An Overview of Parasites in Pediatric Patients
Authors
Joseph U. Becker, MD, Clinical Associate Professor, Department of Emergency Medicine, Stanford University, Palo Alto, CA
Vincent Ndebwanimana, MD, Consultant, Department of Emergency Medicine and Critical Care, Kigali University Teaching Hospital, University of Rwanda
Peer Reviewer
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Keller Army Community Hospital, West Point, NY
Executive Summary
- Parasites are organisms that live on or in another organism and benefit from the relationship, usually nutritionally, while causing harm to the host.
- Entamoeba histolytica is most common in areas with limited sanitation resources and systems. After an incubation period of two to four weeks, the majority of patients experience either dysenteric or non-dysenteric colitis, producing diarrhea with or without the presence of mucus and gross blood. The diagnosis of intestinal E. histolytica infection generally is made via analysis of stool samples with identification of telltale cysts or trophozoites. All patients found to be infected with E. histolytica should be treated, even if asymptomatic.
- Giardia intestinalis (also known as Giardia duodenalis or Giardia lamblia) is a flagellated protozoan capable of causing individual and epidemic outbreaks of diarrhea. Transmission of G. intestinalis is via ingestion of contaminated water or food sources or fecal-oral contact. Common symptoms of G. intestinalis infection include watery diarrhea, abdominal cramping, weight loss, nausea, flatulence, and steatorrhea. Polymerase chain reaction testing for G. intestinalis is now widely available, many times as part of a diarrhea or intestinal pathogen panel. Although the cost typically is higher than that of microscopy and antigen detection testing, sensitivity and specificity are higher at 91% and 95%, respectively.
- Ascariasis, infection with Ascaris lumbricoides, is the most common nematodal (roundworm) infection in humans. Infection begins with the ingestion of embryonated eggs, usually from a soil-contaminated source, such as vegetables. The eggs hatch in the jejunum, releasing larvae that then begin a circuitous anatomic course. The diagnosis of A. lumbricoides infection typically is made via identification of eggs or, occasionally, adult worms during stool microscopy. There is a high rate of coinfection with other parasitic diseases, and consideration should be given to identifying and treating other possible co-infections. Treating uncomplicated A. lumbricoides infection is straightforward. A single dose of albendazole (400 mg) is more than 90% effective and generally is well-tolerated.
- Schistosomiasis (also known as bilharzia) is infection caused by a parasitic blood fluke called Schistosoma that is endemic to many parts of the world. Schistosoma flukes enter the body via contact with contaminated fresh water. Snails are critical to the schistosome life cycle, and multiple species are capable of serving as hosts to schistosome flukes. Symptomatic disease is directly related to the host immune response to egg depositing. Eggs can embolize and become trapped in various tissues and organs, producing local inflammation and scarring. Early laboratory signs of infection often are limited and eosinophilia is the sole abnormal lab finding in Katayama fever. Eosinophilia also may be present in the cerebrospinal fluid in cases with neurologic involvement. Anemia may be present after chronic intestinal or urinary hemorrhage. Eosinophilia may be used as a marker for treatment response, since stool microscopy and serology may remain positive long after treatment.
- There are several main species of human tapeworms, and they generally are associated with their most common food source: Taenia saginata (cows) and Taenia solium and Taenia asiatica (pigs). Diphyllobothrium spp. are the main human fish tapeworm species and can infect humans after ingestion of incompletely cooked freshwater or saltwater fish.
- T. solium is the cause of cysticercosis. T. solium causes two distinct but often concomitantly occurring disease processes: taeniasis, as intestinal infection is known, and cysticercosis, which involves the formation of cysts in many tissues but particularly those of the neurological system. The symptoms of cysticercosis depend on the tissue where the cysticerci are located. In parenchymal neurocysticercosis, seizures are the typical complication. Seizures may begin during the inflammatory phase and persist through the nonviable phase, becoming lifelong.
- There are two species of hookworm that cause human disease: Ancylostoma duodenale (found in the Mediterranean region and across Asia) and Necator americanus (found in North and South America and parts of Southeast Asia and India). The symptoms of hookworm infection depend largely on the phase of infection and, thus, in which the larvae or worms are residing. Initial cutaneous penetration often is associated with pruritic rash at the site of the penetration, sometimes with visible tracks revealing the path of cutaneous migration of the parasite. Passage through the lungs usually is asymptomatic but may be associated with a mild cough. Once the larvae arrive in the intestines and develop into adult worms, intestinal symptoms may be noted, including abdominal pain, nausea, vomiting, diarrhea, and increased flatulence. Major consequences are iron deficiency anemia and nutritional deficiencies.
- Pinworm (Enterobius vermicularis) is the most common helminthic infection in the United States. E. vermicularis infection typically is asymptomatic save for nocturnal perianal itching. Individuals with high worm burdens may experience abdominal discomfort, nausea, and/or vomiting. The treatment of pinworm in children consists of albendazole (400 mg in a single dose) or mebendazole (100 mg as a single dose, followed by a second dose after two weeks).
As we have learned from the recent COVID-19 pandemic, we are susceptible to infections from a diversity of locations. Awareness of infections that may travel to our emergency departments is critical to making an accurate diagnosis and institute appropriate treatment.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Parasitic diseases remain a major public health challenge in many parts of the globe and still occur with frequency among North American populations, especially children. Although the great burden of parasitic disease occurs in Africa, Southeast Asia, and Latin America, immigration, travel, and a stable background frequency of outbreaks and locally occurring parasitic infections assure the presence of these organisms among North American pediatric populations for the foreseeable future. Furthermore, parasitic diseases are some of the most commonly occurring worldwide, with soil-based helminthic infections, such as Ascaris lumbricoides, thought to infect more than a quarter of the human population at any one particular time.1 The United States is not immune to these infections; evidence of antibody responses to several parasites have been found in substantial nonimmigrant portions of the U.S. population.2 Furthermore, returned travelers may manifest evidence of parasitic infection at time periods distant from their return, making their consideration in the differential diagnosis and a travel history important components of the patient interaction.3
Parasitology is a fascinating and massive medical discipline, incorporating and drawing on a variety of medical and scientific specialties, including entomology, veterinary medicine, biology, public health and hygiene, water systems and waste management, and environmental sciences and ecology, to name but a few. Additionally, immunodeficiency or exposure to various wild or domesticated animal species and their potential zoonotic infections widely expands the range of parasites capable of causing human disease. An exhaustive discussion of human parasitology and all its pathogens is beyond the scope of this publication. Instead, the focus for this review will be to survey the intestinal parasitic species most relevant to physicians caring for children and returned travelers in North America while providing discussion and background of some of the major organisms that contribute to the wider global burden of parasitic disease.
Background
The parasite is an organism that lives on or in another organism and benefits from the relationship, usually nutritionally, while causing harm to the host organism. Human parasites can be divided into protozoa and helminths (worms) and encompass an array of organisms with complex life cycles. Protozoa are a diverse group of single-celled organisms and include Plasmodium, Giardia, and Entamoeba. Helminths are large, multi-celled organisms that frequently are visible even to the naked eye in their adult stages. They include flatworms, which can be further divided into trematodes (flukes, such as the multiple species of Schistosoma), cestodes (tapeworms, such as Taenia solum), and roundworms (also known as nematodes, such as Ascaris lumbricoides). Human parasites sometimes are classified regarding their tissue or organ of residence, such as in the bloodstream, soft tissues, or intestinal lumen.
Protozoic Infection
Amoebiasis
Entamoeba histolytica is a protozoa that causes both intestinal and extraintestinal disease. Multiple species of Entamoeba have been implicated in human disease and asymptomatic infection, with E. histolytica thought to be responsible for most symptomatic infections in the United States. Amoebiasis, as infection with Entamoeba species is called, is very common, with an estimated 50 million symptomatic infections occurring yearly across the globe and leading to more than 100,000 deaths annually.4,5 E. histolytica is most common in areas with limited sanitation resources and systems. In some regions, the prevalence of infection may reach as high as 50% of the population, although many infections are asymptomatic.5 In the United States, amoebiasis is seen most often in migrants from endemic areas, returned travelers, and in disease outbreaks among specific communities, including institutionalized populations, men who have sex with men (MSM), and immunocompromised patients.
Pediatric amoebiasis is seen most commonly in the setting of outbreaks at daycare centers or restaurants where hygiene or sanitation practices are inadequate. Infection in children may be more severe than in adults, particularly in the very young and/or immunocompromised.6 However, severe E. histolytica infections and extraintestinal spread have been diagnosed in returned healthy travelers after only brief visits to endemic areas.7,8
Infection with E. histolytica occurs via ingestion of cysts in contaminated food, water, or via fecal-oral contact. In the alkaline environment of the small intestine, the cysts develop into trophozoites, which produce symptoms and infectious complications. Infection usually is asymptomatic or mild, and indeed asymptomatic carriers who chronically shed infectious cysts are important in the cycle of transmission.5 After an incubation period of two to four weeks, the majority of patients experience either dysenteric or non-dysenteric colitis, producing diarrhea with or without the presence of mucus and gross blood.6 Fever is present only in 38% of patients.6,8
Infections may become chronic, leading to symptoms of alternating diarrhea and constipation, which may mimic malabsorptive conditions or irritable bowel syndrome (IBS).6 In a minority of cases, E. histolytica infection may be severe. In susceptible populations, severe intestinal manifestations can lead to fulminant colitis with ulceration and even perforation and resultant peritonitis. Untreated, chronic infection also may result in toxic megacolon.5,6,9 In severe cases, amebomas, which are masses of amoebas and associated granulation tissue, may aggregate in the intestines, usually in the cecum, causing bowel obstruction or appendicitis that may be palpable on exam.
In a minority of cases, E. histolytica infection also may manifest as extraintestinal disease, often without identifiable preceding intestinal symptoms or infection. Extraintestinal E. histolytica infection most commonly involves the liver with the formation of solitary or multiple amoebic abscesses. (See Figure 1.) Patients typically report right-upper quadrant abdominal pain and referred pain to the right shoulder. Fever is variably present. Liver abscesses may rupture, with progression of infection into the lungs, pleural space, or peritoneum.10
Figure 1. Entamoeba histolytica on Magnetic Resonance Cholangiopancreatography |
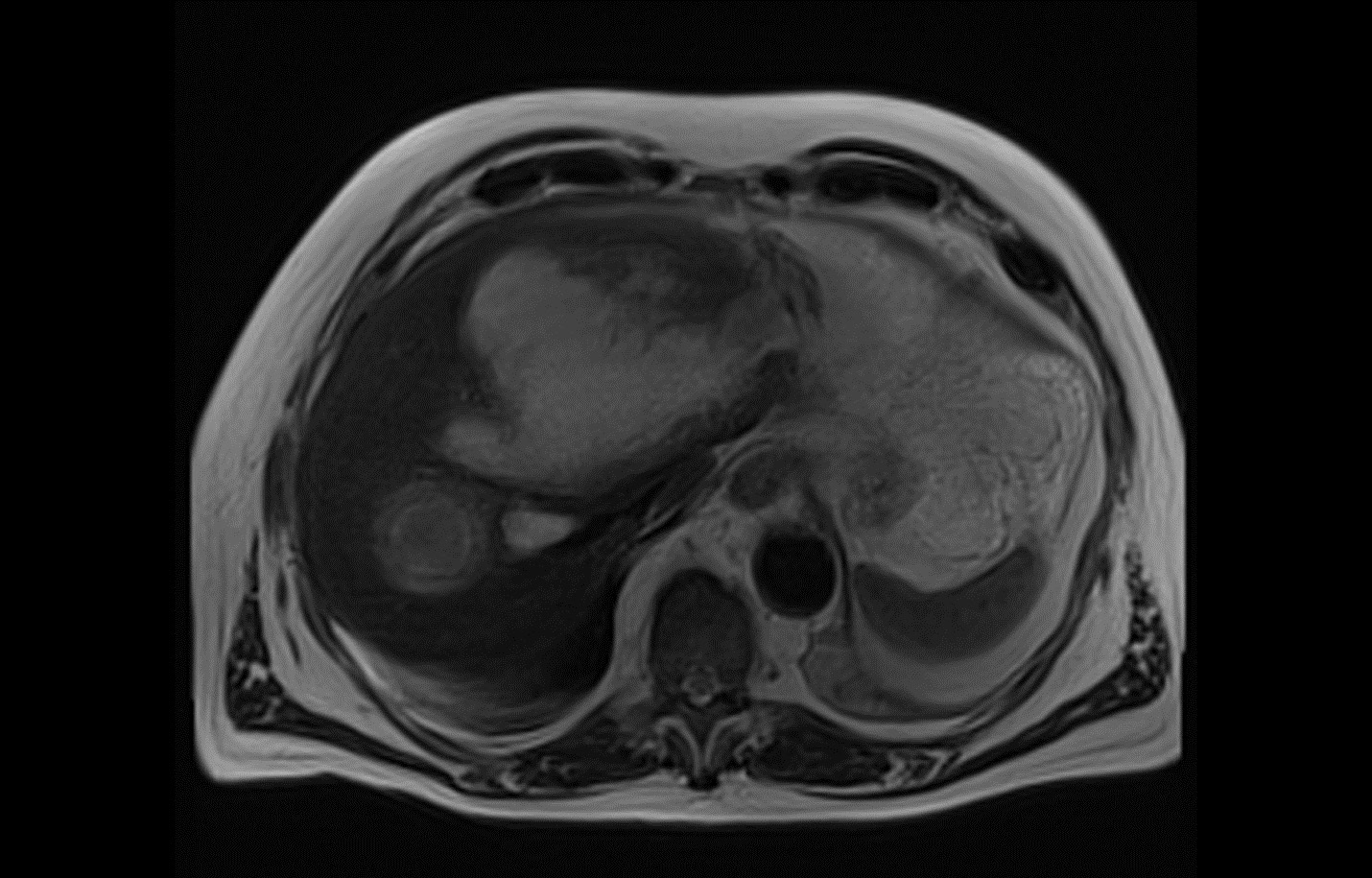 |
Courtesy of Nespola B, Betz V, Brunet J, et al. First case of amebic liver abscess 22 years after the first occurrence. Published June 18, 2015. https://en.wikipedia.org/wiki/File:Parasite150029-fig1_First_case_of_amebic_liver_abscess_22_years_after_the_first_occurrence.tif |
The diagnosis of intestinal E. histolytica infection generally is made via analysis of stool samples with identification of cysts or trophozoites. Given the low sensitivity of stool microscopy and fluctuations in cyst shedding, multiple samples may need to be examined over the course of several days. A skilled microscopist with experience diagnosing intestinal parasitic disease is critical. Microscopy generally is not able to differentiate between different species of Entamoeba, given the similar appearance of trophozoites of different species.11,12
Antigen testing using enzyme-linked immunosorbent assay (ELISA) or immunofluorescence generally has better sensitivity than microscopy and also is available and able to differentiate between E. histolytica and other Entamoeba infections.6 Primers able to specifically diagnose E. histolytica infection via polymerase chain reaction (PCR) have been developed, and this diagnostic modality is becoming more common. PCR testing can differentiate species of Entamoeba, is more sensitive than microscopy or antigen testing, and allows testing for multiple pathogens simultaneously.11,13
Enzyme immunoassay (EIA) kits are available for the serodiagnosis of E. histolytica infection, although patients remain positive long after infection and/or definitive treatment. Studies have shown that up to 35% of the population in endemic areas may have positive serology for E. histolytica, limiting the utility of serology in diagnosing new cases.4 The diagnosis of E. histolytica also may be made via tissue biopsy at colonoscopy or biopsy of abscess aspirates followed by histologic examination, antigen testing, or PCR testing.14 Extraintestinal E. histolytica infection most commonly manifests as hepatic abscesses. Stool studies, including antigen testing, PCR, and/or microscopy, should be considered, although extraintestinal disease frequently does not involve prior or concomitant intestinal symptoms, and stool studies frequently are negative.10 Abscess aspirates or biopsies also may fail to reveal characteristic trophozoites or cysts. Serologic testing may be useful for building evidence to justify treatment if the individual is not from an endemic region. Hepatic abscesses are best visualized via ultrasound, computed tomography (CT) scanning, or magnetic resonance imaging (MRI).
All patients found to be infected with E. histolytica should be treated, even if asymptomatic. In asymptomatic patients, monotherapy with a single agent targeted against intraluminal (intestinal) trophozoites is adequate. Consider a course of paromomycin (25-35 mg/kg per day in three divided doses for seven days), iodoquinol (30 mg/kg to 40 mg/kg per day in three divided doses for children for 20 days), or diloxanide furoate (20 mg/kg/day in three divided doses for children for 10 days).6 Treatment for symptomatic E. histolytica generally involves two agents — one to eliminate organisms in the intestinal lumen and a second agent to address those that may have left the intestinal lumen and invaded tissues. In patients with mild-moderate symptoms, including dysentery, diarrhea, abdominal pain, or fever, systemic therapy with metronidazole (35-50 mg/kg/day in three divided doses for seven to 10 days), tinidazole (50 mg/kg [max 2 g/day]) daily for three to five days), or secnidazole (30 mg/kg/day for one to three days) is administered in addition to an intraluminal agent.6,9,15-17
In patients with severe E. histolytica infection, including bowel perforation, fulminant colitis, or toxic megacolon, appropriate, broad-spectrum antibiotic coverage is required in addition to antiprotozoal therapy. Surgical consultation is necessary when perforation, peritonitis, toxic megacolon, or severe hemorrhage occurs. In patients with extraluminal disease, including hepatic abscess, metronidazole, tinidazole, or secnidazole is indicated. E. histolytica hepatic abscesses typically respond to pharmacologic therapy alone, and drainage or surgical management should be considered only if the abscess size exceeds 10 cm, the abscess is thought to be in imminent danger of rupture, or after the failure of medical therapy.10
Giardiasis
Giardia intestinalis (also known as G. duodenalis or G. lamblia) is a flagellated protozoan capable of causing individual and epidemic outbreaks of diarrhea. It is the most common intestinal parasitic infection in the United States, with an overall stool prevalence of 5% to 7% in the general population. It is the third most common cause of diarrhea in children globally, with an estimated 300 million cases per year.6,18 G. intestinalis infection may account for as much as 15% of the nonrotavirus causes of diarrhea in U.S. children. Although as many as half of G. intestinalis infections are asymptomatic, symptomatic disease is more common in children.19
G. intestinalis is transmitted via ingestion of contaminated water or food sources or fecal-oral contact. Childcare centers have been the origin of many documented outbreaks, and contact with children in diapers has been identified as an independent risk factor for developing giardiasis.20 Given limitations in water quality, hygiene, and sanitation, G. intestinalis infection is endemic in many parts of the world. As such, returned travelers are at heightened risk for infection. Hikers, campers, or anyone who is in contact with inadequately purified drinking water is at elevated risk for infection, and chlorination alone is not an entirely effective means of killing G. intestinalis, and several outbreaks have been associated with the use of recreational water sites, including water parks. MSM also are at increased risk for G. intestinalis infection. Rarely, transmission may occur directly from animals but the majority of Giardia species found in animals are not capable of infecting humans.21
Infection occurs via ingestion of G. intestinalis cysts. Once in the intestines, the cysts mature into trophozoites. (See Figure 2.) After an incubation period of one to three weeks, they cause symptomatic disease by adhering to the intestinal epithelium, although the specific mechanism by which this results in symptoms is not known. Invasion through the intestinal epithelium does not occur, and extraintestinal manifestations of infection are rare. Trophozoites make their way into the colon, re-encyst, and are shed in stool. From there, they may be viable in the environment for as long as three months. Multiple factors seem to influence whether an infection is symptomatic or not. Because children are more commonly symptomatic, it is surmised that acquired immunity may play a role in the severity of symptoms. Some studies have suggested that a fraction of asymptomatic patients shed cysts for a period of time and, thus, can infect those in contact with them.22 Patients with impaired immunity, especially with deficiencies of immunoglobulin, are at increased risk for symptomatic disease.23
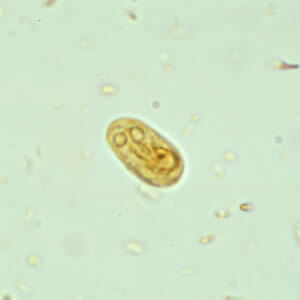
Figure 2. Giardia intestinalis |
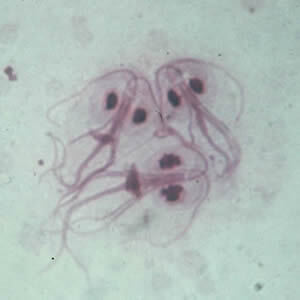 |
Giardia intestinalis cyst (top) and trophozoite (bottom) Centers for Disease Control and Prevention. Giardiasis: Image gallery. Last reviewed Dec. 9, 2017. https://www.cdc.gov/dpdx/giardiasis/index.html |
Common symptoms of G. intestinalis infection include watery diarrhea, abdominal cramping, weight loss, nausea, flatulence, and steatorrhea. Fever is an uncommon symptom. While severe disease is uncommon, watery diarrhea may cause dehydration or electrolyte abnormalities. The majority of individuals clear the infection within three weeks, even in the absence of treatment. However, a substantial group (as much as 50% of patients with symptomatic infection) may develop chronic and persistent symptoms, including watery diarrhea, malaise, weight loss, and nausea.22,24 Studies have demonstrated that individuals infected with G. intestinalis, even when asymptomatic, are at increased risk for the development of lactose intolerance, IBS, chronic fatigue, and malabsorptive conditions. In children, both asymptomatic and symptomatic infection are associated with growth stunting and a range of vitamin deficiencies.22,25,26
The diagnosis of G. intestinalis infection historically was made via stool microscopic identification of trophozoites and/or cysts. However, microscopy largely has been replaced by molecular diagnostic techniques, including stool antigen detection via direct immunofluorescence assays and EIA. These techniques have comparable cost to microscopy but offer a higher sensitivity and are more easily performed by minimally trained laboratory and clinical personnel. PCR testing for G. intestinalis is now widely available, many times as part of a diarrhea or intestinal pathogen panel. Although the cost typically is higher than that of microscopy and antigen detection testing, sensitivity and specificity are higher at 91% and 95%, respectively.27 A limitation of molecular methods of diagnosis is that they are not useful in determining treatment response. Individuals treated for G. intestinalis infection may continue to be antigen- or PCR-positive for weeks after successful treatment. While this is less true for microscopy, some persistent shedding of cysts may continue even after successful treatment. Because G. intestinalis is noninvasive, there are no characteristic abnormalities in the complete blood count (such as eosinophilia) or other laboratory testing. Stool blood and leukocytes typically are negative. While colonoscopy/sigmoidoscopy with biopsy has been used as a diagnostic modality, this usually is unnecessary, given the sensitivity of antigen and PCR testing.
Various agents have antimicrobial activity against G. intestinalis, and treatment depends on the presence or severity of symptoms, pregnancy, the ability to tolerate the regimen, and patient age. The treatment of G. intestinalis in asymptomatic individuals typically is limited to those in contact with immunocompromised or pregnant patients or those who may be contributors to transmission, such as food service or daycare workers. Metronidazole (15 mg/kg orally divided three times a day) for a five- to seven-day course generally is the most available treatment and has a parasitological cure rate of 80% to 100%.9
However, metronidazole is associated with a variety of adverse effects, including nausea, poor taste, and abdominal pain, and it is difficult to tolerate in suspension. Tinidazole (50 mg/kg)is an effective treatment in children aged 3 years and older, has an efficacy of 91%, and has the added benefit of being a single dose. A nitazoxanide oral suspension (100 mg orally twice daily for three days for children aged 1-3 years, 200 mg twice daily for children aged 4-11 years, and 500 mg twice daily for children aged 12 years and older) may be prescribed and is effective against other intestinal parasites. Data are lacking to provide strong recommendations regarding treatment in children younger than 1 year of age, but most sources recommend metronidazole. Paromomycin (10 mg/kg orally three times per day for five to 10 days) typically is prescribed in pregnant patients, given the lack of safety data for the other agents. If symptoms are mild, treatment generally should be delayed until the second trimester to further reduce the risk of complications. Other less commonly available agents with proven activity include secnidazole, ornidazole, and quinacrine, but many outpatient pharmacies may not carry them.6,9
Symptom recurrence or persistence after appropriate treatment may be attributable to several factors, including nonadherence to the initial prescription (more common with metronidazole and longer courses of therapy), microbial resistance, or reinfection. Chronic symptoms in the absence of evidence of ongoing infection are common with G. intestinalis, and many patients experience long-term symptoms, such as diarrhea, lactose intolerance, nausea, bloating, and weight loss. These symptoms may be the result of alterations in gastrointestinal flora or persistent malabsorption.24
Patients with persistent symptoms should undergo repeat antigen or PCR stool testing. If the results are negative, patients likely will not benefit from repeat antimicrobial therapy. If results are positive, a repeat course of the initial regimen (if tolerated the first time) should be prescribed. Alternatively, tinidazole should be considered if it is available, given its high efficacy. Should symptoms persist and ongoing infection is confirmed or suspected, a combination regimen may be used. The most common combination regimens include albendazole with metronidazole or tinidazole. For patients with persistent infection after two courses of treatment, the patient should be evaluated for immunodeficiency.
Prevention of G. intestinalis infection is mainly accomplished via responsible hygiene and adequate water purification. Campers and hikers should use commercially available water purifiers with a pore size of < 0.5 μm or use iodine tablets.28 Symptomatic individuals should be counselled to avoid food preparation and swimming venues. Patients with jobs in the food service or daycare sectors should avoid those settings and responsibilities until adequately treated.
Helminths
Ascariasis
Ascariasis, infection with A. lumbricoides, is the most common nematodal (roundworm) infection in humans, with estimates of more than 1 billion infected people worldwide. Some communities have infection rates in excess of 90%.29,30 Ascariasis is most common among school-age children, although, in the United States, infection in travelers returning from endemic zones also is common. Most Ascaris infections are asymptomatic, but worm burdens may be high, particularly in endemic regions, and may lead to malnutrition, growth stunting, cognitive delay, and vitamin deficiencies in endemic communities. Concomitant infection with other parasites is common.1,6
Infection begins with the ingestion of embryonated eggs, usually from a soil-contaminated source, such as vegetables. The eggs hatch in the jejunum, releasing larvae that then begin a circuitous anatomic course. The larvae initially penetrate the bowel mucosa and make their way via portal circulation to the lungs. In the alveoli, the larvae mature and ascend the bronchi and trachea, where they are swallowed and returned to the small intestine, where they continue their maturation into adult worms ranging in size from 15 cm to 40 cm. The entire process takes between eight to 12 weeks.6 A female worm is capable of producing more than 200,000 eggs daily, which are excreted in the stool. In the absence of a male worm, unfertilized eggs are released.
Adult worms have a lifespan of up to 24 months, and worms are not born within the body; thus, high worm burdens are an indicator of elevated or repetitive exposure. Adult worms may be passed dead or alive in the stool or in vomitus. Once in the stool, fertilized eggs are deposited in the soil through poor hygiene or waste management systems, or, occasionally, as night soil (human waste used as fertilizer). They require a period of at least two weeks to embryonate and become infectious.6,31 (See Figure 3.)
Figure 3. Ascaris lumbricoides Life Cycle |
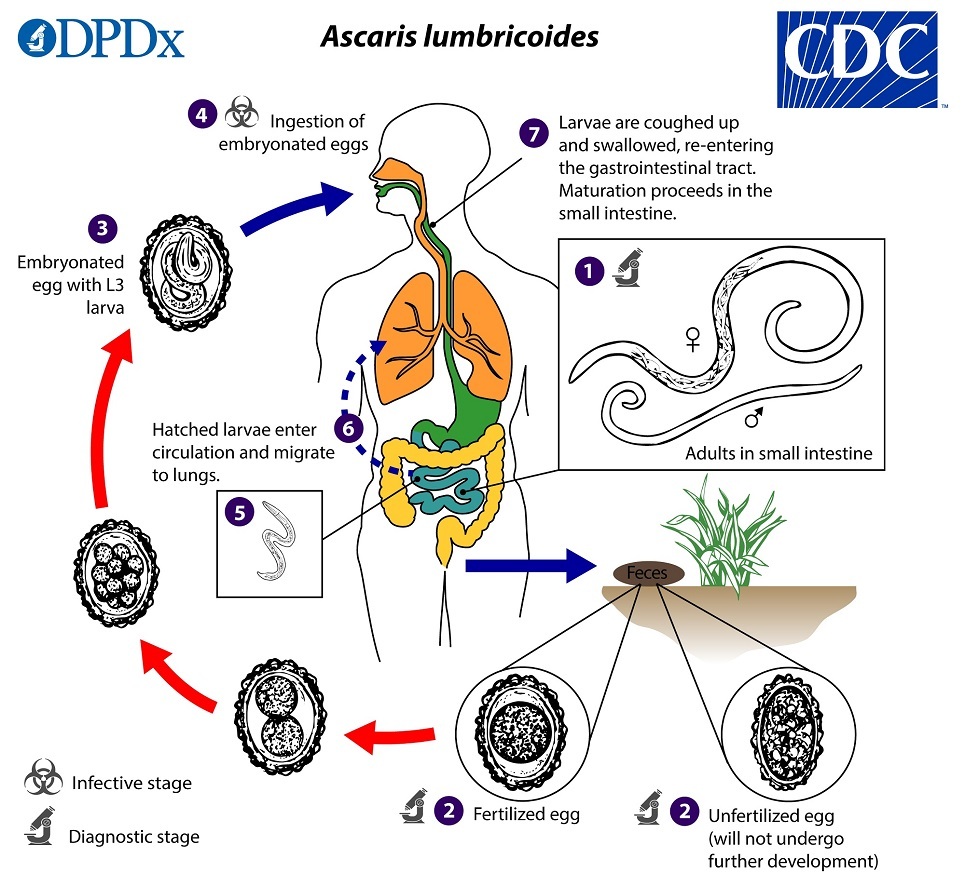 |
Centers for Disease Control and Prevention. Parasites: Ascariasis. Last reviewed July 19, 2019. https://www.cdc.gov/parasites/ascariasis/biology.html |
Most A. lumbricoides infections are asymptomatic. The initial phase of infection, also known as the larval migration phase, normally is not associated with symptoms. However, in previously unexposed, immunologically naïve patients, larval migration may cause an inflammatory pneumonitis, termed Loeffler syndrome, which is similar conceptually to Katayama fever seen in schistosomiasis. Loeffler syndrome is characterized by a dry cough, fever, dyspnea, and eosinophilia. Chest X-ray may reveal patchy round or oval migratory infiltrates. Symptoms usually are short-lived and rarely severe.
During the intestinal phase of infection, when adult worms mature and produce eggs in the small intestine, symptoms are not common, but some patients report abdominal pain, nausea, or diarrhea. High worm burdens in the small intestine may produce complications, including small bowel obstruction, appendicitis, perforation, and peritonitis. Children are at higher risk for bowel obstruction (particularly those aged 1-5 years), given their smaller intestinal lumen diameter. In some regions, ascariasis is a leading cause of bowel obstruction in children, accounting for as much of as one-third of all bowel obstructions.31,32 Additionally, worms may ascend the biliary system, leading to obstructive jaundice, cholangitis, and pancreatitis. Migration of adult worms to extraintestinal sites, such as the skin or other tissues is rare, but it can occur when the worms are under stress, such as with antimicrobial treatment, host illness, or anesthesia.
The diagnosis of A. lumbricoides infection typically is made via identification of eggs or, occasionally, adult worms during stool microscopy. There is a high rate of coinfection with other parasitic diseases, and consideration should be given to identifying and treating other possible co-infections. Loeffler syndrome may be associated with eosinophilia, but in the absence of repetitive exposure, stool studies are not useful because, during the larval migration phase, there are no worms or eggs in the stool. Molecular diagnostic techniques, such as PCR testing, are becoming more commonly available. Serology is of limited use, since it is unable to differentiate acute from chronic infection, and there is cross-reaction of antibodies between multiple parasitic species.
Given the size of adult A. lumbricoides worms, radiography may be a useful diagnostic tool. Adult worm boluses may be visible on plain abdominal radiography as a “whirlpool effect.” Barium- or contrast-assisted radiography may reveal worm-filling defects in the small bowel. Worms also are visible on CT scan and may be visualized in the hepatobiliary system on ultrasound. Magnetic resonance cholangiopancreatography also helps identify worms in the biliary tree. Meanwhile, endoscopic retrograde cholangiopancreatography (ERCP) may be required to remove worms in the bile or pancreatic ducts.31,32
Treating uncomplicated A. lumbricoides infection is straightforward. A single dose of albendazole (400 mg) is more than 90% effective and generally is well-tolerated.6 Alternative regimens include mebendazole (500 mg daily or 100 mg twice daily for three days). In pregnant patients, pyrantel pamoate (11 mg/kg up to 1 g) is recommended, although World Health Organization (WHO) guidelines allow the use of albendazole in the second or third trimester. Given the effectiveness of treatment, follow-up stool testing generally is not required, although rates of reinfection are quite high in endemic areas, and periodic annual or biannual therapy with albendazole or mebendazole is both recommended by the WHO and administered routinely to school-age children by several national health programs. Nitazoxanide and ivermectin are effective against A. lumbricoides, but they are not as effective as albendazole and mebendazole.1,6
Loeffler syndrome treatment is supportive, since the agents discussed in this review do not have activity against the larval stage of development. In patients suspected of having Loeffler syndrome, stool testing should be performed eight to 12 weeks after symptoms appear, which is when egg production is likely to have started. The treatment of small bowel obstruction is a trial of conservative management, including nasogastric decompression and bowel rest. Obtain a surgical consultation if initial treatment is unsuccessful or if peritonitis is suspected. Occasionally, worm boluses require surgical removal. Patients with pancreatic or biliary complications frequently benefit from ERCP removal of adult worms. Prevention of A. lumbricoides infection principally involves the sanitary disposal of human feces. Hand washing and washing of vegetables are critically important.
Schistosomiasis
Schistosomiasis (also known as bilharzia) is infection caused by a parasitic blood fluke called Schistosoma that is endemic to many parts of the world. More than 200 million people globally are thought to be infected, and the sequelae of infection can lead to longstanding illness and disability. Although not common in North America, the organism is acquired by contact with contaminated fresh water, and, thus, schistosomes are of relevance to travelers returning from affected areas where contact with fresh water may occur. Schistosomes are versatile parasites, and schistosomiasis outbreaks have occurred in communities in Europe and in other previously unaffected areas.33
Schistosoma flukes enter the body via contact with contaminated fresh water. Snails are critical to the schistosome life cycle, and multiple species are capable of serving as hosts to schistosome flukes. There are three major species of Schistosoma that cause human disease: Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium (although Schistosoma mekongi is another species that causes a burden of human disease in Southeast Asia). S. japonicum and S. mansoni have a tropism for the human intestinal tract or liver/biliary system, whereas S. haematobium migrates to the human genitourinary tract. S. mansoni and S. haematobium infection occur principally in sub-Saharan Africa and parts of South America and the Caribbean. S. japonicum infection is found in China and Southeast Asia, including parts of the Philippines, Indonesia, and Papua New Guinea.33 There have been no known outbreaks of schistosomiasis in the United States, although there are multiple schistosome-carrying freshwater snails endemic to U.S. lakes and rivers, particularly in the Southwest.34
The Schistosoma life cycle begins with the depositing of eggs into fresh water via contamination with infected urine (in the case of S. haematobium) or feces (S. mansoni and S. japonicum). Some livestock may serve as reservoirs for infection. These eggs hatch free in fresh water and the resulting miracidia seek out the freshwater snail hosts. In the snails, the miracidia mature into cercariae, which are released into the water. Infection occurs when an individual enters a contaminated freshwater source.34-36 Cercariae directly penetrate the skin and enter the bloodstream, where they mature into schistosomes that migrate to the liver and mature into adult worms. In the case of S. japonicum or S. mansoni, the adult worms migrate through the portal venous circulation to the mesenteric venules of the intestines. In the case of S. haematobium, the adult worms migrate to the vesicular venous plexus. Once ensconced in these locations, the female worms begin to produce large numbers of eggs that are eliminated in the urine or feces, and the cycle begins again.
The natural course of schistosomiasis infection is complex and not completely understood. Most individuals who are infected do not develop symptoms and, without reinfection, eventually clear the parasites. The severity of disease seems to depend on multiple factors, including age of exposure, repeat exposures, host genetic susceptibility, concomitant disease, and malnutrition. Adults seem to have lower levels of infection than children and adolescents, which is thought to be related to the development of protective immunity.37
Symptomatic disease is directly related to the host immune response to egg depositing. Eggs can embolize and become trapped in various tissues and organs, producing local inflammation and scarring. The depositing of eggs in the bladder wall leads to fibrosis, pseudopolyps, and hematuria, and chronic infection has been linked to bladder cancer.38 In the liver, periportal fibrosis eventually causes portal hypertension with the associated esophageal varices. This fibrosis, however, does not cause significant injury to the hepatocytes themselves, and hepatic synthetic function usually is preserved. Eggs trapped in the bowel wall may incite inflammation, ulceration, hemorrhage, fibrosis, and, rarely, obstruction. Embolic egg depositing in other organs, such as the brain, spinal cord, lungs, peritoneum, and soft tissues, has been described.39,40 Rarely, adult worms migrate to tissues other than the intestinal or vesicular plexi. The resulting egg depositing in these tissues may lead to severe morbidity and even mortality (in the case of the brain or spinal cord).
Schistosomiasis symptoms may be divided into acute and chronic. Symptoms of acute infection generally are related to the early immune reaction to the organism. Swimmer’s itch is a pruritic dermatitis that often occurs at the site of cercarial penetration of the skin barrier. The skin findings may be subtle, with only urticaria or erythema being noted. Irritation and itching usually begin within several hours of infection, then subside. Katayama fever, or acute schistosomiasis syndrome, is a febrile illness characterized by headache, myalgias, fatigue, headache, and urticaria.6,40
Katayama fever typically occurs between three and 10 weeks after initial exposure, so many returned travelers do not associate their symptoms with their travel. The symptoms and inflammation of Katayama fever are thought to be in response to the beginning of egg production and the significant immune response to proinflammatory egg surface proteins.40,41 Katayama fever typically is quite mild, and symptoms generally resolve within several days. Katayama fever is associated most with acute infection in immunologically naïve subjects, such as travelers, and is on the differential diagnosis for fever in the returned traveler.41
Chronic infection is most common among individuals living in endemic areas who have repeated infection with substantial egg tissue deposits. However, chronic infection in returned travelers with either a single or very few exposures to contaminated water sources does occur.42 In general, the symptoms of chronic schistosomiasis are related to the tropism of the infecting species and the tissue bed(s) where the significant egg depositing and resulting inflammation has occurred. Species with tropism to the intestines, including S. mansoni and S. japonicum, may produce abdominal pain, diarrhea, and melena or hematochezia. In children, abdominal symptoms and chronic blood loss may result in iron deficiency anemia and/or feeding problems and malnutrition. Rarely, fibrosis, scarring, and polyp formation may lead to intestinal obstruction.
Tropism to the bladder venules (S. haematobium) results in hematuria, usually at the end of voiding (terminal hematuria). Early treatment generally leads to an improvement in symptoms, but chronic infection, particularly with a heavy egg burden, can lead to permanent bladder and/or urethral/ureteral fibrosis, calcification, and scarring, with the possibility of resultant obstruction. Individuals with chronic schistosomiasis have an elevated risk for bladder and genitourinary cancers.38 The male and female reproductive tracts may become involved, with egg depositing and resulting inflammation in the fallopian tubes and ovaries leading to scarring and infertility. Genital schistosomiasis may lead to penile or vaginal lesions, particularly in children, which may be reversible with treatment. Vaginal schistosomiasis lesions are thought to increase the risk for human immunodeficiency virus infection.33,40 Renal dysfunction in chronic schistosomiasis is thought to be caused by immune complex glomerulopathy, with resulting renal insufficiency, proteinuria, and nephrotic syndrome.43
Neuroschistosomiasis occurs when embolization of eggs to the spinal cord or cerebral circulations produces an immune response, leading to scarring that can result in serious and permanent neurologic deficits. Rarely, neuroschistosomiasis can present even in returned tourists with a single or minimal exposure and infection burden.44 Lesions in the spinal cord produce a myelitis, which commonly results in lower-extremity motor deficits and bowel and bladder incontinence. Cerebral schistosomiasis is variable in presentation and depends on the cerebral structures and territories affected. However, symptoms may manifest as focal neurologic deficits, seizures, or encephalopathy.44,45
Early laboratory signs of infection often are limited and eosinophilia is the sole abnormal lab finding in Katayama fever. Eosinophilia also may be present in the cerebrospinal fluid in cases with neurologic involvement. Anemia may be present after chronic intestinal or urinary hemorrhage. Eosinophilia may be used as a marker for treatment response, since stool microscopy and serology may remain positive long after treatment.
The treatment of schistosomiasis aims to treat the symptoms of acute disease and prevent chronic complications. In endemic areas where infection and reinfection are frequent, repeat treatment is necessary. Even when complete eradication of adult worms is unsuccessful, treatment has the positive effect of reducing egg load and, thus, the subsequent inflammation and deleterious effects a high egg burden can cause. If caught early, some manifestations of chronic disease may actually improve or reverse after treatment, although significant fibrosis and scarring likely is permanent. The treatment of swimmer’s itch is symptomatic and relies on oral and topical anti-inflammatories and antihistamines. In the vast majority of cases, no subsequent infection occurs, so no specific treatment is necessary.
The treatment of Katayama fever involves both initial suppression of inflammation, generally with corticosteroids, such as prednisone (1 mg/kg/day for five to seven days), and antihelminthic therapy with praziquantel (40 mg/kg with a repeat dose two to four weeks later). Because praziquantel is only effective against adult worms, it should not be administered until at least six weeks after the suspected exposure, with the second dose theorized to eliminate schistosomes that were immature and not susceptible at the time of initial treatment. The eosinophil count and stool microscopy should be monitored, and persistent eosinophilia or visible eggs weeks after treatment should prompt repeat dosing. In endemic areas, where exposure is likely to be ongoing, treatment still has advantages and reduces the parasite burden significantly, although repetitive dosing at intervals may be necessary depending on the frequency of re-exposure.6
Tapeworms: Cysticercosis and Taeniasis
There are several main species of human tapeworms, and they generally are associated with their most common food source: Taenia saginata (cows) and Taenia solium and Taenia asiatica (pigs). Diphyllobothrium spp. are the main human fish tapeworm species and can infect humans after ingestion of incompletely cooked freshwater or saltwater fish. There also are species of tapeworms that primarily infect animals but that may infect humans incidentally. Almost all Taenia infections occur via the ingestion of raw or undercooked meat. This review will focus on T. solium, the cause of cysticercosis. T. solium causes two distinct but often concomitantly occurring disease processes: taeniasis, as intestinal infection is known, and cysticercosis, which involves the formation of cysts in many tissues but particularly those of the neurological system. T. solium is endemic across large parts of the globe, including Latin America, Africa, and Asia. Rates often are higher in areas where pigs are raised or where hygiene practices may be less than optimal. Cysticercosis is seen in U.S. health care settings, typically in immigrants or travelers returning from endemic regions. Consumption of pork is a significant risk factor for disease, although there also is evidence of human-to-human spread, with studies revealing clustering in households that do not share food sources and evidence of disease in nonendemic communities and countries where pork is not consumed.46,47
Transmission of T. solium occurs when an infected individual sheds eggs in stools. In areas where hygiene practices are lacking or sewage treatment is inadequate, the eggs contaminate soil or food sources and are ingested by pigs. Once ingested, the eggs embryonate and invade the pig intestinal wall, after which they migrate to a variety of tissues and organs, including skeletal muscle. In the skeletal muscle, a cysticercus (3-5 mm encapsulated cyst) develops where the tapeworm resides. Humans then ingest the skeletal muscle (meat) and, if it is not adequately cooked, the tapeworm will leave the cyst and attach via the head or scolex to the intestinal wall using hooks or suckers. The attached scolex then will produce and shed proglottids (body segments containing eggs), each one potentially containing thousands of eggs. The proglottids and associated eggs then are shed in the stool and infect the soil or food of pigs, continuing the cycle.
The majority of cases of intestinal T. solium infection (taeniasis) are asymptomatic, although patients may complain of abdominal or intestinal symptoms or may notice proglottids in their stool or feel the motile worms as they are passed. While the scolex generally remains attached to the intestinal wall, proglottids may obstruct the appendix or bile ducts, causing appendicitis, obstructive jaundice, cholangitis, or pancreatitis. However, the main complications of cysticercosis occur not as a result of ingesting undercooked infected pork but via the ingestion of eggs. As such, asymptomatic carriers who shed and may transmit eggs to others are an important component of the transmission cycle and disease development. Once ingested, eggs mature into larvae, which penetrate the intestinal wall and spread hematogenously to various tissues and organs, where they form cysts. Although patients with taeniasis do not automatically develop cysticercosis, they are at elevated risk for auto-contamination with eggs and, thus, frequently do.
Cysticercosis generally is divided into neurocysticercosis (which is further divided into parenchymal and nonparenchymal) and non-neurocysticercosis, depending on the eventual destination of the larvae. The phases of cysticercosis reflect the fate of the cysticerci. The initial phase is termed the viable phase and may persist for several years. The cysticerci at this stage generally do not cause significant inflammation, so this phase usually is asymptomatic. However, after a variable period of time, the cysticerci lose their ability to evade the immune response, and significant, local inflammation occurs. Eventually the cysticerci begin to degenerate and enter a long-term nonviable stage. This stage often is noticed on radiography as calcified granulomas in the brain and other tissues.48,49
The symptoms of cysticercosis depend on the tissue where the cysticerci are located. In parenchymal neurocysticercosis, seizures are the typical complication. Seizures may begin during the inflammatory phase and persist through the nonviable phase, becoming lifelong. In regions with high endemnicity of cysticercosis, neurocysticercosis may be a significant contributor to the overall rate of epilepsy in the community.50,51 In extraparenchymal neurocysticercosis, the cysticerci are established in the ventricles, the subarachnoid space, or the spinal cord and result in elevated intracranial pressure. Ocular cysticercosis requires surgical removal. The majority of nonneurologic cysticerci end up in muscle and soft tissue and cause limited symptoms. Most are noted incidentally on imaging studies. The diagnosis of taeniasis is accomplished by the identification of eggs or proglottids in the stool or on anal swabs. Egg shedding may be intermittent, and multiple stool samples should be examined to raise the sensitivity of analysis. Peripheral eosinophilia may be present. ELISAs exist to detect T. solium antigens in stool. PCR testing also exists for diagnosis and speciation of tapeworm infections.6
The diagnosis of cysticercosis often is made via neuroimaging after seizures or elevated intracranial pressure. Both MRI and CT have roles in the diagnosis of cysticercosis, with CT being superior in identifying calcifications and MRI proving more useful for identifying extraparenchymal cysts.6 In cases where there is persistent uncertainty after appropriate imaging studies have been performed, serology or biopsy may be considered.
Neurocysticercosis treatment is guided by symptoms and imaging findings. Evidence of increased intracranial pressure should be managed either with corticosteroids or surgical placement of a shunt or endoscopic removal of obstructing cysts. Seizures may be controlled with standard anti-epileptic medications. Antiparasitic therapy is associated with reduced seizure frequency and improvement in extraparenchymal symptoms. However, antiparasite therapy risks initial worsening of inflammation associated with the cysticerci with resultant clinical consequences such as seizures or worsening cerebral edema or hydrocephalus. Antiparasite therapy should be reserved for patients with evidence of cysticerci in the viable or inflammatory/degenerating phases.
The commencement of antiparasite therapy is not urgent, and patients should be stabilized before beginning therapy. Antiparasite therapy should not be administered in cases of high cyst burden and diffuse cerebral edema, untreated hydrocephalus, or in those in whom non-viable, calcified lesions only are visualized on imaging.52 For patients with a mild radiologic burden of cysts (one to two cysts), treatment includes albendazole (15 mg/kg/day, max dose 800 mg/day) twice daily for 10-14 days. In patients with more than two cysts visualized, treatment incudes albendazole and praziquantel (50 mg/kg/day for three daily doses). Corticosteroid therapy (prednisone 1 mg/kg/day) should be commenced in all cases prior to antiparasite therapy.6,52 In more severe cases, higher-dose steroid regimens may be considered, in consultation with relevant consultants. Despite adequate treatment, seizures may persist and continuation of anti-epileptic medications should continue until evidence of control exists.52,53
The treatment of taeniasis or intestinal tapeworm infection consists of praziquantel (10 mg/kg in a single dose). Care should be taken in patients with a history of neurologic symptoms or in those who are from T. solium endemic regions, since the possibility of occult neurocysticercosis and resultant antiparasitic therapy associated inflammation and clinical worsening exists.53 As with most parasitic disease, the prevention of taeniasis and cysticercosis centers on hygiene and human waste management. Avoiding consumption of inadequately cooked pork (or fish or beef in the case of T. saginata and T. asiatica, respectively, also is critical.
Hookworm
There are two species of hookworm that cause human disease: Ancylostoma duodenale (found in the Mediterranean region and across Asia) and Necator americanus (found in North and South America and parts of Southeast Asia and India). Hookworm is one of the most commonly occurring parasitic infections, with an estimated 500 million cases globally at any one time. Hookworm is present where soil is contaminated by human fecal waste and where bare human skin comes in contact with contaminated soil. Children are at an elevated risk given their propensity to play and/or walk barefoot in soil.1,54,55
The hookworm life cycle commences when an infected individual passes eggs into the stool, which then contaminates soil. In the soil, the eggs hatch and release larvae, which then penetrate human skin. Once in the skin, the larvae migrate hematogenously to the lungs, where they further develop in the alveoli, and eventually ascend the tracheobronchial tree to be swallowed. In the intestines, the worms mature and attach to the intestinal wall, which results in blood loss and the anemia frequently associated with hookworm infection. Sexual reproduction occurs in the small intestine, and female worms begin shedding eggs into the stool about six to eight weeks after the initial infection with N. americanus infection and up to 40 weeks in the case of A. duodenale. Adult worms have a variable lifespan, but they can persist for several years.56,57
The symptoms of hookworm infection depend largely on the phase of infection and, thus, where the larvae or worms are residing. Initial cutaneous penetration often is associated with pruritic rash at the site of the penetration, sometimes with visible tracks revealing the path of cutaneous migration of the parasite. Passage through the lungs usually is asymptomatic but may be associated with a mild cough. Once the larvae arrive in the intestines and develop into adult worms, intestinal symptoms may be noted, including abdominal pain, nausea, vomiting, diarrhea, and increased flatulence. Rarely, infected individuals note gross rectal bleeding; however, most blood loss is occult. The major consequences of hookworm infection, particularly among children and pregnant women, are anemia and nutritional deficiency. Each adult worm consumes between 0.3 mL and 0.5 mL of blood per day. The resultant loss of iron and protein (mostly in the form of albumin) may result in significant nutritional deficiencies, particularly in heavily infected patients.57,58
A hookworm diagnosis should be investigated in children with evidence of anemia, nutritional deficiencies, or growth retardation. Peripheral blood eosinophilia may be another indicator of hookworm or other parasitic infection. Most diagnoses are established via microscopic examination of the stool for eggs. Examination of the stool during the period of time between skin penetration and the maturation of adult worms in the small intestine will be unhelpful. As with most stool parasite microscopy, multiple specimens are required to elevate the sensitivity. PCR testing for both N. americanus and A. duodenale exists, often as part of a stool parasitology panel.
The treatment of hookworm infection consists of albendazole (400 mg for a single dose), mebendazole (500 mg for a single dose), or pyrantel pamoate (11 mg/kg/day for three days). Successful treatment of hookworm infections results in improvements in hemoglobin level, growth, and even cognitive function. Prevention, as with most parasitic diseases, is centered around improvements in hygiene and human waste management. Regular deworming of school-age children with yearly or twice yearly doses of albendazole is practiced in many countries.1,58
Pinworm and Whipworm
Pinworm (Enterobius vermicularis) is the most common helminthic infection in the United States, with an estimated 40 million Americans infected at any given time.59 The life cycle of E. vermicularis is simple, with female worms exiting the anus of an infected individual (usually at night) to lay eggs on the external perianal skin. The eggs cause an intense pruritis, which assists in transmission and autoinfection by causing the infected individual to scratch the perianal area. Transmission also can occur via surfaces and clothes or linens. Eggs are then ingested by oral contact and eventually hatch in the small intestine, and the larvae make their way to the large intestine where the worms mature and develop. Sexual reproduction occurs in the large intestines, and female worms are capable of producing tens of thousands of eggs daily. Although worm lifespans are only two to three months, autoinfection may contribute to substantial worm burdens.59
E. vermicularis infection typically is asymptomatic save for nocturnal perianal itching. Individuals with high worm burdens may experience abdominal discomfort, nausea, and/or vomiting. E. vermicularis infection also may occur at other sites in the genitourinary system causing a variety of symptoms, including vulvovaginitis, urethritis, and salpingitis.60 The diagnosis of pinworm commonly and famously is made by the “Scotch tape test,” in which an adhesive surface is applied to the perianal area upon awakening and then examined under microscopy. Fingernail scrapings also may be examined. Multiple specimens increase the sensitivity of testing. Occasionally, mature female worms may be identified on the perianal skin. Stool testing often is not helpful, since eggs are not shed in stool.
The treatment of pinworm in children consists of albendazole (400 mg in a single dose) or mebendazole (100 mg as a single dose, followed by a second dose after two weeks). Pyrantel pamoate is effective and preferred in pregnant patients (11 mg/kg as a single dose to be repeated in two weeks) and is available over the counter in the United States. Prevention includes not only testing and treatment but also handwashing and general hygiene practices. Surfaces, clothes, and bedding that have the potential to come into contact with E. vermicularis eggs should be washed.6,61
Whipworm (Trichuriasis trichiuria) is another incredibly common intestinal parasite, and it is estimated that nearly a quarter of the global human population carries this parasite, with some communities in endemic areas testing 90% positive in sampling studies.62 Children are at higher risk of exposure, given their frequent contact with the soil and hand-to-mouth hygiene. The life cycle of T. trichiuria is fairly straightforward — eggs are ingested via food or hands that are contaminated with eggs, usually from the soil. The eggs hatch in the small intestine, and the resulting larvae mature into adult worms. The worms then travel to the colon, where sexual reproduction occurs. There, the female worms produce eggs, which are shed in stool. In areas with limited hygiene, stool contaminates the soil (or food produced in the soil) and continues the cycle of infection. Adult worms can grow to up to 4 cm in length and may live several years. Reinfection is common, and worm burdens can be significant, particularly in children.6
Infection with T. trichiuria often is asymptomatic, with heavy worm burdens contributing to the development of symptoms. Diarrhea (with or without blood), abdominal pain, nausea, and vomiting are common symptoms. Children may manifest anemia, weight loss, or delayed growth or development with heavy worm burdens.63 Diagnosis frequently is made via stool microscopy. As with other intestinal parasites, at least three stool samples may have to be examined to attain adequate sensitivity. PCR testing is becoming more commonplace for T. trichiuria infection because it may have higher sensitivity and may be used more easily for speciation than microscopy.64 Rarely, whipworm is diagnosed on colonoscopy or sigmoidoscopy, with the visualization of adult worms protruding from the colonic mucosa.
T. trichiuria treatment consists of monotherapy with albendazole (400 mg per day orally for three days) or mebendazole (100 mg twice daily for three days). Single-dose regimens of either albendazole or mebendazole are not efficacious. Alternatively, ivermectin and albendazole (400 mg daily for three days) or ivermectin (200 mcg/kg daily for three days) has been shown to have similar or better efficacy.6 Albendazole and mebendazole should be avoided in pregnancy, and, given the usually mild symptoms (particularly in older children, adolescents, and young adults), the risks of treatment should be weighed against the harm of the infection. Post-treatment follow-up stool microscopy generally is good practice, particularly for patients who receive monotherapy.
Conclusion
Intestinal parasitic infections remain a significant threat to the health, growth, cognitive development, and nutritional status of children worldwide. Children remain disproportionally affected by intestinal parasitic diseases, with inadequate hygiene and play and contact with soil and the environment contributing not only to higher burdens of intestinal parasites but also to high rates of reinfection and, often, polymicrobial infections. Given the persistence of inadequate sewage or hygiene systems in many parts of the world, it is likely that substantial human populations will continue to be affected by intestinal parasites for the immediate future. This review provides a brief discussion of several of the commonplace intestinal parasites of relevance to pediatric clinicians. While a medical knowledge of intestinal parasites is important for anyone providing medical care for children, these pathogens will only be controlled through significant investments in education, public health, and the proliferation of effective water and waste management systems.
References
To view the references online, visit https://bit.ly/3PPgOXr.
As we have learned from the recent COVID-19 pandemic, we are susceptible to infections from a diversity of locations. Awareness of infections that may travel to our emergency departments is critical to making an accurate diagnosis and institute appropriate treatment.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
