A Review of Insulin Transition
January 1, 2023
AUTHORS
Suvarchala Kompella, MD,Diabetes Fellow, Touro University College of Osteopathic Medicine, Vallejo, CA
Jay Shubrook, DO, Professor, Primary Care Department, Touro University College of Osteopathic Medicine, Vallejo, CA
Sumera Ahmed, MD, Assistant Professor, Primary Care Department, Touro University College of Osteopathic Medicine, Vallejo, CA
PEER REVIEWER
Emily Beckett, PharmD, BCPS, Family Medicine Residency Clinical Pharmacist, Family Health Center – Broadlawns Medical Center, Des Moines, IA
EXECUTIVE SUMMARY
Since the discovery of insulin by Banting and Best in Toronto a century ago, there has been an explosion of insulins and insulin analogs available, resulting in complexity in the management of insulin transitions.
- Insulins are categorized by mode of administration: subcutaneous or inhaled. Insulin analogs have different pharmacokinetic (PK) and pharmacodynamic (PD) properties. Understanding these PK and PD properties will help practitioners to choose the appropriate insulin therapy for each individual and prevent adverse events.
- Insulins also are classified based on onset, peak, and duration of action in the body. These features are important to consider, since they affect the dosing and safety of these insulins.
- Optimally, insulin is administered to mimic the normal physiological patterns of insulin secretion, which consist of a sustained low level of basal insulin production throughout the day, superimposed with bursts of insulin secretion at each meal.
- Patients using basal/bolus insulin will require 50% of their total daily dose as basal and 50% as prandial.
- The type of insulin prescribed depends on multiple factors, which include patient preference, insurance coverage, cost, and comorbid conditions, such as end-stage kidney disease on peritoneal dialysis.
Introduction
Diabetes mellitus is a collection of chronic metabolic diseases that occur either as the result of insulin deficiency or insulin resistance. Over the past few decades, there have been an increasing incidence and prevalence of diabetes mellitus.1 National diabetes statistics report that about 37.3 million people (11.3% of the U.S. population) have diabetes, with 28.7 million people diagnosed and 8.5 million undiagnosed.2 In 2019, diabetes mellitus was the ninth leading cause of death, with an estimated 1.5 million deaths directly caused by diabetes.1
The years 2021-2022 marks 100 years since the discovery of insulin by Banting and Best in Toronto.3 In the past century, there has been an explosion of insulins and insulin analogs available. Initially, insulin was extracted from animals (beef or pork pancreas). In early 1980, human insulin was first produced synthetically. Researchers, using recombinant deoxyribonucleic acid (DNA) technology, inserted the human insulin gene into the genetic material of bacteria. Escherichia coliis by far the most widely used bacteria. Other than bacteria, the yeast strain Saccharomyces also has been used in the commercial production of insulin.4 Currently, human insulin and insulin analogs are available for insulin replacement therapy. There is ongoing research regarding oral insulin, which might open a new horizon in diabetes management. Currently, once-weekly insulin icodec and basal insulin Fc (BIF) are in Phase III trials.
Insulins are categorized by mode of administration: subcutaneous or inhaled. Insulin analogs have different pharmacokinetic (PK) and pharmacodynamic (PD) properties. Understanding these PK and PD properties will help practitioners to choose the appropriate insulin therapy for each individual and prevent adverse events.5
Insulins also are classified based on onset, peak, and duration of action in the body. (See Figure 1.) These features are important to be aware of, since they affect the dosing and safety of these insulins.
• Onset is the length of time it takes for insulin to reach the bloodstream and begin working by lowering blood glucose.
• Peak time is the time the dose is at its strongest and most effective in lowering blood glucose.
• Duration is the total time the dose is in the bloodstream and lowering blood glucose. There are long-acting, intermediate-acting, short-acting, and ultra-short-acting insulins.
Figure 1. Pharmacokinetic Profiles of Currently Available Insulins |
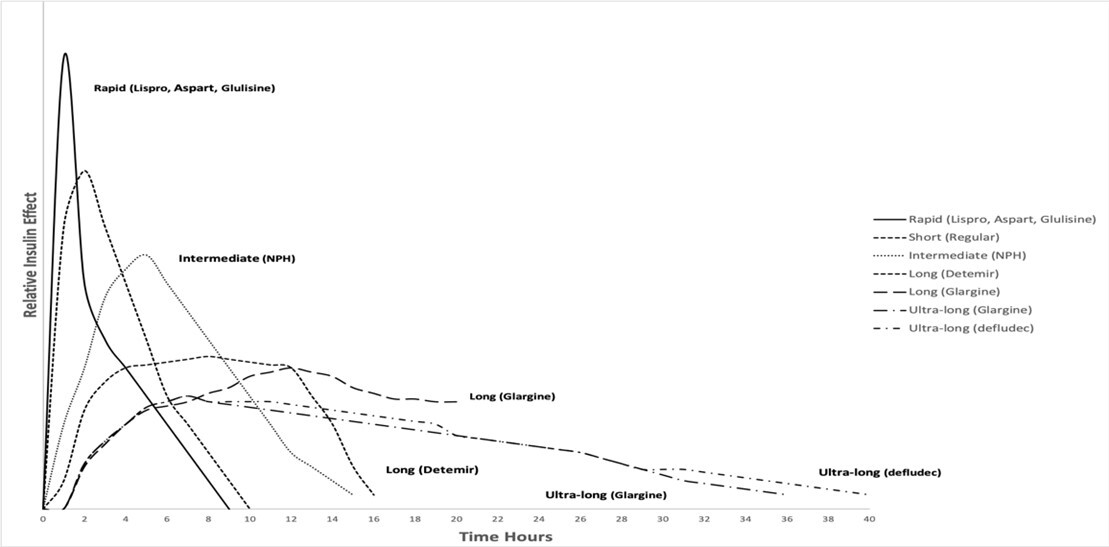 |
Courtesy of Suvarchala Kompella, MD |
One of the primary goals in diabetes treatment includes lowering blood glucose levels sufficiently to prevent microvascular and macrovascular complications.6 Insulin remains the mainstay of treatment in people with type 1 diabetes (T1D) and consists of a basal insulin and another insulin that is administered at mealtimes and for correction. Although most people with type 2 diabetes (T2D) typically are treated initially with oral antidiabetic drugs (OAD), eventually they may need insulin to maintain glycemic control as the disease progresses.
Optimally, insulin is administered to mimic the normal physiological patterns of insulin secretion, which consist of a sustained low level of basal insulin production throughout the day, superimposed with bursts of insulin secretion at each meal. This is termed bolus or prandial insulin and typically covers the glucose spike with each meal (one to three hours).6 Physiologic endogenous insulin secretion limits fluctuation of the narrow and high surge of blood glucose levels.5 The goal with insulin replacement is to do the same.
The total daily dose of exogenous insulin is estimated based on body weight, which typically ranges from 0.4 units/kg/day to 1.0 units/kg/day. People using basal/bolus insulin will require 50% of their total daily dose as basal and 50% as prandial.7
Basal insulin options include neutral protamine Hagedorn (NPH), long-acting insulin analogs, and continuous delivery of rapid-acting insulin via an insulin pump. Prandial insulin options include short-acting, rapid-acting, and ultra-rapid-acting insulins. Table 1 includes currently available insulins and Table 2 is the package insert references for primary care providers.
Table 1. Insulin Pharmacokinetic and Pharmacodynamic Properties | |||||
Type | Name | Onset | Peak | Duration | Availability |
Ultra-rapid-acting | Lispro-aabc (Lyumjev) U100, U200 | 15-17 minutes | 57 minutes | 4.6-7.3 hours | Vials, pens |
Faster aspart (Fiasp) U100 | 15-20 minutes | 63 minutes | 5-7 hours | Vials, pens | |
Rapid-acting | Lispro (Humalog) U100 | 10-20 minutes | 30-90 minutes | 3-5 hours | Vials, pens |
Lispro (Humalog) U200 | 15 minutes | 30-90 minutes | 3-5 hours | Vials, pens | |
Lispro (Admelog) | 15 minutes | 30-90 minutes | 3-5 hours | Vials, pens | |
Aspart (Novolog) U100 | 10-20 minutes | 30-90 minutes | 3-5 hours | Vials, pens | |
Glulisine (Apidra) U100 | 10-20 minutes | 30-90 minutes | 3-5 hours | Vials, pens | |
Afrezza U100 | 12 minutes | 35 minutes | 1.5-4.5 hours | Cartridges | |
Short-acting | Humulin R U100 | 30-60 minutes | 120-240 minutes | 6-8 hours | Vials, pens |
Novolin R U100 | 30 minutes | 80-120 minutes | Up to 8 hours | Vials, pens | |
Intermediate-acting | NPH (N) | About 2 hours | 4-12 hours | 18-26 hours | Vials, pens |
Long-acting | Glargine U100 (Basaglar, Lantus, Semglee) | 3-4 hours | No peak | 24 hours | Vials (only Lantus and Semglee), pens |
Glargine U300 (Toujeo) | 6 hours | No peak | 36 hours | Vials, pens | |
Detemir U100 (Levemir) | 1-2 hours | Small peak, based on the dose | 14-24 hours | Vials, pens | |
Degludec U100, U200 (Tresiba) | 1-2 hours | No peak | > 40 hours | Vials, pens | |
Premixed | NPH 70%/regular 30% | 30-60 minutes | Dual (NPH and regular) | 10-16 hours | Vials, pens |
NPL 50%/lispro 50% | 30-60 minutes | Dual (NPH and lispro) | 10-16 hours | Vials, pens | |
NPL 75%/lispro 25% | 5-15 minutes | Dual (NPL and lispro) | 10-16 hours | Vials, pens | |
NPA 70%/aspart 30% | 5-15 minutes | Dual (NPA and aspart) | 10-16 hours | Vials, pens | |
Degludec 70%/aspart 30% | 15 minutes | Dual (degludec and aspart) | > 40 hours | Vials, pens | |
U500 | Humulin R | 30-45 minutes | 8-24 hours | 8-24 hours | Vials, pens |
NPA: neutral protamine aspart; NPH: neutral protamine Hagedorn; NPL: neutral protamine lispro | |||||
Table 2. Package Insert References |
|
NPH: neutral protamine Hagedorn |
The type of insulin prescribed depends on multiple factors, which include patient preference, insurance coverage, cost, and comorbid conditions, such as end-stage kidney disease on peritoneal dialysis. In clinical practice, insulins frequently are interchanged based on insurance formularies, cost, affordability, availability, patient preferences, treatment intensification, total daily dose (TDD), maximum dose per injection, and adverse effects. Common barriers for initiating or continuing insulin therapy are fear of injections, hypoglycemia, and/or weight gain, which can impair treatment adherence.8
Severe hypoglycemia is most concerning, since it causes anxiety and fear among people with diabetes and their families and adds financial burden to individuals and healthcare systems. The incidence of hypoglycemia is noticed more often with some insulins compared to others because of their different PK and PD properties. For example, when transitioning basal insulin, consider degludec compared to NPH insulin, which is more prone to hypoglycemia.9
Before choosing or transitioning to an appropriate insulin, consider all social determinants (see Figure 2) and individualize the treatment regimen as “one size does not fit all.” For example, if access or cost of the insulin is the reason for difficulty in taking insulin regularly, opting for less expensive regular insulin might improve adherence. People requiring multiple daily injections (MDI) can be transitioned to premixed injections to reduce the MDI burden.
Figure 2. Social Determinants of Health |
Source: Centers for Disease Control and Prevention |
People with T2D with insulin resistance requiring high doses of insulin can be transitioned to concentrated insulins. For example, if a person is requiring more units per volume, they can be transitioned to concentrated insulins, which provide the same units with less volume and avoid MDI.
Table 3 provides the maximum dose of insulin that can be administered per injection. Insulin doses either are increased or decreased based on their PK and PD properties during transitions. Educating patients and their families prior to initiating insulin, during transition, and during intensification will prevent dosing errors and improve adherence. Best practice includes supervision for the first injection, either in the clinician’s office or with a diabetes care education specialist.
Table 3. Maximum Doses Per Injection10 | |
Insulin Type | Maximum Dose |
U100 insulin vial (any product) | 100 units |
U100 lispro (Humalog U-100 KwikPen) | 60 units |
U100 aspart (Novolog FlexPen) | 60 units |
U100 aspart (Fiasp FlexPen) | 80 units |
U100 glulisine (Apidra SoloSTAR Pen) | 80 units |
U100 detemir | 80 units |
U100 glargine | 80 units |
U100 glargine (Basaglar) | 80 units |
U100 glargine (Semglee) | 80 units |
U100 degludec (Tresiba Pen) | 80 units |
U200 degludec (Tresiba Pen) | 160 units |
U200 lispro (Humalog U-200 KwikPen) | 60 units |
U300 glargine (Toujeo SoloSTAR Pen) | 80 units |
U300 glargine (Toujeo Max SoloSTAR Pen) | 160 units |
U500 regular (Humulin R U500) vial | 250 units |
U500 regular (Humulin R U500 KwikPen) | 300 units |
Rapid-Acting Insulin
Rapid-acting insulin analogs have a faster onset and shorter duration of action compared to regular insulin, providing more physiological postprandial glucose (PPG) control when used in basal-bolus regimens.11,12 These PK/PD of rapid acting analogs result in superior PPG control and decrease risk of hypoglycemia.13
Rapid-acting insulins are administered 15 minutes before mealtimes, synchronizing insulin administration with food absorption and the associated increase in blood glucose.13 They also are convenient for people with unpredictable mealtimes and have helped improve quality of life.12,13
Rapid-acting and short-acting insulin are interchanged with a 20% dose reduction.11 Rapid-acting insulin should be injected no more than 15 minutes before the start of a meal.
Short-Acting Insulin
Regular insulin is a short-acting form of synthetic human insulin. Regular insulin is administered 30 minutes before meals. For example, regular insulin 10 units before meals can be switched to aspart 8 units before meals (80% of 10 units = 8 units).11
Inhaled Insulin
Technosphere insulin (TI; brand name, Afrezza) is an ultra-rapid-acting inhaled human insulin taken before meals to cover the prandial rise in blood glucose. Afrezza refers to the drug/device combination product as consisting of TI powder, an inhaler, and cartridges.14 The doses available are four-, eight-, and 12-unit cartridges.
In clinical practice, the equivalent dose of TI is 1.5 to two times the injected insulin to achieve a similar glucose-lowering effect. Supplemental doses are indicated based on one- and two-hour postprandial glucose levels. (See Table 4.)
The STAT study compared TI to aspart in T1D on multiple daily injections. Time in range (TIR) was significantly greater (62.5% ± 2.6% vs. 53.8% ± 1.7%, P = 0.009) and time in hyperglycemia > 180 mg/dL was lower (34.2% ± 2.7% vs. 41.0% ± 1.7%, P = 0.045) in the treatment-compliant TI group.
Table 4. Injected Insulin Transitioned to Inhaled Insulin Based on Mealtime Insulin Requirements | |
Mealtime Insulin | Afrezza Dose |
Up to 4 units | 4 units |
5-8 units | 8 units |
9-12 units | 12 units |
13-16 units | 16 units |
17-20 units | 20 units |
21-24 units | 24 units |
Adapted from https://afrezzahcp.com/dosing/ | |
Postprandial glucose was significantly lower in TI at 60 and 90 minutes post-meal. Postprandial glucose excursions were lower in TI compared to aspart over one to four hours post-meal (P < 0.05). There was weight gain with aspart compared to TI (P = 0.006) despite higher TI dose. Time spent in hypoglycemia (< 60 mg/dL and < 50 mg/dL) was lower in TI compared to aspart.15
To transition from premixed insulin: If changing from premixed insulin, add the dose of premixed insulin for the TDD, then the mealtime insulin injection is one-half (50%) of the TDD. This result is divided into three doses based on three meals of the day. Convert the mealtime insulin injection to the appropriate TI dose noted previously. For example, TDD of premixed insulin 48 units; 24/3 = 8 units of mealtime insulin injection; 8 units of TI.
Before starting TI, assess the baseline pulmonary function tests and spirometry (FEV1). FEV1 should be checked again after six months of initial therapy and then annually. (Afrezza dose calculator: https://afrezzahcp.com/dosing-calculator)
Ultra-Rapid-Acting Insulins
Ultra-rapid-acting insulins are optimal for postprandial coverage because of their faster absorption, rapid onset of action, and shorter duration of action.17 The postprandial glucose control is based on matching the mealtime insulin with carbohydrate intake as well as timing the insulin dose with meals. The PK and PD properties of ultra-rapid-acting insulins are closer to physiological insulin secretion.
Ultra-rapid-acting insulin provides flexibility, allowing injection 15 minutes before or 20 minutes after meals. This is beneficial to people who have difficulty in adhering to pre-meal dosing schedules. The new insulin formulations are ideal options for people with diabetes who are not able to achieve postprandial glycemic targets with other bolus insulins.18
The PRONTO-T2D trial comparing ultra-fasting-acting lispro (URLi; brand name, Lyumjev) and lispro in T1D showed noninferiority in A1c (similar A1c reduction) with an estimated treatment difference (ETD) of 0.06% (95% confidence interval [CI], 0.05; 0.16) and superiority in controlling one- and two-hour postprandial glucose excursions: one-hour ETD, 0.66 mmol/L (95% CI, -1.01, -0.30); two-hour ETD, -0.96 mmol/L (-1.41, -0.52). There were no significant differences in rates of severe or documented hypoglycemia.17
The PRONTO-T1D trial compared URLi and lispro in T2D. URLi was superior to lispro in reducing one- and two-hour postprandial glucose excursions: ETD, 1.55 mmol/L (95% CI, -1.96, -1.14) at one hour and -1.73 mmol/L (95% CI, -2.28, -1.18) at 2 hours (both P < 0.001) and noninferiority in A1c reduction. The rate and incidence of severe, documented, and postprandial hypoglycemia was similar in both treatment groups.19
The overall insulin exposure and insulin action were similar between the URLi and lispro, suggesting no dose conversion is required when transitioning patients from Humalog to URLi.20
The ONSET 1 trial compared faster aspart (Fiasp) to insulin aspart. Faster aspart lowered both postprandial glucose and A1c compared to aspart in people with T1D. The estimated mean changes from baseline A1c levels were -0.08% for faster aspart and 0.01% for aspart. Changes in one-hour postprandial glucose favored faster aspart (P = 0.0002). There was no overall difference in severe and confirmed hypoglycemia.12
The ONSET 2 trial in T2D confirmed faster aspart is noninferior to insulin aspart regarding changes from baseline A1c (ETD, -0.0%; 95% CI, -0.15 to 0.10). Faster aspart improved one-hour postprandial glucose (P = 0.0198) with no difference to two- to four-hour postprandial glucose compared to insulin aspart. The overall severe and confirmed hypoglycemia were similar in both groups except for an increase in zero to two-hour post-meal hypoglycemia with faster aspart (relative risk, 1.60; 95% CI, 1.13-2.27).21
To transition from ultra-rapid-acting insulin to rapid-acting insulin is a simple 1:1 unit conversion. The only thing that may change is the timing with the meal. For example, lispro 10 units can be switched to lispro-aabc 10 units.
Intermediate-Acting Insulin
NPH is an intermediate-acting basal insulin. American Diabetes Association (ADA) guidelines recommend an NPH insulin dose of 0.4 units/kg/day to 1 unit/kg/day subcutaneously in split doses (BID) to manage T1D. A higher dose is necessary during medical illness and puberty.
A starting dose of 0.1 units/kg/day to 0.2 units/kg/day subcutaneously is recommended in T2D, depending on body weight and hyperglycemia.22 NPH is widely used in gestational diabetes. NPH also is available in a premixed formulation with regular insulin.
NPH has a higher risk of hypoglycemia and nocturnal hypoglycemia as a result of the high day-to-day variability in PK/PD profile. Nocturnal hypoglycemia is more frequent with an evening dose of NPH, since insulin peaks at three to eight hours, which occurs around midnight when the body does not require as much insulin.22,23 Intermediate-acting insulin is taken once or twice per day to provide basal insulin needs in between meals and overnight.11
Intermediate-acting insulins include NPH insulin (Humulin N), Novolin N, and ReliOn NPH.
Intermediate-acting insulins are interchanged with another intermediate-acting insulin or long-acting insulin analogs with a 20% dose reduction.11 For example, NPH 20 units daily can be changed to detemir 16 units daily. NPH 34 units in the daytime and 16 units at nighttime (34 + 16 = 50) can be changed to glargine U100 40 units daily.11
For example, glargine U100 50 units daily can be changed to 40 units NPH, divided based on meal frequency. If the patient is eating two meals per day, split one-half of the NPH (20 units) with the first meal of the day and one-half of the NPH (20 units) with the second meal of the day.11
Long-Acting Insulin
Long-acting insulin analogs glargine and detemir are structurally altered human insulins that mimic the PK properties of endogenous insulin more closely than NPH.24 Long-acting insulin is taken once or twice per day to provide basal insulin needs in between meals and overnight.11
Insulin detemir has a less pronounced peak and lower intrasubject variation in PK properties compared with NPH. Insulin detemir provides more consistent insulin levels and predictable glucose control than NPH because of its lower absorption variability.
A study comparing NPH and insulin detemir in T1D patients on basal-bolus therapy showed similar glycemic control between the groups (P = 0.59) and reduced risk of hypoglycemia (P = 0.049) in the detemir group. The mean dose of insulin detemir was 23.5 times higher than NPH.23
A meta-analysis reviewed 285 randomized controlled trials (RCTs) comparing long-acting insulin analogs (glargine and detemir) with NPH in T1D. Long-acting analogs have a small but significant effect in A1c reduction compared with NPH (-0.07 [0.13 ;0.010]; P = 0.026). Long-acting analogs were associated with a reduced risk for nocturnal and severe hypoglycemia (odds ratio [OR], 0.69; 95% CI, 0.55-0.86 and OR 0.73; 95% CI, 0.60-0.89; P < 0.01, respectively).25
A meta-analysis reviewed 24 RCTs comparing glargine and detemir with NPH in T2D. Changes in A1c were comparable for long-acting insulin analogs and NPH insulin.
The incidence of confirmed hypoglycemia and nocturnal hypoglycemia was lower in patients taking long-acting insulin compared to NPH.9
Insulin glargine compared to NPH has a risk ratio (RR) for severe hypoglycemia of 0.68 (95% CI, 0.46-1.01; P = 0.06; absolute risk reduction [ARR], -1.2%; 95% CI, -2.0 to 0). The RR for serious hypoglycemia was 0.76 (95% CI, 0.52-1.09; P = 0.13).9
Treatment with insulin detemir compared to NPH has an RR for severe hypoglycemia of 0.45 (95% CI, 0.17-1.20; P = 0.11). The OR for serious hypoglycemia was 0.16 (95% CI, 0.04-0.61; P = 0.007).9
Second-Generation Longer-Acting Basal Insulin Analogs
Second-generation longer-acting basal insulin analogs insulin degludec U100 and insulin glargine U300 (Gla-300) have smoother PK/PD profiles than glargine U100, with lower variability.26,27
Insulin Glargine U300
Gla-300 has more constant prolonged PK and PD profiles compared to insulin glargine 100 (Gla-100), extending beyond 24 hours. The longer duration of action allows greater flexibility in injection timing while providing equivalent glycemic control and less hypoglycemia.
In trials, a higher dose of Gla-300 is required compared Gla-100 because of the prolonged absorption of Gla-300 from subcutaneous depot, resulting in lower bioavailability related to longer residence time in the subcutaneous depot with degradation by tissue peptidases.8,27-29
The dose difference is consistent with lower 24-hour exposure of Gla-300 compared Gla-100 observed under steady state conditions in PK and PD studies.
The EDITION 1 trial compared Gla-300 to Gla-100 in patients with T2D on basal and mealtime insulin. Gla-300 was noninferior to Gla-100 in A1c reduction. The RR of confirmed or severe nocturnal hypoglycemia was 21% lower with Gla-300 during the first eight weeks of the study and from week 9 to the end of the study (RR, 0.79; 95% CI, 0.67-0.93; P < 0.005). At the end of study period, a 10% higher dose of Gla-300 was required to maintain glycemic control.29
The EDITION 2 trial compared Gla-300 to Gla-100 in patients with T2D using basal insulin plus OAD. Glycemic control was similar in both groups. There was a 23% reduction in the risk of confirmed nocturnal and severe hypoglycemia observed with Gla-300 from week 9 to the end of the treatment period (six months) (RR, 0.77; 95% CI, 0.61-0.99; P = 0.038) and during the first eight weeks. Weight gain was lower with Gla-300 (P = 0. 015). The Gla-300 group required 10% more basal insulin (units/kg/day) than the Gla-100 group.8
The EDITION 4 trial compared Gla-300 to Gla-100 in long duration of T1D. Gla-300 was noninferior to Gla-100 in A1c reduction; the change in A1c was equivalent (difference, 0.04%; 95% CI, 0.10 to 0.19). Similar results were found for morning and evening injection times. Hypoglycemia did not differ, except for the first eight weeks of the study, when severe and confirmed hypoglycemia was lower with Gla-300 (rate ratio, 0.69; 95% CI, 0.53-0.91). Hypoglycemia did not differ by the time of injection. Weight gain was lower with Gla-300 (95% CI, -1.1 to -0.03, P = 0.037). The basal insulin dose was higher for Gla-300 at six months.28
Another study evaluated the safety of seniors (> 65 years of age) with T2D taking Gla-300 compared to Gla-100. There was a comparable reduction of A1c between the two groups. Lower rates of documented symptomatic hypoglycemia were observed in Gla-300 vs. Gla-100. A significant benefit in hypoglycemia reduction was seen in participants aged > 75 years, indicating that Gla-300 is suitable for use in this vulnerable population.30
Insulin Degludec
Insulin degludec is an ultra-long-acting basal insulin with a half-life of 42 hours. Degludec forms a depot of soluble multihexamers that dissociates slowly and consistently, resulting in a flat and stable profile with a prolonged duration of action.31 Degludec has a lower day-to-day variability than glargine U100 and U300.32,33
The BEGIN Basal-Bolus Type 1 trial compared insulin degludec with glargine U100 in T1D patients on basal-bolus treatment with mealtime insulin aspart. Degludec was noninferior to glargine in A1c reduction.
The rates of confirmed or severe hypoglycemia were similar in both groups (episodes per patient-year of exposure; estimated rate ratio [degludec to glargine], 1.07 [0.89-1.28]; P = 0.48). The rate of nocturnal confirmed hypoglycemia was 25% lower with degludec than with glargine (episodes per patient-year of exposure; 0.75 [0.59-0.96]; P= 0.021).34
The BEGIN Basal-Bolus Type 2 trial compared insulin degludec with glargine U100 in T2D patients on basal-bolus treatment with mealtime insulin aspart and with or without OAD. Degludec was noninferior to glargine in A1c reduction. Rates of severe hypoglycemia were similar in the two groups. Rates of confirmed hypoglycemia episodes per patient-year of exposure (estimated rate ratio, 0.82; 95% CI, 0.69-0.99; P = 0.0359) and nocturnal confirmed hypoglycemia episodes per patient-year of exposure (estimated rate ratio, 0.75; 95% CI, 0.58-0.99; P = 0.0399) were lower with degludec.35
The BEGIN Once Long trial compared insulin degludec and glargine U100 in the insulin naïve in T2D. Degludec was noninferior to glargine in A1c reduction, with similar glycemic control between the groups. Overall confirmed hypoglycemia (P = 0.106) and weight changes (P = 0.28) were similar between the two groups. The rate of confirmed nocturnal hypoglycemia was lower by 36% with degludec; the estimated rate ratio of degludec to glargine was 0.64 (95% CI, 0.42-0.98; P = 0.038).31
The SWITCH 1 trial compared insulin degludec with glargine U100 in T1D patients with at least one risk factor for hypoglycemia. During the maintenance period (16 weeks), overall symptomatic hypoglycemia (rate ratio, 0.89; 95% CI, 0.85-0.94; P < 0.001 for noninferiority, P< 0.001 for superiority), nocturnal symptomatic hypoglycemia (rate ratio 0.64; 95% CI, 0.56-0.73; P < 0.001 for noninferiority, P < 0.001 for superiority), and severe hypoglycemia episodes (P < 0.002) were lower with degludec compared to glargine U100.
Degludec was noninferior to glargine U100 in relation in A1c changes. Weight changes were not significant between the two groups. The trial included a population with moderate renal failure (estimated glomerular filtration rate, 30 mL/min/1.73 m2 to 59 mL/min/1.73 m2), one or more severe hypoglycemic episodes, hypoglycemic unawareness, diabetes for more than 15 years, or an episode of hypoglycemia in the last 12 weeks. Post-hoc analysis showed insulin doses were significantly lower with insulin degludec compared to insulin glargine U100.33
The SWITCH 2 trial compared insulin degludec with glargine U100 in T2D with at least one risk factor for hypoglycemia on basal insulin and with or without OAD. During the maintenance period (16 weeks), overall symptomatic hypoglycemia (rate ratio, -0.70; 95% CI, 0.61-0.80; P < 0.001) and nocturnal symptomatic hypoglycemia (rate ratio, -0.58; 95% CI, 0.46-0.74; P < 0.001) were lower in the degludec group compared to the glargine U100 group. Severe hypoglycemia was not statistically different between the groups (P = 0.35).
Degludec was noninferior to glargine U100 in relation to A1c changes. Weight changes were not significant between the groups. The trial included a population with moderate renal failure (estimated glomerular filtration rate, 30 mL/min/1.73 m2 to 59 mL/min/1.73 m2), one or more severe hypoglycemic episodes, hypoglycemic unawareness, exposure to insulin for more than five years, or an episode of hypoglycemia in last 12 weeks. Post-hoc analysis showed insulin doses were significantly lower with insulin degludec compared to insulin glargine U100 (P < 0.001).32
Basal insulin analogs may be interchanged with another basal insulin analog with a 20% dose reduction, except for degludec.11 For example, degludec
80 units or less may be interchanged with another basal insulin analog with a 20% dose reduction.
Degludec dosage greater than 80 units may be interchanged with another basal insulin analog with a 20% dose reduction, but the other basal insulin must be split into two equal doses taken 12 hours apart.
For example, degludec 100 units daily can be changed to glargine U100 40 units every 12 hours (80 units/2).11
The BRIGHT trial compared glargine U300 to degludec U100 in the insulin naïve with uncontrolled T2D on oral agents with or without glucagon-like peptide 1 receptor agonist. Gla-300 was noninferior to degludec U100 in mean A1c change at 24 weeks (mean difference, -0.05%; 95% CI, -0.15 to 0.05; P < 0.0001). There was no difference in incidence and event rate of confirmed hypoglycemia at any time of the day between the groups for the 24 weeks of the treatment period. During the first 12 weeks (during titration period), the incidence and event rate of confirmed hypoglycemia were lower in Gla-300 vs. degludec U100 (rate ratio, 0.65; 95% CI, 0.43-0.98).
The Gla-300 insulin dose was higher by 0.11 units/kg than degludec U100. Glycemic control was similar in the two groups, with relatively low risk for hypoglycemia.27
The CONCLUDE trial compared degludec U200 and Gla-300 in T2D patients on basal insulin and with or without oral glucose-lowering agents with one risk factor for hypoglycemia.
During the maintenance period (36 weeks), there was no significant difference in overall significant hypoglycemia between the groups. The rates of nocturnal symptomatic (rate ratio, 0.63; 95% CI, 0.48-0.84) and severe hypoglycemia (rate ratio, 0.20; 95% CI, 0.07-0.57) were considerably lower with degludec U200 compared with Gla-300 during the maintenance and total treatment period. Similar results were observed in post-hoc analysis. There was a greater increase in body weight with degludec U200 compared with Gla-300.26
In the studies, basal insulin U100 interchanged to insulin U300 based on the frequency of dosing. Examples:
• NPH or U100 once daily can be interchanged to glargine U300 with a 1:1 dose conversion.
• NPH or U100 twice daily (BID) can be interchanged to glargine U300 by a 20% dose reduction. For example, NPH 40 units BID can by interchanged to glargine U300 (Toujeo) 80 units daily.
• After switching glargine U100 to glargine U300, a 10% to 15% increased dose may be required to maintain glycemic control.
• Glargine U300 can be interchanged to glargine U100 by a 20% dose reduction. For example, glargine U300 (Toujeo) 100 units can be interchanged to glargine U100 (Lantus) 80 units.
Premixed Insulins
Premixed insulins are a fixed combination of intermediate-acting or long-acting insulin with rapid-acting or short-acting insulin in a single injection. These formulations include premixed formulation with regular insulin, premixed formulations with analog, and premixed formulations with rapid and ultra-long-acting basal analogs. (See Table 5.)
Premixed insulin analogs were developed to more closely mimic physiological endogenous insulin secretion as well as to reduce the number of injections. Premixed insulin has PK that favor 24-hour efficacy and convenience.
Table 5. Different Types of Premixed Insulins37 | |||
Type | Low-Mix | Mid-Mix | High-Mix |
Regular insulin-NPH |
|
|
|
Insulin analogs |
|
|
|
Coformulations |
| ||
NPH: neutral protamine Hagedorn | |||
Premixed insulins are dosed once, twice, or three times daily based on PK/PD properties.36 (Visit https://dtc.ucsf.edu/types-of-diabetes/type2/treatment-of-type-2-diabetes/medications-and-therapies/type-2-insulin-rx/types-of-insulin/pre-mixed-insulin/ for more information.)
The ratio of the combination is indicated in the name. Premixed insulin can be helpful for people who have trouble taking MDI, drawing insulin out of two different vials, and following different dosage directions.
A fixed-ratio combination has the advantage of simplifying the treatment regimen, which has been shown to improve glycemic control, adherence, and lower overall cost.
The disadvantages of a fixed combination are that it is limited to people who follow the same diet plans and have unchanged physical activity. People need to have a fixed eating schedule and consistent food to match the meals with the insulin. Patients using premixed insulin should be counseled on balanced nutrition and physical activity at treatment initiation and intensification and on avoiding unaccustomed vigorous physical activity within two to three hours after taking the injection.
The premixed insulin starting dose is 10 units or 0.1 unit/kg/day to 0.2 unit/kg/day immediately before or soon after the largest meal. Once-daily doses may be split equally into pre-breakfast and pre-dinner dinner doses if the evening pre-prandial glucose levels or A1c remain high, or dose requirements increase beyond 30 units/day or 0.4 units/kg/day. High premixed insulin is dosed as two-thirds in the morning and one-third in the evening when the once-daily insulin dose exceeds 20 units or 30 units.36
Premixed analog insulins compared to premixed human insulin have better mealtime flexibility, postprandial glycemic control, and weight management.36
Clinical trials of premixed insulin analog twice daily found that it is superior to insulin glargine in lowering A1c in people with T2D.13,36 A meta-analysis of 13 RCTs comparing premixed and basal-bolus regimens showed no significant difference in A1c levels, rates of hypoglycemia, weight change, or daily insulin dose.37,38 There are more reports of an increased risk of minor nocturnal hypoglycemia with premixed insulin than with basal insulin.39 Low-mix formulations compared to mid-mix formulations offer better control of PPG, lesser glucose excursions, and less intense monitoring of blood glucose levels.
Few studies have reported significant reductions in A1c with the initiation and intensification of therapy with mid-mix formulations compared with low-mix formulations.36
Premixed insulins are interchanged to other premixed insulin with a 20% dose reduction. Premixed insulins can be interchanged to NPH with a 20% dose reduction.11
Premixed insulin containing rapid-acting insulin should be injected no more than 15 minutes before meals. Premixed insulins containing regular insulin can be injected up to 30 minutes before the start of a meal.11,36
U500 Regular Insulin
U500 regular insulin is five times more concentrated than U100 regular insulin. The high concentration enables the administration of large doses of insulin in a small volume. U500 insulin frequently is used in severe insulin resistance. U500 regular insulin has distinct PK and PD with delayed onset and longer duration of action, like that of intermediate-acting (NPH) insulin.7 The extended duration of action is the result of continued absorption of insulin from the subcutaneous depot and, potentially, a slower clearance of highly concentrated insulin.40
U500 insulin has dual action, providing both basal and mealtime coverage, allowing it to be used as insulin monotherapy.11 A steady state is reached 24 hours after the first dose is administrated for once-daily, BID, and three times daily (TID) regimens.
Because of fluctuations in PK/PD with once-daily dosing, BID or TID dosing is recommended. TID dosing provides stable insulin activity for 24 hours at a steady state compared to BID dosing.41 U100 is interchanged to U500 if the patient requires more than 200 units of insulin per day or 2 units/kg.7,41
MDI are a common barrier to adherence, which affects glycemic targets and negatively affects clinical and economic outcomes.42,43
A meta-analysis of studies reporting U500R administrated as MDI resulted in significant A1c reduction of 1.59%, weight gain of 4.38 kg, and increase in TDD by 51.9 units.44 The incidence of severe hypoglycemia was either not reported or not different from U100 insulin.40,44
A U500R titration-to-target study compared the safety and efficacy of BID to TID dosing. Mean A1c reductions were similar between BID and TID regimens (1.2% BID vs. 1.1% TID). There was a mean increase in TDD in both of the treatment groups. The incidence of severe hypoglycemia and modest weight gain were similar between the treatment regimens. A higher incidence of non-severe hypoglycemia was observed in the BID regimen. Rates of both severe and mild hypoglycemia were higher in people taking > 300 units/day. The BID regimen scored better in the treatment burden, daily life, compliance, and overall treatment domains of the Treatment-Related Impact Measure–Diabetes questionnaire.40
Humulin R U500 pen dosing is based on A1c and blood glucose levels.46 The TDD of U100 is decreased by 80% if glycated hemoglobin (A1c) is less than or equal to 8% or if self-monitored blood glucose (SMBG) for the past seven days is less than 183 mg/dL.42
If the A1c is more than 8% or SMBG is more than 183 mg/dL, start at 100% of TDD of U100 insulin. U500 is administered as BID (60%/40%) or TID (40%/30%/30%). U500 insulin is administered 30 minutes before meals. For example, TDD of U100 250 units and A1c 10%: Dose for U500 100 units (40%) before breakfast, 75 units (30%) before lunch, and 75 units (30%) before dinner. For TDD of U100 250 units and A1c 10%: dose for U500 150 units (60%) before breakfast and 100 units (40%) before dinner.
Conclusion
Insulin transitions are common in clinical practice. Understanding the PK and PD properties will help providers with calculating insulin transitions. Insulin therapy should be individualized to every person based on glycemic targets, personal preference, cost, and their social determinants of health. Patients should be involved in decision-making when choosing the insulin formulations and appropriate education should be provided when initiating and intensifying insulin therapy.
Initiating the first dose of insulin in the clinic will decrease some of the barriers of insulin therapy, such as fear of injections and needles, and patients and families can better understand the proper technique for administering insulin injections. All the insulin brands have websites that provide information about starting dose, titration, and maximum dose. Research and ongoing trials will bring more advancement in technology and therapies in diabetes management.
Clinical Pearls
• Discuss with every individual and their family the different insulin options available and choose the insulin regimen together.
• Transition to concentrated insulins if the patient requires more units to decrease the volume of insulin injected.
• Transition to premixed insulin in patients taking MDI to decrease the number of injections, as long they follow a regular diet and activity schedule.
• Transition to U500 insulin if the patient requires more than 200 units of TDD.
• Regular follow-up with the patient and educating them about insulin dosing will improve adherence.
References
- World Health Organization. Diabetes. https://www.who.int/diabetes
- Centers for Disease Control and Prevention. Diabetes. Last reviewed Nov. 1, 2022. https://cdc.gov/diabetes
- Lewis GF, Brubaker PL. The discovery of insulin revisited: Lessons for modern era. J Clin Invest 2021;131:e142239.
- Siew YY, Zhang W. Downstream processing of recombinant human insulin and its analogues production from E. coli inclusion bodies. Bioresour Bioprocess 2021;8:65.
- Sharma AK, Taneja G, Kumar A, et al. Insulin analogs: Glimpse on contemporary facts and future prospective. Life Sci2019;219:90-99.
- Niswender KD. Basal insulin: Physiology, pharmacology, and clinical implications. Postgrad Med 2011;123:17-26.
- American Diabetes Association Professional Practice Committee; Draznin B, Aroda VR, Bakris G, et al. 9. Pharmacologic approaches to
glycemic treatment: Standards of medical care in diabetes – 2022. Diabetes Care 2022;45(Suppl 1):S125-S143. - Yki-Jarvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: Glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care 2014;37:3235-3243.
- Semlitsch T, Engler J, Siebenhofer A, et al. (Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev 2020;11:CD005613.
- American Diabetes Association. Devices and Technology. Insulin pens. https://diabetes.org/tools-support/devices-technology/insulin-pens
- American Diabetes Association. Switching between insulin products in disaster response situations. https://diabetes.org/sites/default/files/2019-08/switching-between-insulin.pdf
- Mathieu C, Bode BW, Franek E, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): A 52-week, randomized, treat-to-target phase III trial. Diabetes Obes Metab 2018;20:1148-1155.
- Levy P. Insulin analogs or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetes. MedGenMed 2007;9:12.
- Klonoff DC. Afrezza inhaled insulin: The fastest-acting FDA-approved insulin on the market has favorable properties. Diabetes Sci Technol 2014;8:1071-1073.
- Akturk HK, Snell-Bergeon JK, Rewers A, et al. Improved postprandial glucose with inhaled Technosphere insulin compared with insulin aspart in patients with type 1 diabetes on multiple daily injections: The STAT study. Diabetes Technol Ther 2018;20:639-647.
- Afrezza. For U.S. healthcare professionals only. https://afrezzahcp.com/
- Blevins T, Zhang Q, Frias JP, et al. Randomized double-blind clinical trial comparing ultra rapid lispro with lispro in basal-bolus regimen in patients with type 2 diabetes: PRONTO-T2D. Diabetes Care 2020;43:2991-2998.
- Wong EY, Kroon L. Ultra-rapid-acting insulins: How fast is really needed? Clin Diabetes 2021;39:415-423.
- Klaff L, Cao D, Dellva MA, et al. Ultra-rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: Results from the 26-week PRONTO-T1D study. Diabetes Obes Metab 2020;22:1799-1807.
- Leohr J, Dellva MA, Carter K, et al. Ultra rapid ispro (URLi) accelerates insulin lispro absorption and insulin action vs Humalog consistently across study populations: A pooled analysis of pharmacokinetic and glucodynamic data. Clinical Pharmacokinet 2021;60:1423-1434.
- Bowering K, Case C, Harvey H, et al. Faster aspart versus insulin aspart as part of a basal-bolus regimen in inadequately controlled type 2 diabetes: The onset 2 trial. Diabetes Care 2107;40:951-957.
- Saleem F, Sharma A. NPH Insulin. StatPearls [Internet]. StatPearls Publishing; 2022.
- Hermansen K, Madsbad S, Perrild H, et al. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: A randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care 2001;24:296-301.
- Bradley MC, Chillarige Y, Lee H, et al. Severe hypoglycemia risk with long-acting insulin analogs vs neutral protamine Hagedorn insulin. JAMA Intern Med2021;181:598-607.
- Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab 2009;11:372-378.
- Philis-Tsimikas A, Klonoff DC, Khunti K, et al. Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin-treated patients with type 2 diabetes: The randomised, head-to-head CONCLUDE trial. Diabetologia 2020;63:698-710.
- Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/mL versus degludec 100 units/mL in insulin-naïve type 2 diabetes: The randomized head-to-head BRIGHT trial. Diabetes Care 2018;41:2147-2154.
- Home PD, Bergenstal RM, Bolli GB, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: A randomized phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015;38:2217-2225.
- Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: Glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care 2014;37:2755-2762.
- Ritzel R, Harris SB, Baron H, et al. A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: Results from the SENIOR study. Diabetes Care 2018;41:1672-1680.
- Zinman B, Philis-Tsmikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: A 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care 2012;35:2464-2471.
- Wysham C, Bhargava A, Chaykin L. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 2 diabetes. The SWITCH 2 randomized clinical trial. JAMA 2017;318:45-56.
- Lane W, Bailey TS, Gerety G, et al. Effects of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: The SWITCH 1 randomized clinical trial. JAMA 2017;318:33-44.
- Heller S, Buse J, Fisher M, et al. Insulin degludec, an ultra-long-acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): A phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1489-1497.
- Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-long-acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): A phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1498-1507.
- Kalra S, Czupryniak L, Kilov G, et al. Expert opinion: Patient selection for premixed insulin formulations in diabetes care. Diabetes Ther 2018;9:2185-2199.
- Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 Diabetes: A comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260-265.
- Malone JK, Bai S, Campaigne BN, et al. Twice-daily pre-mixed insulin rather than basal insulin therapy alone results in better overall glycemic control in patients with type 2 diabetes. Diabet Med 2005;22:374-381.
- Strojek K, Bebakar WM, Khutsoane DT, et al. Once-daily initiation with biphasic insulin aspart 30 versus insulin glargine in patients with type 2 diabetes inadequately controlled with oral drugs: An open-label, multinational RCT. Curr Med Res Opin 2009;25:2887-2894.
- Sze D, Goldman J. Human regular 500 units/mL insulin therapy: A review of clinical evidence and new delivery options. Clin Diabetes 2018;36:319-324.
- Kalra S. High concentration insulin. Indian J Endocrinol Metab 2018;22:160-163.
- Chen J, Borra S, Fan L, et al. Treatment patterns and outcomes before and after human regular U-500 insulin initiation via KwikPen® among US veterans with type 2 diabetes mellitus. J Diabetes Complications 2021;35:107995.
- Kabul S, Hood RC, Duan R, et al. Patient-reported outcomes in transition from high-dose U-100 insulin to human regular U-500 insulin in severely insulin-resistant patients with type 2 diabetes: Analysis of a randomized clinical trial. Health Qual Life Outcomes2016;14:139.
- Reutrakul S, Wroblewski K, Brown RL. Clinical use of U-500 regular insulin: Review and meta-analysis. J Diabetes Sci Technol2012;6:412-420.
- Humulin R U-500 insulin human injection. For consumers. https://www.humulin.com
- Diabetes Education Online. Pre-mixed insulin. Diabetes Teaching Center at the University of California, San Francisco. https://dtc.ucsf.edu/types-of-diabetes/type2/treatment-of-type-2-diabetes/medications-and-therapies/type-2-insulin-rx/types-of-insulin/pre-mixed-insulin
