Diagnosis and Management of Pulmonary Embolism in the ICU
November 1, 2023
By Trushil Shah, MD
Assistant Professor of Medicine, University of Texas Southwestern, Dallas
Venous thromboembolism is the third most frequent acute cardiovascular syndrome and leads to approximately 300,000 deaths per year in the United States.1 A significant proportion of these patients (34%) die from sudden cardiac arrest or within a few hours of onset before appropriate treatment can be initiated.1 Furthermore, among those who died of acute pulmonary embolism (PE), 59% were diagnosed after death by autopsy.1 Prompt diagnosis and management of PE in the emergency room and intensive care unit (ICU), therefore, may affect the course of these patients.
DEATH SPIRAL IN PULMONARY EMBOLISM
The anatomic obstruction caused by a large amount of clot in the pulmonary vasculature is the main reason for a sudden elevation in pulmonary vascular resistance (PVR) and obstructive shock resulting from acute right ventricular (RV) failure. However, other mechanisms, such as hypoxic vasoconstriction, neurohumoral activation, and RV ischemia, contribute to further failure of the RV.1 The series of events that takes place after a PE that leads to progressive RV failure is called the death spiral. (See Figure 1.)
Figure 1. Death Spiral in Pulmonary Embolism |
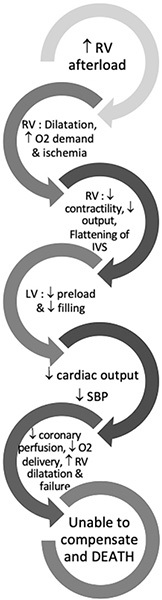 |
DIAGNOSIS
Clinical presentation of acute PE is nonspecific and requires a high index of suspicion. Presentations can vary from cardiac arrest in the field to incidental diagnosis without symptoms. Hemodynamic instability and syncope are rare presentations but very important to recognize and treat because they carry very high mortality.1 Typical presenting symptoms are sudden onset dyspnea, chest pain, tachycardia, and hypoxemia. Leg swelling and concomitant deep vein thrombosis (DVT) often are present. Clinical pretest probability prediction rules, such as Wells or Geneva prediction scores, should be used. Computed tomography (CT) pulmonary angiography is the test of choice if the patient is stable enough to travel to the CT scanner and does not have any other contraindications. However, in critically ill patients, especially those in refractory shock or cardiac arrest, it may not be safe to wait for a CT scan. In such cases, bedside echocardiography with signs of overt RV failure in a high probability patient is adequate to make the diagnosis and initiate treatment.1
MANAGEMENT IN THE ICU
Most patients with PE who need ICU admission generally are high risk (e.g., hemodynamically unstable) or intermediate risk. Pulmonary Embolism Response Teams (PERTs) help expedite decisions and treatment for these patients and are beneficial in providing prompt clinical decision making in real time.2 Some studies also have shown that the presence of a PERT may reduce mortality in PE patients.3
High-Risk PE
High-risk PE refers to hemodynamically unstable patients who are in obstructive shock secondary to PE. Obstructive shock in PE carries a poor prognosis, with mortality rates approaching 30%.4 These patients present in a spectrum of hypotension, shock, or overt cardiac arrest. In patients with high clinical probability of PE and refractory shock or cardiac arrest, immediate diagnosis with a bedside echocardiogram should be established and systemic thrombolysis (ST) should be initiated. Emergent venoarterial extracorporeal membrane oxygenation (VA-ECMO) should be considered as a rescue intervention for these patients with refractory shock/cardiac arrest.1 For patients who fail systemic thrombolysis or have contraindications to ST, surgical embolectomy after stabilization with VA-ECMO should be considered. In some select patients, catheter embolectomy after VA-ECMO support can be considered as well. If neither surgical nor catheter-directed embolectomies can be performed, many patients with high-risk PE who are supported with VA-ECMO can recover with anticoagulation alone.5
For high-risk PE patients who need inotropic support, systemic thrombolysis also should be considered, and the patients should be watched closely for any deterioration. In patients who are not candidates for ST, surgical or catheter-directed embolectomy should be considered. The anticoagulant of choice in patients receiving systemic thrombolysis is unfractionated heparin (UFH) because of easy reversibility in case of bleeding events or need for surgical intervention.
Intermediate-Risk PE
Intermediate-risk PE is further divided into intermediate-high risk and intermediate-low risk based on evidence of right heart strain on echocardiogram or CT scan and elevation of cardiac biomarkers (e.g., troponin, N-terminal prohormone of brain natriuretic peptide [NT-pro-BNP] or brain natriuretic peptide [BNP]).1 Patients with both evidence of RV strain and positive biomarkers are classified as intermediate-high risk, whereas patients with either RV strain or elevated biomarkers are classified as intermediate-low risk. All patients in an intermediate-risk category should be treated with therapeutic anticoagulation. In these patients, low molecular weight heparin (LMWH) is preferred over UFH as initial anticoagulation, since UFH often is associated with significantly higher risk of subtherapeutic anticoagulation levels in the first 48 hours.6
Most patients in the intermediate-low risk category can be managed with anticoagulation alone and do not need advanced therapies, such as systemic thrombolysis or catheter-directed interventions. However, these patients will need to be monitored closely for decompensation, in which case advanced therapies should be considered.1
Management of intermediate-high risk patients is more variable. In this situation, a multidisciplinary PERT team is helpful because of unclear evidence on the use of advanced therapies.
The largest trial of systemic thrombolysis, PEITHO, studied 1,006 intermediate-risk patients and showed that ST led to improvement in the risk of hemodynamic collapse within seven days (1.6% vs. 5%; P = 0.002), but was associated with significantly increased risk of major hemorrhage (6.3% vs. 1.5%; P < 0.001) with no effect on mortality and a 2% incidence of intracranial hemorrhage.7 In practice, the risk of intracranial hemorrhage is around 3% to 5% with ST. Therefore, ST should not be used routinely for intermediate-high risk patients.8
Another studied application of ST is the use of half-dose ST (50 mg of alteplase).9,10 In a recent propensity score-matched study of 3,768 patients who received half vs. full-dose ST, patients in the half-dose group had a higher incidence of treatment escalation (53.8% vs. 41.4%; P < 0.01), which was driven largely by secondary fibrinolysis (25.9% vs. 7.3%; P < 0.01) and catheter-directed therapy (14.2% vs. 3.8%; P < 0.01).11 In these patients, rates of major bleeding and intracranial bleeding were similar. In light of these results, routine use of half-dose alteplase also is not recommended.
Catheter-based therapies are increasingly used for intermediate-high risk PE patients. Catheter-based therapies include catheter-directed fibrinolysis, embolectomy, and pharmaco-mechanical therapy. Current literature studying catheter-based therapies lacks data from randomized controlled trials (RCTs) supporting improvement in clinical outcomes. The most studied of these is ultrasound-assisted catheter thrombolysis, which in multiple studies (OPTALYSE-PE, SEATTLE-2, and an RCT by Kucher et al) showed improvement in RV/left ventricle (LV) ratio and a decrease in clot burden.12-14
Mechanical catheter embolectomy can be helpful in patients with contraindications to fibrinolytic therapy. Currently, three different systems are available: FlowTriever system (Inari Medical), Indigo thrombectomy system (Penumbra), and AngioVac system (Angiodynamics). The FlowTriever and Indigo thrombectomy systems have been studied in single-arm multicenter studies of 106 (FLARE study) and 119 (EXTRACT-PE study) intermediate-risk patients, respectively.15,16 Both studies showed improvement in RV/LV ratio.15,16
Current challenges in determining how to optimally use catheter-directed interventions include the lack of RCTs to compare these to anticoagulation alone and the lack of clinically meaningful outcomes in any of the studies noted earlier.8 Given these limitations, the decision to use these interventions should be individualized by a multidisciplinary team (i.e., PERT).
It should be noted that, in the case of catheter-based procedures, patients preferably should not be intubated for the procedure and should receive minimal sedation, given the high risk of RV failure. Propofol should not be used for anesthesia induction in high- or intermediate-risk patients because it is associated with higher mortality.17
CONCLUSION
Management of PE in the ICU often is complex (see Figure 2) and should include a multidisciplinary team approach to expedite decision-making in these patients with high mortality.
Figure 2. Management of Acute Pulmonary Embolism in the Intensive Care Unit |
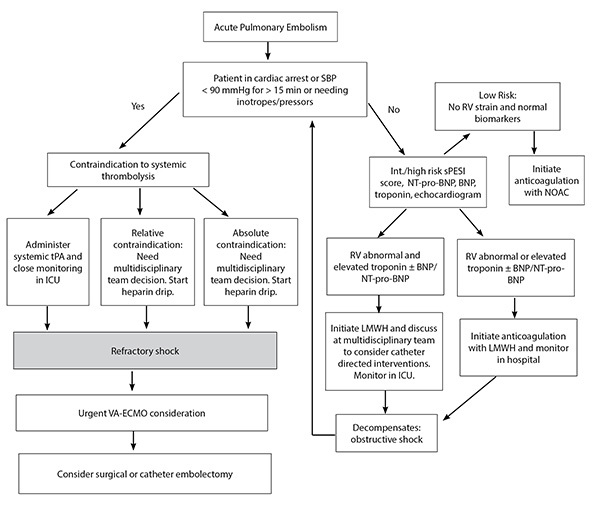 |
SBP: systolic blood pressure; RV: right ventricular; sPESI: simplified Pulmonary Embolism Severity Index; NT-pro-BNP: N-terminal prohormone of brain natriuretic peptide; BNP: brain natriuretic peptide; NOAC: non-vitamin K antagonist oral anticoagulant; tPA: tissue plasminogen activator; ICU: intensive care unit; LMWH: low molecular weight heparin; VA-ECMO: venoarterial extracorporeal membrane oxygenation |
REFERENCES
- Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Respir J 2019;54:1901647.
- Dudzinski DM, Piazza G. Multidisciplinary Pulmonary Embolism Response Teams. Circulation 2016;133:98-103.
- Wright C, Goldenberg I, Schleede S, et al. Effect of a Multidisciplinary Pulmonary Embolism Response Team on patient mortality. Am J Cardiol 2021;161:102-107.
- Secemsky E, Chang Y, Jain CC, et al. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med 2018;131:1506-1514.e0.
- Ghoreishi M, DiChiacchio L, Pesrija C, et al. Predictors of recovery in patients supported with venoarterial extracorporeal membrane oxygenation for acute massive pulmonary embolism. Ann Thorac Surg 2020;110:70-75.
- Aday AW, Beckman JA. Pulmonary embolism and unfractionated heparin: Time to end the roller coaster ride. Acad Emerg Med 2020;27:176-178.
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402-1411.
- Piazza G. Advanced management of intermediate- and high-risk pulmonary embolism: JACC Focus Seminar. J Am Coll Cardiol 2020;76:2117-2127.
- Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: A randomized, multicenter, controlled trial. Chest 2010;137:254-262.
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol 2013;111:273-277.
- Kiser TH, Burnham EL, Clark B, et al. Half-dose versus full-dose alteplase for treatment of pulmonary embolism. Crit Care Med 2018;46:1617-1625.
- Kucher N, Boekstegers P, Muller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014;129:479-486.
- Tapson VF, Sterling K, Jones N, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: The OPTALYSE PE Trial. JACC Cardiovasc Interv 2018;11:1401-1410.
- Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015;8:1382-1392.
- Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: The FLARE Study. JACC Cardiovasc Interv 2019;12:859-869.
- Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of pulmonary embolism: Results of the EXTRACT-PE Trial. JACC Cardiovasc Interv 2021;14:319-329.
- Manchec B, Liu B, Tran T, et al. Sedation with propofol during catheter-directed thrombolysis for acute submassive pulmonary embolism is associated with increased mortality. J Vasc Interv Radiol 2019;30:1719-1724.
Prompt diagnosis and management of pulmonary embolism in the emergency room and intensive care unit (ICU), therefore, may affect the course of these patients.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
