The Fifth Vital Sign: Pulse Oximetry in Noninvasive Respiratory Monitoring
Author: Sharon E. Mace, MD, FACEP, FAAP, Associate Professor, Ohio State University School of Medicine; Faculty, MetroHealth Medical Center/Emergency Medicine Residency; Clinical Director, Observation Unit; Director, Pediatric Education/Quality Improvement, Cleveland Clinic Foundation, Cleveland
Peer Reviewer: David Kramer, MD, FACEP, FAAEM, Program Director, Emergency Medicine Residency, and Vice-Chair, Department of Emergency Medicine, York Hospital, York, Pennsylvania
The fifth vital sign, pulse oximetry, routinely is used in every emergency department (ED) throughout the country. It is used to determine the baseline oxygenation of a patient in respiratory distress, to assess a patient’s response to therapeutic decisions, and to monitor a child during a conscious sedation or resuscitation. It is important to understand how the device functions and the limitations of this routinely used technology.
Understanding that pulse oximetry measures functional saturation will help the clinician understand the limitations of this technology in the setting of a carbon monoxide exposure. It is also very important clinically to understand the limits of pulse oximetry in the setting of high venous pressures (congestive heart failure) or anemia.
Certain clinical factors, such as sickle cell anemia, do not affect pulse oximetry, and the results provide meaningful information. As with every diagnostic test that a clinician performs, the information obtained is useful only if it can be interpreted accurately and applied appropriately to the individual patient.
The author reviews a technology that every clinician uses on a daily basis with special attention to appropriate uses, interpretation of results and limitations that may affect accurate interpretation of the data provided. — The Editor
Introduction
Pulse oximetry is a technology that allows continuous noninvasive monitoring of the arterial oxygen saturation (SaO2) level,1 which is calculated from estimates of the oxygenated hemoglobin (HgbO2) and the reduced hemoglobin (Hgb) levels.2
The oxygenated Hgb and reduced Hgb measurements are derived from photodetector measurements of two wavelengths of light. The two wavelengths of light (red and infrared) are transmitted from two light-emitting diodes (LEDs) through a pulsatile vascular bed (e.g., the finger) and strike a photodetector. (See Figure 1.)
Figure 1.
Pulse Oximetry with Light-Emitting Diodes and Photo Detector

The pulsatile components of red (660 nanometers [nm]) and infrared (990 nm) light absorbencies as they pass through tissue may be used to determine the ratio of HgbO2 to reduced Hgb.3 (See Figure 2.) From this ratio of HgbO2 to reduced Hgb, the SaO2 level can be calculated using calibration curves.4
Figure 2.
Absorbance Spectra Curves
for Various Hemoglobins

Clinical Uses of Pulse Oximetry
Pulse oximetry has been suggested as the fifth vital sign.5,6 It is a form of point-of-care testing that allows for continuous noninvasive bedside monitoring of a patient’s oxygenation status.7
Indications for pulse oximetry are categorized into three groups: 1) a baseline indicator or monitor of a patient’s oxygenation status, 2) evaluation of response to therapy, and 3) monitoring during procedures. (See Table 1.) Pulse oximetry is indicated in patients with cardiopulmonary disease; unstable or critically ill patients; patients in cardiopulmonary arrest; and patients with or the potential for apnea, hypoxia, respiratory distress/failure, or shock.1,7-13 Pulse oximetry has been used in the diagnosis of numerous cardiopulmonary diseases and to evaluate the response to treatment in various cardiopulmonary disorders ranging from asthma, chronic obstructive pulmonary disease, bronchiolitis, and reactive airway disease, to pneumonia, airway obstruction, heart failure, and cyanotic congenital heart disease.7-16
Table 1. Clinical Uses of Pulse Oximetry

Examples of the use of pulse oximetry to monitor a patient’s clinical status include apnea monitoring, evaluation of periodic breathing (in infants), during transport (in the hospital or prehospital setting), and in critical care areas (e.g., the intensive care units and the ED).1,7,8,17-20 Pulse oximetry is used during airway procedures (e.g., intubations) and during lumbar punctures and other invasive procedures, (e.g., central lines), especially in infants.8,21 Pulse oximetry is used for monitoring patients on mechanical ventilation.22-24 In patients with an endotracheal (ET) tube, pulse oximetry may be a clue to a misplaced or blocked ET tube.
Pulse oximetry has become a standard of care during anesthesia since its recommendation by the American Society of Anesthesiologists in 1986.3,25 The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) cited a need for pulse oximetry in locations other than in the operating room in 2003.26 Multiple medical specialty societies, including the American College of Emergency Physicians, the American Academy of Pediatrics, and the American Society of Anesthesiology, have guidelines that suggest pulse oximetry be part of the monitoring of patients undergoing sedation and analgesia.27-31
Advantages of Pulse Oximetry
Pulse oximetry’s advantages over arterial blood gases measurements are obvious: noninvasive, less pain and risk than arterial puncture, less expensive, more real-time or point-of-care testing method (immediately available vs time for obtaining/transporting/ running/reporting a blood gas), and a continuous monitor (rather than an isolated point[s] in time).1,7,8,12,14,18,32 (See Table 2.) The advantages of pulse oximetry include use as a continuous noninvasive marker or warning signal for adverse patient events that can result in hypoxia/arterial desaturation.2-4,16 By detecting those events early, treatment can be initiated sooner with the goal of improving patient outcomes.7,8 Because clinical detection of cyanosis is unreliable, use of pulse oximetry should allow earlier detection of hypoxemia.20,33 Reports from the recovery room and the operating room do indicate a faster detection of hypoxic episodes, a lower incidence and shorter duration of arterial desaturation, and fewer adverse events in the recovery room in cases where pulse oximetry is used.34-36 However, several studies of pulse oximetry in anesthesia/post anesthesia care and in general care units failed to show a difference in patient outcome with its use.36-39 The consensus and expert opinion are that pulse oximetry can and should be used,26-30 and there are many clinical indications for its use.1,2,4,6,11-14
Table 2. Advantages of Pulse Oximetry

Pulse oximetry is easy to use and requires no special training. It can be applied easily and quickly and is inexpensive. It is small, lightweight, and portable; therefore, it can be used in almost any area and requires little space. It can be stand-alone equipment or incorporated into a bedside monitoring unit. It can be used in any age or type of patient from newborns to geriatric patients, in the ED, intensive care units, inpatient floors, operating suite, recovery room, and in prehospital care.
It has become the fifth vital sign and is a standard of care during procedures requiring general anesthesia and during procedural sedation and analgesia.5,26-31
Mechanism of Pulse Oximetry
Pulse oximetry is based upon spectrophotometric principles and on the Beer-Lambert law. All substances have a unique absorbency spectrum. Oxyhemoglobin (HgbO2) and deoxyhemoglobin absorb light differently. (See Figure 2.) HgbO2 absorbs more infrared light (wavelength = 940 nm) and less red light (wavelength = 660 nm) than reduced Hgb.40 The light intensity transmitted (It) or passing through a solution is dependent upon the intensity of the incident light (Io) minus the light absorbed. The amount of light absorbed depends upon the concentration of solute (C), the distance or path length through the solution (D), and the molar extinction coefficient of the solute (k) (a constant for Hgb at a given wavelength), or absorbance = k · C · D = log Io/It. (See Figure 3.) Blood is the solvent and hemoglobin, the solute.
Figure 3.
Schematic Diagram of Absorption Signal
Passing through a Solution (e.g. blood)

Because the amount of tissue is constant, the amount of light transmitted (and the amount of light absorbed) varies with the arterial blood flow. Arterial blood creates a pulsatile flow—and a pulsatile signal—with each heartbeat. The venous blood and nonpulsatile arterial blood flow are fairly constant throughout the cardiac cycle, and the tissue is constant; therefore, the only variation during the cardiac cycle (e.g., one heartbeat) is the arterial pulsatile flow. 22,41,42 This pulsatile arterial blood flow increases the distance or path length through which the red and infrared wavelengths travel. (See Figure 4.)
Figure 4.
Absorption Signal as it Passes through Pulsatile Tissue
(e.g., arterial blood)

According to the Beer-Lambert Law, this results in increased absorbance of light (of both the red and infrared wavelengths) with less light transmitted and striking the photodetector. The pulse oximeter calculates the ratio of the pulsatile and nonpulsatile absorbencies at the 660 nm (red) and 940 nm (infrared) wavelengths. (See Figure 2.) From the absorbencies, the pulse oximeter’s microprocessor calculates the ratio of the HgbO2 and the reduced Hgb levels, and then, plots this ratio against a standardized calibration curve to derive the SaO2 measurement.
Figure 5. Reflectance Oximetry

Pulse Oximetry Equipment
All pulse oximeters contain a probe that consists of two light- emitting diodes (LEDs), a photodetector, and an onboard microprocessor/computer.41 (See Figure 1.) The SaO2 measurement is derived from the ratio using the calibration curves in the computer software.22 The standardized calibration curve is derived from healthy adult volunteers. Across from the LEDs is a photodetector that is placed across a pulsatile vascular bed, (e.g. a finger, toe, earlobe, nose, or forehead).2 (See Figure 1.) If the forehead is used, then reflectance (not transmittance) is used. (See Figure 5.) With reflectance oximetry, the light from the LEDs is reflected from tissue, and the photodetector is located on the same side as the LED.2 Reflectance oximetry is technically more difficult with less accuracy; therefore, it is not used widely.
Pulse oximetry probes usually are configured into a disposable patch or, more commonly, a reusable clip.7 In adults, the finger is the most common probe site, whereas in infants and small children, the great toe is used frequently. Adhesive sensors, which often are used in infants and small children, can be placed over the heel or lateral aspect of the foot and secured with a gauze or Coban wrap.9 To avoid interference with high intensity ambient light, a light barrier (e.g. a dark cloth) can be placed around the probe. However, the author of a recent article found that ambient light had no statistically significant effect on pulse oximetry readings, and any difference due to ambient light was small and clinically unimportant.43 If motion artifact is a problem, then using a different site may help. Also, correlating an electrocardiogram (ECG) monitor waveform with a pulse oximeter signal may help determine whether a reading is a valid pulse oximetry record or a motion artifact.
Pulse oximetry is noninvasive and considered safe, although rare reports of pressure necrosis and burns due to defective probes have been reported.44-47 Pulse oximetry probes can be a source of nosocomial infection if they become contaminated with pathogenic bacteria. However, there are many methods to avoid this possibility: Disposable probes can be resterilized, and protective sheaths can be placed on the probe to allow for multiple uses.
Pulse oximetry has a low failure rate. In one operating room study, the failure rate was less than 5% with a trend toward failure occurring in elderly and sicker patients and during longer surgical procedures.48 Accidental disconnection or probe misplacement are probably the most common causes of pulse oximetry failure.
Pathophysiology
Oxygen Saturation. Oxyhemoglobin (HgbO2) and deoxyhemoglobin absorb light differently, forming the basis for pulse oximetry oxygen saturation measurements. (See Figure 2.) Erythrocytes containing hemoglobin (Hgb) pick up oxygen in the pulmonary capillary beds and become oxyhemoglobin (HgbO2). They travel back to the left side of the heart, then, are transported throughout the body to the systemic capillary beds. Oxygen is released down a gradient to the tissues when the tissue partial pressure of oxygen is lower than that of the arterial blood. After releasing oxygen to the tissues, some of the Hgb—which is now deoxygenated Hgb—may pick up some of the carbon dioxide (CO2) formed as a byproduct of cellular aerobic metabolism to form carbaminohemoglobin. Approximately 5%-10% of CO2 is transported as carbaminohemoglobin; the majority of CO2 (90%-95%) is transported as bicarbonate. CO2 is transported to the lungs where it is released into the alveoli and eliminated in the process of ventilation.49
The SaO2 measurement is the percentage of Hgb bound to oxygen. In normal systemic arterial blood, the SaO2 level is about 97% (> 95%). Thus, the amount or concentration of Hgb bound to oxygen (HgbO2) is 97% of capacity. The hemoglobin saturation (SaO2) (in percent) equals the amount of oxygenated Hgb divided by the total amount of Hgb available for oxygenation (the oxygenated Hgb plus the reduced Hgb) times 100.8
| SaO2 (%) = Oxygenated Hgb x 100 = Total Hgb available
[Oxygenated Hgb] x 100 |
Generally, hypoxemia is defined as an SaO2 level less than 95%, with severe hypoxia being an SaO2 level less than 90% and a pO2 level less than 70 mmHg. The oxygen concentration of the blood (CaO2) is equal to the amount of oxygen bound to Hgb (HgbO2) plus the oxygen dissolved in blood. Hgb can bind chemically 1.34 mL O2/g. Given a normal Hgb of 15 (150 g/L), the O2 capacity of Hgb is 200 mL O2/L (1.34 mL O2/g x 150 g/L Hgb). Because oxygen has a low solubility, the amount of dissolved oxygen under normal conditions is relatively small, less than 2%-3% of the oxygen present. At a pO2 level of 100 mmHg, one liter of blood holds only 3 mL oxygen in solution. When breathing 100% FiO2, the amount of dissolved oxygen is 18-20 mL. The CaO2 level for systemic arterial blood under normal conditions is: CaO2 = Percent SaO2 x O2 capacity of Hgb + dissolved O2.
| CaO2 = [.97 x 200 + 0.03 x 100] mL O2/L = 194 + 3 = 197 mL O2/L.49 |
Oxyhemoglobin Disassociation Curve. The oxygen molecule combines loosely and reversibly with the heme portion of the Hgb molecule. When the PaO2 level is high, oxygen combines with Hgb. Conversely, when the PaO2 level is low, oxygen is released from Hgb, as occurs in the tissue capillaries. The oxyhemoglobin disassociation curve plots the HgbO2 saturation level or SaO2, which is the percentage of Hgb bound to oxygen, against the PaO2 level.50 The SaO2 level is plotted on the Y-axis vs the partial pressure of oxygen (PaO2) on the X-axis. (See Figure 6.) From the graph, the sigmoidal shape of the oxyhemoglobin (HgbO2) disassociation curve demonstrates the nonlinear relationship between SaO2 and PaO2. In the upper flat part of the oxyhemoglobin disassociation curve, minor changes in the SaO2 level result in larger changes in the PaO2 level. In the steep middle part of the curve, large changes in the SaO2 level result in smaller changes in PaO2 levels. As the PaO2 level increases, there is a progressive increase in the percentage of Hgb bound with oxygen.
Figure 6. Oxyhemoglobin Disassociation Curve

Various factors can cause a change in the oxyhemoglobin dissociation curve by shifting the curve to the right or the left. (See Table 3.) When the curve is shifted to the right, there is a decrease in the affinity of oxygen for Hgb; therefore, more oxygen is released to the tissues. Variables that shift the curve to the right include metabolic acidosis (decreased pH), increased temperature (fever), increased pCO2 (respiratory acidosis), and increased 2,3-DPG levels. The 2,3-DPG level is increased with chronic hypoxemia, chronic alkalosis, anemia, high altitude adaptation, and hyperthyroidism. A shift of the oxyhemoglobin disassociation curve to the left results in an increased affinity of oxygen to Hgb at a lower PaO2 level, with less oxygen released to the tissues. Factors resulting in a shift to the left are alkalosis (increased pH), hypocarbia (decreased pCO2), hypothermia (decreased temperature), and decreased 2,3-DPG level.51 (See Table 3 and Figure 6.)
Table 3. Oxyhemoglobin Disassociation Curve

The clinical importance of the sigmoidal shape of the oxyhemoglobin disassociation curve is two-fold. First, in hypoxic patients, because their SaO2 levels are on the steep part of the curve, large changes in SaO2 levels correspond to small changes in PaO2 levels. Secondly, in the high range of oxygenation (i.e., the plateau part of the curve), SaO2 measurements are somewhat insensitive in detecting significant PaO2 changes. (See Figure 6.)
Limitations of Pulse Oximetry
Limitations of pulse oximetry can be categorized into four categories: 1) those based on oxygen saturation level and the oxyhemoglobin disassociation curve, 2) those based on pulse oximetry’s design, 3) technical aspects, and 4) patient factors. (See Table 4.)
Table 4. Limitations of Pulse Oximetry

Oxygen Saturation. Pulse oximetry measures oxygen saturation; therefore, unlike an arterial blood gas or end-tidal CO2 monitoring, it yields no data on pH or PaCO2 levels. Because of the characteristics of the oxyhemoglobin disassociation curve, the SaO2 level is relatively insensitive to PaO2 level changes in the upper flat part of the curve. In the middle steep part of the curve, small changes in PaO2 levels result in large changes in SaO2 levels. The SaO2 level does not necessarily indicate the adequacy of ventilation. Hypercapnia due to hypoventilation may occur before a drop in the SaO2 level is detected (one advantage of end-tidal CO2 monitoring). However, pulse oximetry is an improvement over the clinical assessment of cyanosis because cyanosis is difficult to detect clinically33,51 and is an unreliable clinical sign.2
Design Aspects of Pulse Oximetry. Some limitations of pulse oximetry are attributable to the design of pulse oximeters and its measurement. Pulse oximeters only measure two wavelengths, and thus, functional saturation, not fractional saturation.7 Pulse oximeters measure the functional saturation, which equals the HgbO2 level divided by the reduced Hgb level plus the HgbO2 level times 100 to give a percentage.
| HgbO2 x 100 Functional SaO2 (%) = HgbO2 + Reduced Hgb
HgbO2 x 100 |
Fractional saturation is the HgbO2 level divided by the [HgbO2 + reduced Hgb + other hemoglobins].22 If other dyshemoglobinemias, (e.g., methemoglobin or carboxyhemoglobin) are present, then pulse oximetry (measurement of the functional SaO2 level) gives a falsely high SaO2 measurement.52,53 Co-oximetry should be done if abnormal hemoglobins (e.g., methemoglobin or carboxyhemoglobin) are present because co-oximeters calculate the fractional SaO2 level. However, because it uses only two wavelengths and two hemoglobins, pulse oximetry is less expensive, and the equipment is smaller and lighter and more portable than co-oximeters. Pulse oximeters do remain accurate if fetal hemoglobin is present;54,55 co-oximeters inaccurately report elevated carboxyhemoglobin levels in the presence of fetal hemoglobin. Both fetal hemoglobin and as much as 5% carboxyhemoglobin may be present in the blood of newborns.
Pulse oximetry measurements are affected by various dyes/ pigments.56,57 For example, methylene blue—which is recommended therapy for methemoglobinemia—absorbs light at 660 nm, similar to the absorption rate of reduced hemoglobin. (See Figure 2.) The presence of methylene blue will cause falsely low pulse oximetry saturations.56 Other intravenous dyes (e.g., fluorescein, indocyanine green, and indigo carmine) used for therapeutic or diagnostic purposes also will produce spuriously low pulse oximetry readings.57 (See Table 5.)
Table 5. Factors Affecting Pulse Oximetry Readings

Pulse oximeters have an onboard computer that uses empiric calibration curves derived from data from a small number of healthy adult volunteers.22,55 The calibration curves programmed into the pulse oximeter’s software differ among the various manufacturers, and may vary among pulse oximeters made by the same manufacturer. Whether the use of such calibration curves derived from healthy adults can be generalized for all ages (e.g., neonates, geriatric patients), all types of patients (e.g., male, female, ethnic groups), and all conditions/disease severity levels has not been evaluated extensively.
Again, the trend of pulse oximetry readings in a given patient and the presence or absence of hypoxia are more important than an isolated measurement of oxygen saturation.
Technical Aspects of Pulse Oximetry. Technical problems with pulse oximetry have been divided into two categories: 1) insufficient signal, as occurs with hypoperfusion or poor probe placement, and 2) too much signal with artifact/noise (excessive motion or ambient light).
Improper probe placement can cause a penumbra effect, whereby light from the LED is aimed directly at the photodetector instead of passing through the tissue (e.g., patient’s finger), causing a mistakenly high pulse oximetry oxygen saturation measurement when the arterial blood oxygen saturation level is less than 85%, and a falsely low pulse oximetry oxygen saturation measurement when the arterial blood oxygen saturation level is greater than 85%.2
Excessive motion of the probe causes much artifact and unreadable or unreliable/inaccurate readings. Newer pulse oximeter models have programmed software that can measure and remove the noise components; newer pulse oximetry probes also can eliminate most of the motion artifact.58
The pulse oximeter’s photodetector is nonspecific; therefore, high-intensity ambient light can result in interference, although a recent study documented no clinically or statistically significant variations in pulse oximetry from ambient light.43 This problem is avoided by covering the probe with opaque material.
Interference from electrocautery can be decreased by placing the sensor or probe farther from the surgical site (e.g., increasing the distance from the operative field).7
The wavelengths of light transmitted by the pulse oximeters (660 nm and 940 nm) LEDs may vary by ± 30 nm, which may affect accuracy.2
Patient Variables Affecting Pulse Oximetry. Pulse oximeters correlate best with arterial blood gas saturation measurements when the oxygen saturation level is greater than 80% (with an accuracy of approximately ± 4%-5%).64,65 Similarly, pulse oximeters work best in the middle ranges of heart rates (40 to 180 beats per minute), blood pressure measurements (systolic pressure in the 80-106 range), and hematocrit levels (20%56%). Pulse oximetry has decreased accuracy when severe anemia is present (e.g., Hct < 10%).64,65 The accuracy and precision of pulse oximeters is acceptable; in clinical practice, the presence or absence of hypoxia (and the trend) is more important than to define a specific level.
Pulse oximeters calculate the SaO2 level by computing the ratio of HgbO2 from the ratio of pulsatile to baseline tissue absorption, thus, the requirement for a pulsating vascular bed. When shock, severe hypotension, or vasoconstriction occurs (e.g., use of vasoconstricting drugs, such as epinephrine), the signal from the diminished arterial pulse can not be differentiated from background noise.66,67 Most pulse oximeters will display a message that there is an inadequate pulse signal. Changing the sensor to another location with a higher perfusion, such as from an extremity (finger or toe) to the earlobe, may allow for a better pulse signal. With cold extremities, vasoconstriction, or low perfusion, warming the extremities may generate a measurable signal.
When venous pressure is high, falsely low pulse oxygen saturation measurements may result; the pulse oximeter construes any pulsatile measurement as arterial, including the increased venous pulsations.7, 68 Situations in which there may be a high venous pressure include congestive heart failure, superior vena cava syndrome, traumatic venous obstruction (e.g., tension pneumothorax or pericardial tamponade), and transient iatrogenic causes (e.g., application of a tourniquet or inflation of a manometer cuff). (See Table 5.)
Deeply pigmented skin may decrease pulse oximetry’s accuracy, although the effect is minor.69,70 Using parts of the body with lighter pigmentation (e.g., the fifth finger or the earlobe) may minimize this variable. Fingernail polish has been cited as a variable affecting pulse oximetry readings when a finger or toe probe is used. A nursing recommendation is to remove fingernail polish or artificial nails if a finger or toe probe is used for pulse oximetry.71 However, a recent study documented a small decrease (2%) in pulse oximetry readings occurring only with brown or black fingernail polish,72 which could be eliminated by placing the finger probe side to side instead of top to bottom.72,73 However, there are many variables that do not affect pulse oximetry readings (e.g., fetal hemoglobin,55,74 sickle hemoglobin,8,75 hyperbilirubinemia,69,76 and dialysis grafts [A-V fistulas]).7
Summary
Pulse oximetry has wide usage throughout the hospital—including the ED and in prehospital care— because of its many advantages. It is a user friendly, inexpensive, safe, noninvasive point-of-care method for continuous monitoring of a patient’s oxygenation status. It can serve as an early warning signal of impaired oxygenation in a given patient, while avoiding the pain and risks of arterial puncture. The clinician should be aware of the pathophysiology/background of pulse oximetry to use this technology appropriately. (See Table 6.)
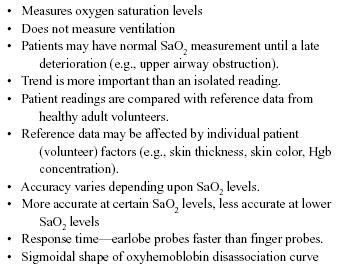
Table 6. Pearls and Pitfalls with Pulse Oximetry
In addition, the clinician should keep in mind scenarios where pulse oximetry readings may give either falsely high readings (e.g., carboxyhemoglobin or anemia) or falsely low readings (e.g., high venous pressures or diagnostic dyes) and use this information appropriately to manage the patient. Clinicians also should be aware of technical aspects of pulse oximetry, such as excessive probe motion and improper probe placement, that may result in inaccurate testing results. Continued improvement and advances in technology have resulted in valuable tools, such as pulse oximetry, that greatly enhance patient management in the acute care setting. Further advances will continue to contribute noninvasive, real-time information that facilitates the clinician's ability to diagnose and manage patients in the ED.
References
1. Murphy MF, Thompson J. Monitoring the emergency patient. In: Marx JA, Hockberger PS, Walls RM, et al (eds). Rosen’s Emergency Medicine Concepts and Clinical Practice. St. Louis:Mosby 2002; 8-33.
2. Hess D. Detection and monitoring of hypoxemia and oxygen therapy. Respir Care 2000;45:65-83.
3. St. John RE, Thompson PD. Noninvasive respiratory monitoring. Crit Care Nurs Clin North Am 1999;11:423-435.
4. Soubani AO. Noninvasive monitoring of oxygen and carbon dioxide. Am J Emerg Med 2001;19:141-146.
5. Neff TA. Routine oximetry. A fifth vital sign? Chest 1988;94 227.
6. Mower WR, Sach C, Nicklin EL, et al. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997; 99: 681-686.
7. Hedges JR, Baker WE, Lanoix R, et al. Use of monitoring devices for assessing ventilation and oxygenation. In: Roberts JR, Hedges JR (eds). Clinical Procedures in Emergency Medicine. Philadelphia: Saunders 2004;29-50.
8. Fuerst RS. Use of pulse oximetry. In: Henretig FM, King C (eds): Textbook of Pediatric Emergency Procedures. Baltimore: Williams and Wilkins 1997;823-828.
9. Salyer JW. Neonatal and pediatric pulse oximetry. Respir Care 2003;48:386-398.
10. Hay WW. Pulse oximetry: as good as it gets? J Perinatol 2000;3: 181-183.
11. Richmond S, Reay G, Abu Harb M. Routine pulse oximetry in the asymptomatic newborn. Arch Dis Child Fetal Neonatal Ed 2002; 87: F83-F88.
12. Maneker AJ, Petrack EM, Krug SE. Contribution of routine pulse oximetry to evaluation and management of patients with respiratory illness in a pediatric emergency department. Ann Emerg Med 1995; 25: 36-40.
13. Mower WR, Sachs C, Nicklin EL, et al. Effect of routine emergency department triage pulse oximetry screening on medical management. Chest 1995;108:1297-1302.
14. Panacek EA. The use of pulse oximetry in the emergency department. J Emerg Med 1995;13:816-817.
15. Mallory MD, Shay DK, Garrett J, et al. Bronchiolitis management preferences and the influence of pulse oximetry and respiratory rate on the decision to admit. Pediatrics 2003;111:e45-e51.
16. Zamarron C, Gude F, Barcala J, et al. Utility of oxygen saturation and heart rate spectral analysis obtained from pulse oximetric recordings in the diagnosis of sleep apnea syndrome. Chest 2003;123: 1567-1576.
17. Kline JA, Hernandez-Nino J, Newgard CD, et al. Use of pulse oximetry to predict in-hospital complications in normotensive patients with pulmonary emboli. Am J Med 2003;115:203-208.
18. Macnab AJ, Susak L, Gagnon FA, et al. The cost-benefit of pulse oximeter use in the prehospital environment. Prehospital Disaster Med 1999;14:245-250.
19. Howes DW, Field B, Leary T, et al. Justification of pulse oximeter costs for prehospital providers. Prehosp Emerg Care 2000;4:151-155.
20. Brown LH, Manring EA, Komegay HB, et al. Can prehospital personnel detect hypoxemia without the aid of pulse oximeters? Am J Emerg Med 1996;14:43-44.
21. Hay WW Jr, Thilo E, Curlander JB. Pulse oximetry in neonatal medicine. Clin Perinatol 1991;18:441-472.
22. Jubran A, Tobin MJ. Monitoring during mechanical ventilation. Clin Chest Med 1996;17:453-473.
23. Jubran A, Tobin MJ. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator dependent patients. Chest 1990;97:1420-1425.
24. Niehoff J, Del Guerico C, La Morte W, et al. Efficacy of pulse oximetry and capnometry in postoperative ventilatory weaning. Crit Care Med 1988;16:701-705.
25. AARC Clinical Practice Guideline. Pulse oximetry. Respir Care 1991;36:1383-1386.
26. Joint Commission on Accreditation of Healthcare Organizations: Comprehensive Accreditation Manual for Hospitals, the Official Handbook. Chicago, Il: JCAHO Publication, 2003.
27. American College of Emergency Physicians. Clinical policy for procedural sedation and analgesia in the emergency department. Ann Emerg Med 1998; 31: 663-677.
28. Committee on Drugs. American Academy of Pediatrics. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: addendum. Pediatrics 2002; 110: 836-838.
29. American Academy of Pediatrics Committee on Drugs. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 1992; 89: 1110-1115.
30. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002;96: 1004-1017.
31. Innes G, Murphy M, Nijssen-Jordan C, et al. Procedural sedation and analgesia in the emergency department. Canadian Consensus Guidelines. J Emerg Med 1999;17:145-156.
32. LeBourdelles G, Estagnasie P, Lenoir F, et al. Use of a pulse oximeter in an adult emergency department: Impact on the number of arterial blood gas analyses ordered. Chest 1998; 113: 1042-1047.
33. Comroe JH Jr, Botelho S. The unreliability of cyanosis in the recognition of arterial hypoxemia. Am J Med Sci 1947;214:1-6.
34. McKay WP, Noble WH. Critical incidents detected by pulse oximetry during anesthesia. Can J Anaesth 1998; 35: 265-269.
35. Morris RW, Buschman A, Warren DL, et al. The prevalence of hypoxemia detected by pulse oximetry during recovery from anesthesia. J Clin Monit 1988;4:16-20.
36. Cooper JB, Cullen DJ, Nemeskal R, et al. Effects of information feedback and pulse oximetry on the incidence of anesthesia complications. Anesthesiology 1987;67:686-694.
37. Bierman MI, Stein KL, Snyder JV. Pulse oximetry in postoperative care of cardiac surgical patients: A randomized controlled trial. Chest 1992;102:13671370.
38. Pedersen T, Moller AM, Pedersen BD. Pulse oximetry for perioperative monitoring: Systematic review of randomized controlled trials. Anesth Analg 2003;96:426-431.
39. Pedersen T, Pedersen D, Moller AM. Pulse oximetry for perioperative monitoring. Cochrane Database Syst Rev 2002; (3).
40. Szocik JF, Barker SJ, Tremper KK. Fundamental principles of monitoring instrumentation. In: Miller RD (ed). Anesthesia. New York: Churchill Livingstone;2000:1053-1086.
41. Oximetric measurement. In: Shapiro BA, Peruzzi WT (eds). Clinical Application of Blood Gases. St Louis:Mosby, 1994;331-342.
42. Continuous oximetry. In: Shapiro BA, Peruzzi WT (eds). Clinical application of blood gases. St Louis:Mosby;1994;263-274.
43. Fluck RR Jr, Schroeder C, Frani G, et al. Does ambient light affect the accuracy of pulse oximetry? Respir Care 2003;48:677-680.
44. Murphy KG, Secunda JA, Rockoff MA. Severe burns from a pulse oximeter. Anesthesiology 1990;73:350-352.
45. Chemello PD, Nelson SR, Wolford LM. Finger injury resulting from pulse oximeter probe during orthognathic surgery. Oral Surg Oral Med Oral Pathol 1990;69:161-163.
46. Bashein G, Syrory G. Burns associated with pulse oximetry during magnetic resonance imaging. Anesthesiology 1991;75:382-383.
47. Stogner SW, Owens MW, Baethge BA. Cutaneous necrosis and pulse oximetry. Cutis 1991;48:235-237.
48. Freund PR, Overanrd PT, Cooper J, et al. A prospective study of intraoperative pulse oximetry failure. J Clin Monit 1991;7:253-258.
49. Transport of oxygen and carbon dioxide: Tissue oxygenation. In: Berne RM, Levy MN (eds). Physiology. St. Louis: Mosby 1998; 561-571.
50. Transport of oxygen and carbon dioxide in the blood and body fluids. In: Guyton AC, Hall JE (eds). Textbook of Medical Physiology. Philadelphia:WB Saunders Co :2000;462-473.
513. Mower WR, Sachs C, Nicklin EL, et al. Effect of routine emergency department triage pulse oximetry screening on medical management. Chest 1995;108:1297-1302.
52. Bozeman WP, Myers RA, Barish RA. Confirmation of the pulse oximetry gap in carbon monoxide poisoning. Ann Emerg Med 1997; 30:608-611.
53. Barker SJ, Tremper KK, Hyatt J. Effects of methemoglobinemia on pulse oximetry and mixed venous oximetry. Anesthesiology 1989;70: 112-117.
54. Mendelson Y, Kent JC. Variations in optical absorption spectra of adult and fetal hemoglobins and its effect on pulse oximetry. IEEE Trans Biomed Eng 1989;36:844-848.
55. Hess D. Respiratory care monitoring. In: Burton GG, Hodgkin JE, Ward JJ (eds). Respiratory Care A Guide to Clinical Practice. Philadelphia: Lippincott:1997;309-332.
56. Sidi A, Paulus DA, Rush W, et al. Methylene blue and indocyanine green artifactually lower pulse oximetry readings of oxygen saturation: Studies in dogs. J Clin Monit 1987;3:249-256.
57. Scheller Ms, Unger RJ, Kelner MJ. Effects of intravenously administered dyes on pulse oximetry readings. Anesthesiology 1986;65: 550-552.
58. Sahni R, Gupta A, Ohira-Kist K, et al. Motion resistance pulse oximetry in neonates. Arch Dis Child Fetal Neonatal Ed 2003;88: F505F508.
59. Severinghaus JW, Naifeh KH. Accuracy of response of six pulse oximeters to profound hypoxemia. Anesthesiology 1987;67:551-558.
60. Severinghaus JW, Naifeh KH, Koh SO. Errors in 14 pulse oximeters during profound hypoxemia. J Clin Monit 1989; 5:72-81.
61. Hannhart B, Haberer JP, Saunier C, et al. Accuracy and precision of fourteen pulse oximeters. Eur Respir J 1991;4:115-119.
62. Webb RK, Ralston AC, Runciman WB. Potential errors in pulse oximetry. II Effects of changes in saturation and signal quality. Anaesthesia 1991;46:207-212.
63. Jensen LA, Onyskiw JE, Prasad NG. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998;27:387-408.
64. Lee S, Tremper KK, Barker SJ. Effects of anemia on pulse oximetry and continuous mixed venous hemoglobin saturation monitoring in dogs. Anesthesiology 1991;75:118-122.
65. Severinghaus JW, Koh SO. Effect of anemia on pulse oximeter accuracy at low saturation. J Clin Monit 1990;6:85-88.
66. Clayton DG, Webb RK, Ralston AC, et al. A comparison of the performance of 20 pulse oximeters under conditions of poor perfusion. Anaesthesia 1991;46: 3-10.
67. Ibanez J, Velasco J, Raurich JM. The accuracy of the Biox 3700 pulse oximeter in patients receiving vasoactive therapy. Intensive Care Med 1991;17:484-486.
68. Sami HM, Kleinman BS, Lonchyna VA. Central venous pulsations associated with a falsely low oxygen saturation measured by pulse oximetry. J Clin Monit 1991;7:309-312.
69. Ralston AC, Webb RK, Runciman WB. Potential errors in pulse oximetry: III. Effects of interferences, dyes, dyshaemoglobins and other pigments. Anaesthesia 1991; 46: 291-295.
70. Zeballos RJ, Weisman IM. Reliability of noninvasive oximetry in black subjects during exercise and hypoxia. Am Rev Respir Dis 1991; 144:1240-1244.
71. Pullen RL. Caring for a patient on pulse oximetry. Nursing 2003; 33:30.
72. Chan MM, Chan MM, Chan ED. What is the effect of fingernail polish on pulse oximetry? Chest 2003;123: 2163-2164.
73. White PF, Boyle WA. Nail polish and oximetry. Anesth Analg 1989; 68:546-547.
74. Harris AP, Sendak MJ, Donham RT, et al. Absorption characteristics of human fetal hemoglobin at wavelengths used in pulse oximetry. J Clin Monit 1988;4:175-177.
75. Kress JP, Pohlman AS, Hall JB. Determination of hemoglobin saturation in patients with acute sickle chest syndrome: a comparison of arterial blood gases and pulse oximetry. Chest 1999; 115:1316-1320.
76. Chelluri L, Snyder JV, Bird JR. Accuracy of pulse oximetry in patients with hyperbilirubinemia. Respir Care 1991;36:1383-1386.
The fifth vital sign, pulse oximetry, routinely is used in every emergency department throughout the country to determine the baseline oxygenation of a patient in respiratory distress, to assess a patients response to therapeutic decisions, and to monitor a child during a conscious sedation or resuscitation. It is important to understand how the device functions and the limitations of this routinely used technology.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
