Diagnosing and Treating Pediatric Urinary Tract Infections
Authors
Victoria Branham, MD, Department of Emergency Medicine, University of North Carolina, Chapel Hill
Ethan Gerdts, MD, Department of Emergency Medicine, University of North Carolina, Chapel Hill
Daniel Migliaccio, MD, FDP, FAAEM, Clinical Assistant Professor, Director of Emergency Ultrasound, Ultrasound Fellowship Director, University of North Carolina, Chapel Hill
Peer Reviewer
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity, Samaritan, Troy, NY
Executive Summary
- Simple urinary tract infections (UTIs) can evolve quickly into widespread infections and are one of the most common causes of pediatric sepsis and bacteremia. Therefore, a prompt and accurate diagnosis and appropriate treatment are essential to prevent substantial mortality. If recurrent UTIs are undiagnosed or unrecognized, this infection can lead to chronic medical problems, such as renal scarring, hypertension, and chronic renal insufficiency.
- Escherichia coli is the most frequently identified pathogen, causing approximately 75% to 95% of cases, but other commonly identified bacteria include members of the Enterobacteriaceae family, such as Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus saprophyticus, and Enterococcus spp.
- Fecal retention can increase the risk of cystitis through the mechanical compression of the full rectum on the bladder, leading to displacement of the bladder and elongation of the urethra. That chain of effects reduces urinary flow, which promotes urinary stasis and pathogen adherence.
- When considering the population 2 years of age and younger, symptoms can be vague and include irritability, poor feeding, lethargy, jaundice, vomiting, and fever. Among this age group, the findings most suggestive of a UTI are the history of previous UTI(s), fever > 40°C, and suprapubic tenderness on examination.
- The simplest method for obtaining urine from non-toilet-trained children involves collecting urine via a sterile bag attached to the child’s perineum. This also is the method that is most frequently contaminated. A positive urine culture from a bagged specimen has a false-positive rate up to 75% from periurethral flora and, therefore, requires a confirmation culture with a catheterized or suprapubic aspiration specimen. The sensitivity and specificity of a catheterized specimen is significantly better than a bagged sample and has a specificity of 83% to 89%.
- Indications of UTI on a dipstick or urinalysis include the presence of leukocyte esterase and nitrites. Positive leukocyte esterase suggests inflammation, and white blood cell presence can be 84% sensitive and 78% specific for diagnosing UTIs. Positive nitrite indicates the presence of bacteria that convert nitrates into nitrites, most commonly gram-negative bacteria, and can be 50% sensitive and 98% specific for diagnosing UTIs.
- The urine culture remains the gold standard for diagnosing UTIs. Positive cultures can be defined as either more than 50,000 CFU/mL on a catheterized or suprapubic aspiration or more than 100,000 CFU/mL in a voided specimen.
- An antibiotic decision should be made based on careful consideration of the most common pathogens, local antibiogram sensitivity data, patient allergy profile, and potential side effects. From current available data, empiric antibiotics should have adequate coverage against E. coli, Klebsiella spp., and Proteus spp. — these all share the same gram-negative rod bacterial structure. Other common pathogens, such as S. saprophyticus and Enterococcus spp., are gram-positive cocci.
Urinary tract infections can be challenging to suspect and diagnose in young patients. Unfortunately, devastating consequences, such as pyelonephritis and bacteremia, are a real risk. It is critical for clinicians to have a high degree of suspicion, obtain optimal urine samples, and be aware of the best practices for treatment in this unique population.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
A urinary tract infection (UTI) is a common bacterial infection in both adult and pediatric patient populations.1 It is defined as the presence of bacteria in the urine, called bacteriuria, and is confirmed by a urine culture that yields at least 50,000 colony-forming units (CFU)/mL in a voided or catheterized specimen, although it often is assumed positive with urinalysis interpretation before culture data are available.2-6 Typically, a UTI can be diagnosed through obtaining a history, performing a physical exam, and interpreting a urinalysis; however, young children and infants are limited in their ability to provide the information required to make an easy diagnosis. A missed infection can have dire consequences, given the high rate of hematogenous spread. This can result in severe infections, such as pyelonephritis and bacteremia.1-3 emergency medicine physicians are challenged with maintaining a high index of suspicion for this diagnosis to prevent missed infections and recurrent symptoms, and to avoid the high morbidity associated with this type of infection.
Epidemiology
UTIs are the second-most common bacterial infection in the pediatric population, after otitis media.7 They account for more than 1.5 million clinician visits per year and cost the healthcare system, including the visit, diagnosis, and treatment, more than $180 million annually.8 The prevalence varies among sex, age, ethnicity, and circumcision status. In most populations, females are more likely to be diagnosed with a UTI than males, except for the first year of life when compared to uncircumcised boys. The incidence among females is an interesting bimodal distribution. It is highest in female toddlers between the ages of 1-2 years and in adolescence.9-10 During the first 12 months of life, the incidence of UTI is approximately 0.7% in girls and 2.7% in uncircumcised boys.1 This difference is even more pronounced among febrile infants during the first two months of life, with an incidence of 5% in girls and 20.3% in uncircumcised boys.1,11 As children age, the presentation reverses. Among prepubertal children, 3% of females and 1% of males are diagnosed with UTI.11 By 16 years of age, 11.3% of girls and 3.6% of boys will have had a UTI at some point in their lives.1
In a retrospective study of 37,450 febrile children presenting to emergency departments (EDs) performed by Bachur and Harper, the prevalence of UTIs was 2.1% overall (2.9% for girls and 1.5% for boys, respectively).12 Race also affects overall prevalence. In the same study, among girls, the prevalence of UTIs was 5.0% in white patients, 2.1% in Hispanic patients, and 1.0% in Black patients.12 Among boys, the prevalence was 2.2% in Hispanic patients, 1.4% in white patients, and 0.8% in Black patients. In terms of race, Hispanic and white children have a two-to-four-fold increased risk of being diagnosed with UTI compared to Black Americans.12
Simple UTIs can evolve quickly into widespread infections and are one of the most common causes of pediatric sepsis and bacteremia. Therefore, physicians are tasked with promptly and accurately diagnosing and treating this infection because it is associated with substantial mortality.1,7 If recurrent UTIs are undiagnosed or unrecognized, this infection can lead to chronic medical problems, such as renal scarring, hypertension, and chronic renal insufficiency.7 Additionally, once a child presents with their first UTI, they are at a 13.6% increased risk for experiencing a recurrent infection, which associates with the negative outcomes of school absenteeism, the need for parental leave, frequent healthcare visits, and likely leads to an increase in healthcare costs.13,14
Pathophysiology
UTIs generally are divided anatomically. An “upper” UTI, which must include the kidneys, is called pyelonephritis, whereas a “lower” UTI, which involves the bladder or distal ureter, is known as cystitis. Cystitis results from the colonization of the periurethral mucosa by bacteria, which is hypothesized to come from nearby structures, such as the rectal or vaginal flora, which then ascend into the urethra and eventually the urinary bladder.15 The severity of infection is a broad spectrum, ranging from a mild dysuria to life-threatening urosepsis and bacteremia.6,11,12 Lower UTIs involve the urethra (urethritis) and bladder (cystitis). Lower UTIs tend to be milder, whereas upper tract infections tend to be more severe, since they involve the ureters and kidneys (pyelonephritis). The majority of infections occur in the lower tract.16 Hematogenous spread of the pathogen usually occurs in debilitated, obstructed, or immunocompromised patients.17
Enteric pathogens tend to be the most common underlying causes of cystitis. Escherichia coli is the most frequently identified pathogen, causing approximately 75% to 95% of cases, but other commonly identified bacteria include species of the Enterobacteriaceae family, such as Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus saprophyticus, and Enterococcus spp.15 Table 1 highlights commonly detected uropathogens. The virulence of uropathogenic E. coli is driven mainly by the presence of lipopolysaccharide and P. fimbriae, also known as pyelonephritis-associated pili. P. fimbriae and lipopolysaccharide, respectively, bind to epithelial cells and transmembrane proteins present on parts of the renal tubule.18 The binding of these proteins leads to the release of proinflammatory cytokines and chemokines and recruitment of macrophages and neutrophils, resulting in inflammation of the urinary system.18 This proinflammatory response initiated by the macrophages and conducted by the neutrophils needs to occur to remove the pathogen. This inflammatory reaction is the etiology of the patient’s fever and dysuria that often accompany the diagnosis of UTI. Unfortunately, it also leads to damaging the surrounding tissue and, possibly, fibrosis and long-term scarring.18,19
Table 1. Common Uropathogens8 |
||||
| Male Inpatient | Male Outpatient | Female Inpatient | Female Outpatient | |
Most common |
Escherichia coli |
Escherichia coli |
Escherichia coli |
Escherichia coli |
Enterococcus |
Enterococcus |
Enterococcus |
Enterococcus |
|
Klebsiella |
Proteus mirabilis |
Klebsiella |
Klebsiella |
|
Pseudomonas aeruginosa |
Escherichia coli |
Pseudomonas aeruginosa |
Proteus mirabilis |
|
Enterobacter |
Pseudomonas aeruginosa |
Enterobacter |
Pseudomonas aeruginosa |
|
Least common |
Proteus mirabilis |
Enterobacter |
Proteus mirabilis |
Enterobacter |
Etiology
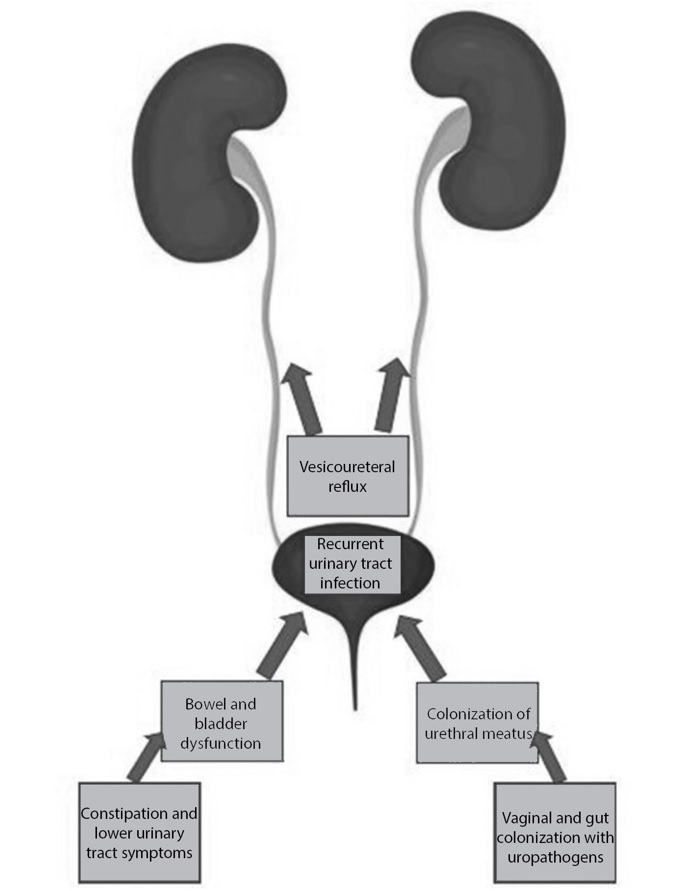
There are predisposing conditions and specific risk factors unique to the pediatric population that must be addressed. As discussed, UTIs typically result from bacteria colonizing the urethral meatus and ascending to cause further infection of the bladder, ureters, and, possibly, the kidneys. The surrounding gastrointestinal and vaginal tracts serve as reservoirs for these common uropathogens.20 Events that cause translocation or local disruption of this bacteria can lead to an increased likelihood of developing a UTI. For example, sexual activity can disrupt bacteria near the urethral orifice, increasing the risk of developing an infection in sexually active patients, most commonly adolescent females.9,10 Other risk factors more common among the adolescent population include a history of diabetes and kidney stones.20 Figure 1 demonstrates a good overview of common risk factors associated with the development of UTIs.
Figure 1. Risk Factors Associated with Urinary Tract Infections |
 |
When considering infant patients, both females and uncircumcised males are at an increased risk of UTIs because of the bacterial skin concentration contained within a wet diaper.10 Female patients have shorter urethras, measured from the urethral meatus to the bladder, compared to males, which makes it easier for bacteria to travel through the urogenital system. Uncircumcised males also are at increased risk because of the increased foreskin surface area, which means more skin flora to infect neighboring areas.10 Antibiotics also can lead to an increased risk of infection because they alter local bacteria strains and wipe out normal flora, which allows abnormal pathogens to colonize and proliferate. For instance, females have protective Lactobacillus species strains within their vagina; however, taking antibiotics can disrupt local flora, leading to reduced protective Lactobacillus counts and, thus, increased enteric bacteria and risk of UTI.13,21
In addition to anatomical risk factors, bowel and bladder dysfunction are highly underdiagnosed pediatric conditions that can lead to an increased risk of developing a UTI.22 Bowel and bladder dysfunction refers to a combination of lower urinary tract symptoms and bowel disorders, such as constipation, encopresis, enuresis, and general incontinence without underlying neurologic disorder or abnormality.13,23 It is important to note, however, that these conditions are in reference to outside of the normal developmental stages in which one would expect a child to not be toilet trained. Any time there is potentially bacteria-laden fluid (e.g., urine and feces) near the urethra meatus, this creates an ample opportunity for infection.
Typically, toilet training begins during the toddler years. In this stage, toddlers learn to hold their bladders to refrain from having any “accidents.” However, intentional voiding postponement can lead to bladder stasis, which also can be a source of a UTI.10,24 An underactive bladder caused by underactive detrusor activity results in increased straining and resistance. This impaired urinary flow against increased bladder pressure leads to increased post-void residual in a stagnant bladder, which provides a rich medium for bacterial replication, adherence, and colonization.13,25
Constipation frequently presents as an issue among the pediatric population. It can be defined as reduced frequency, quantified as fewer than three stools per week, and increased difficulty or discomfort during defecation.26 Fecal retention can increase the risk of cystitis through the mechanical compression of the full rectum on the bladder, leading to displacement of the bladder and elongation of the urethra. That chain of effects reduces urinary flow, which promotes urinary stasis and pathogen adherence.13,26 There also is a theoretical risk of bacterial translocation through the rectovesicular mucosa.
Vesicoureteral reflux (VUR), defined as backflow of urine into one or both ureters, is present in approximately 30% to 40% of children with recurrent UTIs.14 It is a well-established risk factor for cystitis in the pediatric population. This anatomic abnormality is diagnosed through imaging studies and is graded based on severity of reflux. Patients with recurrent episodes should undergo testing for VUR.
Clinical Features
As with any patient who presents to the emergency department, one of the first priorities during a patient encounter is obtaining a thorough history and physical examination to guide the workup and proper treatment of a patient. Unfortunately, infants and most young children do not have the developmental capability to provide physicians with the proper history needed. Therefore, physicians must have high clinical suspicion and focus on certain symptoms and characteristics that would suggest UTI to not miss an occult infection that could lead to dire consequences, such as ascending UTI (pyelonephritis), sepsis, or bacteremia.
When considering the population 2 years of age and younger, symptoms can be quite vague and include irritability, poor feeding, lethargy, jaundice, vomiting, and fever.27 (See Table 2.) Among this age group, the findings most suggestive of correctly identifying UTI are the history of previous UTI(s), fever > 40°C, and suprapubic tenderness on examination.28 Additionally, males within this age group who are not circumcised have increased likelihood of being diagnosed with a UTI as compared to similarly aged circumcised males.28
Table 2. Common Presenting Symptoms of Pediatric Urinary Tract Infection39-44 |
|||
| Preverbal Age | Verbal Age | ||
| Specific | Nonspecific | Specific | |
Malodorous urine |
Fussiness |
Abdominal pain |
Urinary frequency |
Frequent crying |
Fever |
Urinary urgency |
|
Decreased urinary output |
Back pain |
Dysuria |
|
Failure to thrive |
Decreased oral intake |
Sensation of incomplete voiding |
|
Once children get older, they can actively participate in the history and physical exam and provide information that can be more more helpful in identifying UTIs. Verbal children may endorse abdominal pain, dysuria, hematuria, urinary frequency, urinary urgency, and even episodes of incontinence. (See Table 2.) As expected among all age groups, physical exam findings such as suprapubic fullness and tenderness, flank pain, costovertebral angle tenderness, fever, and vomiting can suggest a possible UTI.28 Additionally, among the age group 2-12 years, physicians should perform a focused external genital exam to look for external lesions, discharge, or foreign bodies in patients with recurrent UTI.8 It also has been suggested that patients with mental health disorders and/or developmental delay who are prone to foreign body insertion in other orifices (e.g., ears, nose, and ingestion events) may warrant an external genital exam as well to look for less-common causes of the aforementioned symptoms.8,27,28
The adolescent population is well-versed in localizing pain and articulating symptoms. However, children of this age also may be sexually active. An accurate sexual history should be taken from the patient and should be a part of the differential diagnosis if appropriate, since a sexually transmitted infection can present with symptoms that are almost indistinguishable from a UTI.
Diagnostic Studies
The diagnostic study of choice for a simple UTI is a urinalysis with urine culture. Initially, diagnosing UTIs can be done through the combination of suggestive clinical findings and a positive urinalysis. However, the gold standard for diagnosis is a positive quantitative urine culture, which typically does not result until 24-48 hours after collection.25 The technique used to collect the urine influences the accuracy of the results. Mechanisms of obtaining urine from a child include midstream clean catch (in patients who are able), catheterization, “bagging,” and, less commonly, suprapubic aspiration.25 The age of the child and ability to effectively use the toilet can influence the way the specimen is obtained.
The simplest method for obtaining urine from non-toilet-trained children involves collecting urine via a sterile bag attached to the child’s perineum. This also is the method that is most frequently contaminated. A positive urine culture from a bagged specimen has a false-positive rate up to 75% from periurethral flora and, therefore, requires a confirmation culture with a catheterized or suprapubic aspiration specimen.1,8,28 However, if the results are negative by dipstick and culture, the bagged specimen can be used to rule out UTI without requiring a confirmatory test.8,29 Another noninvasive method would be the midstream clean catch. This method serves as an optimal choice for toilet-trained older children after proper cleansing of the external genitalia.1,29 However, samples obtained via this method still have a contamination rate of approximately 26%.30 This is the most common method of obtaining urine samples in the emergency department.
Children who have an urgent need for antibiotics and cannot provide an adequate voided sample should be catheterized unless there is obvious external genitourinary infection or deformity limiting the ability to do so.29 It provides a safe, fast, and reliable method of obtaining urine and limits contamination rates.8,29 To ensure there is no “dry tap” or an unnecessary catheterization, a quick bedside ultrasound should be performed to ensure there is sufficient urine within the bladder prior to catheterization. Drawbacks of this process include causing discomfort for the child, emotional stress of patients and parents, traumatic injury with resulting hematuria, and the possibility of introducing infection.8,31 However, the sensitivity and specificity of a catheterized specimen is significantly better than a bagged sample and has a specificity of 83% to 89%. Meanwhile, a suprapubic sample with >100,000 CFU/mL approaches 99% specificity.8
If a sample cannot be obtained through the previous methods and a child presents critically ill, then suprapubic aspiration should be considered. It serves as the most accurate, but most invasive, method of obtaining a urine specimen and has a contamination rate of approximately 1%.8,30,32 Complications can arise from this procedure because it requires a puncture through the skin and abdominal wall. These complications include perforation of abdominal viscera, penetration into an incorrect target, post-procedure bleeding, introduction of infection, and transient gross hematuria.1 Successful aspiration rates can be improved with the use of ultrasound.1,8,11
The three methods commonly used for urinalysis testing are dipstick, microscopy, and culture. The cheapest, most accessible, and most easily interpreted method is the dipstick, which is why it is commonly stocked in in medical facilities.8 Indications of UTI on a dipstick or urinalysis include the presence of leukocyte esterase and nitrites. Positive leukocyte esterase suggests inflammation, and white blood cell presence can be 84% sensitive and 78% specific for diagnosing UTIs.8 Positive nitrite indicates the presence of bacteria that convert nitrates into nitrites, most commonly gram-negative bacteria, and can be 50% sensitive and 98% specific for diagnosing UTIs.8 Thus, dipstick analysis with positive leukocyte esterase and nitrite can effectively rule in UTIs. Conversely, negative leukocyte esterase and nitrite can effectively rule out UTIs.33 However, if a dipstick is positive for one and not the other, the results are inconclusive, and further testing, such as a confirmatory culture is required.
Microscopy analysis requires more money, equipment, and skilled analysis compared to dipstick and is only readily available in the hospital or emergency department setting.8 The analysis evaluates the presence of white blood cells, red blood cells, bacteria, and crystals in the sample. The presence of pyuria (> 5 white blood cells/high-power field in a centrifuged sample and > 10 white blood cells/high-power field in an uncentrifuged sample) and bacteria (any) can effectively rule in UTI, while the absence of both can effectively rule out infection.33 Once again, the presence of either pyuria or bacteriuria will require further confirmatory testing.
As previously discussed, the urine culture remains the gold standard for diagnosing UTIs. Positive cultures can be defined as either > 50,000 CFU/mL on a catheterized or suprapubic aspiration or > 100,000 CFU/mL in a voided specimen, although practice patterns among clinicians vary widely.8 Time can be a limiting factor with the interpretation for a urine culture, especially in the ED, since bacteria take about 24 hours to become evident on a sample, and sensitivity will result in approximately 48 hours.1 When testing, some additional workup can be helpful in suggesting the presence of a more severe infection. Blood cultures should be considered only in toxic or hemodynamically unstable children when the physician suspects sepsis or bacteremia. Elevated inflammatory markers, such as neutrophilia, erythrocyte sedimentation rate, and C-reactive protein or elevated procalcitonin levels, can indicate pyelonephritis, but they have low specificity and cannot accurately differentiate lower from upper tract infections.34,35
The reason for obtaining imaging in children with UTIs is to detect underlying urologic abnormalities or complications and guide further management. Imaging should be considered in any child with recurrent UTIs or who is part of an atypical demographic, such as circumcised males. Visual representation of images obtained with commonly used modalities can be found in Desai et al’s Table 2 at http://bit.ly/3G8XK4v. The method of choice for pediatric urinary tract imaging is bladder and renal ultrasound because of its safety, decreased radiation exposure, availability, and noninvasiveness.27 Ultrasound imaging can define kidney size, shape, and position, the echotexture of renal parenchyma, presence of duplication or dilatation of ureters, presence of renal abscess, obstructive uropathy, and structural abnormality of the bladder, all of which can predispose children to UTIs.1,11
Populations that ultrasounds should be considered for include children < 2 years of age with a febrile UTI; children with recurrent UTI or palpable abdominal mass; children with abnormal voiding, hypertension, or hematuria; or those with a family history of renal disease.1,11,36 It also should be obtained for children who do not respond to antibiotic therapy or in children with recurrent UTIs to rule out an obstructive process or abscess.1,36 Neonates younger than 2 months of age with a UTI typically should have an ultrasound performed because they have a higher incidence of renal anomalies.2 Although ultrasound imaging can be helpful in determining the presence of abscesses, obstructions, structural abnormalities, and retention, it cannot be used to localize infection and has a low sensitivity for detecting VUR and scarring.2
A voiding cystourethrogram, which is a contrast-enhanced radiographic imaging, has been deemed the gold standard for diagnosing VUR.1,2 It also can confirm the presence of posterior urethral valves and obstructive uropathies.37 However, this study should not be performed on every child with a UTI because it is invasive and may require the sedation of the child, introduces radiation exposure of ~1 mSV, and requires prophylactic antibiotics, not to mention the need for radiology technicians and physician specialists to read and interpret the findings.36 Additionally, most children with VUR do not require further medical or surgical management.7 Thus, this procedure should be limited to a child after their second febrile UTI, a child with abnormal voiding post-first febrile UTI, or a child with abnormal renal ultrasounds suggestive of scarring, hydronephrosis, or other findings suggestive of vesicoureteral reflux or obstructive uropathy.1,36,37 Most VUR studies are performed on an outpatient basis.
There also are nuclear imaging options to detect renal scarring, VUR, and other various renal issues, including acute infections, such as pyelonephritis. This uses the radioisotope dimercaptosuccinic acid (DMSA) in the kidneys to diagnose abnormalities.1,36,37 This method of imaging is not routinely used because of its limited widespread availability, radiation hazards, cost, high number of false positives in the acute phase of UTI (less than three months post-UTI), and inability to differentiate between old and new scarring.36 However, it can be considered in children with reduced renal function to assess for scarring four to six months after a severe UTI or in the acute phase to assess for pyelonephritis if other diagnostic methods are inconclusive or unavailable.38
Magnetic resonance imaging (MRI) has high sensitivity for detection of pyelonephritis, comparable to DMSA, but it is not used routinely to evaluate young children with UTIs because of its relatively high cost, low availability, and the potential need to sedate younger patients because patients undergoing MRI must lie still for a prolonged period of time.2 Post-contrast computed tomography also can be sensitive for detecting renal abscess and pyelonephritis, but it rarely is used in pediatric patients because of its high radiation load.2
In summary, there is a plethora of options for diagnostic studies divided into urine studies, hematologic/chemistry studies from blood, and imaging studies to help diagnose urinary tract infections and their associated complications. As discussed, a urinalysis and a high clinical suspicion often are all that is necessary, but it is important for providers to know all the laboratory and imaging studies that are available should there be a need for them. Imaging studies often are reserved for recurrent or complicated UTIs and their sequelae, rather for than the diagnosis of acute infection.
Differential Diagnosis
The differential diagnosis for a UTI depends on the patient’s presenting symptom. However, as discussed previously, this can be a challenge, especially for preverbal patients. (See Table 2.) This review will discuss the most common presenting symptoms and their differentials. In preverbal patients, patients most commonly present with fussiness and/or fever, which naturally creates a lengthy differential and reinforces the idea that the clinician must maintain a high index of suspicion for UTI during initial presentation.36
A common misconception is that tooth eruption can be accompanied by a fever, but a documented core temperature > 100.4°F should not be attributed to tooth eruption, although an eruption can certainly cause fussiness.40,41 Fever and fussiness also can be caused by other occult infections, such as pneumonia and upper respiratory tract infection, although these usually are accompanied by a cough. Outside of a viral upper respiratory infection, a UTI is the most common cause of pediatric fever > 39°C (102.2°F).40-45 Other infections, such as pharyngitis, peritonsillar abscess, and retropharyngeal abscesses, more commonly are associated with decreased oral intake and poor feeding, in addition to the fever and fussiness.46 Gastrointestinal infections, whether viral or bacterial, also can cause these symptoms, although they usually are accompanied by vomiting and/or diarrhea, which can help differentiate from a UTI.
A genitourinary exam in neonates/infants is essential during this workup because it can reveal other causes, such as phimosis, hair tourniquet, balanitis, balanoposthitis, or diaper rash that also may be causing similar symptoms.47 A simple urinalysis and culture, in addition to a thorough history and physical exam, will help distinguish between these infections. In verbal patients who can help delineate the time course of onset and characterize the type of symptoms/discomfort they are experiencing, patients usually describe burning or pain with urination (dysuria), urinary frequency, or abdominal pain.48 With the former two symptoms, the differential includes any number of genital irritations, including vulvovaginitis, vaginitis, balanitis, phimosis, yeast infection, or sexually transmitted infections with or without sexual abuse.40,46-48 In menstruating patients, a retained foreign body, such as a tampon, also should be on the differential.
Some special patient populations, particularly patients with certain mental health disorders, incarcerated patients, and certain developmentally delayed populations, occasionally will insert foreign bodies into the urethra. This is known as polyembolokoilamania and is quite rare, but it can cause urinary retention, frequency, urgency, and feelings of incomplete voiding.49-51 The highest predictive factor for this condition is a history of the same or a history of foreign body insertion into other orifices.49,51,52 If this rare presentation is suspected, a thorough collateral history through other historians or the electronic medical record should be obtained. If a foreign body is found to be the culprit of the patient’s UTI (or UTI-like symptoms), a head-to-toe exam and a review of systems should be performed to ensure there are no other concomitant foreign bodies to be removed.
Management
After a UTI is diagnosed with one of the aforementioned imaging or laboratory findings, most commonly leukocyte esterase, nitrite-positive urine, or a urine culture yielding > 50,000 CFU/mL of bacterial growth, treatment should be aimed at empiric antibiotic therapy to eradicate the uropathogen. Usually, culture data are not readily available to help guide empiric antibiotic choice, since culture data take one to three days to result. Therefore, an antibiotic decision should be made based on careful consideration of the most common pathogens, local antibiogram sensitivity data, patient allergy profile, and potential side effects.53-55
From current available data, empiric antibiotics should have adequate coverage against E. coli, Klebsiella spp., and Proteus spp. — these all share the same gram-negative rod bacterial structure.56 Other common pathogens, such as S. saprophyticus and Enterococcus spp., are gram-positive cocci.57 Antibiotic selection also should be guided by presumed disposition of the patient and whether intravenous access has been obtained.48,56,57 (See Table 3.) Careful consideration also should be paid to the patient’s past medical records, since providers can tailor treatment of a current infection based on prior positive urine cultures and their antimicrobial resistance patterns.54
Table 3. Common Antimicrobial Regimens for Treatment for Pediatric Urinary Tract Infections36,40,48,56,57 |
||||
| Class of Medication | Examples of Class | Contraindications and Considerations | ||
Oral antibiotics |
First line |
First-generation cephalosporin |
Cephalexin, cefadroxil |
Developing resistance because of frequent prescriptions |
Second-generation cephalosporin |
Cefuroxime |
Uncommonly prescribed, may not be available |
||
Third-generation cephalosporin |
Cefpodoxime, cefdinir, cefixime, ceftibuten |
Often expensive, may not be available |
||
Sulfonamides |
Trimethoprim/sulfamethoxazole |
Most common antibiotic to produce side effects |
||
Second line |
Penicillin |
Amoxicillin |
High rates of resistance |
|
Nitrofurans |
Nitrofurantoin |
Good coverage against Escherichia coli, but not againts other organisms |
||
Penicillin with beta-lactamase inhibitor |
Amoxicillin-clavulanate |
High rates of resistance |
||
Fluoroquinolone |
Ciprofloxacin |
Side effect profile, except for Pseudomonas aeruginosa infections |
||
Parenteral antibiotics |
First line |
Third-generation cephalosporin |
Ceftriaxone, ceftazidime, cefotaxime |
Most common first-line medication |
Fourth-generation cephalosporin |
Cefepime, cefpirome |
Covers P. aeruginosa |
||
Aminoglycoside |
Gentamicin |
Often prescribed with ampicillin if enterococcal infection is suspected |
||
Second line |
Penicillin with beta-lactamase inhibitor |
Ampicillin/sulbactam |
Often combined with gentamicin for enhanced gram-negative coverage |
|
Piperacillin/tazobactam |
For consideration in indwelling foley catheters/suprapubic tubes |
|||
A third-generation cephalosporin is a common intravenous choice, and certain antibiotics from this class also can be prescribed as an outpatient option, including cefdinir, cefpodoxime, and cefixime. Because these antibiotics have broader coverage, they should be reserved for patients who are at higher risk, including immunocompromised patients, very young patients (between 28 and 90 days of age and appropriate for discharge), and patients with recurrent UTIs or previous culture data that suggest a third-generation cephalosporin is the correct choice.60-63 For empiric outpatient treatment, a first-generation cephalosporin has adequate data suggesting that it is just as effective against E. coli, but likely is less effective against non-E. coli pathogens when compared to a third-generation cephalosporin.56,63 Nitrofurantoin also is a reasonable choice, but it largely is ineffective against non-E. coli pathogens.56 Trimethoprim/sulfamethoxazole is frequently prescribed as well, but it is notably only about 80% effective against uropathogenic E. coli.56,62 Amoxicillin, ampicillin, and amoxicillin-clavulanate should be prescribed less commonly or only after consultation with the provider’s institutional antibiogram, given the relative high rate of resistance against them.56,62,63
Antibiotics ideally should be started within two days of symptom onset because this can help reduce the risk of long-term complications such as renal scarring.53,64 Duration of therapy is up to the discretion of the provider and depends on the age, presenting symptoms, and risk factors of the child. An acceptable duration ranges anywhere from five to 14 days. Some recent data suggest that therapy for as little as three days may be just as effective as longer durations in the afebrile child with a UTI.65 However, it is general practice to prescribe a longer duration for a febrile UTI compared to an afebrile UTI. More data are needed to determine evidence-based guidelines regarding the duration of therapy to help minimize the risk of iatrogenic side effects of prolonged antibiotic therapies.48,65 Clinical response should be assessed frequently because, if there is no change in symptoms within 48 hours, a renal ultrasound is indicated to look for renal abscess or proximal nephrolithiasis that may be hindering source control.48,53,66,67 Should a UTI be the suspected source of infection in an immunocompromised patient, there should be some consideration for the possibility of fungal or viral cystitis. These are rarer than bacterial cystitis, but they should be considered if there is a lack of response to traditional antibiotics or in special populations, such as renal transplant recipients who are chronically immune suppressed.48,68 Viral causes usually are adenovirus or polyomavirus, whereas fungal infections, often caused by an indwelling catheter, typically are Candida species.48,68,69 Source control with removal of an indwelling catheter or replacement with an uninfected one is essential in the management of this type of UTI, along with appropriate antifungals.48,69
Complications
This review proposes a unique breakdown of UTI complications. These complications can be divided into primary complications, meaning the direct result of infection progression; secondary complications, meaning long-term consequences of severe infection; or tertiary complications, meaning iatrogenic illness or disease caused by workup. This is summarized in Table 4. As with other pediatric infections, one can consider the loss of time at school a complication of an acute pediatric illness. Additionally, a caregiver’s missed time at work, the cost of a visit to obtain care and treatment, and patient discomfort are anticipated with common childhood illnesses. Dehydration with secondary electrolyte abnormalities also can be expected from a childhood febrile illness and is not considered unique to pediatric UTIs; however, it would help guide management because it would necessitate admission for intravenous rehydration.1,53
Table 4. Complications of Pediatric Urinary Tract Infection (UTI)39,54,58,59 |
||
| Primary Complications | Secondary Complications | Tertiary Complications |
Pyelonephritis/ascending UTI |
Renal scarring |
Diarrhea (from antibiotics) |
Perinephric abscess |
Chronic kidney disease |
Allergic reaction |
Acute kidney injury |
Hypertension |
Resistant bacteria |
Renal abscess |
Chronic colonization |
Stevens-Johnson syndrome/toxic epidermal necrolysis (from antibiotics) |
Bacteremia |
Pyonephrosis |
Cost of workup/treatment |
Sepsis |
Emphysematous pyelonephritis |
Radiation risk (neoplasm) |
Untreated urinary tract infections are more dangerous than other untreated occult infections. This is partially because UTIs are rarely caused by viruses, so infections often are not self-limited. Compared with viral upper respiratory infections, UTIs usually require antibiotics to help clear the infection and reduce symptoms. UTIs also are more dangerous because the anatomic structure of the kidney lends itself to the feared complication of an untreated UTI — bacteremia, or the presence of bacteria in the blood. The kidney receives 20% of total cardiac output, which is disproportionately large, given the size of the kidneys compared to other organs.70 An untreated infection, given its proximity to an extensive amount of blood flow, can progress to bacteremia via hematogenous spread.71,72 In acutely ill children, blood cultures are the gold standard for diagnosing bacteremia and necessitate treatment with parenteral antibiotics. Urosepsis, or simply a systemic inflammatory response to a UTI, can be life-threatening. Because of the inherent changes in vital signs, such as tachycardia and hypotension, vasopressor support is the primary method that urosepsis treatment differs from that for a nonseptic patient. Otherwise, treatment largely is similar, consisting of parenteral antibiotics, fluid resuscitation, and careful monitoring of laboratory values.40,48,73 Imaging for a patient who is not responding to intravenous antibiotics is paramount to ensure there is no other source of infection, as discussed previously.53,48,67,73
Inflammation of the urinary system because of a UTI also can lead to renal scar formation.1,53,54,64 It remains unclear and debated whether renal scarring results in clinically significant decreased renal function, measured as a decrease in glomerular filtration rate (GFR). Leung et al and Simões e Silva et al both describe renal scarring in detail in their reviews of pediatric UTIs.1,54 To summarize, scarring has been described previously, which theoretically can lead to chronic kidney disease (CKD) and end-stage renal disease (ESRD), but the prevalence of scarring and its progression are not well-known.1,53,54,64 It is described mainly in patients with pyelonephritis rather than simple cystitis. Hypertension and chronic electrolyte abnormalities caused by renal damage/scarring also have been described, but primarily in patients with recurrent infections or structural abnormalities, such as VUR or renal hypodysplasia.1
Complications also can arise from inappropriate antibiotic administration adverse reactions. Ensuring the patient is not already allergic to a prescribed antibiotic goes without saying, but antibiotics also can cause other adverse events, such as diarrhea, abdominal pain, QT interval prolongation, toxic epidermal necrolysis, Stevens-Johnson syndrome, Clostridioides difficile infection, and drug rash eruptions, among others. Recent studies have shown that up to 67% of pediatric patients treated with antibiotics for simple cystitis had negative urine cultures on follow-up.74 Ensuring the appropriate empiric antibiotic is prescribed for the right route, right indication (lower UTI vs. upper UTI), right frequency, and right duration can help decrease unnecessary complications associated with antibiotic administration.74-76 When considering treatment for a pediatric UTI, clinical decision tools also have been published to aid in a clinician’s thought process. They have been published both for febrile girls as well as febrile boys and should be used in clinical practice.77,78 Antibiotic stewardship remains of utmost importance. Adverse events related to a recently initiated antibiotic course (notably for all causes, not just pediatric UTI) accounted for nearly 70,000 pediatric ED visits in one four-year study, which demonstrated that the antibiotics with the highest rates of adverse drug events were amoxicillin and trimethoprim/sulfamethoxazole.79 Shared decision-making with the patient’s family should be used whenever clinically appropriate to ensure caretakers are aware of the potential risks associated with antibiotic use.
Disposition
Disposition of the pediatric patient with a UTI depends on age, clinical appearance, and availability of outpatient monitoring. Ill-appearing or uroseptic patients with unstable vital signs should be admitted regardless of age. However, there are three age cutoffs that require careful consideration of each individual patient to make a disposition decision. (See Table 5.) A child younger than 28 days of age should be admitted, per American Academy of Pediatrics guidelines, for empiric antibiotics and exploration of complications of a UTI, such as bacteremia.80
Table 5. Disposition of Pediatric Patients With of Pediatric Urinary Tract Infections (UTIs)37,40,53,54,80 |
||||
| 0-28 Days of Age | 29-60 Days of Age (Majority Admitted) | > 60 Days of Age (Minority Admitted) | ||
| All Admitted | Discharge Signals | Admission Signals | Discharge Signals | Admission Signals |
Do not discharge; admit per American Academy of Pediatrics recommendations and perform workup for other sources of infection |
Tolerates oral intake |
Ill-appearing |
Well-appearing |
Provider discretion/ill-appearing |
Close outpatient follow-up |
Poor follow-up |
Tolerates oral intake |
Immunocompromised |
|
Reliable caregivers |
Vital sign abnormality |
Reliable follow-up |
Caregiver discomfort |
|
Vital signs within normal limits |
Repeated emergency department visits/failure of outpatient therapy |
Discharge with empiric oral antibiotics |
Recurrent UTI/failure of outpatient therapy |
|
A child between 28 and 60 days of age likely is the most controversial of the demographic groups and should be largely based on the provider’s institutional practices or clinical gestalt. There are some studies suggesting adequate safety for outpatient management, but the minority of patients are discharged from the ED for outpatient management, and there is no specific guideline dictating criteria for discharge.36,40,80 Additional risk factors may warrant immediate admission, such as immunocompromised status, inability to tolerate oral antibiotics, previous failure of outpatient therapy, or a lack of follow-up.36,40,53,80 Lastly, a child older than 60 days of age can be discharged, provided they are not ill-appearing and have normal vital signs and close follow-up as an outpatient. Outpatient follow-up with pediatric urology may be necessary in the patient with recurrent UTIs, as discussed previously.
Summary
Pediatric urinary tract infections remain one of the most common reasons for presentation to the ED. Clinicians need to maintain a high suspicion for this diagnosis, particularly in female pediatric patients and uncircumcised males younger than 2 years of age, since missing an infection frequently leads to ascending UTI, bacteremia, and potential progression to sepsis, with high morbidity and mortality. Treating the infection simply requires antibiotics and usually can be done as an outpatient, but recurrent UTI, failure of outpatient treatment, or known anatomical abnormalities may warrant imaging studies to be performed. Antibiotic choice must be carefully considered to limit the adverse effects of treatment.
References
- Leung AKC, Wong AHC, Leung AAM, Hon KL. Urinary tract infection in children. Recent Pat Inflamm Allergy Drug Discov 2019;13:2-18.
- Karmazyn BK, Alazraki AL, Anupindi SA, et al. ACR Appropriateness Criteria® urinary tract infection-child. J Am Coll Radiol 2017;14:S362-S371.
- Koyle MA, Elder JS, Skoog SJ, et al. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: Current controversies in approach to evaluation. Pediatr Surg Int 2011;27:337-346.
- Lim R. Vesicoureteral reflux and urinary tract infection: Evolving practices and current controversies in pediatric imaging. AJR Am J Roentgenol 2009;192:1197-1208.
- Merguerian PA, Sverrisson EF, Herz DB, et al. Urinary tract infections in children: Recommendations for antibiotic prophylaxis and evaluation. An evidence-based approach. Curr Urol Rep 2010;11:98-108.
- Williams GJ, Hodson EH, Isaacs D, et al. Diagnosis and management of urinary tract infection in children. J Paediatr Child Health 2012;48:296-301.
- Becknell B, Schober M, Korbel L, et al. The diagnosis, evaluation, and treatment of acute and recurrent pediatric urinary tract infections. Expert Rev Anti Infect Ther 2015;13:81-90.
- Schmidt B, Copp HL. Work-up of pediatric urinary tract infection. Urol Clin North Am 2015;42:519-526.
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr Infect Dis J 2008;27:302-308.
- Kaufman J, Temple-Smith M, Sanci L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr Open 2019;3:e000487.
- Simões e Silva AC, Oliveira EA. Update on the approach of urinary tract infection in childhood. J Pediatr (Rio J) 2015;91(6 Suppl 1):S2-S10.
- Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med 2001;155:60-65.
- Khan A, Jhaveri R, Seed PC, et al. Update on associated risk factors, diagnosis, and management of recurrent urinary tract infections in children. J Pediatric Infect Dis Soc 2019;8:152-159.
- Conway PH, Cnaan A, Zaoutis T, et al. Recurrent urinary tract infections in children: Risk factors and association with prophylactic antimicrobials. JAMA 2007;298:179-186.
- Li R, Leslie SW. Cystitis. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Morello W, La Scola C, Alberici I, et al. Acute pyelonephritis in children. Pediatr Nephrol 2016;31:1253-1265.
- Baraboutis IG, Tsagalou EP, Lepinski JL, et al. Primary Staphylococcus aureus urinary tract infection: The role of undetected hematogenous seeding of the urinary tract. Eur J Clin Microbiol Infect Dis 2010;29:1095-1101.
- Stephens GM, Akers S, Nguyen H, et al. Evaluation and management of urinary tract infections in the school-aged child. Prim Care 2015;42:33-41.
- Whiting P, Westwood M, Watt I, et al. Rapid tests and urine sampling techniques for the diagnosis of urinary tract infection (UTI) in children under five years: A systematic review. BMC Pediatr 2005;5:4.
- Russo TA, Stapleton A, Wenderoth S, et al. Chromosomal restriction fragment length polymorphism analysis of Escherichia coli strains causing recurrent urinary tract infections in young women. J Infect Dis 1995;172:440-445.
- Lidefelt KJ, Bollgren I, Nord CE. Changes in periurethral microflora after antimicrobial drugs. Arch Dis Child 1991;66:683-685.
- Halachmi S, Farhat WA. Interactions of constipation, dysfunctional elimination syndrome, and vesicoureteral reflux. Adv Urol 2008;2008:828275.
- Austin PF, Bauer SB, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: Update report from the Standardization Committee of the International Children’s Continence Society. J Urol 2014;191:1863-1865.e13.
- Tullus K. Fifteen-minute consultation: Why and how do children get urinary tract infections? Arch Dis Child Educ Pract Ed 2019;104:244-247.
- Chang S-J, Tsai L-P, Hsu C-K, Yang SS. Elevated postvoid residual urine volume predicting recurrence of urinary tract infections in toilet-trained children. Pediatr Nephrol 2015;30:1131-1137.
- Blethyn AJ, Jenkins HR, Roberts R, Jones KV. Radiological evidence of constipation in urinary tract infection. Arch Dis Child 1995;73:534-535.
- Zorc JJ, Levine DA, Platt SL, et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics 2005;116:644-648.
- Shaikh N, Morone NE, Lopez J, et al. Does this child have a urinary tract infection? JAMA 2007;298:2895-2904.
- Doern CD, Richardson SE. Diagnosis of urinary tract infections in children. J Clin Microbiol 2016;54:2233-2242.
- Tosif S, Baker A, Oakley E, et al. Contamination rates of different urine collection methods for the diagnosis of urinary tract infections in young children: An observational cohort study. J Paediatr Child Health 2012;48:659-664.
- Leung AK, Robson WL. Urinary tract infection in infancy and childhood. Adv Pediatr 1991;38:257-285.
- Pollack CV Jr, Pollack ES, Andrew ME. Suprapubic bladder aspiration versus urethral catheterization in ill infants: Success, efficiency, and complication rates. Ann Emerg Med 1994;23:225-230.
- Whiting P, Westwood M, Bojke L, et al. Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: A systematic review and economic model. Health Technol Assess 2006;10:iii-iv, xi-xiii, 1-154.
- Wald E. Urinary tract infections in infants and children: A comprehensive overview. Curr Opin Pediatr 2004;16:85-88.
- Zhang H, Yang J, Lin L, et al. Diagnostic value of serum procalcitonin for acute pyelonephritis in infants and children with urinary tract infections: An updated meta-analysis. World J Urol 2016;34:431-441.
- Shaikh N, Hoberman A. Urinary tract infections in infants older than one month and young children: Clinical features and diagnosis. UpToDate. Updated Dec. 7, 2021. https://www.uptodate.com/contents/urinary-tract-infections-in-infants-and-children-older-than-one-month-clinical-features-and-diagnosis
- Desai DJ, Gilbert B, McBride CA. Paediatric urinary tract infections: Diagnosis and treatment. Aust Fam Physician 2016;45:558-563.
- Korbel L, Howell M, Spencer JD. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health 2017;37:273-279.
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: A systematic review. Pediatrics 2010;126:1084-1091.
- Allen CH. Fever without a source in children 3 to 36 months of age: Evaluation and Management. UpToDate. Updated Oct. 6, 2022. https://www.uptodate.com/contents/fever-without-a-source-in-children-3-to-36-months-of-age-evaluation-and-management
- Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med 1993; 22:1198.
- Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics 1998;102:e16.
- Hoberman A, Chao HP, Keller DM, et al. Prevalence of urinary tract infection in febrile infants. J Pediatr 1993;123:17-23.
- Finkelstein JA, Christiansen CL, Platt R. Fever in pediatric primary care: Occurrence, management, and outcomes. Pediatrics 2000;105:260-266.
- Wright PF, Thompson J, McKee KT Jr, et al. Patterns of illness in the highly febrile young child: Epidemiologic, clinical, and laboratory correlates. Pediatrics 1981;67:694-700.
- Tebruegge M, Curtis N. Infections related to the upper and middle airways. In: Long SS, Prober CG, Fischer M, eds. Principles and Practice of Pediatric Infectious Diseases. 5th ed. Elsevier Saunders;2018:208-215.
- Buechner SA. Common skin disorders of the penis. BJU Int 2002;90:498-506.
- Palazzi DL, Campbell JR. Acute infectious cystitis: Management and prognosis in children older than two years and adolescents. UpToDate. Updated March 3, 2023. https://www.uptodate.com/contents/acute-infectious-cystitis-management-and-prognosis-in-children-older-than-two-years-and-adolescents
- Unruh BT, Nejad SH, Stern TW, Stern TA. Insertion of foreign bodies (polyembolokoilamania): Underpinnings and management strategies. Prim Care Companion CNS Disord 2012;14:PCC.11f01192.
- Mehta P, Leslie SW, Reddivari AKR. Dysuria. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Bhat AL, Kumar A, Mathur SC, Gangwal KC. Penile strangulation. Br J Urol 1991;68:618-621.
- Rafique M. Intravesical foreign bodies: Review and current management strategies. Urol J 2008;5:223-231.
- Mattoo TK, Shaikh N, Nelson CP. Contemporary management of urinary tract infection in children. Pediatrics 2021;147:e2020012138.
- Simões E Silva AC, Oliveira EA, Mak RH. Urinary tract infection in pediatrics: An overview. J Pediatr (Rio J) 2020;96 Suppl 1(Suppl 1):65-79.
- Wennerstrom M, Hansson S, Hedner T, et al. Ambulatory blood pressure 16-26 years after the first urinary tract infection in childhood. J Hypertens 2000;18:485-491.
- Shaikh N, Hoberman A, Keren R, et al. Predictors of antimicrobial resistance among pathogens causing urinary tract infection in children. J Pediatr 2016;171:116-121.
- Said MS, Tirthani E, Lesho E. Enterococcus infections. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Gebäck C, Hansson S, Himmelmann A, et al. Twenty-four-hour ambulatory blood pressure in adult women with urinary tract infection in childhood. J Hypertens 2014;32:1658-1664.
- Jacobson SH, Eklof O, Eriksson CG, et al. Development of hypertension and uraemia after pyelonephritis in childhood: 27-year follow-up. BMJ 1989;299:703-706.
- Copp HL, Shapiro DJ, Hersh AL. National ambulatory antibiotic prescribing patterns for pediatric urinary tract infection, 1998-2007. Pediatrics 2011;127:1027-1033.
- McGregor JC, Elman MR, Bearden DT, Smith DH. Sex- and age-specific trends in antibiotic resistance patterns of Escherichia coli urinary isolates from outpatients. BMC Fam Pract 2013;14:25.
- 62. McGregor JC, Quach Y, Bearden DT, et al. Variation in antibiotic susceptibility of uropathogens by age among ambulatory pediatric patients. J Pediatr Nurs 2014;29:152-157.
- Edlin RS, Shapiro DJ, Hersh AL, Copp HL. Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J Urol 2013;190:222–227.
- Karavanaki KA, Soldatou A, Koufadaki AM, et al. Delayed treatment of the first febrile urinary tract infection in early childhood increased the risk of renal scarring. Acta Paediatr 2017;106:149-154.
- Michael M, Hodson EM, Craig JC, et al. Short vs. standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database Syst Rev 2003:CD003966.
- Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management; Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011;128:595-610.
- Cheng C-H, Tsai M-H, Su L-H, et al. Renal abscess in children: A 10-year clinical and radiologic experience in a tertiary medical center. Pediatr Infect Dis J 2008;27:1025-1027.
- Hofland CA, Eron LJ, Washecka RM. Hemorrhagic adenovirus cystitis after renal transplantation. Transplant Proc 2004;36:3025-3027.
- Kauffman CA. Candiduria. Clin Infect Dis 2005;41 Suppl 6:S371-S376.
- Dalal R, Bruss ZS, Sehdev JS. Physiology, renal blood flow and filtration. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Alavudeen SS, Asiri AA, Fageeh SA, et al. Evaluation of antibiotic prescribing practices and antimicrobial sensitivity patterns in urinary tract related infectious diseases in pediatric patients. Front Pediatr 2021;9:740106.
- Pokrzywa CJ, Papageorge CM, Kennedy GD. Preoperative urinary tract infection increases postoperative morbidity. J Surg Res 2016;205:213-220.
- Czech K, John E. Approach to pediatric patients with UTI in the PICU. J Pediatr Intensive Care 2016;5:64-68.
- Chardavoyne PC, Kasmire KE. Appropriateness of antibiotic prescriptions for urinary tract infections. West J Emerg Med 2020;21:633-639.
- Percival KM, Valenti KM, Schmittling SE, et al. Impact of an antimicrobial stewardship intervention on urinary tract infection treatment in the ED. Am J Emerg Med 2015;33:1129-1233.
- Spellberg B. The new antibiotic mantra: “Shorter is better.” JAMA Intern Med 2016;176:1254-1255.
- Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics 1998;102:e16.
- Gorelick MH, Shaw KN. Clinical decision rule to identify febrile young girls at risk for urinary tract infection. Arch Pediatr Adolesc Med 2000;154:386-390.
- Lovegrove MC, Geller AI, Fleming-Dutra KE, et al. U.S. emergency department visits for adverse drug events from antibiotics in children, 2011-2015. J Pediatric Infect Dis Soc 2019;8:384-391.
- Pantell RH, Roberts KB, Adams WG, et al; Subcommittee on Febrile Infants. Evaluation and management of well-appearing febrile infants 8 to 60 days old. Pediatrics 2021;148:e2021052228. Erratum in: Pediatrics 2021;148:e2021054063.
Urinary tract infections can be challenging to suspect and diagnose in young patients. Unfortunately, devastating consequences, such as pyelonephritis and bacteremia, are a real risk. It is critical for clinicians to have a high degree of suspicion, obtain optimal urine samples, and be aware of the best practices for treatment in this unique population.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
