EXECUTIVE SUMMARY
This article summarizes for primary care physicians an update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report, used worldwide as a tool to implement effective management programs for chronic obstructive pulmonary disease (COPD).
- COPD was the fourth leading cause of death in the United States in 2018 and is estimated to be responsible for 3 million deaths annually around the world, with an expected increase to 5.4 million annual deaths from COPD and related conditions by 2060. The costs attributable to COPD in the United States over the next 20 years are projected to be $800.9 billion, or $40 billion per year.
- COPD is the end result of complex, dynamic, and cumulative interactions between an individual’s genes and their environment over the course of a lifetime, resulting in damage to their lungs and/or alteration of their normal development or aging processes.
- The most common environmental risk factors include cigarette smoking, biomass exposure (burned as fuel for household fires, particularly in lower-income countries), occupational exposures, and air pollution.
- The best documented genetic risk factor for COPD is the mutation in the SERPINA1 gene, leading to the hereditary of alpha-1 antitrypsin deficiency.
- A forced spirometry that demonstrates a forced expiration volume in 1 second (FEV1)/forced vital capacity (FVC) ratio < 0.7 is mandatory to establish the diagnosis. The FEV1 also is needed to establish the severity of airflow obstruction.
- Exacerbations of COPD are key events in the natural history and progression of the disease. Exacerbations are associated with most of the healthcare costs associated with COPD. The most consistent predictor of the frequency of future exacerbations is the history of previous exacerbations. Other risk factors for frequent exacerbations (defined as two or more per year) include severity of airflow obstruction, poor health status, age, and the presence of emphysema and leukocytosis.
- Pharmacotherapy includes beta-2 agonists, antimuscarinic agents, and anti-inflammatory therapy. Other measures may include supplemental oxygen, surgical and bronchoscopic interventions, pulmonary rehabilitation, and lung volume reduction surgery in selected patients.
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) releases a report annually that is used worldwide as a tool to implement effective management programs for chronic obstructive pulmonary disease (COPD). This article provides an update for primary care physicians based on the GOLD 2023 report.1
Definition
GOLD 2023 defines COPD as a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production, and/or exacerbations) caused by abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that result in persistent, often progressive, airflow obstruction.2
The Burden of COPD
COPD was the fourth leading cause of death in the United States in 2018 and is estimated to be responsible for 3 million deaths annually around the world, with expected increases to 5.4 million annual deaths from COPD and related conditions by 2060.3 The costs attributable to COPD in the United States over the next 20 years are projected to be $800.9 billion, or $40 billion per year.4 For the individual patient, the cost of care increases with the severity of the disease, driven by such factors as the costs of hospitalizations, medications, and ambulatory oxygen and other durable medical equipment. Data from the Global Burden of Disease Study 2017 estimated that COPD was the second leading cause of reduced disability-adjusted life years (DALYs), with ischemic heart disease coming first.5
The Burden of Obstructive Lung Diseases (BOLD) program found the estimated global prevalence of COPD to be 10.3% (95% confidence interval, 8.2%, 8.2%, 12.8%), with prevalence increasing sharply with age.6 The mean prevalence was 11.2% in men and 8.6% in women, and higher in smokers than never-smokers, with a mean population attributable risk for smoking of 5.1% in men and 2.2% in women.
Pathogenesis: Causes and Risk Factors
COPD is the end result of complex, dynamic, and cumulative interactions between an individual’s genes and their environment over the course of their lifetime, resulting in damage to their lungs and/or alteration of their normal development or aging processes.7 The most common environmental risk factors include cigarette smoking, biomass exposure (burned as fuel for household fires, particularly in lower-income countries), occupational exposures, and air pollution.
Cigarette smokers have a higher prevalence of respiratory symptoms and lung function abnormalities than nonsmokers. They also have a greater annual rate of forced expiration volume in 1 second (FEV1) decline and mortality than nonsmokers, and quitting helps to mitigate against some of these effects.8 Only about 50% of smokers develop COPD, pointing to a possible underlying genetic susceptibility to cigarette smoke in some individuals.
Smoking during pregnancy poses a risk to the unborn baby by altering lung growth and development in utero, and possibly priming the immune system for abnormal or enhanced responses to environmental factors in the future.7,9
The best documented genetic risk factor for COPD is the mutation in the SERPINA1 gene leading to the hereditary alpha-1 antitrypsin deficiency (AATD). AATD is rare, with only 0.12% of COPD patients carrying the AATD protein inhibitor ZZ genotype in European populations.10 It is more common in people of northern European descent than in those of eastern European descent. Other genetic variants have been associated with reduced lung function and increased risk for COPD, but these are even more rare than AATD.
Adults diagnosed with asthma have a 12-fold increased risk of developing COPD over their lifetime than those without asthma, after adjusting for smoking.11 Another risk factor is infections, including chronic bronchial infection (particularly with Pseudomonas aeruginosa), tuberculosis, and human immunodeficiency virus.12-14
Poverty consistently is associated with airflow obstruction and with mortality as the result of COPD.15 It is not clear how much of this observation is caused by exposure to household and outdoor air pollutants, overcrowding, poor nutrition, infections, and/or other factors.
Lung Development and Aging
Lungs continue to grow and mature until about 20 to 25 years of age, followed by a short plateau before subsequent progressive, mild decline in lung function as the result of physiological lung aging. This expected trajectory in lung function may be altered by processes during the prepartum and peripartum periods, childhood, and adolescence that may impair lung growth and, hence, diminish peak lung function. It also may be altered by processes later in life that accelerate the aging process.16 Reduced peak pulmonary function in early adulthood increases the risk of developing COPD later in life.
Disadvantages during childhood that may impair lung growth and development include premature birth, low birth weight, maternal smoking during pregnancy, repeated respiratory infections, and poor nutrition.
Pathophysiology
Airflow obstruction, as measured by spirometry, is widely available and is the most readily reproducible test of lung function. In COPD, chronic inflammation, especially in response to chronic irritants such as cigarette smoke, leads to changes in the small airways and lung parenchyma.
These changes lead to airflow obstruction caused by a combination of small airway disease (leading to increased resistance to airflow) and parenchymal destruction (known as emphysema), which impairs the normal elastic recoil of the lung.17 Small airway disease includes luminal narrowing and exudates as well as the loss of the overall number of small airways.18
The consequence of these changes is limited emptying of the lung during forced expiration, with resultant gas trapping and hyperinflation.
Static hyperinflation related to the loss of elastic recoil reduces inspiratory capacity and commonly leads to further (dynamic) hyperinflation during exertion, resulting in limited exercise capacity and exertional dyspnea.
Structural abnormalities in the airways, alveoli, and pulmonary circulation alter the normal ventilation-perfusion distributions. This is the main mechanism of abnormal gas exchange resulting in hypoxemia with or without hypercapnia.19
COPD Diagnosis and Assessment
Diagnosis
A forced spirometry that demonstrates an FEV1/forced vital capacity (FVC) ratio < 0.7 is mandatory to establish the diagnosis. The FEV1 also is needed to establish the severity of airflow obstruction (discussed later in this article).
Clinical Presentation
The most characteristic clinical feature of COPD is chronic dyspnea. Cough with sputum production is present in up to 30% of patients. The diagnosis of COPD should be considered in any patient in whom any of the following clinical indicators are present, keeping in mind that in every instance, spirometry is required to establish the diagnosis:
• Dyspnea that is progressive over time, worse with exercise, and/or persistent/chronic;
• Recurrent wheeze;
• Chronic cough with or without sputum production;
• History of risk factors, including tobacco smoke; smoke from home cooking and heating fuel; occupational dusts, vapors, fumes, gases, and other chemicals; and host factors, including genetic factors, developmental abnormalities, low birth weight, prematurity, and childhood infections.
Dyspnea
Patients describe dyspnea in various ways, such as increased work or effort of breathing, chest heaviness or tightness, or air hunger. Dyspnea is prevalent in all stages of airflow obstruction and is more pronounced with exertion. The five-level modified Medical Research Council (mMRC) scale is used to classify dyspnea in the GOLD clinical scheme. It is important to assess symptoms because patients with higher dyspnea scores use more healthcare resources, incurring higher costs. This scale is discussed in greater detail later.
Chronic Cough
Chronic cough often is the first symptom that prompts patients to seek medical care. It frequently may be discounted by the patient as an expected consequence of their smoking. Initially intermittent, patients eventually experience daily cough, often throughout the day. The cough may be productive or nonproductive of sputum.20
There are many causes of chronic cough, and clinicians should explore the full range of differential diagnoses for each patient. Other common causes of chronic cough include:1
• Intrathoracic: asthma, lung cancer, heart failure, bronchiectasis, interstitial lung disease, cystic fibrosis, tuberculosis;
• Extrathoracic: gastroesophageal reflux, allergic rhinitis, postnasal drip syndrome, medications (e.g., angiotensin-converting enzyme inhibitors).
Sputum Production
Chronic bronchitis has a longstanding, somewhat arbitrary classic definition that includes regular production of sputum for three or more months in two consecutive years.21 This definition does not reflect the full spectrum of sputum production encountered in COPD. Patients commonly produce small amounts of tenacious sputum with cough, but sputum production is difficult to estimate because patients may swallow rather than expectorate it, a habit that is subject to cultural and even gender norms. Production of copious amounts of sputum may point to a diagnosis of comorbid bronchiectasis. In one meta-analysis, the mean prevalence of bronchiectasis in patients with COPD was 54.3%.22
Other Symptoms
Other symptoms of COPD include:
• Fatigue: A subjective feeling of exhaustion or lack of energy that affects a patient’s ability to perform activities of daily living and affects their quality of life.
• Wheezing and chest tightness: Tends to vary from day to day and with exertion. Also may be present in patients with asthma.
• Weight loss, sarcopenia, and anorexia: Also may be seen in patients with severe COPD. These symptoms are prognostic, but also may point to other underlying diagnoses, such as lung cancer, and should be investigated accordingly.23,24
Physical Examination
Physical examination findings have low sensitivity and specificity for COPD, and, indeed, signs of airflow obstruction may not be present until after significant impairment has set in.25 Despite these limitations, clinicians should perform a physical examination in every instance, since it may lead to detection of complications of COPD, as well as potential comorbidities and/or differential diagnoses. Expected findings would include barrel chest, finger clubbing, cyanosis, decreased breath sounds, wheezing, prolonged expiration, and the use of accessory muscles of respiration.
Spirometry
Spirometry is the most objective assessment of airway obstruction. It is a test that is readily reproducible, widely available, noninvasive, and relatively cheap. Clinicians who care for patients with COPD should endeavor to ensure that they have ready access to spirometry in their practice settings. Peak expiratory flow measurements have good sensitivity, but their weak specificity limits their usefulness as the primary diagnostic test.26,27 Typically, spirometry is performed both before and after the administration of bronchodilators to assess for any degree of reversible airway obstruction, which is more consistent with asthma than with COPD.
In a healthy individual, the forced spirometry tracing typically will show a sharp rise in the volume of exhaled air (in liters) with increasing time (in seconds) until they reach the maximum amount of air they can exhale. The two most essential parameters measured during this test are:
• FEV1: The volume of air forcefully exhaled in the first second of the test.
• FVC: The total volume of air exhaled forcefully from full lung inspiration to maximum expiration.
A normal spirometry tracing will show a well-defined, rapid rise in volume during the first second, reaching close to the FVC, indicating that a significant amount of air can be expelled quickly from the lungs. (See Figure 1.)
Figure 1. Spirometry Flow Volume Loop |
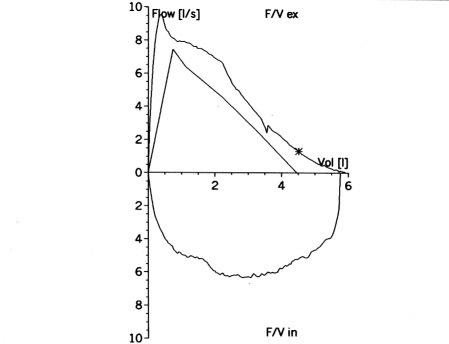 |
The X axis represents air flow (liters per second), the Y axis represents volume (liters). The top of the graph is maximum expiration, and the bottom is the following minimum inspiration. |
Source: https://commons.wikimedia.org/wiki/File:Flow-volume-loop-small.png. Creative Commons Attribution-Share Alike 3.0 Unported license. Credit: Silvermask at the English-language Wikipedia. |
In patients with COPD, the spirometry tracing will show a decreased FEV1 because of airflow obstruction, and, to a lesser extent, FVC as the result of air trapping.
The spirometric criterion for airflow obstruction is a post-bronchodilator ratio or FEV1:FVC of < 0.7. This criterion is independent of reference values, since it relies on variables measured in the same individual at the same time. An alternative to the use of a fixed FEV1:FVC ratio to define airway obstruction is to use a cut-off based on the lower limit of normal (LLN) values for FEV1:FVC. LLN values are based on normal distribution and classify the bottom 5% of the healthy population as abnormal. Furthermore, LLN values have not been validated in longitudinal studies across different populations. The use of the LLN values does not result in improved diagnostic and prognostic accuracy over the use of the fixed ratio, while the fixed ratio offers diagnostic simplicity and consistency.28 Finally, the fixed ratio has been used in all the clinical trials that form the evidence base on which treatment recommendations are drawn. As a result, GOLD favors the use of the fixed ratio over LLN values.1
A single post-inhaled bronchodilator FEV1:FVC ratio between 0.60 and 0.80 should be confirmed by repeat spirometry on a separate occasion.1 Diagnostic instability, with patients requiring reclassification as either having COPD or not based on subsequent spirometry findings, is common among patients close to the diagnostic cut-off, particularly if they quit smoking. In one study, up to one-third of symptomatic ex-smokers with baseline obstruction on diagnostic spirometry had shifted to non-obstructed when routinely re-tested after one or two years.29,30 Current smokers who are just above the baseline of normal also are more likely to progress to overt obstruction over time. In addition, spirometry should not be assessed during an acute exacerbation of the patient’s COPD symptoms.
Although post-bronchodilator spirometry is the standard for diagnosis and subsequent assessment of COPD, measurement of the degree of reversibility of airflow obstruction (measuring FEV1 before and after inhaled bronchodilator or corticosteroid therapy) to inform therapeutic decisions no longer is recommended.31 The degree of reversibility in a single individual fluctuates over time and does not differentiate between the diagnosis of COPD from asthma or help to predict the response to long-term treatment with corticosteroids or inhaled bronchodilators.32 Spirometry is useful in COPD for diagnosis, assessment of degree of airflow obstruction (for prognosis), follow-up assessment (therapeutic decisions, need for nonpharmacological interventions, and identification of rapid decline).
Interpretation of the severity of lung function impairment is dependent on having appropriate reference values. However, studies have identified differences in lung function between different regions of the world, and across different generations living in the same region.33,34 Accordingly, lung reference values should be revised periodically.
GOLD advocates for active case finding (actively seeking out patients with symptoms and/or risk factors for COPD and performing spirometry in the primary care setting), but not screening spirometry of the general population.35 This approach (case finding) leads to increasing rates of diagnoses of COPD, particularly in patients with mild or minimally symptomatic disease, but there are limited data to suggest a significant effect on patient outcomes.36,37
Initial Assessment
After the diagnosis of COPD has been confirmed by spirometry, the clinician must focus on four important aspects to guide therapy: severity of airflow obstruction, nature and magnitude of the patient’s symptoms, previous history of exacerbations, and the presence and type of comorbidities.
Severity of Airflow Obstruction
The severity of airflow limitation is based on the post-bronchodilator value of FEV1 (as a percentage of the reference) and reported as GOLD grades 1 through 4, representing increasing severity of airflow obstruction. (See Table 1.)
Table 1. GOLD Grades and Severity of Airflow Obstruction Based on Post-Bronchodilator FEV1 in COPD Patients (FEV1:FVC < 0.7) |
||
GOLD 1 |
Mild |
FEV1 ≥ 80% predicted |
GOLD 2 |
Moderate |
50% ≤ FEV1 80% predicted |
GOLD 3 |
Severe |
30% ≤ FEV1 < 50% predicted |
GOLD 4 |
Very severe |
FEV1 < 30% predicted |
GOLD: The Global Initiative for Chronic Obstructive Lung Disease; FEV1: forced expiration volume in 1 second; COPD: chronic obstructive pulmonary disease; FVC: forced vital capacity |
||
Symptoms
There is only a weak correlation between the severity of airflow obstruction (GOLD grades 1-4) and the severity of symptoms experienced by the patient or the magnitude of impairment to their health status.38,39 Consequently, a formal assessment of the patient’s symptoms using a validated questionnaire is needed. The more comprehensive disease-specific health status questionnaires, such as the Chronic Respiratory Disease Questionnaire, are important tools in research but are too complex to use in routine practice.
The mMRC dyspnea scale measures breathlessness, a key, but often unrecognized, symptom in patients with COPD. (See Table 2.) The mMRC score correlates well to other multidimensional health status questionnaires and predicts future mortality risk even better than the degree of airflow obstruction.40
Table 2. Modified Medical Research Council (mMRC) Dyspnea Scale |
|
mMRC Grade 0 |
Breathlessness with strenuous exercise |
mMRC Grade 1 |
Shortness of breath when hurrying on a level surface or walking up a slight hill |
mMRC Grade 2 |
Walking slower than people the same age (on a level surface) because of breathlessness or stopping for breath when walking at own pace (on a level surface) |
mMRC Grade 3 |
Stopping for breath after walking about 100 meters (300 yards) or after a few minutes on a level surface |
mMRC Grade 4 |
Too breathless to leave the house or breathlessness when dressing or undressing |
Adapted from Global Initiative for Chronic Obstructive Lung Disease. 2023 Gold Report. https://goldcopd.org/2023-gold-report-2/ |
|
The COPD Assessment Test (CAT) is an eight-item questionnaire that also has been extensively documented and is more comprehensive than the mMRC, incorporating other symptoms, including sputum production, chest tightness, ability to walk up a hill, and limitation of household activities. However, it is more difficult to use in a busy primary care setting, particularly with patients with limited health literacy.
Exacerbation Risk
Exacerbations of COPD are key events in the natural history and progression of the disease that significantly affect the patient’s immediate health status, accelerate the rate of decline in lung function, and worsen the prognosis of the patient. Exacerbations are associated with most of the healthcare costs related to COPD. The most consistent predictors of the frequency of future exacerbations is the history of previous exacerbations.41 Other risk factors for frequent exacerbations (defined as two or more per year) include the severity of airflow obstruction, poor health status, age, and the presence of emphysema and leukocytosis.42
Combined Initial COPD Assessment
GOLD recommends combining three components in the initial assessment of the patient with COPD: severity of airflow obstruction (spirometric GOLD grade 1-4), severity of symptoms (mMRC or CAT), and history of moderate and severe exacerbations.
The patient’s final categorization would be reported as GOLD grade X group X, with grades ranging from 1-4 and groups including A, B, or E. See https://erj.ersjournals.com/content/erj/61/4/2300239/F2.large.jpg for further information.
Imaging
Although chest X-ray is not useful to establish the diagnosis of COPD, it is valuable in excluding alternative diagnoses. Computed tomography (CT) is valuable in documenting additional information on structural abnormalities present in COPD. The use of low-density CT to screen for lung cancer in patients with a history of smoking has increased the number of patients requiring chest CT for evaluation of pulmonary nodules. These imaging modalities in turn provide further information, such as the presence and severity of emphysema and the presence of comorbid conditions. In addition to those who meet criteria for lung cancer screening, GOLD 2023 recommends chest CT in COPD patients with persistent exacerbations, symptoms out of proportion to degree of airflow obstruction, and severe airflow limitation with significant hyperinflation and gas trapping.
AATD Testing
The World Health Organization (WHO) recommends that all patients with COPD be screened once for AATD, particularly in areas with a high prevalence of AATD.43 COPD associated with AATD is not related to smoking, and screening should not be limited only to nonsmokers. Furthermore, screening should not be limited to young (younger than age 45 years) patients only, since the diagnosis often is delayed until later in life. A low concentration of alpha-1 antitrypsin (< 20% of normal) is highly suggestive of homozygous deficiency. Family members should be screened and referred, together with the index patient, to specialized centers for advice and management.
Management of Stable COPD
After the diagnosis and severity of COPD have been established, management involves both pharmacological and nonpharmacological measures.
Smoking Cessation
Approximately 40% of patients with COPD are current smokers. Smoking cessation has the greatest capacity to influence the natural history of COPD. Smoking has an adverse effect on prognosis and progression of the disease.44,45 With dedication of effective resources and time to smoking cessation, long-term quit rates of up to 25% can be achieved.46 A combination of behavioral and pharmacologic therapies is most effective. Legislative bans also are effective in reducing harm caused by second-hand smoke exposure and increasing quit rates.
Pharmacotherapies for Smoking Cessation
Nicotine replacement therapies include gum, inhalers, nasal spray, lozenges, transdermal patches, and sublingual tablets. These reliably increase long-term abstinence better than placebo. E-cigarettes are not recommended as nicotine replacement therapy because of concerns regarding vaping-associated lung injury.
Pharmacological products for smoking cessation include bupropion and nortriptyline. Varenicline tartrate is the most effective pharmacotherapy.
Counseling
Counseling delivered by physicians and other health professionals increases quit success rates, with more intense (time per session and frequency of sessions) yielding better results than brief advice.47 The five-step program provides a useful framework for clinicians to use when discussing smoking cessation with their patients.48 (See Table 3.)
Table 3. Strategies to Help the Patient Willing to Quit Smoking |
|
Ask |
Implement a system to query and document smoking status for every patient during every clinic visit. |
Advise |
Strongly urge all tobacco users to quit. |
Assess |
Determine willingness and rationale of patient’s desire to make a quit attempt. |
Assist |
Aid the patient in quitting by providing them with a quit plan, practical counseling, social support, and approved pharmacotherapy. |
Arrange |
Schedule follow-up contact either in person or via telephone. |
Adapted from Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: A prospective cohort study. Lancet Respir Med 2013;1:43-50. |
|
Vaccinations
Various vaccinations can reduce the morbidity and mortality associated with COPD. Influenza vaccination reduces serious illness, such as lower respiratory tract infections requiring hospitalization and death in patients with COPD.49 It also reduces the number of COPD exacerbations.50 The pneumococcal vaccine has been shown to reduce the incidence of community-acquired pneumonia and exacerbations in people with COPD. The Centers for Disease Control and Prevention (CDC) recommends one dose of 20-valent pneumococcal conjugate vaccine (PCV20) or one dose of PCV15 followed by 23-valent pneumococcal polysaccharide vaccine (PPSV23). For more information, visit https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf.
SARS-CoV-2 (COVID-19) vaccination is recommended by the WHO and CDC for all patients with COPD. For pertussis, the CDC recommends tetanus, diphtheria, and pertussis (Tdap) dTaP/dTPa vaccination to people with COPD who were not vaccinated as adolescents. The CDC recommends zoster vaccine to protect against shingles in all people with COPD who are 50 years of age or older.
Pharmacotherapy for Stable COPD
Pharmacologic therapy for COPD is aimed at reducing symptoms and the severity and frequency of exacerbations, and improving exercise tolerance and health status.
The classes of medications available to treat COPD include bronchodilators, anti-inflammatories, and others.
Bronchodilators
Bronchodilators act by relaxing the airway smooth muscle. Beta-2 agonists include short-acting beta agonists (SABAs), such as albuterol and levalbuterol, with a duration of action of four to six hours. Long-acting beta agonists (LABAs), such as formoterol, indacaterol, salmeterol, arfomoterol, and olodaterol, have a duration of action exceeding 12 hours.
Antimuscarinic agents include short-acting muscarinic antagonists (SAMAs), such as ipratropium, and long-acting muscarinic antagonists (LAMAs), such as aclidinium, glycopyrrolate, tiotropium, umeclidinium, and revefenacin.
LAMAs and LABAs significantly improve lung function, dyspnea, and health status and reduce exacerbation rates. LAMAs have a greater effect on exacerbation reduction and the decrease of hospitalizations compared to LABAs, but LAMA/LABA combinations are more efficacious than monotherapy with either type of drug class in increasing FEV1 while reducing symptoms and exacerbations.51-53 Although regular use of short-acting bronchodilators is not recommended, combinations of SABA and SAMA are superior compared to either medication alone in improving FEV1 and symptoms.
There are various combinations of bronchodilators available, and the choice of one over the other should be driven by cost and patient comfort with the unique delivery system and device. Wherever possible, single inhaler therapy should be prescribed over multiple inhalers to increase convenience and effectiveness.
Anti-Inflammatory Therapy in Stable COPD
The addition of inhaled corticosteroids (ICS) to bronchodilator therapy should be considered in those with elevated eosinophil counts and/or frequent or severe exacerbations. Regular treatment with ICS increases the risk for pneumonia, and de-escalation of therapy should be considered when appropriate. Monotherapy with ICS is not recommended in patients with COPD. The patients with the greatest likelihood of benefit from additive ICS therapy are those with blood eosinophil counts ≥ 300 cells/microliter. Patients who have blood eosinophil counts < 100 cells/microliter are at greatest risk for developing pneumonia and should not be treated with long-term ICS therapy.54 (See Table 4.)
Table 4. Considerations for ICS Treatment in Addition to Dual LAMA/LABA Therapy |
|
Strongly consider use |
|
Consider use |
|
Do not use |
|
*Despite appropriate long-acting bronchodilator maintenance therapy ICS: inhaled corticosteroids; LAMA: long-acting muscarinic agent; LABA: long-acting beta agonist; COPD: chronic obstructive pulmonary disease |
|
See Figure 3 from the Global Initiative for Chronic Obstructive Lung Disease 2023 Report (https://erj.ersjournals.com/content/erj/61/4/2300239/F3.large.jpg) for further information on initial pharmacological treatment of COPD.
Regarding other anti-inflammatory drugs:
• Long-term use of oral glucocorticoids has numerous side effects with minimal evidence of benefits. Systemic glucocorticoids are beneficial in treating acute exacerbations in hospitalized patients.
• For patients with chronic bronchitis, severe to very severe COPD, and a history of exacerbations, phosphodiesterase-4 (PDE-4) inhibitors such as roflumilast improve lung function and reduce moderate and severe exacerbations.
• Long-term azithromycin or erythromycin reduces the risk of exacerbations over one year but increases the risk of resistant bacteria. There is a possibility for QT interval prolongation with these drugs, which may lead to potentially fatal arrhythmias.
• Regular treatment with mucolytics, such as erdosteine, carbocysteine, and N-acetylcysteine, reduces the risk of exacerbations in select populations (particularly those not receiving ICS).
Rehabilitation, Education, and Self-Management
Pulmonary rehabilitation improves dyspnea, health status, and exercise tolerance in stable patients; reduces hospitalization among patients with recent (less than four weeks) exacerbation; and leads to a reduction in symptoms of anxiety and depression.
Education alone not has not been shown to be effective. Self-management intervention with communication with a healthcare professional improves health status and decreases hospitalizations and emergency room visits.
Other Treatments
Long-term administration (> 15 hours per day) of supplemental oxygen therapy has been shown to increase survival in individuals with severe resting hypoxemia (arterial partial pressure of oxygen [PaO2] < 55 mmHg or oxygen saturation level measured by blood analysis [SaO2] < 88%) but not in those with moderate resting or exercise-induced hypoxemia (SpO2 89% to 93%).55,56 Supplemental oxygen should be titrated to keep SaO2 ≥ 90%.
Primary care physicians should refer patients with severe COPD to specialized centers for consideration of various bronchoscopic and surgical interventions that may alleviate symptoms and improve health status. Patients with large bullae may benefit from bullectomy. Patients who are not candidates for surgical or endoscopic interventions may be considered for lung transplantation.
Therapies Shown to Reduce Mortality in COPD
Whereas oxygen supplementation historically has been the only intervention shown to reduce mortality in patients with COPD, there are several other interventions also now proven to reduce mortality:
• LAMA + LABA + ICS in patients with a history of frequent and/or severe exacerbations;
• Smoking cessation in asymptomatic or mildly asymptomatic patients;
• Pulmonary rehabilitation in patients recently hospitalized for exacerbation of COPD;
• Long-term oxygen therapy in patients with severe hypoxemia;
• Noninvasive positive pressure ventilation in stable COPD with marked hypercapnia;
• Lung volume reduction surgery in patients with upper lobe emphysema and low exercise capacity.
Supportive, Palliative, End-of-Life, and Hospice Care
The goal of palliative care is to prevent and relieve suffering, and to support the best possible quality of life for patients and their families, regardless of the stage of disease.57 COPD is a highly symptomatic disease, and patients experience fatigue, dyspnea, depression, anxiety, and insomnia, which require palliative management.
Palliative Treatment for Dyspnea
A major goal of care for people with COPD is to relieve dyspnea during daily life activities. Interventions that have been postulated to relieve breathlessness include opiates, fans blowing air onto the face, neuromuscular electrical stimulation, and chest wall vibration.
Nutritional Support
Low body mass index and particularly low fat-free mass is associated with increased exacerbation risk and mortality in people with COPD.58 In patients with malnutrition, nutritional supplementation promotes weight gain and leads to significant improvements in respiratory muscle strength and overall quality of life.59
Panic, Anxiety, and Depression
Pulmonary rehabilitation, cognitive behavioral therapy, and mind-body interventions (mindfulness-based therapy, yoga, and relaxation) may reduce anxiety symptoms, whereas the efficacy of antidepressants in people with COPD is inconclusive.60
Fatigue
Self-management education, pulmonary rehabilitation, nutritional support, and mind-body interventions all may mitigate excessive fatigue in people with COPD.61
Summary
COPD is a common, preventable, and treatable disease, but extensive underdiagnosis and misdiagnosis lead to patients not receiving treatment or receiving incorrect treatment. Primary care physicians should know the clinical presentation, risk factors, diagnosis, and management of COPD. Diagnosis and management of COPD exacerbations will be discussed in a future article.
References
- Agustí A, Celli BR, Criner GJ, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med 2023;207:819-837.
- Global Initiative for Chronic Obstructive Lung Disease. 2023 Gold Report. https://goldcopd.org/2023-gold-report-2/
- Heron MP. Deaths: Leading causes for 2018. Natl Vital Stat Rep 2021;70:1-115. https://stacks.cdc.gov/view/cdc/104186
- Zafari Z, Li S, Eakin MN, et al. Projecting long-term health and economic burden of COPD in the United States. Chest 2021;159:1400-1410.
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020;8:585-596.
- Burney P, Patel J, Minelli C, et al. Prevalence and population-attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med 2021;203:1353-1365.
- Agustí A, Melén E, DeMeo DL, et al. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene-environment interactions across the lifespan. Lancet Respir Med 2022;10:512-524.
- Kohansal R, Martinez-Camblor P, Agustí A, et al. The natural history of chronic airflow obstruction revisited: An analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 2009;180:3-10.
- Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 1995;152:977-983.
- Blanco I, Diego I, Bueno P, et al. Prevalence of α1-antitrypsin PiZZ genotypes in patients with COPD in Europe: A systematic review. Eur Respir Rev 2020;29:200014.
- Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004;126:59-65.
- Martínez-García MÁ, Faner R, Oscullo G, et al. Chronic bronchial infection is associated with more rapid lung function decline in chronic obstructive pulmonary disease. Ann Am Thorac Soc 2022;19:1842-1847.
- Fan H, Wu F, Liu J, et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ann Transl Med 2021;9:390.
- Hernandez Cordero AI, Yang CX, Obeidat M, et al. DNA methylation is associated with airflow obstruction in patients living with HIV. Thorax 2021;76:448-455.
- Townend J, Minelli C, Mortimer K, et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J 2017;49:1601880.
- Agustí A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med 2019;7:358-364.
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645-2653.
- McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-1575.
- Rodríguez-Roisin R, Drakulovic M, Rodríguez DA, et al. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol (1985) 2009;106:1902-1908.
- Cho SH, Lin HC, Ghoshal AG, et al. Respiratory disease in the Asia-Pacific region: Cough as a key symptom. Allergy Asthma Proc 2016;37:131-140.
- [No authors listed]. Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965;1:775-779.
- Ni Y, Shi G, Yu Y, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease with comorbid bronchiectasis: A systemic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2015;10:1465-1475.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005;82:53-59.
- Rutten EP, Calverley PM, Casaburi R, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: Do they influence patient-related outcomes? Ann Nutr Metab 2013;63:239-247.
- Holleman DR Jr, Simel DL. Does the clinical examination predict airflow limitation? JAMA 1995;273:313-319. (Erratum in: JAMA 1995;273:1334).
- Perez-Padilla R, Vollmer WM, Vázquez-García JC, et al. Can a normal peak expiratory flow exclude severe chronic obstructive pulmonary disease? Int J Tuberc Lung Dis 2009;13:387-393.
- Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: Cross sectional survey. BMJ 2003;327:653-654.
- Bhatt SP, Balte PP, Schwartz JE, et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA 2019;321:2438-2447.
- Aaron SD, Tan WC, Bourbeau J, et al. Diagnostic instability and reversals of chronic obstructive pulmonary disease diagnosis in individuals with mild to moderate airflow obstruction. Am J Respir Crit Care Med 2017;196:306-314.
- Schermer TR, Robberts B, Crockett AJ, et al. Should the diagnosis of COPD be based on a single spirometry test? NPJ Prim Care Respir Med 2016;26:16059.
- Albert P, Agustí A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax 2012;67:701-708.
- Hansen JE, Porszasz J. Counterpoint: Is an increase in FEV1 and/or FVC ≥ 12% of control and ≥ 200 mL the best way to assess positive bronchodilator response? No. Chest 2014;146:538-541.
- Duong M, Islam S, Rangarajan S, et al. Global differences in lung function by region (PURE): An international, community-based prospective study. Lancet Respir Med 2013;1:599-609.
- Allinson JP, Afzal S, Çolak Y, et al. Changes in lung function in European adults born between 1884 and 1996 and implications for the diagnosis of lung disease: A cross-sectional analysis of ten population-based studies. Lancet Respir Med 2022;10:83-94.
- Jordan RE, Adab P, Sitch A, et al. Targeted case finding for chronic obstructive pulmonary disease versus routine practice in primary care (TargetCOPD): A cluster-randomised controlled trial. Lancet Respir Med 2016;4:720-730.
- Bertens LC, Reitsma JB, van Mourik Y, et al. COPD detected with screening: Impact on patient management and prognosis. Eur Respir J 2014;44:1571-1578.
- Yawn BP, Duvall K, Peabody J, et al. The impact of screening tools on diagnosis of chronic obstructive pulmonary disease in primary care. Am J Prev Med 2014;47:563-575.
- Jones PW. Health status and the spiral of decline. COPD 2009;6:59-63.
- Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: A prospective cohort study. Lancet Respir Med 2013;1:43-50.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434-1440.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128-1138.
- Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: Risk factors and outcomes in the ECLIPSE cohort. Chest 2015;147:999-1007.
- Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: Diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur Respir J 2017;50:1700610.
- Montes de Oca M, Laucho-Contreras ME. Smoking cessation and vaccination. Eur Respir Rev 2023;32:220187.
- Montes de Oca M. Smoking cessation/vaccinations. Clin Chest Med 2020;41:495-512.
- van Eerd EA, van der Meer RM, van Schayck OC, Kotz D. Smoking cessation for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016;2016:CD010744.
- Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013;2013:CD000165.
- Manley MW, Epps RP, Glynn TJ. The clinician’s role in promoting smoking cessation among clinic patients. Med Clin North Am 1992;76:477-494.
- Wongsurakiat P, Maranetra KN, Wasi C, et al. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: A randomized controlled study. Chest 2004;125:2011-2020.
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2000;CD002733.
- Cazzola M, Molimard M. The scientific rationale for combining long-acting beta2-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther 2010;23:257-267.
- Han MK, Ray R, Foo J, et al. Systematic literature review and meta-analysis of US-approved LAMA/LABA therapies versus tiotropium in moderate-to-severe COPD. NPJ Prim Care Respir Med 2018;28:32. (Erratum in: NPJ Prim Care Respir Med 2021;31:49.)
- Ray R, Tombs L, Naya I, et al. Efficacy and safety of the dual bronchodilator combination umeclidinium/vilanterol in COPD by age and airflow limitation severity: A pooled post hoc analysis of seven clinical trials. Pulm Pharmacol Ther 2019;57:101802.
- Singh D, Agusti A, Martinez FJ, et al. Blood eosinophils and chronic obstructive pulmonary disease: A Global Initiative for Chronic Obstructive Lung Disease Science Committee 2022 review. Am J Respir Crit Care Med 2022;206:17-24.
- Crockett AJ, Cranston JM, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2000;CD001744.
- Long-Term Oxygen Treatment Trial Research Group; Albert RK, Au DH, Blackford AL, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016;375:1617-1627.
- American Academy of Hospice and Palliative Medicine; Center to Advance Palliative Care; Hospice and Palliative Nurses Association; Last Acts Partnership; National Hospice and Palliative Care Organization. National Consensus Project for Quality Palliative Care: Clinical Practice Guidelines for quality palliative care, executive summary. J Palliat Med 2004;7:611-627.
- Putcha N, Anzueto AR, Calverley PM, et al. Mortality and exacerbation risk by body mass index in patients with COPD in TIOSPIR and UPLIFT. Ann Am Thorac Soc 2022;19:204-213.
- Collins PF, Stratton RJ, Elia M. Nutritional support in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Am J Clin Nutr 2012;95:1385-1395.
- Farver-Vestergaard I, Jacobsen D, Zachariae R. Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Psychother Psychosom 2015;84:37-50.
- Payne C, Wiffen PJ, Martin S. Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database Syst Rev 2012;1:CD008427. (Update in: Cochrane Database Syst Rev 2017;4:CD008427.)
The Global Initiative for Chronic Obstructive Lung Disease 2023 report defines chronic obstructive pulmonary disease (COPD) as a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production, and/or exacerbations) caused by abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that result in persistent, often progressive, airflow obstruction. COPD was the fourth leading cause of death in the United States in 2018 and is estimated to be responsible for 3 million deaths annually around the world, with expected increases to 5.4 million annual deaths from COPD and related conditions by 2060.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.

