Complications of Implantable Cardioverter Defibrillators in the Emergency Department Setting
July 15, 2023
Related Articles
-
Echocardiographic Estimation of Left Atrial Pressure in Atrial Fibrillation Patients
-
Philadelphia Jury Awards $6.8M After Hospital Fails to Find Stomach Perforation
-
Pennsylvania Court Affirms $8 Million Verdict for Failure To Repair Uterine Artery
-
Older Physicians May Need Attention to Ensure Patient Safety
-
Documentation Huddles Improve Quality and Safety
AUTHORS
Kathleen McMahon, DO, Attending Physician, St. Luke’s University Health Network, Bethlehem, PA
Guhan Rammohan, MD, FACEP, Emergency Medicine Faculty, St. Luke’s Hospital, Bethlehem, PA
PEER REVIEWER
William J. Brady, MD, FACEP, FAAEM, Professor of Emergency Medicine and Medicine, Medical Director, Emergency Preparedness and Response, University of Virginia Operational Medical Director, Albemarle County Fire Rescue, Charlottesville, VA; Chief Medical Officer and Medical Director, Allianz Global Assistance
EXECUTIVE SUMMARY
- Implantable cardioverter defibrillators (ICDs) with or without pacemaker capabilities are designed to reverse ventricular arrhythmias by delivering a shock directly to the ventricle or to overdrive pace the heart.
- Complications seen shortly after placement include bleeding in the area of the insertion or pericardial bleeding, pneumothorax/hemothorax seen one to four days post insertion, and cardiac perforation.
- Longer-term complications include Twiddler’s syndrome (often leading to lead fracture), infection (Staphylococcus aureus or Streptococcus epidermidis), thrombosis, lead failure, or battery depletion.
- Patients may present with repeated shocks over a short period of time. While this may be due to recurrent ventricular arrhythmias, it also may be due to oversensing by the ICD. Interrogation of the ICD should take place early in the evaluation of the patient.
Case Presentation
A 70-year-old male patient with a history of tobacco use, hypertension, type II diabetes mellitus, and ischemic cardiomyopathy presents to the emergency department via ambulance after a witnessed syncopal episode at his home. Emergency medical services reports that his family witnessed him collapse while standing in his living room without warning or preceding complaints. The patient arrives appearing lethargic but arousable and is complaining of lightheadedness, nausea, and shortness of breath. He denies any recent illnesses or travel, changes in medications, or history of similar symptoms. On presentation, his heart rate is 170 beats per minute, blood pressure is
80/45 mmHg, oxygen saturation 96% on 2 L nasal cannula, respiratory rate is 14 breaths per minute, and temperature is 37.5°C. On physical exam, he is noted to have palpable but thready peripheral pulses, tachycardia with a regular rhythm on auscultation of the heart, clear breath sounds bilaterally, no abdominal tenderness or distension, and a non-focal neurologic examination. A subcutaneous device pocket is noted in the left upper chest without overlying skin changes or tenderness on palpation. An initial electrocardiogram (ECG) shows ventricular tachycardia.
Two large-bore peripheral intravenous (IV) catheters are placed and 1 L of crystalloid is infused while the patient is connected to an external cardiac defibrillator. Given his clinical instability in the presence of ventricular tachycardia, the patient is electrically cardioverted one time with 100 Joules of synchronized electricity. The patient’s heart rate improves to 90 beats per minute, with a blood pressure of 130/80 mmHg and an ECG remarkable only for a left bundle branch block and Q waves across the precordium consistent with prior ECGs. His initial laboratory studies are remarkable for a troponin level of 0.3 ng/mL, while chemistry and complete blood count studies are within normal limits. On chest X-ray, the patient has clear lungs with cardiomegaly similar to prior radiographs, and an implantable cardioverter defibrillator (ICD) was noted with device leads intact.
Upon interrogation of the patient’s ICD, it is noted that the patient had a prolonged run of ventricular tachycardia, initiated at the time the patient lost consciousness at home. No shocks or overdrive pacing was performed by the device. The battery is noted to be depleted. Electrophysiology is consulted, and the patient is taken later that day to the catheterization laboratory for replacement of the device battery. He is admitted to the hospital for continued telemetry monitoring and further workup.
During his hospitalization, his troponin is down-trending and returns to the normal range. It is learned that his ICD had been alarming intermittently in the weeks leading up to his admission; however, the device had been placed in his former country of residence and he no longer had contact or follow-up with the specialists who had placed his device. The patient was reminded of the importance of close follow-up with electrophysiology and educated on device alarms prior to discharge. The patient was able to return home in his previous state of health.
Introduction
A significant subset of patients presenting to the emergency department have cardiac implantable electronic devices (CIEDs), a term that encompasses permanent pacemakers (PPMs) and ICDs. During the lifespan of the device, up to 8% of patients face at least one complication related to the device.1 Patients with ICDs have an even higher risk of overall complications compared to those with PPMs at nearly 10% of all patients over 16 months.2 While many CIED-associated complications are common to both PPMs and ICDs, there are certain conditions more prevalent in patients with ICDs. Given their prevalence in emergency department patients, knowledge of potential complications related to ICDs is critical for proper diagnosis and management of potentially life-threatening conditions.
Overview of Structure and Indications
Common indications for ICD placement include history of cardiac arrest, sustained ventricular arrhythmias, hypertrophic cardiomyopathy, adult congenital heart disease (ACHD), and heart failure with a left ventricular ejection fraction (LVEF) less than or equal to 35%.2-5 These devices recognize and deliver treatment for ventricular arrhythmias in the form of overdrive pacing (also known as anti-tachycardia pacing or ATP) or a synchronized or asynchronized shock.3
Multiple types of devices exist. Traditional ICDs are inserted transvenously and may have one or more leads. (See Figure 1.) Single-chamber devices have one ventricular lead that both senses and provides therapy (electrical shocks) at the ventricular level. Dual-chamber devices have an additional lead in the atrium that senses atrial activity. A third type of device is a biventricular device with an additional lead that provides cardiac resynchronization therapy (CRT-D).3 Subcutaneous ICDs without transvenous leads have been developed and are under investigation as an option for specific patient populations without need for an artificial pacemaker.6 Importantly, these devices can only deliver shocks and cannot perform ATP.
Figure 1. Implantable Cardioverter Defibrillator |
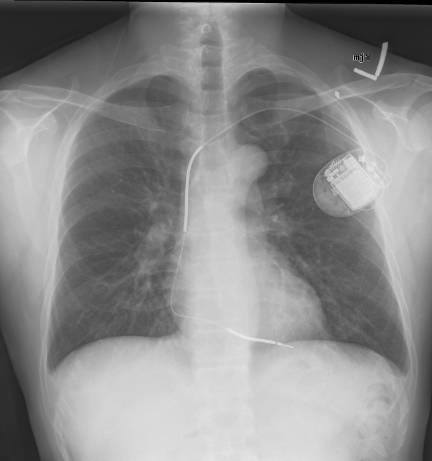 |
Source: Gregory Marcus, MD, MAS, FACC. Wikimedia Commons https://creativecommons.org/licenses/by/3.0/deed.en. |
Although many patients with ACHD are at increased risk for malignant arrhythmias and sudden cardiac death, not all are candidates for ICD placement. Altered cardiac and central vascular anatomy may make traditional ICD placement impossible.4 These patients also may have abnormal electrical activity and unusual ECG patterns at baseline that prevent ICDs from appropriately sensing significant arrhythmias and delivering appropriate therapy. This is especially true for single-chamber devices that are only able to sense intrinsic electrical activity at the level of the ventricle.4,7
The baseline ECG of a patient with an ICD not receiving active electrical therapy from the device should not demonstrate any abnormal electrical spikes unless the patient has a combination device with a permanent pacemaker. A single shock delivered by the device will be represented by a narrow spike on the ECG, likely preceded by an arrhythmia if the device is functioning appropriately. (See Figure 2.) Overdrive pacing by an ICD is seen on ECG as narrow spikes at regular intervals. (See Figure 3.)
Figure 2. Implantable Cardioverter Defibrillator Firing |
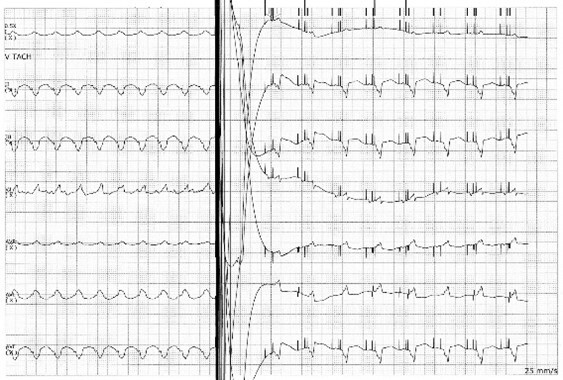 |
Image courtesy of Guhan Rammohan, MD. |
Figure 3. Electrocardiogram Showing Overdrive Pacing |
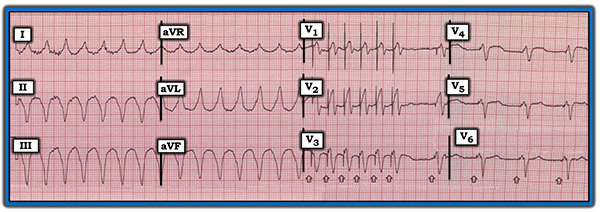 |
Image used with permission from ECGGuru.com |
Epidemiology of ICD Complications
As many as 9% to 11% of patients are noted to experience complications within a year of ICD placement, often soon after placement because of complications of vascular access (such as hematoma, pneumothorax, and cardiac perforation), device malfunction, or lead displacement.2,8 Some suggest that the rate of complications may be even higher due to physician under-reporting in some registries.2 All-cause readmission rates have hovered around 13% for many years, although a causal relationship to the ICD is not necessarily demonstrated in these data sets.8 The incidence of complications is of particular importance, given that as many as two-thirds of patients who receive an ICD never receive a therapeutic shock.8
Multiple factors are associated with higher risk for ICD complications. Younger cohorts (age 65-69 years or even younger) are more likely to experience complications than older cohorts, possibly because of the increased time of the device in situ.8 This is an important consideration in patients with ACHD who may require ICD therapy for many decades. Female sex and Black race are associated with overall increased incidence of complications.1,8 Increased complexity of the device itself also carries a higher risk for complications. Patients who receive ICDs at centers that perform fewer than 750 procedures annually or by operators who perform fewer than 50 procedures annually also are at higher risk for complications.1
An increased risk of future hospitalization and all-cause mortality has been noted in patients who experience ICD complications within 90 days after implantation; however, a causal relationship is uncertain.9
Mechanical and Procedural Complications
Bleeding Complications
Periprocedural and postprocedural bleeding, including pocket hematoma development, is a major risk associated with ICD placement.2,10,11 The risk of bleeding complications is increased by chronic anticoagulation therapy that many ICD placement candidates are already taking for other indications.10 Bleeding from the venous puncture site may occur and pericardial bleeding may occur, and may be exacerbated by continued anticoagulant therapy.2,3,12 In severe cases of significant blood loss, blood transfusions are required.12 Pericardial bleeding may require surgical management.11 The risk of pocket hematoma is higher when the device is placed subpectorally vs. those that are placed subcutaneously.13 Patients with lower body mass index (BMI) and underweight patients have a higher incidence of minor hematoma formation at the implant site.1
Pocket hematomas, or collections of blood in the subcutaneous pocket into which the device is inserted, are not only cosmetically displeasing but can lead to device malfunction and act as a nidus of infection. They often present early after device insertion. The risk is higher for patients receiving ICDs than PPMs, likely because of device complexity.13 Depending on the subgroup of patients and individual risk factors for bleeding, incidence ranges between 1% and 15% of patients undergoing ICD placement.2,6,13 Large or persistent pocket hematomas may require percutaneous drainage or surgical excision.12 Monotherapy with novel oral anticoagulant medications is not associated with increased risk of early pocket hematoma development in retrospective data sets.10 Conversely, use of antiplatelet agents (single or dual agent therapy), vitamin K antagonists, and any combination of multiple antiplatelet or anticoagulant medications is associated with increased risk of pocket hematoma formation.10 Continued perioperative warfarin administration is associated with increased risk for pocket hematoma and also with longer length of hospitalization.14
Superficial bleeding may present with pain, swelling, redness, or sanguineous discharge at the incision or pocket site. Systemic bleeding is much less common. It should be suspected in a patient with continued anticoagulant therapy who has any of the typical signs and symptoms of blood loss, such as tachycardia, hypotension, pallor, poor perfusion of the extremities, altered mental status, or overt bleeding from other locations.
Management of local bleeding may include local pressure application to promote clot formation in the setting of active bleeding. Many pocket hematomas are managed by watchful waiting, since evacuation or aspiration of the collection may increase the risk of infection.13 More significant hematomas, which may cause local tissue necrosis or become primarily infected due to local tissue abnormalities in the setting of a large fluid collection, are sometimes aspirated or surgically evacuated.13 There is sparse literature regarding the use of locally injected tranexamic acid (TXA) or other prothrombotic agents. In severe cases the device may need to be explanted.13 Electrophysiologist (EP) consultation is warranted when a pocket hematoma is noted to ensure continued device integrity and effectiveness.
Management of systemic bleeding includes potential reversal of anticoagulant and antiplatelet medications, transfusion of blood products, and source control. Coordination with appropriate consultants, including cardiology, is warranted. Disposition is guided by the patient’s clinical stability, presence of ongoing bleeding, and severity of symptoms.
Pneumothorax and Hemothorax
Pathologic collections of air and/or blood may result from inadvertent pleural or pulmonary puncture during ICD insertion. Pneumothorax incidence is approximately 1%.2,15
Women and patients with chronic obstructive pulmonary disease (COPD) or lower BMI experience pneumothorax more frequently because of anatomic differences, making inadvertent pulmonary puncture harder to avoid.1,15 Placement of the larger, less flexible magnetic resonance imaging (MRI)-compatible leads may be associated with higher risk of pleural penetration when compared with other leads. Pneumothorax and hemothorax usually occur ipsilateral to the device. Rarely, if attempts to place the device on one side of the chest are aborted and the device is inserted on the other side, bilateral pleural penetration may occur.15
Symptoms usually present between one and four days after the procedure.15 Pneumothorax and hemothorax should be suspected in patients during or soon after ICD placement procedures who experience chest pain, shortness of breath, or cough. Tachycardia, tachypnea, hypoxia, and unilaterally decreased breath sounds on auscultation of the lungs may be noted. Subcutaneous crepitus is uncommonly observed but is possible in the case of a large pleural defect. Large pneumothoraces may result in tension physiology and resulting hemodynamic collapse.
Chest X-ray or bedside ultrasound support the diagnosis. Lack of lung sliding or a lung point may be observed with ultrasound of the lungs in the case of a pneumothorax. In the case of a hemothorax, a pleural effusion may be observed using ultrasound. Small air or fluid collections may be seen only with computed tomography (CT) scan of the chest. Function of the ICD itself may not be altered, and results of device interrogation may be normal.
Treatment of intrapleural air or blood is similar to other pathologic processes. While very small pneumothoraces may be managed expectantly with supplemental oxygen administration and supportive care, larger pneumothoraces and hemothoraces require chest tube placement for drainage.12 The decision to place a chest tube is guided by the size of the effusion or air collection (< 10% of pleural space involvement is unlikely to require chest tube) and severity of the patient’s symptoms.15
Cardiac Perforation
Perforation of the atrial or, less commonly, ventricular free wall may occur during CIED placement.12 While symptomatic free wall penetration is estimated to occur in 0.3% of patients, some suggest that asymptomatic free wall penetration occurs in up to 15% of patients with right atrial leads.15 This occurs more commonly in women and in patients receiving MRI-compatible leads or who are undergoing emergent CIED placement.1,15 Patients who have had previous cardiac surgery may be at lower risk of symptomatic hemopericardium or pneumopericardium due to formation of intrapericardial adhesions. In extremely rare cases, lead advancement during the procedure may cause penetration of the myocardium and pulmonary tissue resulting in pneumopericardium.15
Presentation ranges from chest pain and shortness of breath to cardiovascular collapse from myocardial free wall rupture and/or cardiac tamponade. The severity of symptoms and cardiac compromise depends on both the size of the effusion and rapidity of accumulation, as in other cases of pericardial fluid collections.
Identification of associated clinical features and a high index of suspicion are key to making the diagnosis. Echocardiography may show a pericardial effusion. Observation of right ventricular diastolic collapse on echocardiography is indicative of cardiac tamponade.
Treatment decisions are guided by the severity of the patient’s signs and symptoms and are made in conjunction with electrophysiology and cardiothoracic surgery. Isolated right atrial perforation in a hemodynamically stable patient may be managed expectantly with close monitoring.15 Symptomatic patients likely require surgical consideration. Patients with evidence of cardiac tamponade should undergo emergent pericardiocentesis while awaiting definitive surgical intervention. Further resuscitation should occur in other cases of obstructive shock due to pericardial tamponade.
Twiddler’s Syndrome
Certain patients who manipulate their device resulting in displacement or damage to their CIED experience a condition known as “Twiddler’s syndrome.” Movement of the device in the subcutaneous pocket may cause lead fracture or dislodgement and damage to the generator.3,16 In the case of ICDs, this may result in failure to appropriately defibrillate. Manipulation of the device also may contribute to the formation of pocket hematomas or prevent hematoma resorption.3
The diagnosis of Twiddler’s syndrome is made based on appropriate clinical history, indication of “twiddling” behavior by report or observation, and imaging. Chest radiography may demonstrate abnormal positioning of the device in the chest, lead dislodgement, or lead breakage. Untreated ventricular arrhythmias may be noted on ECG or on manufacturer reports from device interrogation, or certainly may be noted clinically if a hemodynamically unstable or lethal arrhythmia develops.
Treatment for Twiddler’s syndrome involves repair of damaged device components and device replacement in some cases. Placing anchoring sutures in the pectoralis fascia or use of antimicrobial pouches may decrease device mobility in the device pocket.16 Despite these efforts, Twiddler’s syndrome often is recurrent because of the behaviors that lead to the initial occurrence.16 Non-urgent psychiatric consultation often is indicated.
Long-Term Complications
Thrombotic Complications
Upper extremity deep venous thrombosis or venous occlusion is a known complication of CIED placement. Vascular injury during the procedure may lead to early stenosis or clot formation within the first several postoperative weeks. ICD leads may act as a nidus for clot formation, resulting in vascular stenosis or thrombosis as well. Clot formation occurs frequently after ICD placement but often is asymptomatic.17 Approximately 20% of patients fall into each category of minor, moderate, and severe occlusion, with another 22% experiencing complete venous occlusion related to ICD leads based on one large study; however, the estimated incidence ranges more widely.18 Upper extremity vessels, such as the left-sided subclavian and brachiocephalic veins, are involved most commonly, likely because of the typical left-sided approach for device insertion.2,11 In rare cases, the superior vena cava (SVC) may be involved, and SVC syndrome may occur.19 Risk for thrombosis is higher in patients with multi-lead devices.12
Venous stenosis or thrombosis is suggested by upper extremity pain, swelling, erythema, and/or fatigue. If robust collateral venous circulation is present, symptoms may be minimal or absent.18 Should thromboembolism to the lungs occur, signs and symptoms consistent with pulmonary embolism may be present. Workup for upper extremity venous pathology related to ICD leads begins with vascular imaging, such as ultrasound or venography.
Management of ICD lead-related venous obstruction is multifactorial and patient-specific based on potential future need for transvenous pacing leads, central lines, venous ports, and arteriovenous fistulas for hemodialysis. In some cases, a new device may simply be implanted on the other side of the chest.12 Contrast venography is used to map potential vessels for ICD reinsertion or other central venous access when left-sided vessels are compromised.12 Transvenous lead extraction (TLE) often is required.12,19 Venoplasty is undertaken in some cases.12,19
SVC syndrome related to ICD leads can be managed as in other cases of lead-related venous obstruction, with TLE and venoplasty as needed.12,19,20 Fortunately, a history of treated ICD lead-related SVC syndrome is not a contraindication to ICD replacement or permanent pacemaker insertion if clinically indicated.20
Device-Associated Infection
Infections related to CIEDs range from mild superficial infections to fulminant systemic infections. The overall incidence of infection is 1% to 2% of patients with CIEDs.3 The risk of infection after device or lead replacement is higher than that following primary device insertion.1 Staphylococcus aureus and Streptococcus epidermidis are the most commonly associated pathogens.21 Patients who experience a bleeding-related complication in the first month following device implantation seem to be at higher risk of subsequent infectious complications.22 A risk score dubbed the Shariff score has been developed to characterize risk of infection associated with CIED implantation. A score greater than 4 indicates a higher risk of infection and may be an indication for prophylactic periprocedural antibiotics.23
Superficial and pocket infections usually occur in the early postoperative period and are likely due to bacterial translocation into the pocket or subcutaneous tissue at the time of device placement.11,12 Clinical presentation may include redness, swelling, pain, and/or purulent drainage from the implantation site. Any suggestion of erosion of the device through the skin mandates evaluation and treatment for device-associated infection.3,12 Blood cultures are negative in isolated superficial and pocket infections.12 Treatment with antibiotics targeted toward S. aureus and S. epidermidis species often is sufficient. Device extraction may be required unless infection is confined to the soft tissue along the incision and does not involve the device pocket.11,12
Superficial infection may progress along the device leads to involve deeper structures. Deep local tissue infections and infection that spreads along the device leads may infect the heart valves, resulting in endocarditis, or cause bacteremia with severe systemic infection.11,12 The modified Duke criteria may suggest endocarditis as in other patient populations; however, sampling of intracardiac leads or identification of vegetations involving the leads is critical to formally diagnose device involvement. In these cases, device extraction and prolonged courses of antibiotics guided by blood culture results are warranted.12
Endocarditis may result from migration of infection along leads or from seeding of device components from a remote site of infection. Management of endocarditis depends on many variables, and treatment plans should be formulated in conjunction with all relevant subspecialists, including electrophysiology. Vegetations smaller than 2 cm often can be managed percutaneously, while larger vegetations require open heart surgery for optimal management.24
Device-related endocarditis is associated with an increased risk for stroke, particularly in patients with a patent foramen ovale (PFO) or other left to right shunt physiology to act as a pathway for septic emboli to enter systemic circulation.13 This is especially true during TLE when emboli may break off from vegetations more easily. Stroke occurs at a rate of about 1% in patients with endocarditis undergoing TLE, with approximately 50% of these patients having a PFO.13 Patients with larger and more globular vegetations are at even higher risk than those with smaller or nonglobular vegetations.24
The management of patients with a CIED and bacteremia without clear device involvement is not straightforward. Rates of bacteremia relapse are higher in patients whose devices are not removed. Bacteremic patients who do not undergo device explantation have a higher mortality rate (35% in one year in one study) than those whose devices are removed.25 Empiric CIED removal may be considered in some patients in conjunction with consultations with infectious disease and electrophysiology specialists.
Patients with uncomplicated superficial infections likely can be discharged after discussion with the patient’s electrophysiologist and with instructions to return for signs of progressing infection. Patients with systemic infection or suspicion for direct device involvement require admission for further workup and treatment.
Functional Complications
MRI Compatibility
Not all CIEDs are able to withstand exposure to the magnetic field generated by MRI without consequence. Devices that are able to undergo this sort of exposure are dubbed “MRI-conditional,” whereas older devices that cannot be exposed to a strong magnetic field without ensuing dysfunction are “MRI non-conditional.”26 Non-conditional devices may develop increased thermal energy in a magnetic field, which then can damage the leads, device generator, and even the myocardium by way of thermal burns.26 For patients with MRI non-conditional devices who must undergo MRI, transvenous lead extraction must be considered.12
Some noticeable, although not always clinically significant, changes may occur even to MRI-conditional devices during thoracic and non-thoracic imaging procedures.27 Uncommon ICD malfunctions related to MRI exposure include reset of the device to backup mode, change in sensing or output parameters, increases in atrial and/or ventricular capture thresholds, temporary decrease in battery voltage, and change in lead impedance.26,27 The malfunctions typically do not require device reprogramming or removal.27
Lead Failure
Lead failure results in impaired ability of ICD leads to transmit electrical impulses, including both sensing and defibrillating capabilities. It is expected that after a given number of years the leads may fail, since they are considered to have a finite reliable lifespan.28 The mechanical and chemical stresses to which the leads are subjected may result in both structural and electrical dysfunction. Lead failure occurs more commonly in younger, more physically active patients and patients with less advanced heart failure based on New York Heart Association (NYHA) classification in whom lead dislodgement and wearing down of the leads over time is more likely.29
Both structural and electrical dysfunction of the leads may lead to inability to sense endogenous myocardial electrical impulses and deliver pacing or shock therapy appropriately. Lead failure initially may go unnoticed until a clinically significant arrhythmia or sudden death occurs.29,30
In a patient with an ICD, ventricular arrhythmias that are not aborted by the device may be noted clinically and on ECG. Chest radiography, including chest X-ray, may show lead fracture or displacement if the cause of lead failure is mechanical. Evidence of lead failure may be evident on device interrogation reports.
A consult to electrophysiology should be made in all cases of suspected lead fracture. Patients with ventricular arrhythmias or cardiac arrest should be managed in the interim based on standard Advanced Cardiac Life Support (ACLS) protocols and treatment of the underlying arrhythmia. Disposition decisions for stable patients with suspected lead dysfunction should be made in conjunction with electrophysiology.
Inappropriate Shock
Uncommonly, an ICD may deliver a shock in response to a trigger other than a ventricular arrhythmia. Many cases are due to oversensing, particularly of the T wave portion of the ECG.31 The ICD notes the large T wave and mis-identifies it as another QRS complex; thus, the device “believes” that the heart rate is double the actual rate. Patients with a history of hypertrophic cardiomyopathy and atrial fibrillation seem to be at increased risk.31 The consequences of inappropriate shocks include discomfort, anxiety, initiation of a malignant arrhythmia, and myocardial injury.
Stable patients reporting a recent ICD shock should have their device interrogated and electrophysiology consulted. Significantly symptomatic and unstable patients should be resuscitated according to standard procedures in addition to ICD interrogation and specialist consult. (See Figure 4.) Disposition is guided by the patient’s symptoms, results of ICD interrogation, and treatment recommended by electrophysiology.
A key element of ICD interrogation is discrimination among appropriate shock, inappropriate shock, and phantom shock. Note that an appropriate shock is a defibrillation delivered in response to a malignant dysrhythmia, an inappropriate shock is for situations other than a malignant dysrhythmia, and a “phantom shock” is a perceived shock without actual shock delivery by the device.3 After ECG and device interrogation, patients experiencing a phantom shock require reassurance and appropriate outpatient follow-up as needed.
Figure 4. Evaluation of Reported Implantable Cardioverter Defibrillator Shock |
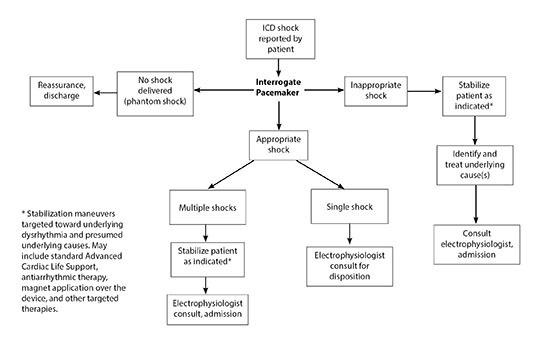 |
ICD: implantable cardioverter defibrillator |
Electromagnetic Interference
ICDs may be negatively affected by nearby electronic devices causing electromagnetic interference (EMI). Common sources of EMI include left ventricular assist devices (LVADs), deep brain and spinal cord stimulators, and even myopotentials from local muscle groups.32,33 Interference from nearby endogenous myopotentials may occur during exercise, seizure activity, or myoclonic activity following administration of depolarizing neuromuscular blocking agents for rapid sequence intubation. Procedures such as endoscopy, bronchoscopy, and surgeries using electrocautery also may cause EMI.32 Exposure to strong electrical alternating currents or some appliances has caused EMI in some cases as well.33 Propensity of a device to experience significant EMI may be in part based on the device manufacturer.32
EMI may cause inappropriate ICD shocks. More commonly, EMI may interfere with device interrogation.32
Increasing distance between the ICD and the source of EMI should resolve the problem.32 In the case of an in-situ device such as an LVAD, raising the device within its pocket or even moving the device entirely may be required. A Faraday cage also may be placed over the LVAD to decrease EMI.32
Battery Depletion and Generator Replacement
One study demonstrated that there are 3.9 repeat operations on CIEDs for reasons other than complications per 100 patient years, generally for device upgrade or replacement.8
The lifespan of an ICD battery typically is four to seven years and depends both on device activity and the manufacturer.8,34 Certain device settings, programmed by an electrophysiologist, may decrease overall device activity and increase battery lifespan.8 Conversely, a higher amount of device activity, such as frequent pacing or defibrillation due to innate arrhythmias or oversensing, can prematurely deplete the battery.
Signs of battery depletion include failure to deliver appropriate ATP or defibrillation therapy and/or audible alarms for some devices. Workup includes device interrogation, which is diagnostic in most cases. Cases of suspected battery depletion should be discussed with electrophysiology.
Device batteries must be replaced surgically.34 Given the risks associated with repeat CIED operations, patients and electrophysiologists should discuss the risks and benefits of different device types to balance the need for therapeutic ICD shock and the risk of future procedures. The risk of infection following generator replacement is higher than that following primary device insertion.1 Dual-chamber devices have an overall higher risk of implantation complications (38%) than single-chamber devices and are associated with a four times greater risk for reoperation for reasons other than complications as well.7,8 Opting for the less complex device, therefore, may confer a lower risk of complications as a result of reoperations in appropriately selected patients, while dual-chamber devices may continue to confer greater benefit to patients who experience atrial arrhythmias.7 Patients who received no therapeutic intervention from the device during its lifespan may not require device replacement, since the risks of reoperation may outweigh the benefits.34
Transvenous Lead Extraction
In some cases, ICD leads may need to be removed. Lead fracture or failure, device replacement, and device explantation due to associated infection are some common indications for TLE.12 While performed endovascularly, TLE is associated with numerous complications. Approximately 2% of patients undergoing TLE experience a clinically significant complication, 16% of which required urgent surgical intervention in one review.35
Certain factors are associated with higher risk for TLE complications. Female sex, extraction of three or more leads, longer implant duration, deep device-associated infection or endocarditis, and lead extraction for cardiac perforation are associated with such increased risk.35
Major complications include stroke from embolization of septic or aseptic clots, tricuspid valve damage and tricuspid regurgitation, cardiac perforation, and vessel injury.12 Focal neurologic symptoms following TLE should be assumed to be stroke until proven otherwise. Hemodynamic decline or collapse should prompt workup for cardiac or vascular damage with imaging such as bedside echocardiography. Complications are managed supportively in the emergency department with the appropriate specialists, such as electrophysiology, neurology, and cardiac surgery, as warranted. Admission generally is indicated.
Wearable Cardiac Defibrillators
Wearable external defibrillators or wearable cardiac defibrillators (WCDs) are used in certain patient populations at high risk for sudden cardiac death, typically as a bridge to definitive therapy.5,36 Indications for use include ischemic cardiomyopathy with LVEF less than 35%, new-onset nonischemic cardiomyopathy or myocarditis with LVEF < 35%, and as a bridge to ICD placement or cardiac transplant.5,36-38 WCD use may be preferable to ICD insertion in cases where underlying pathology is likely to resolve over time, such as patients with recent myocardial infarction, myocarditis or peripartum cardiomyopathy, or patients whose comorbidities preclude safe ICD insertion.5,37-39 In patients who have undergone ICD explantation, WCDs can be used to bridge the patient until a new ICD can be implanted.
While they may be easily taken on and off, strict compliance is critical because lethal cardiac events may occur when the device is off.36,39 Older patients tend to have better compliance with WCD use, which is fortuitous given that patients older than 65 years of age experience higher rates of ventricular tachycardia and ventricular fibrillation treated with WCD shock.40 Older patients also are more likely to ultimately undergo ICD placement.40
Younger populations, including pediatric patients, are shown to have adequate compliance with WCD use despite the fact they can be removed by the patient.41 They are effective at terminating atrial and ventricular arrhythmias in this cohort.41
Because these devices are worn externally, the primary adverse effect associated with WCDs is the occurrence of lethal arrhythmia if the device is not worn correctly.
Conclusion
A growing number of patients, particularly in the United States, undergo ICD implantation each year. These patients are at risk for a variety of complications ranging from minor to life-threatening. It is critical for providers in emergency department settings to be aware of the risks associated with ICD placement and management of ICD malfunctions and complications.
References
- Kirkfeldt RE, Johansen JB, Nohr EA, et al. Complications after cardiac implantable electronic device implantations: An analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186-1194.
- Ezzat VA, Lee V, Ahsan S, et al. A systematic review of ICD complications in randomised controlled trials versus registries: Is our ‘real-world’ data an underestimation? Open Heart 2015;2:e000198.
- Iqbal AM, Butt N, Jamal SF. Automatic Internal Cardiac Defibrillator. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated May 23, 2022. https://www.ncbi.nlm.nih.gov/books/NBK538341/
- Zormpas C, Silber-Peest AS, Eiringhaus J, et al. Eligibility for subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. ESC Heart Fail 2021;8:1502-1508.
- Al-Khatib S, Stevenson W, Ackerman M, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. J Am Coll Cardiol 2018;72:e91-e220.
- Knops RE, Olde Nordkamp LRA, Delnoy PHM, et al; PRAETORIAN Investigators. Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526-536.
- Borne RT, Varosy P, Lan Z, et al. Trends in use of single- vs dual-chamber implantable cardioverter-defibrillators among patients without a pacing indication, 2010-2018. JAMA Netw Open 2022;5:e223429.
- Ranasinghe I, Parzynski CS, Freeman JV, et al. Long-term risk for device-related complications and reoperations after implantable cardioverter-defibrillator implantation: An observational cohort study. Ann Intern Med 2016;165:20-29.
- Kipp R, Hsu JC, Freeman J, et al. Long-term morbidity and mortality after implantable cardioverter-defibrillator implantation with procedural complication: A report from the National Cardiovascular Data Registry. Heart Rhythm 2018;15:847-854.
- Notaristefano F, Angeli F, Verdecchia P, et al. Device-pocket hematoma after cardiac implantable electronic devices. Circ Arrhythm Electrophysiol 2020;13:e008372.
- Ghzally Y, Mahajan K. Implantable Defibrillator. In: StatPearls [Internet]. StatPearls Publishing; 2022 Jan-. Updated Oct. 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK459196/
- Boarescu PM, Roşian AN, Roşian ŞH. Transvenous lead extraction procedure-indications, methods, and complications. Biomedicines 2022;10:2780.
- Lee HM, Park JW, Na YC, et al. Effectiveness of aspiration in treating cardiac implantable electronic device-induced pocket hematoma and characteristics of patients with pocket hematoma. J Wound Management Res 2022;18:31-37.
- Afzal MR, Mehta D, Evenson C, et al. Perioperative management of oral anticoagulation in patients undergoing implantation of subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2018;15:520-523.
- Nantsupawat T, Li JM, Benditt DG, Adabag S. Contralateral pneumothorax and pneumopericardium after dual-chamber pacemaker implantation: Mechanism, diagnosis, and treatment. HeartRhythm Case Rep 2018;4:256-259.
- Osoro M, Lorson W, Hirsh JB, Mahlow WJ. Use of an antimicrobial pouch/envelope in the treatment of Twiddler’s syndrome. Pacing Clin Electrophysiol 2018;41:136-142.
- Safi M, Akbarzadeh MA, Azinfar A, et al. Upper extremity deep venous thrombosis and stenosis after implantation of pacemakers and defibrillators; A prospective study. Rom J Intern Med 2017;55:139-144.
- Duijzer D, de Winter MA, Mijkeuter M, et al. Upper extremity deep vein thrombosis and asymptomatic vein occlusion in patients with transvenous leads: A systematic review and meta-analysis. Front Cardiovasc Med 2021;8:698336. .
- Arora Y, Carrillo RG. Lead-related superior vena cava syndrome: Management and outcomes. Heart Rhythm 2021;18:207-214.
- Gabriels J, Chang D, Maytin M, et al. Percutaneous management of superior vena cava syndrome in patients with cardiovascular implantable electronic devices. Heart Rhythm 2021;18:392-398.
- Khairy TF, Lupien MA, Nava S, et al. Infections associated with resterilized pacemakers and defibrillators. N Engl J Med 2020;382:1823-1831.
- Nichols CI, Vose JG. Incidence of bleeding-related complications during primary implantation and replacement of cardiac implantable electronic devices. J Am Heart Assoc 2017;6:e004263.
- Balla C, Brieda A, Righetto A, et al. Predictors of infection after “de novo” cardiac electronic device implantation. Eur J Intern Med 2020;77:73-78.
- Arora Y, Perez AA, Carrillo RG. Influence of vegetation shape on outcomes in transvenous lead extractions: Does shape matter? Heart Rhythm 2020;17:646-653.
- Nakajima I, Narui R, Tokutake K, et al. Staphylococcus bacteremia without evidence of cardiac implantable electronic device infection. Heart Rhythm 2021;18:752-759.
- Russo RJ, Costa HS, Silva PD, et al. Assessing the risks associated with MRI in patients with a pacemaker or defibrillator. N Engl J Med 2017;376:755-764.
- Nazarian S, Hansford R, Rahsepar AA, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med 2017;377:2555-2564.
- Swerdlow CD, Kalahasty G, Ellenbogen KA. Implantable cardiac defibrillator lead failure and management. J Am Coll Cardiol 2016;67:1358-1368.
- Khattak F, Gupta A, Alluri K, et al. Rate and predictors of electrical failure in non-recalled defibrillator leads. Indian Pacing Electrophysiol J 2019;19:100-103.
- Ellenbogen KA, Wood MA, Shepard RK, et al. Detection and management of an implantable cardioverter defibrillator lead failure: Incidence and clinical implications. J Am Coll Cardiol 2003;41:73-80.
- Olde Nordkamp LR, Brouwer TF, Barr C, et al. Inappropriate shocks in the subcutaneous ICD: Incidence, predictors and management. Int J Cardiol 2015;195:126-133.
- Yalcin YC, Kooij C, Theuns DAMJ, et al. Emerging electromagnetic interferences between implantable cardioverter-defibrillators and left ventricular assist devices. Europace 2020;22:584-587.
- Pai RK, Abedin M, Rawling DA. Inappropriate ICD shocks for inappropriate reasons. Indian Pacing Electrophysiol J 2008;8:69-71.
- Lewis KB, Stacey D, Carroll SL, et al. Estimating the risks and benefits of implantable cardioverter defibrillator generator replacement: A systematic review. Pacing Clin Electrophysiol 2016;39:709-722.
- Sood N, Martin DT, Lampert R, et al. Incidence and predictors of perioperative complications with transvenous lead extractions: Real-world experience with national cardiovascular data registry. Circ Arrhythm Electrophysiol 2018;11:e004768.
- Bodin A, Bisson A, Fauchier L. When is a wearable defibrillator indicated? Expert Rev Med Devices 2021;18(sup1):51-56.
- Ashraf S, Ilyas S, Siddiqui F, et al. Keeping up to date: A current review of wearable cardioverter defibrillator use. Acta Cardiol 2020;75:695-704.
- Röger S, Rosenkaimer SL, Hohneck A, et al. Therapy optimization in patients with heart failure: The role of the wearable cardioverter-defibrillator in a real-world setting. BMC Cardiovasc Disord 2018;18:52.
- Kutyifa V, Vermilye K, Daimee UA, et al. Extended use of the wearable cardioverter-defibrillator in patients at risk for sudden cardiac death. Europace 2018;20:f225-f232.
- Daimee UA, Vermilye K, Moss AJ, et al. Experience with the wearable cardioverter-defibrillator in older patients: Results from the Prospective Registry of Patients Using the Wearable Cardioverter-Defibrillator. Heart Rhythm 2018;15:1379-1386.
- Spar DS, Bianco NR, Knilans TK, et al. The US experience of the wearable cardioverter-defibrillator in pediatric patients. Circ Arrhythm Electrophysiol 2018;11:e006163.
It is critical for providers in emergency department settings to be aware of the risks associated with implantable cardioverter defibrillator (ICD) placement and management of ICD malfunctions and complications.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
