Diagnosis and Management of Abscesses
AUTHORS
Trahern W. ("TW") Jones, MD
Assistant Professor, Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City
Paighton Noel
Medical Student, Spencer Fox Eccles School of Medicine, University of Utah, Salt Lake City
Arnel Besic
H.B.S Student, Department of Chemistry, University of Utah, Salt Lake City
PEER REVIEWER
Daniel Migliaccio, MD, FDP, FAAEM
Clinical Assistant Professor, Director of Emergency Ultrasound, Ultrasound Fellowship Director, University of North Carolina, Chapel Hill
EXECUTIVE SUMMRAY
- Folliculitis is caused by a superficial infection of hair follicles. Risk factors include tight clothing that traps sweat and heat, soaking in a pool or hot tub that is not well-maintained, damage to the hair follicles, dermatitis or hyperhidrosis, and immunocompromising conditions. It occurs most commonly secondary to methicillin-resistant Staphylococcus aureus (MRSA) or methicillin-sensitive Staphylococcus aureus (MSSA).
- Neonates also are susceptible to mastitis and breast abscess in the first six weeks of life, which can be a consequence of exposure to maternal estrogen, resulting in physiologic breast hypertrophy.
- Risk factors for transmission of MRSA include situations with crowding, frequent skin-to-skin contact, sharing of personal items (e.g., towels or razors), and contexts like athletic, incarceration, or military training facilities.
- Malassezia (pityrosporum) folliculitis is a fungal acneiform condition caused by yeasts of the genus Malassezia, formerly Pityrosporum.
- Hot tub folliculitis is an opportunistic infection associated with hot tubs, whirlpools, and swimming pools and classically is caused by Pseudomonas aeruginosa, which is known to colonize and proliferate in wet and hot environments.
- A much rarer cause of hot tub folliculitis is Aeromonas hydrophila, which also has been associated with poorly chlorinated home spas and pools and moreover has been documented in several case reports as an independent cause of purulent folliculitis.
- Paronychia most commonly is attributed to infections with gram-positive bacilli S. aureus and Streptococcus pyogenes (Group A Streptococcus).
- Point-of-care ultrasound technology can establish a diagnosis quickly in clinical scenarios, where potential differentiation between an abscess and a more superficial skin or soft tissue infection is unclear. In addition, it may help distinguish the feasibility of bedside incision and drainage for more superficial collections vs. those that are deeper or more complex (bordering important anatomic structures) that would benefit from operative intervention.
- The Infectious Diseases Society of America practice guidelines recommend adjunctive antibiotic treatment following incision and drainage based on the presence or absence of systemic symptoms, such as tachycardia, temperature > 38°C or < 36°C, tachypnea, and leukocytosis > 12,000 cells/μL or severe leukopenia with < 400 cells/μL. Immunocompromised patients and other high-risk populations, such as patients with human immunodeficiency virus/acquired immunodeficiency syndrome, undergoing chemotherapy, or taking immunosuppressive medications, may require antibiotics to manage even mild or moderate infections because of their increased susceptibility to bacterial infections and may have limited clinical signs of more severe infection, such as with demonstrating systemic inflammatory response syndrome criteria.
- For patients with recurrent MRSA infections, a decolonization regimen of twice-daily mupirocin administered intranasally, chlorhexidine washes, and personal decontamination of clothes, sheets, and other personal items daily may be recommended.
Abscesses are a common complication of skin and soft tissue infections that frequently are encountered in the emergency department. The authors discuss current considerations in the diagnosis and management of abscesses, including recurrent abscesses and the role of ultrasound and antibiotics.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Case Study
An 18-month-old, previously healthy girl presents to an emergency facility with a one-day history of swelling and redness on her buttock. Her mother reports that the child had a similar lesion about three months earlier that required outpatient incision and drainage, followed by a short course of oral antibiotics. She does not remember which antibiotics were used at that time.
The girl’s temperature is 38°C, but her vital signs are otherwise normal. On examination, the child is fussy and uncomfortable but consolable. There is a discrete 1.5-cm-sized spot of erythema with underlying fluctuance on the left buttock under her diaper. The lesion is exquisitely tender. She has no other rashes or skin findings.
Introduction
Abscesses are a common complication of skin and soft tissue infections (SSTIs) in children and adults. Providers frequently may encounter SSTIs with such attendant complications in emergency department, urgent care, primary care, and inpatient settings. Skin and soft tissue abscesses may be characterized by their routes of inoculation and invasion, anatomic sites and associated pathogenesis, the presence or absence of specific microbial agents, and recommended management options, including incision, drainage, and empiric and targeted antibiotic therapy.
This review will discuss all of these considerations along with specific diagnostic steps and considerations regarding unique hosts and the management of new and recurrent abscesses.
Pathogenesis and Clinical Manifestations of Skin and Soft Tissue Abscesses
SSTIs may result from a variety of processes in which bacterial pathogens invade the skin and its supporting structures.1 Most commonly, this process is instigated by trauma or disruption to the integument. Such traumatic inoculation of bacteria may occur following even minor scrapes and abrasions. In other situations, bacteria my become introduced to the underlying integument through inflamed or ingrown hair follicles, obstruction of lactiferous ducts in the case of breast abscesses, traumatized nail folds and beds, or as a post-surgical complication following major and minor procedures.
Following the initial invasion, the host organism musters an inflammatory response that consists of the recruitment of neutrophils at or near the portal of entry.2 The ensuing inflammatory process may generate a collection of dead white blood cells, more commonly referred to as pus, and the formation of which may generate a fluid-filled cavity within the inflamed integument and surrounding structures. A fibrous capsule subsequently develops that surrounds the dead cells to contain the infection and its inflammatory consequences.2 In some situations, and particularly in the immunocompromised, systemic spread of bacteria may follow this initial complication, leading to bacteremia and sepsis.
While an abscess is a general term for a hollow, intraparenchymal pus-filled cavity, there are many different types and causes of abscesses.
Folliculitis is caused by a superficial infection of hair follicles. Risk factors include tight clothing that traps sweat and heat, soaking in a pool or hot tub that is not well-maintained, damage to the hair follicles, dermatitis or hyperhidrosis, and immunocompromising conditions.3 Symptoms in the affected region may include multiple pustular lesions that may burst and crust over, an itchy and burning sensation, and painful and tender skin.3 (See Figure 1.)
Figure 1. Hot Tub Folliculitis |
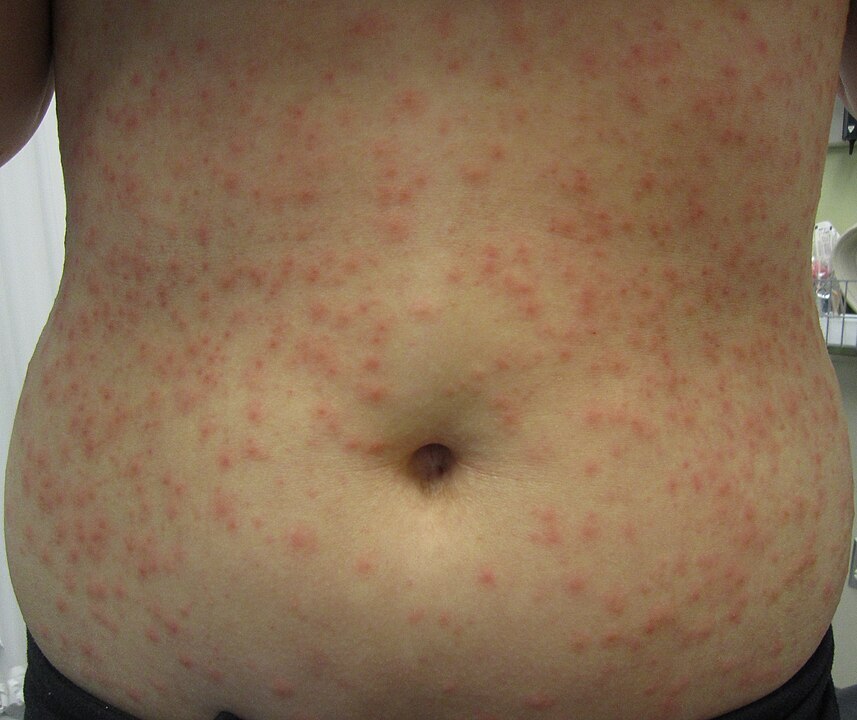 |
Source: Heilman J. Hot tub folliculitis. Published March 8, 2013. https://en.wikipedia.org/wiki/Hot_tub_folliculitis#/media/File:Folliculitis.JPG. CC BY-SA 3.0 |
Furunculosis likewise is an infection of the hair follicle, but it penetrates deeper into the skin.3 Its presentation is similar to folliculitis, and typically begins as such, but it may develop central necrosis and rupture through the skin with subsequent drainage to the surface. (See Figure 2.)
Figure 2. Furunculosis |
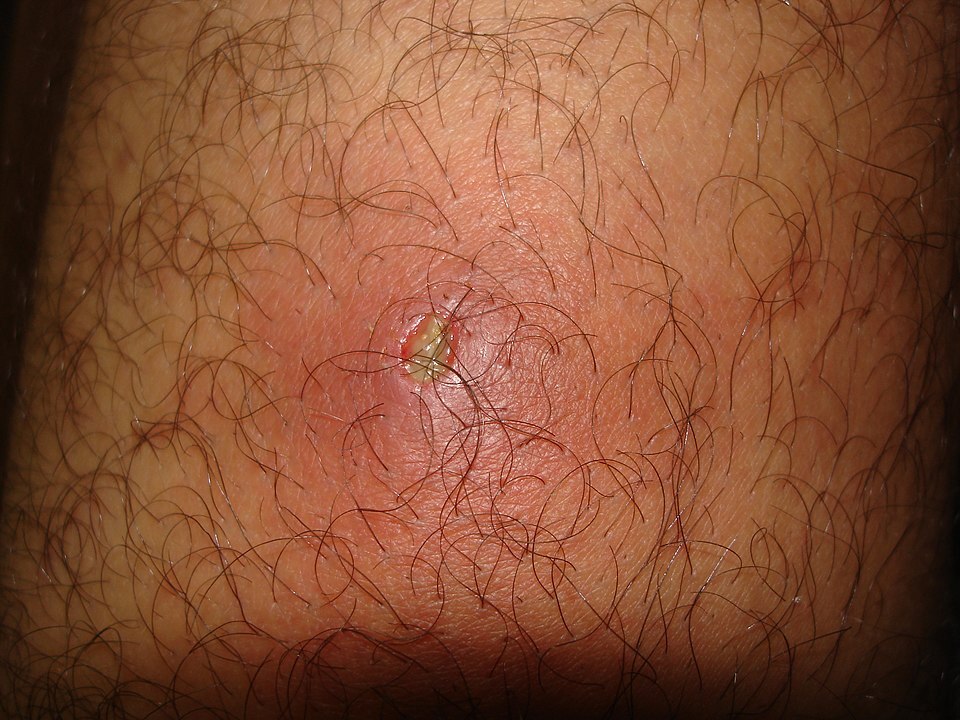 |
Source: Mahdouch. [Example of a boil.] Published March 8, 2013. https://en.wikipedia.org/wiki/Boil#/media/File:Furoncle.jpg. CC-BY-1.0 |
Paronychia is an infection of the fingernail and toenail folds. It can occur due to trauma or may occur spontaneously. It can be classified as acute paronychia, which lasts less than six weeks, or chronic, which is more common in immunocompromised individuals or those with other risk factors, including repeated trauma to the nails and/or prolonged submersion in water — such as may be found among dishwashers, housekeepers, and manual laborers. Other forms of trauma to the nailbeds and periungual regions, such as manicures, artificial nails, or nail-biting, also may lead to infection. Paronychia overall is more common in women, with middle-aged women at the highest risk of infection.4 (See Figure 3.)
Figure 3. Paronychia |
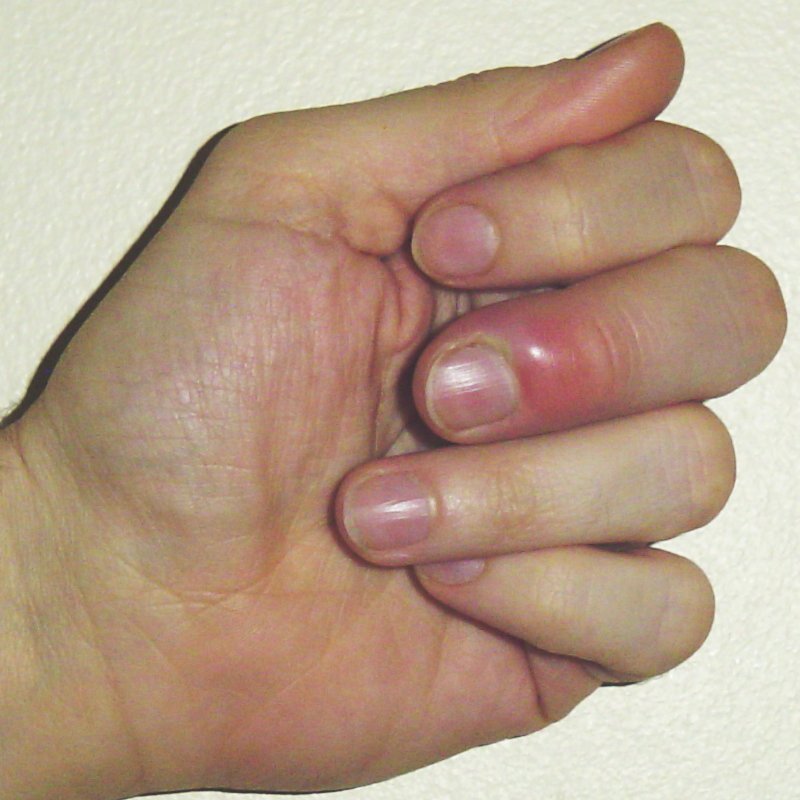 |
Source: Craig C. Paronychia. Published March 29, 2007. https://commons.wikimedia.org/wiki/File:Paronychia.jpg |
Breast abscesses are most common in lactating women, but they can occur in non-lactating women and neonates as well. Abscesses are caused as a result of the lactiferous ducts becoming obstructed, usually due to increased keratin production, which leads to abscess formation. Trauma to the nipple and surrounding tissue caused by a breastfeeding infant can act as the portal of entry. Women are encouraged to continue expressing milk from the affected ducts. As for non-lactating women, there is a wider range of causes. This includes strong association with diabetes and/or smoking. A more insidious etiology, such as carcinoma, also may be implicated.5, 6 Neonates also are susceptible to mastitis and breast abscess in the first six weeks of life, which can be a consequence of exposure to maternal estrogen, resulting in physiologic breast hypertrophy.7
Pilonidal abscesses are caused by infections in the crease of the buttocks near the coccyx.8 Much like folliculitis, these abscesses may develop as complications to infections introduced by friction/trauma, heat, or sweat that is trapped against the skin. It also can be related to folliculitis of thick, stiff hair in the area. Risk factors for such infections include poor hygiene, obesity, sitting for long periods of time, and/or a sedentary lifestyle. Up to 75% of patients with pilonidal cysts are men, and young men aged 20 to 25 years are at greatest risk. Prevention includes regular washing, maintaining a healthy weight, shaving of the affected area, and avoiding prolonged sitting.
SSTIs with abscesses also are potentially a result of traumatic inoculation from surgical or other bedside procedures. Poor wound care following procedures increases the likelihood of such complications, but immunocompromising conditions or systemic diseases that compromise wound healing, such as diabetes or tobacco use, also increase risk significantly.9
Microbiology of Skin and Soft Tissue Abscesses
The majority of all cutaneous abscesses are due to Staphylococcus aureus.10 A significant, albeit smaller percentage of these infections can be ascribed to methicillin-resistant S. aureus (MRSA) strains. Risk factors for transmission of MRSA include situations with crowding, frequent skin-to-skin contact, sharing of personal items (e.g., towels or razors), and contexts like athletic, incarceration, or military training facilities.11
However, some abscesses may be polymicrobial in nature, or other specific pathogens may be implicated. To adequately manage skin and soft tissue abscesses, providers should understand how various pathogens may be implicated depending on the location and circumstances of these infections.
Folliculitis
Superficial folliculitis is most commonly caused by infection of superficial hair follicles with S. aureus bacteria. Both MRSA and methicillin-sensitive (MSSA) strains are known to cause superficial folliculitis, with potential association of either strain with carriage via the nasal cavity and skin of the host organism.12 Deep folliculitis (furunculosis) can form carbuncles, which also are most often caused by S. aureus, again with a proposed association of skin and nasal carrier status, especially in cases of chronic, recurrent furunculosis.13
Malassezia (pityrosporum) folliculitis is a fungal acneiform condition caused by yeasts of the genus Malassezia, formerly Pityrosporum. These species usually represent part of the normal skin flora that has experienced overgrowth, resulting in acneiform eruptions of the skin. This commonly is misdiagnosed as acne vulgaris and is most commonly related to immunosuppression or antibiotic use. Of note, Malassezia also is implicated in various dermatological diagnoses, including head and neck dermatitis, seborrheic dermatitis, and pityriasis versicolor.14
Hot tub folliculitis is an opportunistic infection associated with hot tubs, whirlpools, and swimming pools and classically is caused by Pseudomonas aeruginosa, which is known to colonize and proliferate in wet and hot environments. A much rarer cause of hot tub folliculitis is Aeromonas hydrophila, which also has been associated with poorly chlorinated home spas and pools and moreover has been documented in several case reports as an independent cause of purulent folliculitis.15,16
Other bacterial causes of folliculitis include lactose-fermenting, gram-negative rods, including Klebsiella, Escherichia, and Serratia species. Cases associated with Citrobacter and Morganella species, among other organisms of the Enterobacterales family, also have been described, although less commonly. Notably, Proteus species may invade the skin more deeply, resulting in larger, more suppurative abscess formation and the propensity for cystic lesion formation.16
As previously described, eosinophilic folliculitis and pseudofolliculitis barbae do not have an identified microbial etiology.
Paronychia
Paronychia most commonly is attributed to infections with gram-positive bacilli S. aureus and Streptococcus pyogenes (Group A Streptococcus). However, Pseudomonas pyocyanea and Proteus vulgaris also have been infrequently implicated in cases of paronychia. For patients with risk factors for exposure to oral flora (such as nail-biting), oropharyngeal anaerobes and gram-negative bacteria also may be a possibility.17
Breast Abscess
S. aureus is the most common isolated pathogen with relation to breast abscesses, with recent studies showing increasing incidence in community-acquired MRSA infections. Additional pathogenic organisms to be considered include coagulase-negative Staphylococcus, diphtheroid skin flora, P. aeruginosa, Proteus mirabilis and, rarely, anaerobes like Cutibacterium acnes and Peptostreptococcus anaerobius. Breast abscess formation also can occur with idiopathic granulomatous mastitis followed by polymicrobial infection with skin flora.18,19
Pilonidal Abscess
The most commonly reported bacteria cultured from pilonidal abscesses differ by study. Frequently implicated species include polymicrobial anaerobic, gram-negative and gram-positive enteric species, such as Bacteriodes and enterococci, as well as S. aureus.20
Clinical Manifestations and Pertinent History
Patients with skin and soft tissue abscesses typically experience localized symptoms, such as erythema, edema, warmth, tenderness, and pain. They also may observe purulent drainage. In more severe cases, systemic signs of infection, including chills, fever, and malaise, may be present.
Physical examination may disclose signs of inflammation, such as localized erythema, warm skin, and edema with induration. They also may identify fluctuance, indicating the presence of a fluid-filled cavity within the skin and integument. Tenderness and pain upon palpation are expected. The severity of pain sometimes can provide insights into the extent or seriousness of the infection, although this assessment is subjective.
Furthermore, it is crucial to examine the size, shape, and surrounding skin of the abscess. Concurrent SSTIs, such as cellulitis, should be evaluated. Close attention should be paid to fistulization or other openings of the abscess, since they may aid in diagnosis depending on the location. If present, practitioners should inspect any purulent drainage for color, consistency, and odor. Lymphatic streaking, regional lymphadenopathy, tenderness, or firmness also should be assessed, since they may indicate the spread of infection to nearby lymph nodes.
In cases of deeper infections, there may be limited physical examination findings, emphasizing the importance of obtaining a detailed history and discussing the patient's pain and any systemic symptoms.
When gathering the history, providers should inquire about the location of pain, warmth, discoloration, and any changes in size noted by the patient. Providers also should be sure to ask whether the patient recalls any trauma to the area because of the possibility of contamination or inoculation with unusual bacterial agents. For breast abscesses, information about routine breast cancer screenings, family history of breast cancer, breastfeeding status, and potential causes of fat necrosis injury to the area is relevant to determine the likelihood of inflammatory breast carcinoma or benign causes of breast masses, as opposed to abscess formation.
Determining whether this is the first instance of an abscess or skin/soft tissue infection is important. If not, providers should explore prior resolution or treatment of previous infections, their location, and their frequency and severity. Furthermore, recent surgeries, travel, hobbies, insect bites, and exposures to people, animals, and irritants should be investigated. Reviewing the patient's current medications and supplements, particularly chronic antibiotic therapy or immunosuppressive agents, is essential. Additionally, a thorough assessment of the patient's family, social, and medical/surgical history can provide valuable insights, such as risk factors for acute bacterial SSTIs. Risk factors include obesity, diabetes mellitus, intravenous drug use, MRSA nasal carriage, and tobacco use.21
Diagnosis
The approach to a patient with an SSTI with or without concern for abscess requires careful integration of history, physical examination, laboratory studies, and potentially imaging to develop a narrow, reasonable differential diagnosis. Initial laboratory studies, such as a complete blood cell count with differential (CBCd) and a complete metabolic panel (CMP) can provide insight into systemic involvement based on the presence of leukocytosis or potential acid-base disturbances as well as baseline liver function tests in preparation for potential antibiotic therapy.
For uncomplicated bacterial SSTIs, such as superficial skin abscesses and definitive cellulitis, imaging is not required for diagnosis. However, in situations of recurrence, failure of treatment, less definitive superficial skin abscesses or uncertainty over diagnosis, imaging can provide better clarity. Plain radiographs, while generally a poor option for viewing soft tissue pathology, may be able to discern gas in subcutaneous or deeper surrounding tissues, which would increase concern for the presence of anaerobic bacterial infection. Compared to X-rays, however, computed tomography (CT) and magnetic resonance imaging (MRI) provide much better visualization of abscess cavities, areas of involvement, possible sites of necrosis that may warrant debridement, involvement of adjacent bone or joints, and inflammation in deeper soft tissues, particularly when performed with administration of intravenous contrast.22
Ultrasound has proven to be a useful and dynamic imaging modality with the ability to guide needle drainage of abscesses.21 Moreover, point-of-care ultrasound technology can establish a diagnosis quickly in clinical scenarios, where potential differentiation between an abscess and a more superficial skin or soft tissue infection is unclear. In addition, it may help distinguish the feasibility of bedside incision and drainage for more superficial collections vs. those that are deeper or more complex (bordering important anatomic structures) that would benefit from operative intervention.
On ultrasound imaging, an abscess will appear as a spherical or oblong anechoic to hypoechoic organized collection with hyperechoic debris and potentially a hyperechoic rind around the collection.23,24 Color flow also will help distinguish abscesses from vascular phenomena, like pseudoaneurysms, which could confound diagnosis and management in intravenous drug users and other groups.
Incision and Drainage
Most patients with an abscess should have an incision and drainage performed accompanied with antibiotics. In the case of smaller pus collections, manual expression of pus and conservative management in the use of antibiotics may be considered.25 It should be noted that medical management alone may lead to failure of therapy, often because of the inability of achieving adequate inhibitory concentrations of antibiotics within the center of the fluid collection.
Large and deep abscesses and those in areas that make it difficult to perform an incision and drainage should receive surgical consultation. This includes sensitive areas, such as the face, palms, and soles, as well as areas with a high potential for complications, such as perirectal and periareolar abscesses, which could develop fistulas. A specialist also should evaluate neck abscesses, which may have developed from preexisting cystic lesions.25
The procedure generally can be performed by a single clinician. Support staff may be warranted, especially with young children or neurodevelopmentally delayed individuals who are unable to tolerate the procedure. Child life specialists may be helpful support because their presence has been demonstrated to decrease emotional distress.26
Consent should be obtained, and the risks of the procedure should be explained to the patient and their family, which include bleeding, pain, and possible scar formation. Tetanus immunization should be verified. The clinician performing the procedure should undertake universal and contact precautions by donning a gown, gloves, and a face shield.25 The site should be adequately prepared and anesthetized.
Using a scalpel with a steady grip, an incision should be created directly over the center of the abscess until pus is expressed. This should be performed parallel to skin tension lines to minimize scarring. A curved hemostat can be used thereafter for blunt dissection to disrupt loculations within the cavity. Manual expression may help facilitate drainage. After the drainage, the wound should receive copious irrigation with normal saline.
Wound packing is not recommended for abscesses that are 5 cm or less in diameter, since it has not been shown to affect outcomes of the procedure but may lead to increased pain and does not decrease the risk of abscess recurrence.27,28 The site then should be covered with sterile dressing and tape. A packed wound should receive a follow-up two to three days after the procedure to remove the packing. Wounds then are left to close by secondary intention.25
Needle aspiration may be used instead of incision and drainage, but the procedure is overall less effective than an incision and drainage. The success of needle aspiration drainage was 26% compared to an 80% success rate for patients who underwent an incision and drainage according to a randomized, controlled study.29
Topical antibiotics may be used to treat folliculitis when the number of abscesses is limited, but they are especially effective when combined with incision and drainage. Systemic antibiotics should be used when symptoms such as fever, lymphadenitis, or widespread inflammation and abscesses occur.14 Phototherapy, using a monochromatic excimer light at 308 nm with a minimal erythema dose between 0.5-2, may be used for superficial folliculitis with only mild adverse effects, such as localized erythema, reported.30
Similar to folliculitis, furunculosis may be treated effectively with incision and drainage, especially when accompanied with local warming to increase drainage, followed by antibiotic therapy. The recurrence of furuncles can be prevented by using a chlorhexidine and isopropyl scrub.31
Paronychia should be incised and drained, while using warm soaks before and during the procedure to increase drainage. This should be followed by a course of antibiotics. If paronychia presents with inflammation with no clear abscess, then warm soaks with water or an antiseptic solution may be used along with antibiotics. Chronic paronychia may be treated with a topical antifungal along with avoiding trauma and friction to the affected area.4
Most breast abscesses, and especially those presenting with systemic symptoms, should receive surgical intervention and potential wound packing. Likewise, periareolar abscesses warrant surgical consultation.5
Pilonidal abscesses can be treated by incision and drainage, with postprocedural wound care being a paramount consideration. Regular wound care, such as dressing changes, washing, and shaving, should be employed to prevent recurrence. Because of their generally high rate of recurrence, outpatient surgical consultation often is necessary and should be considered.32
Antibiotic Therapy
Treatment guidelines primarily are based on the Infectious Diseases Society of America (IDSA) practice guidelines for SSTIs. For the treatment of purulent SSTIs, such as cutaneous abscesses, furuncles/boils, and carbuncles, providers should obtain specimens from any pus expressed during drainage to perform Gram stains and aerobic and anaerobic culture to direct therapy. However, empiric therapy without diagnostic studies also is reasonable for typical or mild presentations.
The IDSA practice guidelines recommend adjunctive antibiotic treatment following incision and drainage based on the presence or absence of systemic symptoms, such as tachycardia, temperature > 38°C or < 36°C, tachypnea, and leukocytosis > 12,000 cells/µL or severe leukopenia with < 400 cells/µL. Immunocompromised patients and other high-risk populations, such as patients with human immunodeficiency virus/acquired immunodeficiency syndrome, undergoing chemotherapy, or taking immunosuppressive medications, may require antibiotics to manage even mild or moderate infections because of their increased susceptibility to bacterial infections and may have limited clinical signs of more severe infection, such as with demonstrating systemic inflammatory response syndrome (SIRS) criteria, as noted previously. An additional special group to consider is patients presenting with carbuncles or abscesses who have failed initial treatment. For these specific groups of patients (patients with SIRS criteria and hypotension, immunocompromised patients, and patients with previously failed antibiotic treatment), empiric antibiotic therapy should include coverage against MRSA.10
The IDSA has additional treatment guidelines for recurrent skin abscesses. In these cases, searching for a local cause of recurrence, such as pilonidal cysts, hidradenitis suppurativa, or foreign encapsulated material, is recommended with early drainage and culture of the abscess. Treatment in the setting of recurrence should be initiated following obtaining cultures with a plan for a five- to 10-day course of antibiotics with activity against the pathogen isolated.
For patients with recurrent MRSA infections, a decolonization regimen of twice-daily mupirocin administered intranasally, chlorhexidine washes, and personal decontamination of clothes, sheets, and other personal items daily may be recommended. Additionally, patients with recurrent or abnormally severe suppurative infections may warrant evaluation for primary immunodeficiencies and other neutrophil disorders.10
Empiric regimens should be tailored to the type of infection and the most likely organisms associated with each, as discussed previously. (See Table 1.) For infections suspicious for MSSA, first-generation cephalosporins, such as oral cephalexin or intravenous cefazolin, will suffice. Adequate coverage also typically is provided by clindamycin, trimethoprim-sulfamethoxazole, or even amoxicillin-clavulanate.
Table 1. Empiric Antibiotic Therapy Choices for Abscesses Associated with Suspected Pathogens33-38 |
||
| Abscess Type/Location | Suspected Pathogens | Options for Oral Empiric Antibiotic Therapy* |
Skin/soft tissue: Includes folliculitis, furunculosis, paronychia, breast abscess, and postoperative wound-associated abscesses |
MSSA |
Cephalexin 25 mg/kg/day to 100 mg/kg/day divided every 6 to 8 hours (max dose 4,000 mg per day) OR TMP-SMX** 8 mg to 12 mg TMP/kg/day divided every 12 hours (max dose 320 mg TMP) OR Clindamycin*** 25 mg/kg/day to 30 mg/kg/day divided every 8 hours (max oral dose 450 mg) |
MRSA |
TMP-SMX 8 mg to 12 mg TMP/kg/day divided every 12 hours (max dose 320 mg) OR Clindamycin*** 30 mg/kg/day to 40 mg/kg/day divided every 8 hours (max dose 600 mg) OR Doxycycline**** ≤45 kg: 4 mg/kg/day divided every 12 hours; > 45 kg: 100 mg twice daily for 5 to 10 days |
|
Pseudomonas |
Ciprofloxacin 20 mg/kg/day to 40 mg/kg/day divided every 12 hours (max 1,500 mg/day) OR Levofloxacin 6 months to < 5 years of age: 8 mg/kg/day to 10 mg/kg/dose twice daily; ≥ 5 years of age: 10 mg/kg/dose once daily (max dose: 750 mg/day) |
|
Pilonidal cysts |
Enteric gram-negative pathogens, enteric anaerobes |
Amoxicillin-clavulanate 7:1 formulation: 25 mg to 45 mg amoxicillin/kg/day divided every 12 hours (max 1,750 mg/day) |
MSSA: methicillin-sensitive Staphylococcus aureus; TMP-SMX: trimethoprim-sulfamethoxazole; MRSA: methicillin-resistant Staphylococcus aureus *Antibiotic therapy always should be modified based on isolate susceptibilities and local resistance patterns. In uncertain cases or highly resistant organisms, consultation with infectious disease specialists may be necessary. **Not for use in children 2 months of age and younger ***Should be used with caution/in conjunction with susceptibility data in areas where Staphylococcus aureus clindamycin resistance rates exceed 10% to 15% ****Should not be used in pregnant patients; the American Academy of Pediatrics advises that the risk of tooth staining is minimal for courses of less than 21 days in children younger than 8 years of age |
||
For infections suspicious for MRSA, oral empiric treatment options include trimethoprim-sulfamethoxazole, doxycycline, and clindamycin. It should be noted that local resistance rates for MRSA against clindamycin may obviate this option — some experts recommend avoiding empiric clindamycin use for MRSA infections in areas where resistance rates exceed 10% to 15%.36 Additionally, contraindications to specific antibiotic choices (e.g., pregnancy in the case of doxycycline) should be acknowledged. More severe infections may necessitate intravenous antibiotic therapy against MRSA, which includes vancomycin, daptomycin, linezolid, televancin, or ceftaroline.
While tooth discoloration caused by tetracycline use in young children frequently is cited as a potential contraindication, it should be noted this side effect is exceptionally rare when using the antibiotic class for short durations; the American Academy of Pediatrics advises that courses of doxycycline shorter than 21 days are not contraindicated in children younger than 8 years of age.37,38
Concern for infection caused by Pseudomonas requires additional coverage with oral fluoroquinolones, such as ciprofloxacin or levofloxacin.10 Intravenous coverage in more severe or resistant pseudomonal infections can be provided by ceftazidime, cefepime, or carbapenem-class antibiotics.
As discussed previously, certain situations will implicate the potential for enteric anaerobes and gram-negative bacteria, such as pilonidal cysts. In these cases, amoxicillin-clavulanate often will provide sufficient coverage for these species. Another option includes cephalexin and metronidazole, which will provide excellent MSSA and anaerobic coverage. In patients with allergies or contraindications against beta-lactams, fluoroquinolones or trimethoprim-sulfamethoxazole combined with metronidazole also may be a reasonable option for empiric therapy.
It is important to note that the choice of empiric treatment may vary depending on local resistance patterns and specific patient factors, such as allergies or medication intolerance. Culture and sensitivity results, when available, always should guide the selection of antibiotics based on the identified pathogens and their susceptibility profiles.
Duration of therapy ultimately should be based on an individual's clinical response, but most patients typically require five to seven days for straightforward infections. Treatment up to 14 days is reasonable in the case of severe infection, recurrence, immunosuppression, or slow clinical response to treatment. Of note, recent clinical trials have provided evidence to support adjunctive antibiotic use following incision and drainage with demonstration of significantly higher likelihood of clinical cure. Additional analyses also have found that courses of antibiotics beyond five to seven days, in conjunction with incision and drainage, were associated with significantly decreased incidence of abscess recurrence or new formation of abscess at approximately six weeks follow-up. These findings have been suggested to be secondary to decreasing S. aureus burden or eradicating prior colonization that may lead to recurrence or new infection.39
Considerations in Recurrent Infections
Patients with recurrent abscesses may warrant further work-up. Such patients may require screening for a primary immunodeficiency. More commonly, secondary immunodeficiency may complicate these patients’ presentations, including a variety of medical conditions, such as metabolic syndrome and diabetes mellitus. Iatrogenic causes of secondary immunodeficiency, such as disease-modifying antirheumatic drugs, drug-induced leukopenia, and chronic steroid use, also should be interrogated. Finally, mimics of skin and soft tissue infections, such as cutaneous ulcerations secondary to venous insufficiency and pyoderma gangrenosum, should be considered as potential misleading conditions.
While uncommon, patients with recurrent abscesses and certain historical findings warrant further work-up for primary immunodeficiency as the underlying etiology. Onset of recurrent cutaneous abscesses during infancy or a history of recurrent abscesses at an early age should increase clinical suspicion for a primary immunodeficiency. One important consideration is chronic granulomatous disease, a functional neutrophil defect that can be interrogated through nicotinamide adenine dinucleotide phosphate oxidase testing. A history of atopic dermatitis with recurrent infections can raise suspicion for hyper-immunoglobulin E syndrome and Wiskott-Aldrich syndrome in male patients. Additional clues for immune deficiency can arise with the failure of an expected response to antimicrobial therapy. Specific sites of infection, such as recurrent perianal abscesses, or concern for enterocutaneous abscess formation may suggest underlying inflammatory bowel disease, rather than immunodeficiency.40 For patients with a suspicious clinical course and history for primary immunodeficiency, referral to an infectious disease or immunology specialist can be especially helpful.41
Finally, unusual presentations or microbiologic etiologies with recurrences may warrant differential consideration of a psychogenic etiology, such as factitious disorder, if immunologic and other work-up for recurrence proves negative.42,43
Recurrence specifically in the same location, either with recurrent breast abscesses in the setting of potential granulomatous mastitis or recurrent pilonidal cyst formation, often warrants evaluation for surgical intervention. There are little to no data to support the use of chronic prophylactic antibiotics in the setting of recurrent skin abscesses.44
Conclusion
Providers frequently will encounter abscesses as complications of SSTIs. While bedside incision and drainage frequently are mainstays of management, the considerations discussed in this review will help determine when surgical consultation is recommended, which empiric antibiotics may be helpful, and when further work-up or concern for immunodeficiency should be considered.
Case Study Conclusion
A point-of-care ultrasound is performed, confirming the presence of a 2-cm diameter loculated fluid collection with associated cellulitis over the left buttock, with no uptake on color Doppler. The physician recommends an incision and drainage of the lesion, followed by a course of empiric antibiotics. After initially discussing the risks and benefits of sedation vs. local anesthesia, the parents agree to local anesthesia with assistance from child life specialists. The procedure is completed uneventfully, and the physician is able to drain several milliliters of purulent material and break up internal loculations appropriately. The child tolerates the procedure well, and the wound is dressed. The purulent material is sent for aerobic and anaerobic cultures, and the child initially is sent home on oral cephalexin.
After 24 hours, the laboratory reports that the child’s cultures are growing MRSA. The physician calls the family and the child’s primary care provider, and it is agreed that the patient should be switched to trimethroprim-sulfamethoxazole to complete a five- to seven-day course of antibiotics. Given the recurrence of abscesses for this child, the family also is recommended to undergo household MRSA decolonization. The primary care provider agrees to follow up with an immunologist regarding whether the child warrants further testing for a primary immunodeficiency.
References
- Ramakrishnan K, Salinas RC, Agudelo Higuita NI. Skin and soft tissue infections. Am Fam Physician 2015;92:474-483.
- Long B, Gottlieb M. Diagnosis and management of cellulitis and abscess in the emergency department setting: An evidence-based review. J Emerg Med 2022;62:16-27.
- James WD, Elston D, Treat JR, Rosenbach MA. Bacterial infections. Andrews’ Diseases of the Skin. 13th ed. Elsevier; 2020.
- Dulski A, Edwards CW. Paronychia. StatPearls [Internet]. Updated Aug. 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK544307/
- Toomey A, Le JK. Breast abscess. StatPearls [Internet]. Updated June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK459122/
- [No authors listed]. Practice bulletin No. 164: Diagnosis and management of benign breast disorders. Obstet Gynecol 2016;127:e141-e156.
- Stricker T, Navratil F, Sennhauser FH. Mastitis in early infancy. Acta Paediatr 2005;94:166-169.
- Surrell JA. Pilonidal cyst and abscess: Current management. In: Fowler GC, ed. Pfenninger and Fowler’s Procedures for Primary Care. 4th ed. Elsevier; 2020:211-212.
- Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surgery 2017;152:784-791.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10-e52.
- Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Staphylococcus aureus. Red Book 2021-2024 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics;2021:678-691.
- Toshkova K, Annemüller C, Akineden O. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol Lett 2001;202:17-24.
- Lin H-S, Lin P-T, Tsai Y-S, et al. Interventions for bacterial folliculitis and boils (furuncles and carbuncles). Cochrane Database Syst Rev 2018;2018:CD013099.
- Saunte DM, Gaitanis G, Hay RJ. Malassezia-associated skin diseases, the use of diagnostics and treatment. Front Cell Infect Microbiol 2020;10. doi: https://doi.org/10.3389/fcimb.2020.00112
- Olszewski AE, Karandikar MV, Surana NK. Aeromonas as a cause of purulent folliculitis: A case report and review of the literature. J Pediatric Infect Dis Soc 2017;6:e1-e3.
- Tarlow MM. Gram-negative folliculitis. Medscape. Updated Dec. 14, 2022. https://emedicine.medscape.com/article/1055221-overview
- Rigopoulos D, Larios G, Gregoriou S, Alevizos A. Acute and chronic paronychia. Am Fam Physician 2008;77:339-346.
- Taylor GB, Paviour SD, Musaad S, et al. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between Corynebacteria infection and granulomatous mastitis. Pathology 2003;35:109-119.
- Moazzez A, Kelso RL, Towfigh S. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch Surg 2007;142:881-884.
- Brook I. Microbiology of infected pilonidal sinuses. J Clin Pathol 1989;42:1140-1142.
- Watkins RR, David MZ. Approach to the patient with a skin and soft tissue infection. Infect Dis Clin North Am 2021;35:1-48.
- Saber AA. Abdominal abscess clinical presentation. Medscape. Updated July 6, 2022. https://emedicine.medscape.com/article/1979032-clinical
- Euerle BD. Abscess evaluation. ACEP Sonoguide. Published Aug. 18, 2020. https://www.acep.org/sonoguide/procedures/abscess-evaluation
- O’Rourke K, Kibbee N, Stubbs A. Ultrasound for the evaluation of skin and soft tissue infections. Mo Med 2015;112:202-205.
- Pastorino A, Tavarez MM. Incision and Drainage. StatPearls [Internet]. Updated June 25, 2022. https://www.ncbi.nlm.nih.gov/books/NBK556072/
- Hall JE, Patel DP, Thomas JW, et al. Certified child life specialists lessen emotional distress of children undergoing laceration repair in the emergency department. Pediatr Emerg Care 2018;34:603-606.
- Leinwand M, Downing M, Slater D, et al. Incision and drainage of subcutaneous abscesses without the use of packing. J Pediatr Surg 2013;48:1962-1965.
- Kessler DO, Krantz A, Mojica M. Randomized trial comparing wound packing to no wound packing following incision and drainage of superficial skin abscesses in the pediatric emergency department. Pediatr Emerg Care 2012;28:514-517.
- Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med 2011;57:483-491.e1.
- Nisticò SP, Saraceno R, Carboni I, Chimenti S. Treatment of folliculitis with monochromatic excimer light (308 nm). Dermatology 2008;218:33-36.
- Esposito S, Pagliano P. Bacterial skin infections. In: Rezaei N, ed. Encyclopedia of Infection and Immunity. Elsevier;2022:404-413.
- Humphries AE, Duncan JE. Evaluation and management of pilonidal disease. Surg Clin North Am 2010;90:113-124.
- Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Tables of antibacterial drug dosages. Red Book: 2021 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics; 2021:876-897.
- Jackson MA, Schutze GE; Committee on Infectious Diseases. The use of systemic and topical fluoroquinolones. Pediatrics 2016;138:e20162706.
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: Executive summary. Clin Infect Dis 2011;52:285-292.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med 2007;357:380-390. [Erratum in: N Engl J Med 2007 27;357:1357.]
- Todd SR, Dahlgren FS, Traeger MS, et al. No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr 2015;166:1246-1251.
- Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Antimicrobial agents and related therapy: Introduction. Red Book 2021-2024 Report of the Committee on Infectious Diseases. 32nd ed. American Academy of Pediatrics;2021:863-867.
- Lake JG, Miller LG, Fritz SA. Antibiotic duration, but not abscess size, impacts clinical cure of limited skin and soft tissue infection after incision and drainage. Clin Infect Dis 2020;71:661-663.
- Johnston SL Clinical Immunology Review Series: An approach to the patient with recurrent superficial abscesses. Clin Exp Immunol 2008;152:397-405.
- Fernandez J. Approach to the patient with suspected immunodeficiency - immunology; allergic disorders. Merck Manual Professional Version. Updated January 2023. https://www.merckmanuals.com/professional/immunology-allergic-disorders/immunodeficiency-disorders/approach-to-the-patient-with-suspected-immunodeficiency
- Flohr S, Agyeman PKA, Deppenthaler A. Recurrent Mycobacterium chelonae skin infection unmasked as factitious disorder using bacterial whole genome sequence analysis. Open Forum Infectious Diseases 2020;7:ofaa506.
- Yates GP, Feldman MD. Factitious disorder: A systematic review of 455 cases in the professional literature. Gen Hosp Psychiatry 2016;41:20-28.
- Spelman D, Baddour LM. Skin abscesses in adults: Treatment. UpToDate. Upated April 27, 2023. https://www.uptodate.com/contents/skin-abscesses-in-adults-treatment
Abscesses are a common complication of skin and soft tissue infections that frequently are encountered in the emergency department. The authors discuss current considerations in the diagnosis and management of abscesses, including recurrent abscesses and the role of ultrasound and antibiotics.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
