By Rebecca H. Allen, MD, MPH, Editor
The prevalence of human immunodeficiency virus (HIV) infection in the United States is approximately 1.2 million persons.1 In 2021, there were 32,100 new infections. Men who have sex with men (MSM) accounted for 77% of new infections, heterosexual contact made up 22%, and intravenous drug use contributed 8%. People assigned female at birth accounted for 24% of new infections.1 Therefore, providers of sexual healthcare should be familiar with ways to prevent HIV acquisition.
SCREENING
First, the Centers for Disease Control and Prevention (CDC) recommends screening for HIV at least once among all persons between the ages of 13 and 64 years of age.2 Patients at higher risk for HIV, such as those with the following risk factors, should be screened annually: people who inject drugs and their sex partners; people who exchange sex for money or drugs; sex partners of people with HIV; sexually active gay, bisexual, and other MSM; heterosexuals who themselves or whose sex partners have had one or more sex partners since their most recent HIV test; and people receiving treatment for hepatitis, tuberculosis, or a sexually transmitted infection.
Of course, pregnant patients also should be screened at their first prenatal visit and then rescreened in the third trimester if they are high-risk.2 The aim of this is to decrease mother-to-child transmission of HIV, which can be reduced to 1% with the use of antiretrovirals. There were only 53 children younger than age 13 years diagnosed with HIV in 2021 in the United States as a result of this screening and treatment policy.1
Clearly, knowing one’s HIV status can help patients take steps to prevent transmission. Male and female condoms are options for preventing HIV during sexual intercourse.2 These products are widely available over the counter in retail stores and pharmacies and their use should be encouraged for preventing sexually transmitted infections. Yet, there is another option available, pre-exposure prophylaxis (PrEP), for patients to prevent HIV that many providers have not yet added to their armamentarium. Therefore, this special feature will review the indications for PrEP, its use, and the medications recommended.
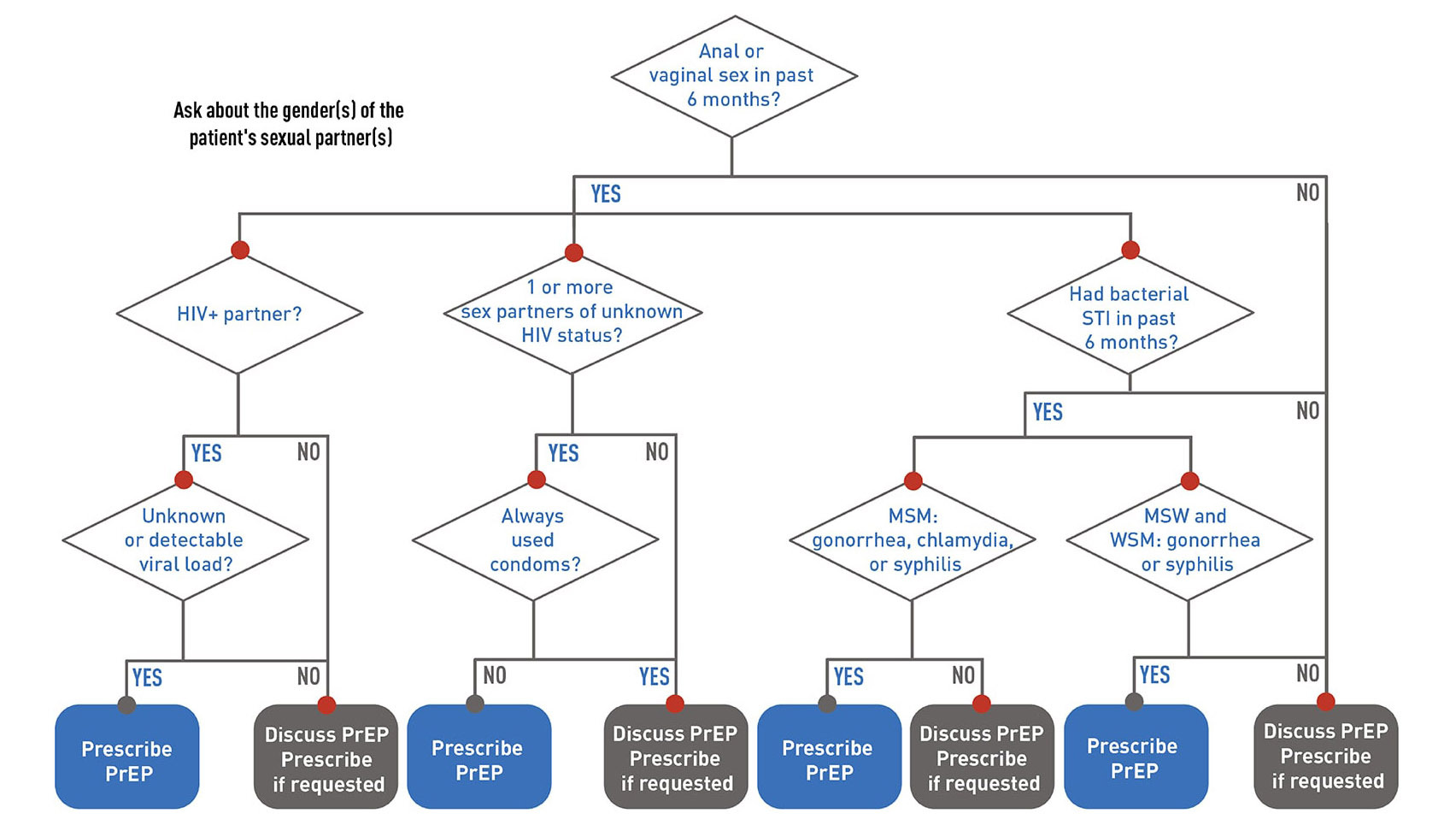
According to the CDC, any sexually active person is a candidate for PrEP, and clinicians should introduce the topic to their patients.3 Figure 1 shows the pathway by which PrEP should be introduced to patients. Patients who have injected drugs in the past six months and share equipment also should be offered PrEP. However, patients who have behaviors of lower risk — for example, consistently using condoms for vaginal and anal sex and being in a monogamous relationship — generally do not benefit from PrEP.
After the patient has asked for or agreed with the use of PrEP, it is important to obtain a baseline HIV test. If they report possible HIV exposure or signs or symptoms of acute HIV infection in the past four weeks, then they may need additional testing to clarify their status, such as an HIV-1 ribonucleic acid (RNA) assay. Additional screenings include baseline chlamydia, gonorrhea, hepatitis B, and syphilis testing. Finally, depending on the medications used, baseline renal function and lipid testing may be required as well.4
CURRENTLY AVAILABLE MEDICATIONS
There are three main options for PrEP medications.3 The first, emtricitabine (F) 200 mg in combination with tenofovir disoproxil fumarate (TDF) 300 mg (F/TDF), brand name Truvada, is an oral tablet taken daily. This can be used to prevent HIV among all people with sex or injection drug use risk factors. Renal function needs to be measured because of the rare risk of acute kidney failure. This drug is approved for use in people with an estimated creatinine clearance (eCrCl) > 60 mL/min. This drug also can be used in pregnancy and breastfeeding if necessary, and it is the preferred regimen for those who may become pregnant.4
The second oral medication option taken daily is emtricitabine (F) 200 mg in combination with tenofovir alafenamide (TAF) 25 mg (F/TAF), brand name Descovy. F/TAF can be used in patients with decreased renal function (eCrCl ≥ 30 mL/min). Lipid levels should be checked before starting this medication. However, F/TAF is not an option for patients at risk of HIV through receptive vaginal sex, since it has not yet been studied in that population.3
The final option is an intramuscular injection of cabotegravir (CAB) 600 mg, which can be used for all types of patients. The injection is given every month for two doses and then every two months thereafter.4 Renal and lipid studies are not necessary for this medication, and it has the advantage of the patient not having to remember to take a daily pill. However, the patient does have to return for injection visits.
EFFECTIVENESS AND FOLLOW-UP
The effectiveness of PrEP in reducing HIV acquisition is estimated at 99% from sexual encounters and at 74% from injection drug use, as long as high adherence rates are maintained.3 PrEP is considered safe to use, with few complications. The CDC recommends rechecking an HIV test every three months and only prescribing a 90-day supply for those taking oral medications. For those using the injectable, an HIV test is recommended prior to each injection. Repeat screening for other sexually transmitted infections also should be conducted every six months.4
Kidney function also may need to be monitored at six months if the patient is 50 years of age or older or started out with a lower CrCl. None of the PrEP medication interacts with hormonal contraception. At 12 months, all patients on oral medications should have repeat renal function testing. In sum, PrEP clearly requires patient cooperation and adherence to both taking medication and to frequent visits.
PrEP visits and counseling certainly can take time, and this should be allowed for in the clinical schedule. In June 2019, the U.S. Preventive Services Task Force recommended PrEP for adults and adolescents.5 This means that most health insurance companies are required to cover PrEP visits, lab tests, and medications.
For patients who do not have insurance coverage, there are assistance programs available. PrEP is an underused but powerful tool to prevent HIV infection. As sexual health providers, we should become more accustomed to offering PrEP to our patients and the follow-up requirements for its use.
REFERENCES
- HIV.gov. U.S. statistics. Updated Dec. 7, 2023. https://www.hiv.gov/hiv-basics/overview/data-and-trends/statistics
- Centers for Disease Control and Prevention. Sexually Transmitted Infections Treatment Guidelines, 2021. Last reviewed July 22, 2021. https://www.cdc.gov/std/treatment-guidelines/toc.htm
- Centers for Disease Control and Prevention. Clinical guidance for PrEP. Published May 6, 2024. https://www.cdc.gov/hivnexus/hcp/prep/index.html
- Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States – A clinical practice guideline. Published December 2021. https://stacks.cdc.gov/view/cdc/112360
- U.S. Preventive Services Task Force. Prevention of acquisition of HIV: Preexposure prophylaxis. Published Aug. 22, 2023. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/prevention-of-human-immunodeficiency-virus-hiv-infection-pre-exposure-prophylaxis