbach M, Dittberner A, Boeger D, et al. Hospitalization for epistaxis: A population-based healthcare research study in Thuringia, Germany. Eur Arch Otorhinolaryngol 2020;277:1659-1666.
- Côrte FC, Orfao T, Dias CC, et al. Risk factors for the occurrence of epistaxis: Prospective study. Auris Nasus Larynx 2018;45:471-475.
- Masoudian P, McDonald JT, Lasso A, Kilty SJ. Socioeconomic status and anterior epistaxis in adult population. World J Otorhinolaryngol Head Neck Surg 2017;4:263-267.
- Riemann R. [Tips & Tricks — epistaxis management in consideration of contamination]. [Article in German]. Laryngorhinootologie 2016;95:11-14.
- Florescu C, Mustafa ER, Târtea EA, et al. Antiplatelet therapy in secondary ischemic stroke prevention — a short review. Rom J Morphol Embryol 2019;60:383-387.
- Musgrave KM, Powell J. A systematic review of anti-thrombotic therapy in epistaxis. Rhinology 2016;54:292-391.
- Sawant R. Management of epistaxis in patients on anti-platelet and/or anticoagulant medication. J Med Dent Sci Res 2017;4:43-48.
- Williams A, Biffen A, Pilkington N, et al. Haematological factors in the management of adult epistaxis: Systematic review. J Laryngol Otol 2017;131:1093-1107.
- Gökdoğan O, Akyildiz I, Sayin BY, et al. The rate of epistaxis incidence in new- generation anticoagulants and perioperative approach in otorhinolaryngological practices. J Craniofac Surg 2017;28:e178-e182.
- Glikson E, Chavkin U, Madgar O, et al. Epistaxis in the setting of antithrombotic therapy: A comparison between factor Xa inhibitors, warfarin, and antiplatelet agents. Laryngoscope 2019;129:119-123.
- Stadler RR, Kindler R, Holzmann D, Soyka MB. The long-term fate of epistaxis patients with exposure to antithrombotic medication. Eur Arch Otorhinolaryngol 2016;273:2561-2567.
- Escabasse V, Bequignon E, Vérillaud B, et al. Guidelines of the French Society of Otorhinolaryngology (SFORL). Managing epistaxis under coagulation disorder due to antithrombotic therapy. Eur Ann Otorhinolaryngol Head Neck Dis 2017;134:195-199.
- Newton E, Lasso A, Petrcich W, Kilty SJ. An outcomes analysis of anterior epistaxis management in the emergency department. J Otolaryngol Head Neck Surg 2016;45:24.
- Esmonde S, Sharma D, Peace A. Antiplatelet agents in uncertain clinical scenarios — a bleeding nightmare. Cardiovasc Diagn Ther 2018;8:647-662.
- Tersalvi G, Biasco L, Cioffi GM, Pedrazzini G. Acute coronary syndrome, antiplatelet therapy, and bleeding: A clinical perspective. J Clin Med 2020;9:2064.
- Bouget J, Balusson F, Viglino D, et al. Major bleeding risk and mortality associated with antiplatelet drugs in real-world clinical practice. A prospective cohort study. PLoS One 2020;15:e0237022.
- Buchberger AMS, Baumann A, Johnson F, et al. The role of oral anticoagulants in epistaxis. Eur Arch Otorhinolaryngol 2018;275:2035-2043.
- Chaaban MR, Zhang D, Resto V, Goodwin JS. Factors influencing recurrent emergency department visits for epistaxis in the elderly. Auris Nasus Larynx 2018;45:760-764.
- Darling CE, Martindale JL, Hiestand BC, et al. An emergency medicine–focused summary of the HFSA/SAEM/ISHLT clinical consensus document on the emergency management of patients with ventricular assist devices. Acad Emerg Med 2020;27:618-629.
- Brown CS, Abi-Hachem R, Jang DW. Management of epistaxis in patients with ventricular assist device: A retrospective review. J Otolaryngol Head Neck Surg 2018;47:48.
- Dell’Era V, Dosdegani R, Valletti PA, Garzaro M. Epistaxis in hospitalized patients with COVID-19. J Int Med Res 2020;48:300060520951040.
- Womack JP, Kropa J, Jimenez Stabile M. Epistaxis: Outpatient management. Am Fam Physician 2018;98:240-245.
- Thamboo A, Lea J, Sommer DD, et al. Clinical evidence based review and recommendations of aerosol generating medical procedures in otolaryngology - head and neck surgery during the COVID-19 pandemic. J Otolaryngol Head Neck Surg 2020;49:28.
- Cui C, Yao Q, Zhang D, et al. Approaching otolaryngology patients during the COVID-19 pandemic. Otolaryngol Head Neck Surg 2020;163:121-131.
- Kravchik L, Hohman MH, Pester JM. Anterior epistaxis nasal pack. In: StatPearls [Internet]. May 26, 2023. Stat Pearls Publishing. 2024 Jan.
- Amini K, Arabzadeh A, Jahed S, Amini P. Topical tranexamic acid versus phenylephrine-lidocaine for the treatment of anterior epistaxis in patients taking aspirin or clopidogrel: A randomized clinical trial. Arch Acad Emerg Med 2020;9:e6.
- Passali D, Damiani V, Passali FM, et al. An international survey on the pragmatic management of epistaxis. Acta Biomed 2020;91:5-10.
- Gottlieb M, Long B. Managing epistaxis. Ann Emerg Med 2023;81:234-240.
- Bellew SD, Johnson KL, Nichols MD, Kummer T. Effect of intranasal vasoconstrictors on blood pressure: A randomized, double-blind, placebo-controlled trial. J Emerg Med 2018;55:455-464.
- Gottlieb M, DeMott JM, Peksa GD. Topical tranexamic acid for the treatment of acute epistaxis: A systematic review and meta-analysis. Ann Pharmacother 2019;53:652-657.
- Runyon MS. Topical tranexamic acid for epistaxis in patients on antiplatelet drugs: A new use for an old drug. Acad Emerg Med 2018;25:360-361.
- Whitworth K, Johnson J, Wisniewski S, Schrader M. Data on the hemostasis in epistaxis with topically administered TXA versus topical oxymetazoline spray. Data Brief 2020;58:211-216.
- Sanderson M, Powell J, Lang E. Topical tranexamic acid for the treatment of epistaxis in patients using antiplatelet agents. CJEM 2018;20:774-776.
- Morgenstern J, Rangarajan S, Heitz C, et al. Hot off the press: Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs. Acad Emerg Med 2018;25:1062-1064.
- Zahed R, Mousavi Jazayeri MH, Naderi A, et al. Topical tranexamic acid compared with anterior nasal packing for treatment of epistaxis in patients taking antiplatelet drugs: Randomized controlled trial. Acad Emerg Med 2018;25:261-266.
- Reuben A, Appelboam A, Stevens KN, et al. The use of tranexamic acid to reduce the need for nasal packing in epistaxis (NoPAC): Randomized controlled trial. Ann Emerg Med 2021;77:631-640.
- Hosseinialhashemi M, Jahangiri R, Faramarzi A, et al. Intranasal topical application of tranexamic acid in atraumatic anterior epistaxis: A double-blind randomized clinical trial. Ann Emerg Med 2022;80:182-188.
- Joseph J, Martinez-Devesa P, Bellorini J, Burton MJ. Tranexamic acid for patients with nasal haemorrhage (epistaxis). Cochrane Database Syst Rev 2018;12:CD004328.
- Meccariello G, Georgalas C, Montevecchi F, et al. Management of idiopathic epistaxis in adults: What’s new? Acta Otorhinolaryngol Ital 2019;39:211-219.
- Jamingas L, Daniel M. Management of epistaxis in the emergency department. Emerg Med Rep 2006;27:237-252.
- Alshehri WM, Alwehaibi WM, Ahmed MW, et al. Merocel surgicel wrap technique to manage diffuse epistaxis in patients with comorbidities. Int J Otolaryngol 2020;2020:8272914.
- Murray S, Mendez A, Hopkins A, et al. Management of persistent epistaxis using Floseal hemostatic matrix vs. traditional nasal packing: A prospective randomized control trial. J Otolaryngol Head Neck Surg 2018;47:3.
- Biggs TC, Baruah P, Mainwaring J, et al. Treatment algorithm for oral anticoagulant and antiplatelet therapy in epistaxis patients. J Laryngol Otol 2013;127:483-488.
- Kobayashi Y, Komazawa Y, Yuki M, et al. Use of anticoagulant or antiplatelet agents is not related to epistaxis in patients undergoing transnasal endoscopy. Endosc Int Open 2018;6:E104-E110.
Although the complaint of epistaxis often is perceived as less severe when compared to other emergency department complaints, it still may pose a challenge requiring expertise in its acute management.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
