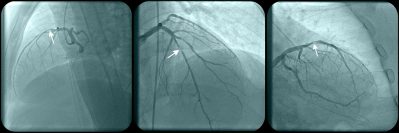
In this large, multicenter, randomized trial, use of the angiography-based quantitative flow ratio method to guide revascularization of intermediate coronary stenoses resulted in a higher incidence of major adverse cardiac events at one year compared with pressure wire-based fractional flow reserve.
Article Limit Reached
You have reached your article limit for the month. Subscribe now to access this article plus other member-only content.
- Award-winning Medical Content
- Latest Advances & Development in Medicine
- Unbiased Content