Renal Failure
Author: Jonathan Glauser, MD, FACEP, Associate Chair, Operations, Department of Emergency Medicine, Cleveland Clinic Foundation, Faculty, Residency Program in Emergency Medicine, MetroHealth Medical Center, Cleveland, OH.
Peer Reviewers: Allan B. Wolfson, MD, FACEP, FACP, Professor of Emergency Medicine, University of Pittsburgh, PA; and Albert Weihl, MD, Section of Emergency Medicine, Department of Surgery, Yale University School of Medicine, New Haven, CT.
Acute renal failure (ARF), characterized by sudden loss of the ability of the kidneys to excrete waste, concentrate urine, conserve electrolytes, and maintain fluid balance, is a frequent clinical problem.1 Renal failure affects approximately 5% of all general hospital patients. The mortality from all forms of ARF is greater than 50% and has not decreased significantly over the past 30 years.2 The odds ratio for dying in patients with ARF was 4.9 compared to patients without ARF in one report.3
During the bombing of London in World War II, Bywaters and Beall described an acute loss of kidney function that occurred in severely injured crush victims.4 They termed this entity acute tubular necrosis because of histologic evidence for patchy necrosis of renal tubules at autopsy. The term acute tubular necrosis (ATN) has persisted, even though the presence of tubular necrosis upon histological examination of the kidney is seen only occasionally.5
Acute hemodialysis first was used during the Korean War in 1950 to treat military casualties, leading to a decrease in mortality of ARF from approximately 90% to about 50%.6,7 Fluid resuscitation on the battlefield and more rapid evacuation of casualties reduced the incidence of ischemic ARF from one in 200 in the Korean War to one in 600 in the Vietnam War.8 This suggested that early intervention, including avoidance of prolonged renal vasoconstriction, could prevent progression to established ARF.
More recently, there were 73,000 new cases of end stage renal disease (ESRD) in 1996 in the United States, with 284,000 patients treated for ESRD in that year, an incidence of 10.4 per 10,000.9 The estimated medical costs exceed $50,000 per year per case of ESRD. Chronic renal failure is a major cause for mortality in the United States, with a five-year survival rate of 35% for patients undergoing dialysis.10 ARF occurs in approximately 19% of patients with moderate sepsis and in 51% of patients with septic shock when blood cultures are positive. The combination of ARF and sepsis is associated with a 70% mortality.11 Among patients who develop a rise in serum creatinine greater than 2.0 mg/dL and survive, the length of hospitalization is nine days longer than for patients who do not develop ARF.12—The Editor
Acute Renal Failure: Definitions and Overview of Renal Function
It is useful to recall that many renal functions are shared with other organs: acid-base control with the lung; blood pressure control via the renin-angiotensin-aldosterone axis with the liver, adrenals, and lung; and hormonal control of erythropoiesis to maintain hemoglobin levels, to name a few. There are only two physiological functions, however, that easily are measured in the emergency setting, that are unique to the kidney, and that are clinically important: production of urine and the excretion of water soluble waste products of metabolism.
ARF represents a sudden decline in renal function resulting in azotemia with an accumulation of nitrogenous waste products (creatinine and urea) over hours to weeks. While definitions vary by author, the following have been cited to define ARF:
- Decline in creatinine clearance of 50%;
- Increase in creatinine of 50%;
- Any renal insult causing the patient to require dialysis.
An absolute cut-off of 4 mg/dL has been suggested to define ARF, when superimposed upon a baseline level of renal insufficiency,13 while a sliding scale also has been proposed to define renal insufficiency:14
- Increase in serum creatinine (Cr) of 0.5 mg/dL or greater in patients with baseline Cr less than 1.9 mg/dL;
- Increase in serum Cr of 1 mg/dL or greater in patients with a serum Cr of 2-4.9 mg/dL;
- Increase in serum Cr of 1.5 mg/dL or greater in patients with a baseline Cr of greater than 5 mg/dL.
Renal failure further may be categorized as oliguric, with fewer than 500 mL/day of urine produced, or nonoliguric, producing more than 500 mL urine/day. Oliguria classically has been defined by weight as urine output fewer than 5 mL/kg/day or 0.5 mL/kg/hour. Anuria indicates production of fewer than 50-100 mL urine/day.15 Urine output very commonly is used in the emergency and critical care setting as a parameter of renal function. However, it is not specific unless urine output is severely reduced or absent. Renal failure frequently exists despite normal urine output. The mortality of nonoliguric renal failure is substantially lower than that of oliguric and anuric renal failure.16,17 In nonoliguric renal failure, nitrogenous waste cannot be excreted despite adequate urine output, and the urine usually is iso-osmotic, with a specific gravity approximating 1.010.15
Renal solute excretion is the result of glomerular filtration and the glomerular filtration rate (GFR). The most commonly used equation for calculating GFR is as follows:
In males: GFR= (140 - age) × (weight in kg)/ 72 × serum creatinine in mg/dL;
In females: GFR = 0.85 times GFR in males.
GFR varies, however, not simply as a function of disease, but also as a function of normal physiology. Subjects on a vegetarian diet, for example, may have a GFR of 45-50 mL/min, while the same person with normal kidneys may have a GFR of 140-150 mL/min on a diet with large animal protein intake.18 GFR also decreases with age. A general rule of thumb is that there is a 7.5 mL/min reduction in GFR for every decade older than 30 years.19 Baseline GFR is a function of functional renal mass. A male patient who has undergone a nephrectomy may have a baseline GFR that corresponds to his maximal GFR under unrestricted conditions. That is, if a moderate protein restriction is applied to his diet, his baseline GFR may decrease, revealing the actual decrease in his renal reserve.13 There is a linear relationship between GFR and the inverse of the serum creatinine, so that a doubling of serum creatinine (Cr) corresponds to an approximate decrease in GFR of 50%. Recall that creatinine is formed from the non-enzymatic dehydration of creatine in the liver, and that 98% of the creatine pool is in muscle. Critically ill patients may have abnormalities in liver function and markedly diminished muscle mass. Factors that increase creatinine production include trauma, fever, and immobilization. Conditions of decreased production include liver disease, decreased muscle mass, and aging. A serum creatinine of 1.5 mg/dL at steady state, for example, corresponds to an approximate GFR of 36 mL/min in an 80-year-old female, and approximately 77 mL/min in a 20-year-old male.13 A 90-year-old with a serum creatinine level of 1.0 mg/dL would have a GFR of approximately 50 mL/min, or 50% of normal. Therefore, a change in creatinine is more significant than one isolated value in determining the presence of ARF. As for measurement of GFR itself, a more accurate determination of GFR requires measurement of inulin clearance, or of radio-labeled compounds—testing not routinely available in the emergency department.20 Determining the exact GFR rarely is necessary, and determination of whether renal function is getting worse or better usually can be determined by monitoring serum creatinine alone.21 With established renal failure, the GFR generally is less than 10 mL/min, and the amount of nitrogenous waste in the blood is a function of how long the GFR has been lower than 10 mL/min.
Other markers of renal injury may be mentioned for completeness, although as yet have no clinical applicability. Kidney injury molecule-1 (KIM-1) can be detected in the urine of patients with ARF as an indicator of proximal tubule injury.22 Cystatin-C is a cysteine proteinase inhibitor excreted by the glomerulus, closely reflecting GFR. It is produced by nucleated cells at a constant rate. Cystatin-C may be a better marker of GFR than creatinine, but is not widely available.23 Cystatin-C may permit the detection of acute renal failure one to two days earlier than serum creatinine. Since early detection of ARF can provide time to prevent its progression, this marker may attain increased significance in the future.24
The necessity for clear definitions of ARF has resulted in the development of new criteria endorsed by the Acute Dialysis Quality Initiative. These guidelines are based upon change in the GFR from baseline, change in urine output, and duration of these changes. The stratified definition of ARF is denoted by the term RIFLE, denoting respectively, renal Risk, Injury, Failure, Loss, and End stage renal disease. For example, ARF class R for risk entails a 50% increase in serum creatinine, with 25% loss in GFR. Renal failure implies a 75% loss in GFR, with a three-fold rise in creatinine or a creatinine greater than 4 mg/dL.25
Pre-renal azotemia is a term that generally refers to rises in serum creatinine with intact tubular function. Volume depletion or advanced cardiac or liver failure may induce renal vasoconstriction.
Post-renal azotemia applies to an azotemic state due to urinary tract obstruction. This may be ruled out by identifying a post-void residual bladder urine of less than 50 mL, and excluding pyelocalyceal dilatation using renal ultrasonography.1
Clinical considerations that apply include:
- In the oliguric patient, serum potassium climbs 0.3-0.5 mEq/L/day unless exogenous causes for hyperkalemia such as sepsis or crush injury cause a more rapid climb.
- In anuric patients, serum creatinine climbs 1-2 mg/dL/day; it climbs less if some renal function is preserved, and more if rhabdomyolysis is a complicating factor.
- In distinguishing acute renal failure from chronic renal failure, the emergency physician may have to consult old records, look for secondary signs of ESRD such as small kidneys on imaging, or bony changes of hyperparathyroidism.26
Etiology of renal failure. Renal failure may result from intrinsic renal causes, including ischemic injury, glomerular injury, tubular injury, or iatrogenic causes. Among the latter, non-steroidal anti-inflammatory drugs, (NSAIDs) intravenous contrast agents, angiotensin-converting enzyme inhibitors (ACEIs), and aminoglycosides are prominent. ACEIs and angiotensin receptor blockers (ARBs) prevent post-glomerular arteriolar vasoconstriction. Medications and contrast media accounted for approximately 11% of ARF cases in one report.27 The most common general category of ARF is pre-renal, accounting for 55% of ARF in hospitalized patients,15 often resulting from intravascular volume depletion from hemorrhage, diuretic use, or gastrointestinal fluid losses. Heart failure may be associated with functional intravascular volume depletion, or with dehydration from diuretic therapy. Pre-renal azotemia most quickly may be distinguished from other causes of ARF either by noting a fractional excretion of sodium less than 1% or a spot urine sodium less than 20 mEq/L (see below). In pre-renal azotemia, the kidney compensates for an overall decrease in effective circulating volume by avidly re-absorbing sodium. See Table 1 for a categorization of etiologies for acute renal failure.
Table 1. Etiologies for Acute Renal Failure

The History in the Evaluation of the Acute Renal Failure Patient. There may be elements of the history that are suggestive of predisposing factors for acute renal failure. Hypovolemia is suggested by any of the following: thirst, decreasing urine output, vomiting, diarrhea, sweating, hemorrhage, burns, pancreatic disease, or liver disease. The patient may have a history of restricted access to fluids. A cardiac etiology may present with complaints of paroxysmal nocturnal dyspnea, orthopnea, or dyspnea on exertion.
Medication history may be critical. Iatrogenic causes include medication with angiotensin-converting enzyme inhibitors (ACEIs, ARBs) and diuretics, NSAIDs, aminoglycosides, radiocontrast agents, or cisplatin. Administration of any of the following may cause tubular obstruction by crystals: acyclovir, methotrexate, sulfonamides, uric acid, or ethylene glycol. Certain antibiotics, such as methicillin, predispose patients to interstitial nephritis.
Medical illnesses predisposing patients to renal insufficiency or renal failure include cirrhosis and malnutrition. Sickle cell disease, diabetes mellitus, and pyelonephritis predispose patients to papillary necrosis. A history of prior throat or skin infections suggests a predisposition to glomerulonephritis. Patients with possible rhabdomyolysis predisposing to ARF include those with recent coma, seizures, drug/alcohol abuse, or limb ischemia.
Urinary obstruction from prostatism may elicit a history of urgency, frequency, or hesitancy in a male. The following pre-existing conditions are known to cause neurogenic bladder: diabetes mellitus, spinal cord disease, Parkinson’s disease, anticholinergic drugs, multiple sclerosis, and alpha-adrenergic antagonists.
The Physical Examination: Keys to Diagnosis in ARF. Glomerular diseases may be suggested by edema or other signs of the nephrotic syndrome with heavy proteinuria. Renal vasculitic syndromes may present with rash or evidence for involvement of the retina or lung. Patients may present with evidence for volume depletion or fluid overload. The cardiac examination may reveal evidence for dysrhythmia, endocarditis, or congestive heart failure (CHF). The neurologic examination may reveal alterations in level of consciousness, seizures, or asterixis. The abdomen should be evaluated for aneurysm, flank tenderness, or bladder distention. (See Table 2.)
Table 2. Physical Examination: Possible
Findings in Acute Renal Failure

Prevention of Acute Renal Failure
There are no FDA-approved drugs for the prevention or treatment of clinical ARF. The use of loop diuretics has been a frequently used strategy for this purpose, attempting to induce diuresis and avoid the need for renal replacement. However, no benefit to diuretic therapy has been shown in several studies, and there is evidence that such diuretic strategies may be detrimental to critically ill patients.30 Others have disputed that loop diuretics are associated with higher mortality in ARF.31 Nonetheless, the clinician often has an opportunity for prevention of renal insufficiency and ARF. The following have been proposed:
- Restoration of renal blood flow via early and adequate volume replacement;32
- Judicious use of nephrotoxic agents, with avoidance of further toxicity, for example giving aminoglycosides once daily for less nephrotoxicity;33
- Saline infusion and N-acetylcysteine for prevention of contrast media-induced ARF;
- Use of calcium channel blockers may preserve GFR, renal graft function, and possibly ameliorate renal insufficiency from radiocontrast agents and, possibly aminoglycosides and amphotericin B;34
- Dopamine (Intropin) 0.5-2 mcg/kg/min with or without furosemide (Lasix) may be efficacious (not consistently) in converting oliguric to nonoliguric renal failure.2 Low-dose vasopressin (Pitressin) may be more efficacious than low-dose dopamine in preserving renal blood flow in sepsis.35
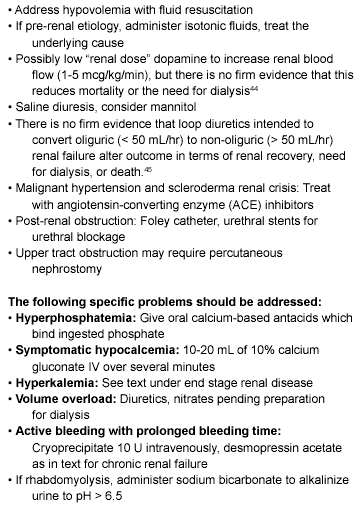
Contrast nephropathy is worthy of special mention because of the frequent need for radiocontrast agents for imaging in the emergency department. Contrast nephropathy is defined as an increase in creatinine of 0.5 mg/dL 48-72 hours after administration of the contrast agent, and has an incidence of approximately 150,000 cases per year.36 Its incidence varies from 5% in patients with mild renal insufficiency to 50% in patients with severe renal dysfunction and diabetes mellitus. The risk is enhanced in the presence of amyloidosis, hypo-proteinuric states, and multiple myeloma, and with the administration of larger doses of contrast agents. Risk factors also include high versus low osmolarity contrast, ionic versus non-ionic contrast, and repeat exposure to contrast within 72 hours. Prevention has entailed ensuring that the patient is hydrated adequately prior to administration of contrast. Prophylactic use of the antioxidant acetylcysteine (NAC), a scavenger of reactive oxygen species, was shown to prevent the reduction by contrast of renal function in patients with chronic renal insufficiency.37 The suggested dose is 600 mg of oral acetylcysteine twice daily for 48 hours.38 Recently it has been argued that data are insufficient to recommend that NAC become the standard of care for patients receiving radiocontrast.39 The ease of administration, low cost, and general availability of NAC are compelling reasons to use it. The vasodilator fenoldopam has been utilized as well. The role of hydration and avoidance of nephrotoxic drugs such as NSAIDs has clear relevance to the emergency physician ordering contrast studies.36 Treatment options in the management of ARF are listed in Table 3.
Table 3. Treatment Options for Developing ARF
Diagnostic Evaluation for the Patient with Acute Renal Failure
Traditional diagnosis has started with observation of the patient response to a fluid challenge. The aim is to prevent progression of acute renal vasoconstriction to established ARF. Decreasing levels of blood urea nitrogen (BUN), therefore, indicated reversible vasoconstriction, while uncontrolled accumulation of the nitrogenous products BUN and creatinine indicated established ARF. Frequently this approach led to fluid overload, pulmonary congestion, hypoxia, and the premature need for dialysis and mechanical ventilatory support.1
Evaluation of urine chemistries is a more useful way of discriminating between established ARF and renal vasoconstriction with intact tubular function. The fraction of filtered sodium that rapidly is reabsorbed by normal tubules of the vasoconstricted kidney is more than 99%. When nitrogenous waste accumulates in the blood due to a fall in GFR secondary to renal vasoconstriction, with intact tubular function, the fractional excretion of sodium is less than 1%. This is calculated by :
FENa = (urine sodium x plasma creatinine)/ (plasma sodium x urine creatinine).
When FENa is less than 1, tubular function generally will be intact unless the patient has a reason to have decreased tubular reabsorption of sodium, as occurs with glycosuria or diuretic administration. There is approximately an 80% diagnostic specificity of distinguishing azotemia associated with renal vasoconstriction/hypovolemia from ARF with tubular dysfunction. There are, however, causes of ARF that are not infrequently encountered in emergency medicine and are associated with an FENa of less than 1, including contrast-induced nephropathy and myoglobinuria.40
With established ARF, the ability of the kidneys to concentrate urine is abolished. Urine specific gravity approaches 1.010. In a patient with rising BUN and creatinine, measurement of urine osmolality may complement the use of FENa in the diagnostic separation of pre-renal azotemia from established ARF.1
Renal imaging in ARF traditionally has relied upon ultrasound, including duplex technique. Ultrasound is widely available and relatively inexpensive in evaluating renal arterial perfusion, venous drainage, and their alterations. Ultrasound may reveal dilatation of the collecting system or shrunken kidneys from chronic disease. More recently, magnetic resonance imaging (MRI) has shown promise in the diagnosis of ARF by demonstrating loss of corticomedullary differentiation.41
The urine analysis itself may show proteinuria from a variety of causes. In glomerulonephritis, red blood cell casts are diagnostic of glomerular inflammation. Intrinsic renal disease, including acute tubular necrosis, is associated with white blood cell, red blood cell, renal tubular cell, or muddy brown cast formation. If the urine dips positive for blood with no red blood cells seen under microscopic examination, myoglobinuria should be suspected. The presence of plasma and urinary eosinophils with peripheral evidence for allergy suggests the diagnosis of interstitial nephritis, and white blood cell casts may be seen.15 Table 4 summarizes possible diagnostic testing for ARF.
Table 4. Diagnostic Testing to Consider
for Acute Renal Failure

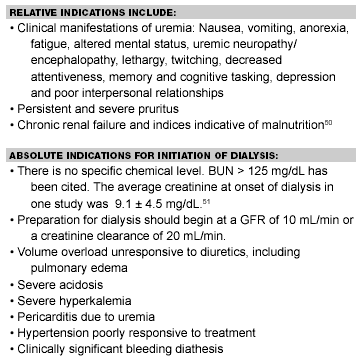
Indications for Emergency Hemodialysis in ARF
Usual indications for acute dialysis include hypervolemia and fluid overload. CHF is the most common reason.46 For this, hemofiltration alone is an option. Hyperkalemia and severe acidemia are the most commonly cited metabolic abnormalities requiring rapid treatment.47
Uremic induced encephalopathy and bleeding may necessitate emergency dialysis. Hypertensive crisis, pericarditis, and pericardial effusion may require intensive dialysis.
Toxins infrequently require dialysis. These include: salicylates, lithium, theophylline, methanol, ethylene glycol. Dialysis has been described for poisoning by Cortinarius and Amanita smithiana mushrooms.48 (See Table 5.)
Table 5. Indications for Initiation of Long-Term Dialysis
Intensive dialysis in ARF has been defined as dialyzing to a goal of a BUN less than 60 mg/dL and serum creatinine of less than 9 mg/dL. Non-intensive dialysis entails dialyzing to a BUN of less than 100 mg/dL and creatinine of less than 9 mg/dL. One study indicated no advantage to intensive dialysis as opposed to non-intensive dialysis in the emergency setting.49
The most common causes of death from ARF are sepsis, pulmonary failure, and cardiac death from volume overload. In the long term, if patients do not experience recovery from ARF, they will require renal replacement. Of dialyzed patients, 83% end up on hemodialysis and 17% on peritoneal dialysis. Transplantation is a long-term consideration.
Admission Criteria for Acute Renal Failure
Anyone who meets the definition of acute renal failure should be admitted to the hospital. As noted earlier, the definition of ARF may vary by clinical setting. Any patient meeting criteria for admission with ESRD, including alteration in mental status, symptomatic uremia, or suspected sepsis also should be hospitalized. Criteria for hospitalizing patients with ESRD follow. Furthermore, extensive laboratory work-up may be needed. This generally is best performed in the in-patient setting.
Prognosis for Recovery in Acute Renal Failure
The mean duration of ARF, defined as an increase in serum creatinine greater than 3 mg/dL, has been reported to be 12 days in those patients who do not require dialysis. In those patients with ARF who require some sort of renal replacement therapy, approximately 10% remain dialysis-dependent, and 40% develop chronic kidney failure.52 In another recent study of patients requiring dialysis for ARF who were followed for up to 9 years, 16% remained dialysis-dependent.53
Chronic Renal Failure and End Stage Renal Disease
Definitions. Chronic renal failure entails renal impairment with a glomerular filtration rate less than 60 mL/min,15 but decreased by no more than 75%. There may be evidence of structural or functional kidney abnormalities that persist for at least three months, and reduction of GFR may be over months to years.10 The patient may be asymptomatic.
ESRD implies that renal function has diminished to a very low level. This usually is associated with signs and symptoms of uremia, and often is reserved for renal impairment with a GFR of less than 10 mL/min. Serious life-threatening complications can be expected without dialysis or transplantation.54
Perspective. The population with ESRD in the United States is composed of approximately 200,000 patients who undergo dialysis and another estimated 70,000 patients with functioning kidney transplants.55 The life expectancy for patients with ESRD is fewer than 10 years.56 The five-year survival rate has been listed at 18-38%. For ESRD patients the mortality rate was 22% per year as of 1996.
When dealing with chronic renal failure or ESRD, in general, the clinician need not be as concerned with delineating the cause for the renal failure or with reversal of the process as with ARF. There may be a question as to whether renal failure is acute or chronic. If no history is available, small kidneys on plain films or ultrasonography, waxy casts on microscopic urine, and the presence of renal osteodystrophy on plain films of the hands or clavicles favors the diagnosis of chronic renal failure over ARF.54
Etiology of ESRD. Causes of ESRD parallel those of acute renal failure, with diabetes mellitus and hypertension being the most prominent. (See Table 6.)
Table 6. Causes of ESRD

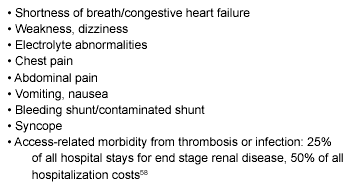
Factors causing problems in patients with renal insufficiency and ESRD generally are related to one of a handful of broad considerations: inability to excrete excess water, inability to excrete toxic metabolites, inability to control electrolyte levels, failure of hematopoiesis, or failure of vitamin D activation. Because 85% of erythropoietin is produced in the kidneys, anemia occurs without erythropoietin replacement. Vitamin D deficiency contributes to secondary hyperparathyroidism and development of renal bone disease. The most common presenting complaints of the ESRD patient to the emergency department generally reflect these principles. (See Table 7.)
Table 7. Most Common Presenting Complaints
to the Emergency Department57
While the emergency physician may not manage chronic problems, certain medications should be recognizable. These include:
- Calcium acetate (PhosLo): phosphate binder;
- Calcitriol (Rocaltrol, Calcijex): Vitamin D analogue (suppresses parathyroid hormone, minimizes consequences of renal osteodystrophy);
- Water soluble vitamins (Nephro-Vite);
- Erythropoietin (Epogen, Procrit); and
- Iron dextran (Infed, Dexferrum): Parenteral iron supplement.
Complications of Renal Failure. Both acute and chronic renal failure may lead to uremia, the advanced form of azotemia in which multiorgan system derangements become evident. These include electrolyte disturbances, volume disturbances, bleeding, metabolic acidosis, and anemia. Hyperkalemia may be life-threatening, as pericarditis can be. Persistent nausea and vomiting can lead to dehydration and shock. Management of these will be considered in turn.
There are numerous common causes for death in ESRD. Myocardial infarction is frequent, and may result from the same underlying disorders, such as hypertension and diabetes mellitus, that caused renal failure in the first place. Other cardiac causes for death include dysrhythmia, tamponade, medication toxicity (digitalis), ischemic or hypertrophic cardiomyopathy, conduction system disease, and acetate cardiotoxicity. Cardiac causes account for approximately 50% of ESRD deaths.59
Infection, especially Staphylococcus aureus sepsis, may be related to impaired neutrophil, T, and B lymphocyte function. Pulmonary infections are common.
Withdrawal from dialysis/suicide has been implicated in approximately 20% of deaths, with a slightly higher rate of withdrawal from therapy in patients older than 65 years. Cerebrovascular disease, including intracranial hemorrhage, occurs frequently, as does malignancy.
Electrolyte disturbances, including hyperkalemia and hypercalcemia, may be life-threatening.55 Gastrointestinal (GI) bleeding may be from peptic ulcer disease, colonic diverticula, or arteriovenous malformations. Other potentially lethal GI events include bowel infarction and ischemia.60 Dementia frequently contributes to morbidity and mortality.
Important Elements of the History in ESRD Patients. It is important to determine the underlying cause for the patient’s ESRD, as an underlying disease such as diabetes mellitus may have an impact on the presenting complaint. The dry weight is critical in the evaluation of a patient’s volume status, as well as whether it was achieved last dialysis.
It is critical to know the schedule of the patient’s dialysis (M-W-F, T-Th-S), as well as whether any sessions were missed and why. Recall that 20% of dialysis patients withdraw from therapy before death. It is important to know whether there has been retention of the patient’s native kidneys, as a source for hypertension, nephrolithiasis, or infection.
Since intra-dialysis hypotension is a common presenting complaint, knowledge of baseline vital signs may become critical. Bone pain may indicate fractures as a sign of renal osteodystrophy. Coughing may be a clue to the presence of CHF. Clues to inadequate dialysis may include anorexia, nausea, vomiting, diarrhea, weakness, or impaired alertness.
Essentials of the Physical Examination. The physical examination of the ESRD patient may focus on certain specific items. The significance of vital signs is self-explanatory. Vascular access should be evaluated for a bruit or thrill. Shunt thrombosis usually is from outflow stenoses in the venous circulation. The site should be examined for erythema, warmth, swelling, tenderness, or discharge. Infection, if present, may be subtle.
The dermatologic examination may reveal yellowish skin, urea deposition (“frost”), or uremic fetor. The cardiac examination should focus on murmurs, distant heart sounds possibly indicative of pericardial effusion, or evidence for fluid retention indicative of CHF. The neurologic examination may reveal neuropathy, asterixis, lethargy, somnolence, hiccups, myoclonic twitching, or altered mental status. As bleeding always is a consideration in uremic patients with suboptimal platelet function, subdural hematoma should be suspected with focal deficits or depressed level of consciousness. A rectal examination may be performed to diagnose GI bleeding.
Clinical features of chronic renal failure may be manifest in a variety of systems. Possible central nervous system abnormalities include neuropathy (peripheral and autonomic), lethargy, fatigue, seizures, singultus, coma, or dementia. Uremic encephalopathy typically shows memory loss, decreased attentiveness, slurred speech, or asterixis. Dialysis dementia may occur after two years of dialysis, possibly related to oral or dialysate aluminum. Subdural hematoma may be related to bleeding diathesis, anticoagulant use, excessive ultrafiltration, or hypertension, and should be suspected in any ESRD patient with focal neurologic signs or depressed level of consciousness. Other neuromuscular findings may include peripheral neuropathy, myoclonus, or asterixis.
Possible cardiopulmonary presentations of ESRD include CHF, pulmonary edema, pericarditis, hypertension, or pericardial effusions. High output CHF may be related to anemia or the presence of an AV fistula.
Gastrointestinal features of uremia may include nausea, vomiting (mandating a check for hypercalcemia or hyponatremia), anorexia, symptoms of peptic ulcer disease, uremic gastroparesis, or constipation.
Dermatologic manifestations of uremia include pallor (anemia), pruritus, or crystallized urea from sweat (uremic frost).
The medical care of the patient with chronic renal failure should focus on delaying progression of renal disease. This may involve aggressive control of blood glucose, blood pressure, and hyperlipidemia. Diabetics with microalbuminuria may derive benefit from ACEIs or ARBs. Protein restriction may reduce uremic complications, but should be balanced against the potential to develop malnutrition. Physicians generally are aware of the need for dosage reductions for renally excreted medications. The largest category of drugs requiring dosage reductions is antibiotics, although digitalis is prominent among these as well. Metformin should be avoided because of the risk of life-threatening lactic acidosis. Metabolic abnormalities that may require attention include hyperkalemia, hyperphosphatemia, hypocalcemia (osteodystrophy), metabolic acidosis, hyperuricemia, and hyperamylasemia. (See Table 8.)
Table 8. Some Suggested Laboratory Testing
for the ESRD Patient in the ED

Renal Replacement Therapy: Hemodialytic Management of Acute Renal Failure
The most common intermittent and continuous renal replacement therapies (RRT) in clinical practice are, respectively, intermittent hemodialysis, continuous venovenous hemofiltration/ hemodiafiltration (CVVH), and peritoneal dialysis. They consist of diffusive and convective techniques that employ various degrees of sophisticated equipment.61 The clinician may encounter a variety of means for ensuring dialysis.
Intermittent hemodialysis is widely available and is an efficient way of removing solute and volume from the circulation. Unfortunately, dialysis-associated hypotension is frequent and may compromise remaining renal function. CVVH is able to remove larger volumes, as it continues around the clock. Peritoneal dialysis tends not to induce hypotension.
Access to the patient’s bloodstream became practical with the development of the Quinton and Scribner external arteriovenous shunt in 1960.62 The internal radiocephalic AV fistula in 1966 was developed by Brescia and Cimino.63 This created an anastomosis of the radial artery and cephalic vein at the wrist. An alternative is a brachiobasilic fistula between the brachial artery and cephalic vein above the elbow. Dacron and polytetrafluorethylene (PTFE) grafts for subcutaneous AV conduits now constitute most of the permanent access in the United States.58 Although the rates of thrombosis of autogenous fistulas is substantially lower than for synthetic grafts, fistula maturation to allow frequent needle cannulation takes 8-16 weeks, and primary failure rates of these autogenous fistulas approach 50%.64 Graft fistulae may be constructed from autogenous saphenous vein, bovine carotid artery, polyethylene terephthalates (Dacron), or PTFE/ Gore-Tex grafts.65 Alternative dialysis accesses that the emergency physician may encounter include percutaneous venous catheters typically placed in the internal jugular or subclavian veins (Hickman).66
Of all access systems, the external arteriovenous fistula (Cimino-Brescia) has the highest survival rate, with a two-year survival rate of greater than 75%.56 Graft occlusion is very common. Access salvage may be possible via embolectomy balloon, mechanical thrombolysis, or pulsed urokinase. Monitoring techniques prospectively prior to graft occlusion include measurements of dynamic and static venous pressure, and direct intra-access measures of blood flow via ultrasound dilution and duplex color flow Doppler.58
Currently the mean length of hemodialysis treatment typically is 3.5 hours three times per week. In the future, patients may undergo Daily Nocturnal Home Hemodialysis (DNHH): two hours of daily treatment at a blood flow rate of 200 mL/min. This requires the availability of a dialysis machine with a second person at home to assist with vascular access.
Clinical Points to Keep in Mind for the ESRD Patient. The following rules apply in general regarding care of the patient with a dialysis line:
- Do not draw blood from a central dialysis line or fistula if possible.
- In general, try to avoid venipuncture in the nondominant arm and in the upper part of the dominant arm of patients with ESRD.
- The involved arm should not be used for blood pressure determination.
- If the patient is in extremis, and the fistula or graft must be used:
- Cleanse the site scrupulously;
- After the puncture, apply firm but nonocclusive pressure for at least 10 minutes;
- Document a thrill both before and after the puncture.54
- In general avoid the use of meperidine (Demerol) for analgesia, as its metabolites accumulate in patients with renal failure and may induce seizures.
- Avoid magnesium-containing cathartics or phosphate-containing enemas (danger of hypermagnesemia, hyperphosphatemia).
- Troponin levels may be elevated in patients with elevated creatinine levels (false positives). Caregivers should know the limits of normal for their laboratory.
- Maturation of a shunt takes 6 weeks historically, though PTFE grafts mature in 3-4 weeks.58 This has implications for pseudoaneurysm formation following puncture.
- Autogenous AV fistulas mature in 8-16 weeks, but may have a longer maturation time (3-6 months, possibly up to 1 year), necessitating nephrology referral when serum creatinine reaches 3-4 mg/dL to allow for a long lead time pre-dialysis. Problems with early thrombosis and inadequate maturation often make synthetic grafts preferable for nephrologists.
- Ultrafiltration affords fast and efficient fluid removal. Negative hydrostatic pressure on the dialysate side can be manipulated to achieve desired fluid removal.
In terms of adequacy of dialysis, this may be assessed clinically or quantitatively.
A widespread numerical index of dialysis adequacy is the concept of Kt/V, where dialyzer clearance = K; duration of dialysis = t; volume of distribution of urea in the body = V. The Kt/V should be greater than 1.2 per session.46
Most patients require 9-12 hours of dialysis/week broken into several sessions (M-W-F, T-Th-S)
Treatment of Specific Medical Problems. Intradialysis hypotension is the most frequent complication of dialysis, affecting 10-30% of hemodialysis patients. The most common cause is excessive rate or amount of ultrafiltration with hypovolemia. Other causes for volume loss such as bleeding or GI fluid losses should be sought. Treatment generally starts with 250-500 cc normal saline challenge intravenously in 100-200 cc increments. Consider causes other than hypovolemia: sepsis, bleeding, hyperkalemia, pericardial tamponade. Hyper-acetatemia (vasodilatation, headache, cardiac toxicity) has been minimized now with use of bicarbonate dialysate, which allows the elimination of acetate as the alkali source.66
Other considerations in the patient with refractory hypotension include ruling out:
- Cardiac causes: LV dysfunction, hypoxia, coronary ischemia, dysrhythmias, pericardial tamponade;
- Electrolyte disorders: magnesium, potassium, calcium;
- Air embolism, which is rare with current technology;
- Hypoxemia;
- Drugs (narcotics, antihypertensives/beta-blockers, anxiolytics);
- Excess heat retention (prevented with reduced temperature dialysis bath (35°C);
- Hypersensitivity reaction to ethylene oxide (sterilizes the dialyzer) or polyacrylonitrile (membrane material), especially if the patient is taking ACE inhibitors (block breakdown of kinins);
- Autonomic neuropathy; and
- Acetate-based dialysate.56
Hyperkalemia. The kidneys normally excrete 90-95% of the total potassium intake.67 In the absence of potassium supplementation, hyperkalemia develops only when the glomerular filtration rate falls to less than 20-25 mL/min. Symptoms referable to cardiac toxicity may include weakness from brady- or tachyarrhythmias, with possible hypotension or cerebral hypoperfusion. Patients may present with paralysis or intestinal ileus. Suicidal thoughts may suggest missed dialysis. Progressive electrocardiogram (ECG) changes include increase (tenting) in amplitude of T waves in the precordial leads V2, V3, II, and III, with lengthening of the PR interval. Later findings that are more ominous include widening of the QRS complex, disappearance of P waves, and the presence of a sine wave pattern preterminally.
In general, serious hyperkalemia should be uncommon unless one of the following is involved: dietary indiscretion, inadequate dialysis, red cell lysis associated with a large hematoma or GI bleeding, or rhabdomyolysis (from seizure or severe exercise). Medications that may exacerbate hyperkalemia include digitalis, propranolol and other beta-blockers, succinylcholine, potassium salts including potassium penicillin, and ACE inhibitors.
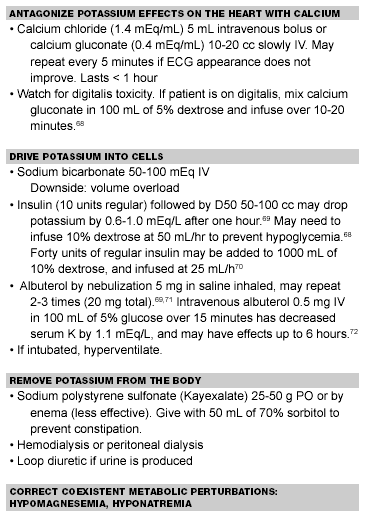
The management of hyperkalemia entails the use of intravenous calcium for cardiac protection. IV calcium works immediately to normalize the ECG, but its effects are of brief duration. Bicarbonate, insulin, and glucose work within approximately 20 minutes and redistribute potassium from the extracellular to the intracellular space for up to two hours.15 Sodium polystyrene sulfonate (Kayexalate) orally or via retention enema will work within 90-240 minutes to lower potassium by enhanced excretion. As with insulin-glucose combinations, beta-agonist therapy serves to redistribute potassium to the intracellular space and may be used even in patients without reactive airway disease. (See Table 9.) Patients ultimately may need dialysis, making these measures temporizing ones until the patient can be dialyzed.
Table 9. Management of Hyperkalemia
Miscellaneous Electrolyte Disturbances. Hypocalcemia, hyperphosphatemia, and secondary hyperparathyroidism are common in chronic renal failure. Secondary hyperparathyroidism may develop into tertiary hyperparathyroidism or an autonomously hyperfunctioning parathyroid gland. Persistently elevated levels of parathyroid hormone may result in osteomalacia and osteitis fibrosa cystica, high bone turnover lesions. Many ESRD patients for this reason have had parathyroid gland removal.
Hypermagnesemia may present early with hyporeflexia, vasodilatation, flaccidity, and profound muscle weakness. More severe elevations of serum magnesium (Mg > 10 mEq/dL) present with bradyarrhythmias, flaccidity, vasodilatation, hypotension, respiratory failure, or asystole. Treatment includes calcium chloride or calcium gluconate as temporizing measures until dialysis, with later dietary modification.
Hypercalcemia may present with nausea, vomiting, constipation, generalized weakness, hypertension, decline in level of consciousness, or seizures. Treatment options include hemodialysis (with a low calcium bath), synthetic salmon calcitonin, or hydration with normal saline if the patient is volume depleted.
A mixed anion and non-anion gap metabolic acidosis develops with chronic renal failure because the kidneys are unable to produce sufficient ammonia in the proximal tubules to excrete a daily acid load. Accumulation of phosphates, sulfates, and other organic anions causes an elevation of the anion gap. Sever acidosis may necessitate dialysis.73
Cardiac Arrest. This may be handled in standard fashion. However, strong consideration should be given to the possibility of life-threatening hyperkalemia. Consider empiric treatment with calcium chloride, bicarbonate, glucose/insulin infusions. The possibility of uremic pericardial effusions or cardiac tamponade should be considered.74
Alteration in Mental Status. While patients with altered mental status in general should not be discharged, with or without focal neurologic findings, in the ESRD patient the overriding principle must be to look for metabolic disorders and to perform computed tomography (CT) scanning early. Subdural hematomas and intracranial bleeds are frequent. Other diagnoses must be considered as well. These include other structural lesions detectable on head CT: cerebrovascular accident (CVA), epidural hematoma, malignancy with central nervous system (CNS) metastases or intracerebral abscess.
Metabolic causes for altered mental status should be sought as well. Sepsis/infection, dialysis dementia, hypoperfusion, hypovolemia, hypertensive encephalopathy, meningitis/encephalitis, drug toxicity or withdrawal, glucose or electrolyte abnormality including hypercalcemia, dialysis disequilibrium, or aluminum toxicity are all considerations.
Disequilibrium syndrome occurs during or within 12 hours of dialysis. Symptoms include headache, malaise, nausea, vomiting, muscle cramps, hypertension, restlessness/confusion, altered mental status, seizures, and death. It is thought to be related to elevated intracranial pressure and rapid lowering of serum osmolarity during dialysis.
Treatment may include anticonvulsants as needed, mannitol, possibly hypertonic saline, or hyperventilation. Other pathology should be ruled out, including malignancy, CVA, subdural hematoma, intracerebral abscess, meningitis, sepsis, or hypertensive encephalopathy.
Hypertension. Uncontrolled blood pressure may be treated with a variety of agents, including adrenergic-blocking agents, and angiotensin-converting enzyme inhibitors, especially if there is accelerated hypertension from scleroderma. Vasodilators such as hydralazine (Apresoline) or minoxidil (Loniten), or clonidine (Catapres) patches often are effective. Hemodialysis should be considered for malignant hypertension and volume overload. Fluid overload should be addressed aggressively, with accurate, up-to-date target weights.
Pulmonary Edema. Fluid overload and pulmonary edema occur frequently in chronic renal failure patients. Diagnosis is facilitated when the patient knows his/her dry weight, and is suggested by the following: excess weight over dry weight by more than 5 pounds, chest x-ray, physical examination, missed dialysis, or dietary indiscretion. B-type natriuretic peptide measurement is a sensitive and specific diagnostic marker for CHF,75 but is elevated at baseline in the ESRD patient. Treatment may entail a variety of modalities, not all of which are dependent upon urine output or diuresis. (See Table 10.)
Table 10. Treatment for Pulmonary Edema
in the ESRD Patient

Note that in one report, an intravenous line was established in only 29/46 patients presenting with acute CHF from fluid overload, and only 6 of these received any parenteral medications. It is possible to manage CHF in the dialysis patient without an intravenous line.81 Endotracheal intubation, positive end expiratory pressure (PEEP), mask non-invasive pressure support ventilation (BiPAP), or continuous positive airway pressure (CPAP) may be performed as needed.
Uremic Pericarditis, Pericardial Tamponade. Symptoms of uremic pericarditis may include dyspnea, cough, positional chest pain, and possibly malaise or fever. Physical examination may demonstrate: jugular venous distention (JVD), pulsus paradoxus greater than 10 mmHg, tachycardia, hypotension, tachypnea, narrow pulse pressure, or friction rub. The ECG may show electrical alternans, or low voltage. Diagnosis is made by echocardiography or by invasive demonstration of equal left and right atrial pressures.
Treatment entails aggressive volume support, intensive hemodialysis, and possibly pericardiocentesis. If the patient is unstable, a pericardial window or partial pericardiectomy may be required. Other causes of pericarditis such as acquired immuno-deficiency syndrome or systemic lupus erythematosus may need to be considered.
Infection. If infection is suspected, blood should be drawn for cultures as above. Urine culture should be obtained, if the patient produces urine. It may be desirable to get one culture from percutaneous dialysis catheter with involvement of a nephrologist. Antibiotics such as vancomycin and an aminoglycoside (2-5 mg/kg) pending culture results should be administered. Alternative antibiotics for sepsis include pipericillin/tazobactam (Zosyn), ticarcillin/clavulanate (Timentin), imipenem (Primaxin), meropenem (Merrem), or a third generation cephalosporin with anti-pseudomonas activity, such as ceftazidime.
Sites of infection to consider include: blood access sites both new and old (Staphylococcus aureus and epidermidis), endocarditis, septic pulmonary emboli, osteomyelitis, septic arthritis, and urinary tract infections (especially in patients with polycystic kidney disease). Pneumonia may present as dyspnea or malaise. Consider odontogenic, extremity cellulitis, and perirectal abscess as possible sites of infection.
Anemia. Anemia treatment in the ESRD patient includes erythropoietin 50 U/kg SQ three times weekly, and iron to obtain a transferrin saturation greater than 20%. Folate should be given as indicated. The target hematocrit is 36-40% in general.82 Transfusion may be required in an emergency, but often is best avoided because of possible sensitization to HLA antigens, making transplantation less successful.
Vascular Access Problems. There are several possibilities, discussed in turn.
Infection. Vancomycin should be administered with or without gram-negative coverage (gentamicin). Findings may be subtle and may include malaise, intermittent fever, relative hypotension, or elevated WBC as clues. This may be a source for sepsis. Pulmonary emboli, empyema, bacterial meningitis, septic arthritis (wrist, knee, shoulder), and endocarditis should be considered, especially of the aortic valve.
Thrombosis (intimal hyperplasia). The physician should document presence or absence of a thrill or bruit over the access site, and whether the patient needs emergent or urgent (within 24 hours) dialysis.
Venous stenosis or thrombosis. If a double lumen catheter is used in subclavian vein aneurysms, pseudoaneurysms may cause pain, impingement on surrounding nerves, or marked thinning or erosion of overlying skin.
Ischemia of the arm from a “steal” syndrome may result in pain with a pale cool distal extremity.
Prolonged Bleeding Times. These may result from uremia-induced platelet dysfunction or from heparin anticoagulation. A normocytic normochromic anemia frequently complicates renal failure and is multifactorial. Decreased erythropoietin production, blood loss, and hemolysis are major considerations.15 Treatment chronically entails erythropoietin injections to keep the hematocrit greater than 25%. Transfusion generally is not necessary for asymptomatic patients whose hematocrit is greater than 18%.
Prolonged bleeding times may be treated with desmopressin (Desamino-8-D-arginine vasopressin or DDAVP) 0.3 mcg/kg IV or SQ,82 or 3 mcg/kg intranasally. Onset of effect is 1-2 hours. It lasts 4-8 hours and works by stimulating the release of stored factor VIII and von Willebrand factor. It may be repeated in 8-12 hours. Alternatively, cryoprecipitate 10 units over 10-15 minutes may be administered every 12-24 hours. This corrects bleeding time for approximately 4 hours. Conjugated estrogens such as Premarin 25 mg/day (or 0.6 mg/kg acutely) for 7 days have effects lasting 21 days. When ESRD patients have evidence for coagulopathy, bleeding sites including gastrointestinal, retroperitoneal, and pleural, should be considered.
Vascular access hemorrhage should be managed in general with direct pressure at the puncture site for 5-10 minutes. In the unusual event that pressure alone does not suffice and over-anti-coagulation is suspected, protamine 10-20 mg (0.01 mg/ IU of heparin, assuming 1000-2000 units of heparin used on the average) may be given. Cryoprecipitate 10 units every 12-24 hours may be effective, as it is rich in von Willebrand factor. It is mandatory to check for a bruit after pressure or protamine administration, and it is advisable to observe for 1-2 hours for evidence of rebleeding.
Air Embolism. This is an acute cardiorespiratory or neurologic complication of dialysis. Patients may present with dyspnea, tachypnea, chest pain, hypotension, confusion, or cardiac arrest. A “millwheel” cardiac murmur may be audible. Treatment includes clamping the access/venous bloodline and placing the patient supine or left lateral decubitus in Trendelenburg. Hyperbaric oxygen (HBO) therapy may be considered. If the patient is in cardiac arrest, the physician may aspirate RV outflow tract, followed possibly by HBO.
Miscellaneous Problems. AV fistulas causing high-output CHF may require surgical binding of the fistula to reduce blood flow.
GI bleeding may result from peptic ulcer disease, angiodysplasias of the stomach and duodenum, or esophagitis. Recommended disposition depends upon hemodynamic status and stability.
Dysrhythmias should be addressed by normalizing electrolytes K, Mg, and Ca. Hypoxemia and possible pericardial disease should be investigated and treated.
Hypersensitivity reactions may result from ethylene oxide, formaldehyde, or a dialyzer membrane. Therapy includes volume infusion as needed, antihistamines, epinephrine, or steroids.
Admission Criteria for the ESRD Patient
The ESRD patient should be hospitalized for any indication for emergency hemodialysis, as in ARF.
If pericardial tamponade is suspected, air embolism is suspected, or the patient developed chest pain during dialysis, the patient should be observed in the hospital pending further testing. Neurologic presentations precluding discharge include altered mental status, uremic encephalopathy as manifested by asterixis, myoclonic twitching, lethargy, or new onset seizure.
Hyperkalemia may be managed as above. However, if the serum potassium is over 7 or there are electrocardiographic changes from baseline, the patient should be admitted to a monitored bed. Patients with lower potassium may be discharged if treated with sodium polystyrene sulfonate (Kayexalate), dialysis is planned within 8 hours, and the patient is reliable. Any patient requiring emergency treatment with a modality that redistributes potassium, including calcium, albuterol, insulin-glucose, or bicarbonate, should be admitted.
It is advisable to confine patients with hemodynamically significant GI bleeding, including bright red blood from stool and melena.
Febrile ESRD patients with the following presentations should be considered for hospitalization: ill-appearing, dyspnea, hypotension, unreliable home environment, or specific infection (pneumonia, urosepsis, obvious shunt site infection). If a shunt infection is merely suspected, it may be acceptable to discharge after culture of the blood and access site, with IV antibiotics and follow-up with the patient’s nephrologist within 24 hours.65
A list of other admission criteria in the ESRD patient includes:
- Suspected disequilibrium syndrome, as this can proceed to seizures, coma, and death;
- Persistent vomiting from any cause (inability to take oral fluids);
- Refractory severe hypertension (diastolic > 130 despite treatment), scleroderma renal crisis;
- Malignant hypertension, especially with hypertensive encephalopathy or cardiovascular decompensation;
- Dysrhythmia related to electrolyte abnormality, or which would otherwise warrant hospitalization;
- Symptomatic pulmonary edema after treatment. If dialysis or hemofiltration is available in the ED, hospitalization may not be indicated;
- Symptomatic hypermagnesemia with or without cardiotoxicity;
- Hypercalcemia with cardiovascular dysfunction or acute neurologic symptoms; and
- Inability to control bleeding from dialysis site.
Peritoneal Dialysis
Overview. While peritoneal dialysis (PD) first was accomplished in 1923, the Tenckhoff silicon rubber peritoneal catheter developed in 1968 simplified the procedure significantly. In the United States, 16-17% of those who need dialysis undergo PD. PD utilizes a slow rate of solute and volume removal and is therefore useful in hemodynamically unstable patients. Continuous ambulatory peritoneal dialysis (CAPD) was developed in 1976. It generally is repeated four times per day, with dwell times of 4-6 hours. Intermittent peritoneal dialysis (IPD) is characterized by rapid cycling of dialysis fluid at intervals of 60 minutes or less. Continuous cycling peritoneal dialysis (CCPD) consists of rapid fluid exchanges during the night using an automated cycler. There is a greater incidence of peritonitis with CAPD relative to IPD or CCPD.65 PD obviates the need for anticoagulation, although the peritoneal access is a potential source of complications such as catheter leaks and peritonitis.61 Dialysate can be performed with varying glucose formulations to control fluid volume via ultrafiltration.
The focused physical examination in the PD patient should especially examine the abdomen for hernia, bowel sounds, and rebound. The peritoneal catheter and surrounding skin may show evidence for hernia or tunnel infection. Problems specific to the PD patient are discussed in turn.
Peritonitis is always a major concern. It may present with abdominal pain, cloudy dialysate (over 100 white blood cells in dialysate, with greater than 50% polymorphonuclear leukocytes), fever, nausea, and vomiting. The incidence is approximately once per 15 patient-months of dialysis.83 Commonly isolated organisms include bacteria such as S. epidermidis, S. aureus, Streptococcus, and Gram-negative bacteria (Acinetobacter, Pseudomonas, Enterobacteriaceae), although fungal (candida) and mycobacterial infections occur as well.
Treatment may include several rapid exchanges of fluid, followed by antibiotics in the dialysate: cephalothin 200-500 mg/L of dialysate, or vancomycin (Vancocin) (1 g IV loading, then 30-50 mg/kg intraperitoneal dose per 2 L exchange if methicillin-resistant Staphylococcus aureus (MRSA). Some try to avoid these agents due to vancomycin-resistant enterococcus (VRE) emergence. Gentamicin (Garamycin) IM/IV, then intraperitoneal (8 mg/liter first exchange, followed by 4 mg/liter subsequently) has been employed successfully.84 Treatment lasts for 7-14 days. Heparin 500-2500 U/liter prevents fibrin formation.
Ceftazidime (Ceptaz, Fortaz, Tazidime, Tazicef) 1 g intraperitoneally (IP) or IV is an acceptable alternative, with Aztreonam (Azactam) 3 g IP or IV if gram-negative organisms are suspected.85 Patients may be treated on an out-patient basis, depending upon their appearance.
Catheter infections (exit site and tunnel infections) present with localized pain, erythema, swelling, or discharge. Frequent causative bacteria include S. aureus and P. aeruginosa on culture around the catheter. The diagnosis may require ultrasound. Treatment may require incision and drainage, and oral antibiotics (first generation cephalosporin or dicloxacillin).
Catheter leaks present with abdominal wall edema and clear fluid from the catheter ostium. Treatment entails stopping peritoneal dialysis, necessitating short-term hemodialysis. Dialysate fluid culture should be cultured, and a nephrologist should be consulted for consideration of empiric therapy for peritonitis. Besides antibiotic therapy, surgical correction may be required.
Abdominal or inguinal hernias are risks due to increased intraperitoneal pressure. They should be referred for surgical repair, as incarceration poses a potential risk.
Hydrothorax is a rare complication of peritoneal dialysis. Effusions generally are right-sided. Diagnosis may be made by methylene blue injection into the peritoneal fluid. Therapy includes temporary discontinuance of PD.
A comparison of PD to continuous renal replacement therapies indicates that patients who were critically ill with ARF had a higher mortality if PD was utilized.86 Since continuous renal replacement therapies also appear superior to PD in solute and volume removal and in correction of acid base disturbances, it has been proposed that PD is best utilized in hemodynamically unstable patients in whom vascular access has been exhausted and in those with limited disease severity.61
Admission Criteria for the PD Patient. Patients with leaks as a mechanical complication of their PD should be confined. PD patients with peritonitis should be admitted if the following are present: severe pain, vomiting, hypotension, ileus, hemodynamic instability, high fever, inability to administer antibiotics at home, or unreliable follow-up within 24-48 hours.
Psychosocial considerations may preclude optimal care as an out-patient. Patients should be admitted if there is suspected visceral perforation, such as fecal material in peritoneal drainage.
Other considerations for admission in the PD patient include: incarcerated incisional or inguinal hernia, pain or vomiting from suspected bowel obstruction, or severe hyperglycemia in diabetics from hyperosmolar dialysate.
Conclusions
Renal failure continues to cause a rate of morbidity and mortality in the United States. With the aging population, and survival from medical problems such as sepsis, diabetes, and hypertension, it is expected that the prevalence of renal failure will increase in the United States with time. The emergency physician should recognize various techniques for renal replacement therapy and be prepared to manage a variety of metabolic and structural disorders that occur in this population.
References
1. Schrier RW, Wang W, Poole P, et al. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest 2004;114:5-14.
2. Alkhunazi AM, Schrier RW. Management of acute renal failure: New perspectives. Amer J of Kidney Diseases 1996;28:315-328.
3. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality: A cohort analysis. JAMA 1996;275:1489.
4. Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br Med J 1941;1:427-432.
5. Racusen LC, Nast CC. Renal histopathology, urine cytology, and cytopathology in acute renal failure. J Nephrol 1999 In Atlas of Diseases of the Kidney, RX Schreier editor, Blackwell Science, Philadelphia:1-12.
6. Teschan PE. Post-traumatic renal insufficiency in military casualties. I. Clinical characteristics. Am J Med 1955;18:172-186.
7. Smith LH Jr. Post-traumatic renal insufficiency in military casualties II. Management, use of an artificial kidney, prognosis. Am J Med 1955;18: 187-198.
8. Whelton A, Donadio JV Jr. Post-traumatic acute renal failure in Vietnam. A comparison with the Korean War experience. Johns Hopkins Med J 1969;124:95-105.
9. United States Renal Data System: Chapter II: Incidence and Prevalence of ESRD. Am J Kidney Dis 1998;32 (suppl 1):S38.
10. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1 (suppl 1).
11. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 2004;351:159-169.
12. Chertow GM, Burdick E, Homour M, et al. Toward an evidence-based definition of hospital-acquired acute renal failure. J Am Soc Nephrol 2003;8:1042A.
13. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: Physiological principles. Intensive Care Medicine 2004;30:33-37.
14. Hou SH, et al. Hospital-acquired renal insufficiency: A prospective study. Am J Med 1983;74:243.
15. Costa J, Crausman RS, Weinberg MS. Acute and chronic renal failure. J Am Podiatr Med Assoc 2004;94:168-176.
16. Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med 1996;334:1448-1453.
17. Corwin HL, Teplick RS, Schreiber MJ, et al. Prediction of outcome in acute renal failure. Am J Nephrol 1987;7:8-12.
18. Bosch JP, Lauer A, Glabman S. Short-term protein loading in assessment of patients with renal disease. Am J Med 1984;77:873-879.
19. Rodriguez-Puyol D. The aging kidney. Kidney Int 1998;54:2247.
20. Brandstrom E, Grzegorczyk A, Jacobsson L, et al. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant 1998;13:1176-1181.
21. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem 1992;38:1933-1953.
22. Han WK, Bailly V, Aichandani R, et al. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62:237-244.
23. Jovanovic D, Krstivojevic P, Obradovic I, et al. Serum cystatin C and beta2-microglubulin as markers of glomerular filtration rate. Ren Fail 2003;25: 123-133.
24. Rosenthal SH, Marggraf G, Husing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney International 2004;66:1115-1122.
25. Ronco C, Kellum JA, Mehta R. Acute dialysis quality initiative (ADQI). Nephrol Dial Transplant 2001;16:1555-1558.
26. Wolfson AB, Israel RS. Renal function evaluation and the approach to the patient with acute renal failure. In: Emergency Medicine Concepts and Clinical Practice, Volume III, 4th Ed. Mosby, St. Louis, 1998: 2262-2276.
27. Schmekal B, Pichler R, Biesenbach G. Causes and prognosis of nontraumatic acute renal failure requiring dialysis in adult patients with and without diabetes. Renal Failure 2004;26:39-43.
28. Kaufman J, Dhakal M, Patel B, et al. Community-acquired acute renal failure. Am J Kidney Dis 1991;17:191.
29. Sinert R. Acute renal failure and emergencies in renal failure and dialysis patients, in American College of Emergency Physicians Study Guide, 5th Ed. 611-625.
30. Noble DW. Acute renal failure and diuretics: Propensity, equipoise, and the need for a clinical trial. Crit Care Med 2004;32:1794-1795.
31. Uchino S, Doig GS, Bellomo R, et al. Diuretics and mortality in acute renal failure. Crit Care Med 2004;32:1669-1677.
32. Conger JD. Interventions in acute renal failure: What are the data? Am J Kidney Dis 1995;26:565, 565-576.
33. Hatala R, Dinh T, Cook DJ. Once-daily aminoglycoside dosing in immunocompetent adults: A meta-analysis. Ann Intern Med 1996;124:717-725.
34. Epstein M. Calcium antagonists and renal protection. Current status and future perspectives. Arch Intern Med 1992;152:1573-1584.
35. Holmes CL. Is low-dose vasopressin the new reno-protective agent? Crit Care Med 2004;32:1972-1974.
36. Asif A, Epstein M. Prevention of radio-contrast-induced nephropathy. Am J Kidney Dis 2004;44:12-24.
37. Tepel M, Van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000;343:180-183.
38. Raven QL, Walton T, Howe AM, et al. Role of acetylcysteine in the prevention of contrast-media-induced nephrotoxicity. Am J Health-Syst Pharm 2003;60:2232-2235.
39. Pannu N, Manns B, Lee H, et al. Systematic review of the impact of N-acetylcysteine on contrast nephropathy. Kidney Int 2004;65:1366-1374.
40. Esson ML, Schreier RW. Diagnosis and management of acute tubular necrosis. Ann Intern Med 2002;137:744-752.
41. Dagher PC, Herget-Rosenthal S, Ruehm SG, et al. Newly developed techniques to study and diagnose acute renal failure. J Am Soc Nephrol 2003;14:2188-2198.
42. Somers MJ, Daouk GH, McCluskey RT. Case 11-2004: A boy with rash, edema and hypertension. N Engl J Med 2004;350:1150-1159.
43. Mehta RL, Chertow GM. Acute renal failure definitions and classification: time for change? J Am Soc Nephrol 2003;14:2178-2187.
44. Chertow GM, Sayegh MH, Allgren RI, et al. Is the administration of dopamine associated with adverse or favorable outcomes in acute renal failure? Auriculin Anaritide Acute Renal Failure Study Group. Am J Med 1996;101:49-53.
45. Shilliday IR, Quinn KJ, Allison ME. Loop diuretics in the management of acute renal failure: A prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant 1997;12:2592, 2592-2596.
46. Luyckx VA, Bonventre JV. Dose of dialysis in acute renal failure. Seminars in Dialysis 2004;17:30.
47. Sacchetti A, Stuccio N, Panebianco P, et al. ED hemodialysis for treatment of renal failure emergencies. Am J Emerg Med 1999:17:305-307.
48. Warden CR, Benjamin DR. Acute renal failure associated with suspected Amanita smithiana mushroom ingestions: A case series. Acad Emerg Med 1998;5:808-812.
49. Gillum DM, Dixon BS, Yanover MJ, et al. The role of intensive dialysis in acute renal failure. Clin Nephrol 1986;25:249-255.
50. Hakim RM, Lazarus JM. Initiation of dialysis. J Am Soc Nephrol 1995;6:1319, 1319-1328.
51. Wish JB, Webb RL, Levin AS, et al. Medical appropriateness of initiating chronic dialysis in the U.S.: Results of the revised medical evidence report pilot study. J Am Soc Nephrol 1994;5:345.
52. Morgera S, Kraft AK, Siebert G, et al. Long-term outcomes in acute renal failure patients treated with continuous renal replacement therapies. Am J Kidney Dis 2002;40:275-279.
53. Bhandari S, Turney JH. Survivors of acute renal failure who do not recover renal function. Qjm 1996;89:415-421.
54. Wolfson AB. Chronic Renal Failure and Dialysis. In: Rosen, Barkin, eds. Emergency Medicine: Concepts and Clinical Practice, 4th ed. Mosby; St. Louis:1998: 2278-2292.
55. Renal Data System. USRDS 1997 annual data report. Bethesda, Md.: National Institute of Diseases and Digestive and Kidney Diseases, 1997.
56. Pastan S, Bailey J. Dialysis therapy. N Engl J Med 1998:338:1428-1438.
57. Sacchetti A, Harris R, Patel K, et al. Emergency department transportation of renal dialysis patients: Indications for EMS transport directly to dialysis centers. J Emerg Med 1991;(9):141-144.
58. Schwab SJ. Vascular access for hemodialysis. Kidney International 1999;55: 2078-2090.
59. Levey AS, Eknoyan G. Cardiovascular disease in chronic renal disease. Nephrol Dial Transplant 1999;14:828.
60. Cloonan CC, Gatrell CB, Cushner HM. Emergencies in continuous dialysis patients: Diagnosis and management. Am J Emerg Med 1990;8:134-148.
61. Teehan GS, Liangos O, Jaber BL. Update on dialytic management of acute renal failure. J Intensive Care Med 2003;18:130-138.
62. Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs 1960;6:104-113.
63. Brescia MJ, Cimino JE, Appel K, et al. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med 1996;275:1089-1092.
64. Allen M, Robbin ML. Increasing arteriovenous fistula in hemodilysis patients: Problems and solutions. Kidney Int 2002;62:1109-1124.
65. Hodde LA, Sandroni S. Emergency department evaluation and management of dialysis patient complications. J Emerg Med 1992;10:317-334.
66. Uribarri J. Past, present, and future of end-stage renal disease therapy in the United States. Mt Sinai J Med 1999;66:14-19.
67. Allon M. Treatment and prevention of hyperkalemia in end-stage renal disease. Kidney Int 1993;43:1197-1209.
68. Perazella MA. Approach to hyperkalemic end-stage renal disease patients in the emergency department. Connecticut Medicine 1999;63:131-136.
69. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int 1990;38:869-872.
70. Kliger AS. Complications of dialysis: Hemodialysis, peritoneal dialysis, CAPD. In: Arief AI, DeFronzo RA, eds. Fluid Electrolyte and Acid-Base Disorders, Vol 2, New York, NY, Churchill Livingstone 1985; 803.
71. Kemper MJ, Harps E, Muller-Wiefel DE. Hyperkalemia: Therapeutic options in acute and chronic renal failure. Clin Nephrol 1996;46:67-69.
72. Montoliu J, Lens XM, Revert L. Potassium-lowering effect of albuterol for hyperkalemia in renal failure. Arch Intern Med 1987;147:713-717.
73. Alpern RJ, Sakhaee K. Clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 1997;29:291-295.
74. United States Renal Data System: Chapter VI: Causes of death. Am J Kidney Dis 1998;32 (suppl 1):S81.
75. Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B- type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol 2001;37:379-385.
76. Schmieder RE, Messerli FH, deCarvalho JGR, et al. Immediate hemodynamic response to furosemide in patients undergoing chronic hemodialysis. Am J Kidney Dis 1987;9:55-59.
77. Eiser AR, Lieber JJ, Neff MS. Phlebotomy for pulmonary edema in dialysis patients. Clin Nephrol 1997;47:47-49 abstract.
78. Colucci WS, Elkayam U, Horton DP, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. N Engl J Med 2000;343:246-253.
79. McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure. Circulation 2002;106:416-422.
80. Anderson CC, Shahvari MB, Zimmerman JE. The treatment of pulmonary edema in the absence of renal function: A role for sorbitol and furosemide. JAMA 1979:241:1008-1010.
81. Sacchetti A, McCabe J, Torres M, et al. ED Management of acute congestive heart failure in renal dialysis patients. Am J Emerg Med 1993;11:644-647.
82. Ifudu O. Care of patients undergoing hemodialysis. N Engl J Med 1998;339: 1054-1062.
83. Bloembergen WE, Port FK. Epidemiological perspective on infections in chronic dialysis patients. Adv Ren Replace Ther 1996;3:201-207.
84. Keane WF, Alexander SR, Bailie GR, et al. Peritoneal dialysis-related peritonitis treatment recommendations: 1996 update. Perit Dial Int 1996;16: 557-573.
85. Mion CM. Practical use of peritoneal dialysis. In: Drukker W, et al, eds. Replacement of Renal Function by Dialysis (ed 2). Boston, Ma, Martinus Nijhoff, 1986:472.
86. Phu HN, Hien TT, Mai NTH, et al. Hemofiltration and peritoneal dialysis in infection associated acute renal failure in Vietnam. N Engl J Med 2002;347: 895-902.
