
‘Doc, I Can’t See’: The Emergency Medicine Approach to Acute Atraumatic Vision Loss
September 1, 2024
AUTHORS
Dustin B. Williams, MD, FACEP, Associate Professor, Program Director, Emergency Medicine Residency, University of Texas Southwestern, Dallas
Avi Ruderman, MD, Assistant Professor, University of Texas Southwestern, Dallas
Anthony Han, MD, Chief Resident, University of Texas Southwestern, Dallas
Walter Green, MD, FACEP, Professor, University of Texas Southwestern, Dallas
Larissa I. Velez, MD, FACEP, Professor of Emergency Medicine, Vice Chair for Education, Associate Dean for GME, University of Texas Southwestern, Dallas
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity Health, Albany, NY
EXECUTIVE SUMMARY
- Acute vision loss is a frequently encountered patient presentation in the emergency department and requires an expedited, methodical evaluation by the emergency provider.
- A thorough history and detailed physical exam are crucial in the diagnosis of emergent vision-threatening emergencies.
- Examples of orbital causes of vision loss include acute angle closure glaucoma, retinal detachment, vitreous hemorrhage, corneal issues (abrasions, keratitis, and ulcers), hyphema, uveitis, endophthalmitis, and cataracts.
- Important neurovascular causes of vision loss include central retinal artery occlusion (CRAO), central retinal vein occlusion, amaurosis fugax, optic neuritis, giant cell arteritis, and idiopathic intracranial hypertension.
- Central etiologies of vision loss include pituitary apoplexy, cortical blindness, and posterior reversible encephalopathy syndrome.
- Prompt diagnosis and initiation of treatment are crucial in patients with acute angle closure glaucoma and include rapid provision of intraocular drops and intravenous medications to lower the intraocular pressure with concomitant ophthalmologic specialty consultation.
- Patients presenting with suspected amaurosis fugax or CRAO should be evaluated as stroke equivalents, and appropriate stroke workup/activations should be initiated immediately.
- Point-of-care ultrasound should be considered in the evaluation of patients with acute vision loss because it can facilitate the diagnosis of retinal and/or vitreous detachment as well as CRAO.
- Discussion with ophthalmology consultants early in the evaluation of patients with acute vision-threatening pathologies will help guide disposition planning.
Introduction
Emergency medicine clinicians encounter eye complaints on a regular basis, with data showing that about 3% of emergency department (ED) visits in the United States are related to eye complaints.1 Acute vision loss is the loss or reduction in vision from one or both eyes that can be transient, lasting from minutes to days, or permanent. It can be painful or painless, and many educational materials categorize vision loss in that way. Painless vision loss results from conditions such as central retinal artery occlusion (CRAO), central retinal vein occlusion (CRVO), ischemic optic neuropathies (IONs), and vitreous hemorrhage (VH).2 Causes of painful vision loss include acute angle closure glaucoma, iritis, and optic neuritis.3 While most of the causes of acute vision loss are intrinsic to the eye, conditions external to the eye also can result in vision loss. These include toxins (methanol poisoning is an example) and hypertension (posterior reversible encephalopathy syndrome, or PRES) as examples.4-7 This article will discuss the various emergent causes of vision loss, including necessary diagnostic testing, imaging, and needed interventions and consultations. Most importantly, emergency medicine clinicians must be sensitive to the goal of restoration and preservation of as much vision as possible.
Epidemiology and Etiologies
Sudden vision impairment can be a frightening and anxiety-provoking condition for patients. These individuals frequently present to the ED, and it is essential for them to receive rapid assessment and intervention to preserve visual acuity. Vision frequently is reported as the most valued sense, and visual acuity loss is among the top four worst things that patients report could happen to them.8,9 According to recent studies, the estimated prevalence of people in the United States living with visual acuity loss was 7.08 million in 2017, with 1.1 billion people affected worldwide.9,10 The prevalence of vision impairment and eye disease increases with age and also can vary with factors including race, ethnicity, family history, gender, socioeconomic status, and geographic location.11
The epidemiology of sudden vision loss includes a wide range of etiologies, from relatively benign causes such as migraines or transient ischemic attacks (TIAs) to more serious conditions like retinal detachment, CRAO, acute angle closure glaucoma, or stroke. These diverse etiologies arise due to the complex nature of the neuro-ophthalmic structures involved in image formation and perception. The visual system, which converts light into neural impulses processed by the brain to form images, can be compromised at any point along the neuro-ophthalmologic pathway, leading to visual impairment. Hundreds of diseases, conditions, and injuries can affect vision, with the impact varying based on the involved structures and the extent of damage or dysfunction. This article will focus solely on the non-traumatic etiologies of sudden vision loss.
Vision loss can be characterized by its onset (sudden or gradual), laterality (monocular or binocular), duration (transient or permanent), and scope (total or partial, central and/or peripheral). The pathology of vision loss can be categorized in five broad categories: orbital/ocular, neurovascular, brain, functional, and toxic/metabolic. Table 1 includes a sampling of some of the more common non-traumatic conditions that result in acute vision loss.
Ocular etiologies of sudden vision loss include acute angle closure glaucoma, retinal detachment and tears, vitreous hemorrhage, hyphema, corneal dysfunction, endophthalmitis, and lens dislocation or opacification. Neurovascular etiologies include retinal vessel occlusion (CRAO and CRVO), optic neuritis, anterior ischemic optic neuropathy (arteritic and non-arteritic), and idiopathic intracranial hypertension (previously known as pseudotumor cerebri). Intrinsic brain etiologies include pituitary apoplexy, PRES, and stroke. Functional vision loss, also referred to as non-physiologic vision loss, is always a diagnosis of exclusion and reserved for specialists after ruling out vision-threatening emergent conditions. Understanding the diverse etiologies and their associated risk factors is crucial for accurate diagnosis and prompt management in the ED.
Clinical Presentation/History
The emergency physician must begin the assessment of sudden visual loss with a focused, organized, and detailed history and physical examination to properly select the appropriate laboratory testing and imaging. A differential diagnosis cannot be accurate and inclusive without gathering the proper information at the onset of the patient evaluation.
Any providers at triage must be aware of emergent conditions that require emergent and expedited care. Orbital trauma with visual loss, eye exposure to any potentially caustic substance, or transient visual loss that can be classified as a TIA, also known as amaurosis fugax, will require immediate intervention and care managed under the ED’s trauma protocols; toxicology treatment with immediate eye irrigation; or initiation of the ischemic stroke intervention pathway, respectively.
The history should begin by determining if the vision loss is acute and has occurred in the last 24 hours to a few days, or if the vision loss is long-standing and only recently discovered. Confirm that there is no history of trauma or projectile, exposure to caustic substances, or that there was a transient visual loss which could represent a TIA. The use of corrective lenses, reading glasses, or contact lenses is important to ascertain early in the evaluation. Determine if the symptoms are unilateral or bilateral, whether the process is painless or painful, and if the patient notes pain on eye movement. The patient should be specifically questioned about any foreign body sensation. Pain with a sharp sensation indicates the anterior structures are more likely the etiology, while dull pain more typically originates from increased intraocular pressure, vitreous inflammation, or with extra-orbital disease. (See Table 2.)
Table 2. Important Historical Elements in Vision Loss |
Triage History
History: Important Elements
|
Other important features to ascertain include if the patient has had any eye discharge (noting the color and consistency), matting of the lids, excess tearing, itching, and/or redness. Similarly, crucial history taking will include the description of any floaters (myodesopsia), flashing lights (photopsia), blind spots (scotoma), or any visual field deficit. In addition, systemic symptoms of nausea, vomiting, fever, night sweats, headache, fatigue, weight loss, or tinnitus may indicate other disease processes that can affect the optic nerve.
Past medical history should include questions concerning past eye examinations, the use of ophthalmologic medications, and any ocular surgery that was performed or recommended. The presence of diabetes, hypertension, arteritis, and pituitary adenomas and other intracranial masses should be reviewed with the patient (and, when feasible, searched for in the electronic health record), since each can help to accurately direct a differential diagnosis.
The physical examination should be methodical and thorough. Specific findings will be discussed later in this article. Every eye examination should begin with a visual acuity test using either an eye chart at 20 feet or a hand-held eye card, documenting the visual acuity in each eye and then with both eyes. (See Table 3.) Corrective lenses should be worn if available. If the patient is older than 40 years of age or is missing their corrective lenses, use a pinhole to test their corrected acuity if using a near card. If the patient fails using either of these methods, finger counting, hand motion, or simple light perception should be documented. Next, visual inspection of the orbit and palpation of the periorbital structures should be documented, including the appearance of the lids, signs of trauma, edema, lid lag, ptosis, or any facial droops. The temporal artery should be palpated for tenderness and lack of pulsations to screen for temporal arteritis. The conjunctivae should be examined for the presence of conjunctival or limbal injection, foreign bodies, and cobblestoning. One should look for focal tenderness, and the position of the globe, noting any proptosis or sunken appearance (enophthalmos).
Table 3. Comprehensive ED Eye Examination and Ancillary Testing |
||
Test |
Purpose |
Description |
Visual Acuity |
Assess clarity of vision at different distances |
|
Visual Field Testing |
Assess peripheral vision and detect visual field deficits (using confrontation) |
|
Pupil Exam |
Evaluate pupil size, shape, and reactivity to light |
|
Slit Lamp Exam |
Examine the anterior segment of the eye |
|
Fundoscopy |
Examine the interior surface of the eye |
|
Tonometry |
Measure intraocular pressure |
|
ED: emergency department; RAPD: relative afferent pupillary defect; CRAO: central retinal artery occlusion; CRVO: central retinal vein occlusion; RD: retinal detachment; VH: vitreous hemorrhage; IOP: intraocular pressure; AACG: acute angle closure glaucoma |
||
Extraocular muscle integrity and movement should be documented, noting any muscle entrapment or signs of cranial nerve deficits.
Visual field testing using confrontation is performed with the examiner directly in front of the patient. Position yourself such that when you extend your arm diagonally in front of you, your fingers are midway between you and the patient so that your peripheral visual fields match theirs. Have the patient fix their vision on your face. Testing one eye at a time, flash a number of fingers in each quadrant to test their vision. The provider should evaluate for hemineglect/extinction by testing diagonal quadrants at the same time. Finally, one should evaluate the patient’s central vision by having the patient look directly at the provider’s face and noting if the patient describes any abnormalities such as blurriness and/or a skewed image.
Pupillary size, reactivity to light, and consensual reaction should be evaluated. It is important to note pupil symmetry, ensuring they both are round, reactive to light, and equal to each other. The provider should evaluate pupillary constriction to accommodation. One must evaluate for the presence of a relative afferent pupillary defect (RAPD), in which one pupil fails to constrict as robustly to direct light as the other. The pupil with an RAPD, however, will constrict consensually. If one pupil is fixed or irregular, evaluate if the mobile pupil dilates when you swing your light from the mobile pupil to the fixed pupil. The presence of an RAPD indicates retinal or optic nerve pathology and also is known as the Marcus Gunn phenomenon.28
Attempt a funduscopic evaluation in the undilated pupil. Fundoscopy and retina visualization, although difficult in the undilated pupils, should begin with obtaining a red reflex and proceed to note the optic disc and retinal vessels. Fundoscopy can be performed with or without pupillary dilation. Using a cycloplegic involves a risk of precipitating acute angle closure glaucoma and generally is avoided in the ED unless an ophthalmologist is available. First, evaluate for vitreous hemorrhage. This will appear as a dark haze that can obscure the fundus. Next, evaluate the optic disc to see if it is swollen or pale, indicating optic nerve pathology. Evaluate the retinal blood vessels for cotton-wool spots and bleeding. Evaluate the macula for bleeding or detachment. Finally, evaluate the peripheral retina for tears, holes, bleeding, and detachment. A “blood and thunder” appearance of the retina is consistent with a CRVO. A pale retina with a “cherry red” macula is consistent with a CRAO. (See Figure 1.) A “cottage cheese and thunder” or “pizza-like” appearance of the retina can be caused by cytomegalovirus (CMV) retinitis.
Figure 1. “Cherry Red Spot” Consistent with Central Retinal Artery Occlusion |
 |
Dilated fundoscopic exam showing pale retina with “cherry-red spot” consistent with central retinal artery occlusion (CRAO). Courtesy of Dustin Williams, MD |
After adequate topical anesthesia, fluorescein staining and evaluation with a Wood’s lamp should precede tonometry testing. This is in an effort to confirm the integrity of the globe and evaluate the cornea and globe for any penetrating injury. If no penetrating wound is suspected or noted, the upper lids should be everted to inspect for hidden foreign bodies, and the pressures in the anterior chambers should be documented. The intraocular pressure (IOP) then should be measured. The normal IOP ranges between 10 mmHg and 20 mmHg. A pressure greater than 40 mmHg is most concerning for acute angle closure glaucoma. Intermediate pressures can be seen in open angle glaucoma or ocular hypertension.
Next, slit lamp examination and further evaluation using cobalt blue light and white light should follow. Importantly, the slit lamp will allow for more detailed evaluation of the conjunctiva, cornea, and anterior chamber. If time has elapsed before the prior application of topical anesthetic, one can use a fluorescein strip with a dab of topical anesthetic to smear over the patient’s lower fornix. After having the patient blink to spread the fluorescein, the provider should evaluate the cornea with a cobalt blue light using a thin beam. If the provider notes an area of fluorescein uptake, that area then should be evaluated with the regular light setting on the slit lamp to assess for an associated corneal infiltrate. Subsequently, evaluation of the cornea with a regular light with a thin beam can assist in the detection of foreign bodies, which may not stain with fluorescein. Using a 1 mm square beam, the provider should shine the light from the side of the eye to examine the anterior chamber for cell and flare. As well, the use of a slit beam allows the provider to evaluate for hypopyon and hyphema. Lastly, a thorough examination of the conjunctiva for injection and hemorrhage should be performed.
The use of point-of-care ultrasound (POCUS) to evaluate the posterior segments of the globe, including the optic nerve, retina, and vitreous, has become integral to the practice of emergency medicine. Ophthalmologists have been early implementors of ocular ultrasound, leveraging its capabilities because the eye and periocular tissues are highly suitable for detailed ultrasound imaging. They use two distinct ultrasound modalities: A-scan and B-scan. The A-scan, or time-amplitude modulated scan, provides a one-dimensional representation of tissues based on the amplitude of reflected sound waves, primarily used for measurements and assessing intraocular distances. This is not a modality commonly used in the ED. Conversely, B-scan, or brightness-modulated scan, offers a two-dimensional cross-sectional view of the eye, allowing for detailed visualization of the globe’s internal and surrounding structures, and is equivalent to the ocular POCUS performed in the ED. POCUS has a sensitivity of 96.9% and a specificity of 88.1% in detecting retinal detachments.29 In addition, ocular ultrasonography can facilitate the diagnosis of CRAO, retinal tears, vitreous hemorrhages, cataracts, and endophthalmitis.30 POCUS also may aid in the diagnosis of giant cell arteritis (GCA), demonstrating what is called the “halo sign” around the temporal artery.31,32
Pearl: POCUS is an integral tool for the diagnosis of several ocular conditions.
Diagnostic Evaluation
Additional methods of evaluating the posterior segment of the eye may include digital fundus photography and ultrasound. Digital fundus photography has been shown to have reasonable sensitivity and high specificity and can be interpreted remotely or asynchronously by an ophthalmologist, when linked into the electronic health record.33 A detailed fundoscopic exam can detect evidence of vascular disease, such as a Hollenhorst plaque, which can lead the clinician toward a diagnosis of amaurosis fugax secondary to ischemia. As mentioned earlier, POCUS has demonstrated high sensitivity and specificity for diagnosing retinal detachments and also can be used to evaluate for other ocular pathologies.30,34 Using POCUS, retinal detachments will appear as wavy lines tethered to the posterior segment of the eye. (See Figure 2.) Vitreous hemorrhages will display a sign called the “washing machine sign,” in which the echogenic blood will swirl when the globe is moved from side to side and typically is not tethered to the posterior segment. (See Figure 3.) To see an example of this, visit: https://emottawablog.com/2022/08/ocular-pocus-keep-your-prize-on-the-eyes/#:~:text=Vitreous%20hemorrhage%20occurs%20when%20extravasated,a%20“washing%20machine%20sign”.
Figure 2. Ocular POCUS Showing Retinal Detachment |
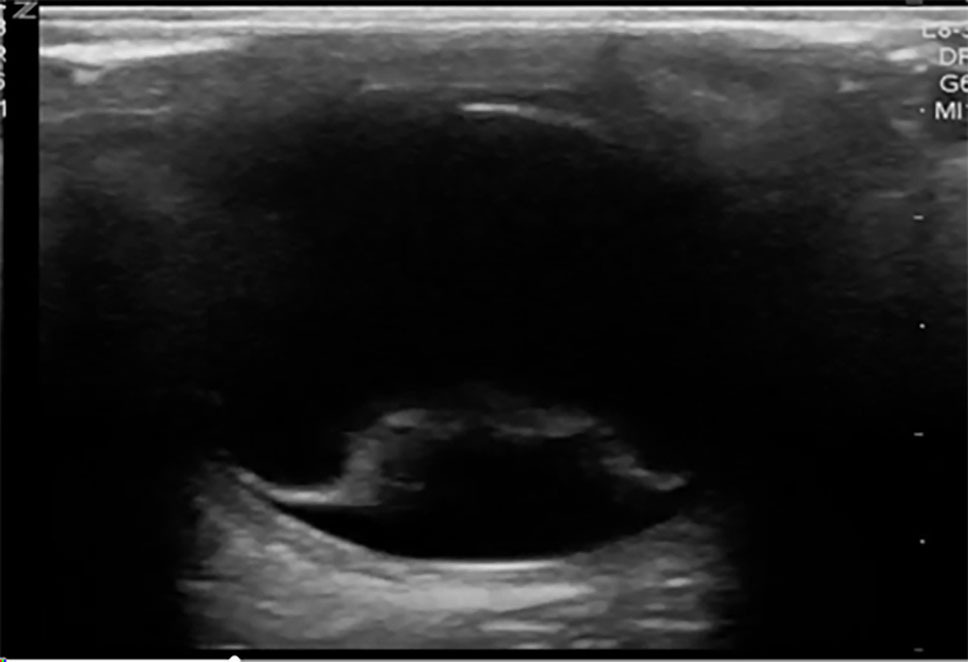 |
Point-of-care ultrasound (POCUS) showing retinal detachment with wavy hyperdense line tethered to the posterior segment of the eye Courtesy of Jodi Jones, MD |
Figure 3. Vitreous Hemorrhage |
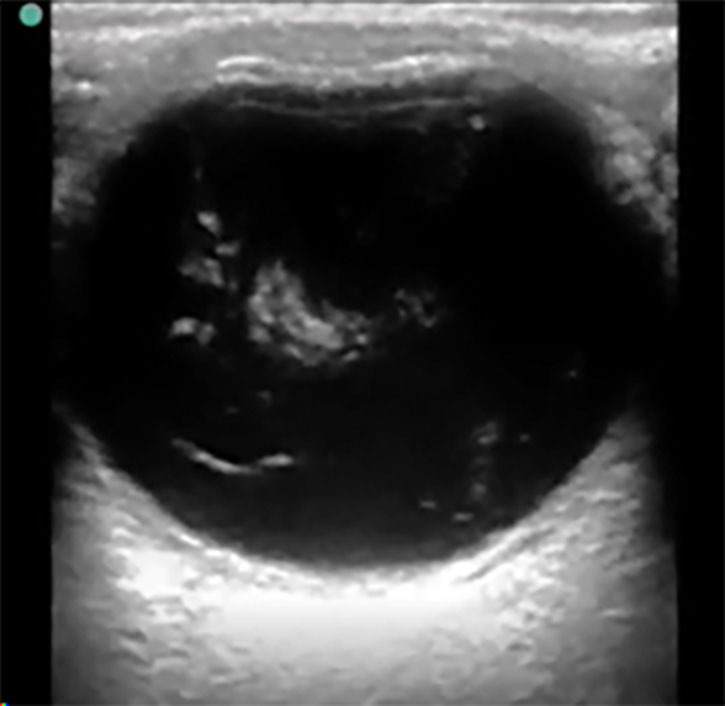 |
Ocular point-of-care ultrasound (POCUS), or B-scan, showing swirling hyperdense echogenic densities seen in vitreous hemorrhage Courtesy of Jodi Jones, MD |
In addition to POCUS, a consultative carotid duplex ultrasound to evaluate the vasculature may be an important tool in the diagnosis amaurosis fugax. Computed tomography (CT) angiography of the neck vessels is another imaging modality that can rapidly evaluate vasculature if duplex sonography is unavailable. In patients presenting with symptoms concerning for posterior brain circulation ischemia, such as binocular vision loss, dizziness, vertigo, or ataxia, neuroimaging with brain magnetic resonance imaging (MRI) should be strongly considered.
In CRAO, a dense retrobulbar spot sign (RBSS) can be seen immediately posterior to the globe as a hyperdense embolism visualized in the central retinal artery. It is the equivalent of the dense middle cerebral artery sign seen in strokes, and in studies has a sensitivity of 83% and a specificity of 100%.35,36 (See Figure 4.)
Figure 4. Retrobulbar Spot Sign Consistent with Central Retinal Artery Occlusion |
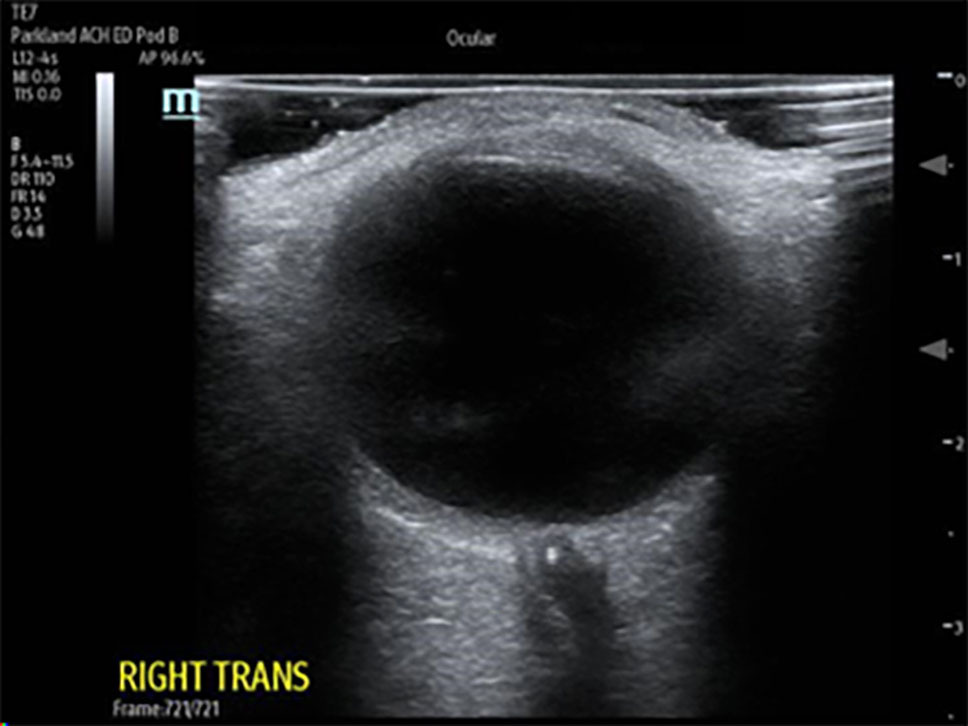 |
Ocular point-of-care ultrasound (POCUS) showing dense retrobulbar spot sign, consistent with central retinal artery occlusion (CRAO) Courtesy of Dustin Williams, MD |
Additional diagnostic testing may include an erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and platelet count if giant cell arteritis is suspected.32 Thyroid function tests, including a thyroid stimulating hormone (TSH), should be ordered when symptoms or physical findings suggest thyroid eye disease. A rapid plasma reagin (RPR) can be ordered when syphilis is suspected. Additionally, an electrocardiogram should be obtained if there is concern for stroke and/or embolic phenomena to evaluate for arrhythmias such as atrial fibrillation.
Initial Management and Pharmacologic Considerations
Management for all vision-threatening conditions should be conducted with consideration of the level of risk for imminent, irreversible vision loss. If this risk is high, emergent interventions, whether by procedure or medications, are essential. Examples of conditions that can lead to rapid irreversible vision loss include but are not limited to acute angle closure glaucoma, retinal detachment, CRAO, CRVO, and giant cell arteritis. These conditions require emergent consultation with ophthalmologists, who will help direct early administration of medications and any procedures. Table 1 summarizes these conditions, along with their emergency management. When ophthalmology consultation is not readily available, treatment for acute angle closure glaucoma must be initiated in the ED as soon as possible with pressure-lowering eye drops along with an intravenous (IV) carbonic anhydrase inhibitor, such as acetazolamide. (See Table 4.) Prompt recognition and treatment in the ED can improve vision outcomes.
Table 4. Medications to Lower Intraocular Pressure in Acute Angle Closure Glaucoma |
||
Medication |
Mechanism of Action |
Dose, Route, Frequency |
Acetazolamide or Dorzolamide 2% |
|
|
Timolol 0.25% or 0.5% |
|
|
Apraclonidine 1% or Brimonidine 0.15% |
|
|
Pilocarpine 2% to 4% |
|
|
Latanoprost 0.005% |
|
|
Prednisolone acetate 1% |
|
|
Systemic analgesia |
|
|
Antiemetics |
|
|
Hyperosmotic agents |
|
|
PO: orally; IV: intravenous; IOP: intraocular pressure |
||
Retinal detachment is another diagnosis that requires emergent consultation with ophthalmology. Often after consultation, the ophthalmological specialist may arrange for patients to have urgent surgery in an outpatient setting, where specialized equipment is available. CRAO is a true ocular emergency, and treatment outcomes are better with early intervention, with some guidelines recommending treating the condition akin to a stroke. If the patient is within a 4.5-hour window, the American Heart Association (AHA) recommends administering tissue plasminogen activator should no other contraindications be present, at 0.9 mg/kg, not to exceed 90 mg, with 10% of the dose given over one minute and the remainder over one hour.14 In individuals who are not candidates for thrombolysis, other procedures can be performed if deemed to be appropriate by a consultant.
CRVO has no effective emergent management strategies. Its treatment is largely guided by the consultant’s preferences, so emergent ophthalmology evaluation is always warranted. Clinical trials have used intravitreal corticosteroids for macular edema and antivascular endothelial growth factor agents to prevent neovascularization and reduce the risk of rebleeding. In some cases, surgical options can be used to preserve vision. These options include pars plana vitrectomy and laser pan-retinal photocoagulation.
Finally, the management of patients with GCA is largely driven by the degree of vision loss at presentation and generally should be directed by ophthalmology. For patients without signs of acute vision loss on presentation, high-dose oral glucocorticoids are a mainstay of treatment, at a fixed starting dose of 40 mg to 60 mg oral prednisone daily until the symptoms resolve.35 A patient with signs of vision loss on diagnosis also may require high-dose IV glucocorticoids (500 mg/day to 1,000 mg/day for two to five days), with an oral course to follow (ranging from 26- to 52-week tapers, starting with a 60 mg/day dose).37 Research shows that administration of glucocorticoids appears to lower the risk of subsequent vision loss to nearly zero if none is present initially. However, reversal of vision loss at the time of diagnosis is rare, making prompt recognition and treatment even more essential. In patients with contraindications to taking glucocorticoids, tociluzumab may be added to their treatment regimen.
Pearl: A central retinal artery occlusion must be treated as an acute stroke.
Pain management typically is multimodal. Topical ophthalmic anesthetics such as tetracaine 0.5% ophthalmic solution or proparacaine 0.5% ophthalmic solution frequently are used to facilitate the physical exam in the ED and provide temporary pain relief, especially for superficial causes of pain.38,39 While use in the ED may be beneficial to relieve acute pain, prolonged use or abuse may lead to corneal epithelial toxicity and may manifest as epithelial defects, which may progress to permanent corneal damage, so use is limited to the ED setting. Patients can be counseled to take over-the-counter pain medication during the acute phase of recovery, should the patient be stable for discharge. Pain resulting from causes such as iritis can be managed with mydriatics, cycloplegics, and steroids, but only in consultation with an ophthalmologist. Pain management for acute angle closure glaucoma should be centered around immediate lowering of the IOP. Systemic analgesia and systemic nausea control are described in Table 1.40
Pearl: To improve vision outcomes, the management of acute angle closure glaucoma must start in the ED by interventions that lower the IOP.
Disposition and Follow-Up Considerations
For all conditions discussed in this article as immediate sight threats, an emergent consultation with ophthalmology is required. (See Table 2.) In the case of CRAO, a stroke evaluation, such as a code stroke activation or equivalent, must be pursued if the patient is within the treatment window. Likewise, cases of acute angle closure glaucoma also require immediate management and ophthalmology consultation. For those with stable conditions, close follow-up with ophthalmology and strict return precautions are recommended. In areas that have fewer resources and have no immediate ophthalmology coverage (either in person or by tele-consult), clinicians should evaluate the patient on a case-by-case basis. Providers should consider transfer to a higher level of care if there is concern for acute vision loss or if there is a possibility for acute worsening of the patient’s condition. Discharged patients should be provided strict return precautions and counseled on specific symptoms or signs to monitor for that would require emergent reevaluation. Examples of these return guidelines include worsening of vision loss, intractable headache or eye pain, and/or any new neurologic changes, such as numbness or weakness.
Special Populations
Special populations such as pediatric, pregnant, and older adult patients, require additional consideration when presenting with vision complaints. Pediatric patients may present unique challenges, including difficulty in articulating symptoms and participating in the ocular examination. Ocular and visual exams can be challenging depending on the patient’s age and cognitive ability. Pediatric-specific causes of vision loss, like retinoblastoma, should be considered in pediatric patients presenting with vision concerns. Corneal pathologies (i.e., corneal abrasions) should be considered in the inconsolable infant when no other identifiable cause is apparent.
In pregnant and recently postpartum patients, hormonal changes can lead to preeclampsia, which may present with vision changes in up to 40% of cases.41
Older adult patients are at increased risk for ophthalmologic emergencies, such as acute angle closure glaucoma, giant cell arteritis, diabetic retinopathy, cataracts, and vascular occlusions. Many of these conditions are due to the physiologic changes associated with aging. Older adult patients also may have multiple medical comorbidities and may be prone to polypharmacy, both of which complicate their assessment and management. Cognitive impairments and communication barriers, such as hearing difficulties and dementia, can further challenge the accurate diagnosis in this population.
Patients with diabetes also warrant particular attention when presenting with vision complaints. Diabetes can lead to a wide range of ophthalmologic complications, resulting from microvascular insults related to chronic hyperglycemia, that then results in neovascularization of tissues. This neovascularization is prone to intraocular bleeding complications. It is crucial for emergency medicine physicians to consider the possibility of diabetic retinopathy in patients with diabetes who report vision changes. Additionally, neovascular glaucoma is another significant diabetic eye condition that could exacerbate acute vision problems. For all of these special populations, prompt recognition of these conditions with a tailored approach and thorough understanding of pathophysiology is essential to ensure optimal emergency care and outcomes and prevent long-term visual impairment.
Pearl: Up to 40% of the patients with preeclampsia have acute vision changes as part of their ED presentation.
Conclusion
Sudden vision loss is an acute medical emergency that requires expeditious evaluation to exclude serious etiologies and preserve vision. These patients need a comprehensive eye examination, which often is not possible outside a dedicated eye clinic. However, even with a lack of specialized equipment, emergency clinicians need to be adept at the basic ocular examination techniques, including tonometry, slit lamp, and POCUS.39 When combined with a thorough history and physical examination, these techniques are essential to evaluate for vision-threatening conditions. In patients with acute, non-traumatic vision loss, a broad differential should be considered. Many acute ocular conditions can be diagnosed and/or treated at the bedside by the emergency physician. Some conditions, such as acute angle closure glaucoma, retinal detachment, CRAO, CRVO, and giant cell arteritis, require immediate intervention and an emergent consult with an ophthalmologist. The overall goal in emergency medicine is to preserve as much vision as possible. Other urgent causes of vision loss require close communication and follow-up with an ophthalmologist, and patients should be given strict emergency return instructions.
REFERENCES
- Lahham S, Ali Q, Palileo BM, et al. Role of point of care ultrasound in the diagnosis of retinal detachment in the emergency department. Open Access Emerg Med 2019;11:265-270.
- Beran DI, Murphy-Lavoie H. Acute, painless vision loss. J La State Med Soc 2009;161:214-216, 218-223.
- Bagheri N, Mehta S. Acute vision loss. Prim Care 2015;42:347-361.
- Mukhi SV, Lincoln CM. MRI in the evaluation of acute visual syndromes. Top Magn Reson Imaging 2015;24:309-324.
- Miller R, Wagner S, Hammond J, et al. Posterior reversible encephalopathy syndrome in the emergency department: A single center retrospective study. Am J Emerg Med 2021;45:61-64.
- Dhingra D, Kaur S, Ram J. Illicit drugs: Effects on eye. Indian J Med Res 2019;150:228-238.
- Karimi S, Arabi A, Shahraki T. Alcohol and the eye. J Ophthalmic Vis Res 2021;16:260-270.
- Raharja A, Whitefield L. Clinical approach to vision loss: A review for general physicians. Clin Med (Lond) 2022;22:95-99.
- Flaxman AD, Wittenborn JS, Robalik T, et al. Prevalence of visual acuity loss or blindness in the US: A Bayesian meta-analysis. JAMA Ophthalmol 2021;139:717-723.
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob Health 2021;9:e144-e160.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Public Health Approaches to Reduce Vision Impairment and Promote Eye Health; Welp A, Woodbury B, McCoy MA, Teutsch SM, eds. Making Eye Health a Population Health Imperative: Vision for Tomorrow. National Academies Press; 2016.
- Ibrar A, Panayiotis M, Mohamed EA. Recognising and managing retinal detachments. Br J Hosp Med (Lond) 2021;82:1-11.
- Lahham S, Shniter I, Thompson M, et al. Point-of-care ultrasonography in the diagnosis of retinal detachment, vitreous hemorrhage, and vitreous detachment in the emergency department. JAMA Netw Open 2019;2:e192162.
- MacGrory B, Schrag M, Biousse V, et al. Management of central retinal artery occlusion: A scientific statement from the American Heart Association. Stroke 2021;52:e282-e294.
- Flaxel CJ, Adelman RA, Bailey ST, et al. Retinal and ophthalmic artery occlusions Preferred Practice Pattern®. Ophthalmology 2020;127:P259-P287.
- Kapoor KM, Kapoor P, Heydenrych I, Bertossi D. Vision loss associated with hyaluronic acid fillers: A systematic review of literature. Aesthetic Plast Surg 2020;44:929-944.
- Mac Grory B, Nackenoff A, Poli S, et al. Intravenous fibrinolysis for central retinal artery occlusion: A cohort study and updated patient-level meta-analysis. Stroke 2020;51:2018-2025.
- Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: Local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology 2010;117:1367-1375 e1.
- Tadi P, Najem K, Margolin E. Amaurosis fugax. In: StatPearls. Aug. 7, 2023. StatPearls Publishing; 2024.
- Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains Eye Study. Arch Ophthalmol 2006;124:726-732.
- Patel A, Nguyen C, Lu S. Central retinal vein occlusion: A review of current evidence-based treatment options. Middle East Afr J Ophthalmol 2016;23:44-48.
- Arbunea-Ghenoiu S, Ciubotaru GV, Dumitrascu A, et al. Pituitary apoplexy: A retrospective study of 36 cases from a single center. Cureus 2022;14:e29769.
- Mergen S, Long B, Matlock A. Posterior reversible encephalopathy syndrome: A narrative review for emergency clinicians. J Emerg Med 2021;61:666-673.
- Zelaya JE, Al-Khoury L. Posterior reversible encephalopathy syndrome. In: StatPearls. May 1, 2022. StatPearls Publishing; 2024.
- Baj J, Forma A, Kobak J, et al. Toxic and nutritional optic neuropathies — An updated mini-review. Int J Environ Res Public Health 2022;19:3092.
- Wasinska-Borowiec W, Aghdam KA, Saari JM, Grzybowski A. An updated review on the most common agents causing toxic optic neuropathies. Curr Pharm Des 2017;23:586-595.
- Muttray A, Wolers V, Jung D, Konietzko J. Effects of high doses of toluene on color vision. Neurotoxicol Teratol 1999;21:41-45.
- Simakurthy S, Tripathy K. Marcus Gunn Pupil. In: StatPearls. Aug. 25, 2023. StatPearls Publishing; 2024.
- Kim DJ, Francispragasam M, Docherty G, et al. Test characteristics of point-of-care ultrasound for the diagnosis of retinal detachment in the emergency department. Acad Emerg Med 2019;26:16-22.
- Skidmore C,Saurey T, Ferre RM, et al. A narrative review of common uses of ophthalmic ultrasound in emergency medicine. J Emerg Med 2021;60:80-89.
- Luqmani R, Lee E, Singh S, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016;20:1-238.
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol 2022;74:1881-1889.
- Teismann N, Neilson J, Keenan J. Quality and feasibility of automated digital retinal imaging in the emergency department. J Emerg Med 2020;58:18-24.
- Propst SL, Kirschner JM, Strachan CC, et al. Ocular point-of-care ultrasonography to diagnose posterior chamber abnormalities: A systematic review and meta-analysis. JAMA Netw Open 2020;3:e1921460.
- Altmann M, Ertl M, Helbig H, et al. Low endogenous recanalization in embolic central retinal artery occlusion — the retrobulbar “spot sign.” J Neuroimaging 2015;25:251-256.
- Usheva E, Williams D, Musgrave H, Zhou S. Sonographic retrobulbar spot sign in diagnosis of central retinal artery occlusion: A case report. J Educ Teach Emerg Med 2023;8:V5-V8.
- Dua AB, Husainat NM, Kalot MA, et al. Giant cell arteritis: A systematic review and meta-analysis of test accuracy and benefits and harms of common treatments. ACR Open Rheumatol 2021;3:429-441.
- Bartfield JM, Holmes TJ, Raccio-Robak N. A comparison of proparacaine and tetracaine eye anesthetics. Acad Emerg Med 1994;1:364-367.
- Knoop K, Trott A. Ophthalmologic procedures in the emergency department — Part II: Routine evaluation procedures. Acad Emerg Med 1995;2:144-150.
- Flores-Sánchez BC, Tatham AJ. Acute angle closure glaucoma. Br J Hosp Med (Lond) 2019;80:C174-C179.
- Stern EM, Blace N. Ophthalmic pathology of preeclampsia. In: StatPearls. Oct. 24, 2022. StatPearls Publishing; 2024.
This article will discuss the various emergent causes of vision loss, including necessary diagnostic testing, imaging, and needed interventions and consultations. Most importantly, emergency medicine clinicians must be sensitive to the goal of restoration and preservation of as much vision as possible.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
