Nephrolithiasis: Diagnosis and Management in the Emergency Department
Authors: Catherine Marco, MD, FACEP, Clinical Professor, Department of Surgery, Division of Emergency Medicine, Medical College of Ohio; Attending Physician, St. Vincent Mercy Medical Center, Toledo, OH; and Brock Boscovich, MD, Resident, Emergency Medicine Residency, St. Vincent Mercy Medical Center, Toledo, OH.
Peer Reviewers: Steven M. Winograd, MD, FACEP, Attending Physician, Emergency Department, St. Joseph Health Center, Warren, OH, and St. Elizabeth Health Center, Youngstown, OH.; and Stephen Crabtree, DO, FACEP, Northside Emergency Associates, Atlanta, GA.
Kidney stones (nephrolithiasis) have plagued mankind for centuries. Scientists have found kidney stones in mummies dating more than 7000 years old.1 The pain associated with this disease is uniformly severe and has been compared with that of child birth and other severe pain. Urolithiasis refers to stones found anywhere along the urinary tract and is a common etiology for abdominal and flank pain in patients presenting to the emergency department (ED). Data from 1997 estimate that the prevalence of kidney stones in the United States is 7% in men and 3% in women.2 This number is likely increasing with the higher consumption of animal protein seen today. The prevalence of kidney stones in the southeastern United States (the “stone belt”) is disproprotionately high, which is thought to be related to ambient temperature and sunlight exposure.3 Recurrence rates reach approximately 50% within 10 years of the first kidney stone, and up to 80% of patients have recurrent stones within 20-30 years.4 They can present at any age, but peak incidence occurs between 20 and 50 years of age.5 Prevalence of kidney stones has been linked to multiple factors including, but not limited to, climate, nutrition, heredity, and anatomic defects.6
There have been multiple advances in evaluation and management of this disease in recent years. This article will provide the emergency physician with an understanding essential for timely diagnosis, management, and disposition of kidney stones in the ED.—The Editor
Pathophysiology and Dynamics
There are multiple factors that contribute to the formation of urinary calculi, including urinary supersaturation, paucity of inhibitory factors for stone formation, and facilitatory conditions for crystallization, such as hyperoxaluria or hypercalciuria.2
With high solute loads and low urine volume, urine becomes a saturated solution in which no more solute can be dissolved and crystals begin to form. Most of these crystals travel down the collecting systems into the ureters and bladder, and are too small to cause any clinical symptoms. Some crystals can adhere to tubular cells, allowing more time for growth into pathologic stones.7 When other factors such as low urine flow from dehydration or obstruction, absence of crystal inhibitors (citrate, nephrocalcin, uropontin, pyrophosphate, and others8), and hypercalciuria are present, growth of these crystals into kidney stones is facilitated.
Spontaneous passage of kidney stones depends mainly on stone size and, to a lesser extent, stone location. Miller and Kane studied the time to stone passage, measuring variables such as stone size, location, gender, age, and pain medication requirements. They found that stones 2 mm or smaller in diameter passed spontaneously at a mean time of 8.2 days, with 4.8% requiring intervention. Stones between 2 mm and 4 mm passed spontaneously at an average of 12.2 days, with 17% requiring intervention. Finally, a mean of 22.1 days was required for spontaneous passage of stones measuring 4-6 mm, with 50% requiring intervention.9 A number of articles have addressed the relationship of stone size to spontaneous passage, all of which have varying results. Simply stated, larger stones decrease the chances for spontaneous passage. Stones 4-5 mm in size have about a 50% chance of spontaneous passage, while stones greater than 6 mm typically require urologic intervention 95% of the time.10
Kidney stones rarely cause complete obstruction of the ureter.11 If present, complete obstruction may cause significant irreversible damage to the ipsilateral kidney within 1-2 weeks.12 After complete or partial obstruction occurs, renal pelvic pressures elevate. This causes a decrease in the glomerular filtration in the ipsilateral kidney and ultimately results in redistribution of blood flow to the opposite kidney for compensation. Because of the contralateral compensation, kidney failure is rare in patients with normal renal anatomy and obstructive kidney stones. Obstruction also leads to urinary stasis, predisposing the patient to infection of the urinary tract. Presence of infection in the face of obstruction is considered by some to be a urologic emergency, and timely urologic consultation should be obtained.
Types of Stones
Urinary calculi may be classified into four different types: calcium-derived stones, struvite stones, uric acid stones, and cystine stones. (See Table 1.) Calcium-derived stones account for 70-80% of all stones. Most are a combination of calcium oxalate and calcium phosphate (37%); however, pure calcium oxalate and calcium phosphate stones make up a significant percentage (26% and 7%, respectively).13 Hyperexcretion of calcium and/or oxalate are major factors in the formation of calcium-containing stones. Many patients suffer from idiopathic hypercalciuria, the cause of which has yet to be found. This appears to be a familial disease and more common in men. Obesity, high protein and salt intake, hyperparathyroidism, and peptic ulcer disease also have been associated with higher urinary calcium excretion.
Struvite stones, or infection stones, are composed of magnesium ammonium phosphate and make up 15-20% of all kidney stones. These are formed by urea-splitting organisms including, but not limited to, Proteus and Providencia species as well as some species of Pseudomonas, Serratia, and Klebsiella. Patients presenting with pure struvite stones rarely have idiopathic hypercalciuria and often have impaired renal function and contralateral kidney involvement. These often present as “staghorn” calculi, described for their appearance of a staghorn as the stone grows up through the calices from the renal pelvis. Prolonged antibiotic therapy has been proven ineffective because of poor penetration into the stones (which act as foreign bodies), necessitating surgical removal of many of these stones.14
Uric acid stones account for 5-10% of all stones and are caused by excess levels of uric acid in the urine. Because uric acid is a weak acid, it also becomes less soluble when the urine becomes more acidic (seen with excess intake of animal protein15), promoting sedimentation and formation of stones. Interestingly, 25% of patients suffering from gout have uric acid nephrolithiasis.16 Currently there is limited objective data regarding dietary recommendations for patients suffering from uric acid nephrolithiasis; however, decreasing meat intake typically is suggested.
Cystine stones comprise only 1% of kidney stones. The disorder leading to cystine stone formation has been described as an autosomal recessive inborn error of metabolism that results in increased urinary excretion of cystine, lysine, arginine, and ornithine.17 Unlike lysine, arginine, and ornithine, cystine has very low solubility in urine, and is further precipitated into solute by acidic, supersaturated urine. Patients with cystine stones usually present in the second or third decades of life. The stones are radiopaque and, like struvite stones, may form staghorn calculi.
History and Predisposing Factors
Certain historical factors are important in the evaluation of nephrolithiasis. Risk factors associated with nephrolithiasis should be sought, including previous history of nephrolithiasis, family history of nephrolithiasis, conditions resulting in urinary stasis (including anatomic abnormalities, prostatic hypertrophy), metabolic abnormalities, abnormal urine pH, recent urinary tract infection, low urine output, and environmental factors such as excessive heat resulting in dehydration, fluid restriction, or occupation. History of previous episodes of nephrolithiasis, previous stone size, previous interventions, and stone composition should be sought, if applicable. Numerous medications are associated with the development of nephrolithiasis. (See Table 2.) Medication-induced calculi have been estimated to represent 1-2% of all nephrolithiasis cases.18 Medications may cause nephrolithiasis by several mechanisms. Some medications such as triamterene, indinavir, and sulfadiazine have poor solubility resulting in crystallization in the urine. Medications such as calcium or vitamin D supplements can cause calculi formation as a result of metabolic effects. Calculi formation also is influenced by urine output, urine pH, and other factors.

Physical Examination
The physical examination of patients with suspected nephrolithiasis should be focused on relevant examination features. Vital signs should be performed immediately, and patients with fever, hypotension, or tachycardia may require early intervention with fluid resuscitation. Other potential causes of flank pain should be addressed in the physical examination, including examination of the lung fields and thorough abdominal examination. Presence of costovertebral tenderness should be sought. Evidence of trauma should be evaluated. Assessment of pain should be performed for all patients to facilitate appropriate pain management.
Diagnostic Studies
Urinalysis. All patients presenting to the ED with complaints of flank or lower abdominal pain should undergo urinalysis. Those with urinary calculi often will exhibit microscopic hematuria; however, it has been shown that 7-12% of kidney stones may present without hematuria.19 As previously discussed, urinary pH also is important in the formation of kidney stones, particularly cystine and uric acid stones, which have a tendency to form in more acidic environments. Conversely, struvite stones more often are seen with alkaline urine. The presence of leukocyte esterase, white blood cells, or nitrites on urinalysis are suggestive of a concomitant urinary tract infection. If a urinary tract infection is suspected, urine culture should be sent. A limited amount of pyuria can exist; however, as stones cause an inflammatory response along the urinary tract.20 Microscopic examination of the urine also may elucidate other findings, such as crystals that may aid in identification of the type of stone present.
Serum Studies. Some physicians recommend a complete blood count (CBC) with differential for patients with possible infection. Urosepsis in the presence of urinary tract obstruction is an indication for emergent urology consultation and intervention. Unfortunately, the CBC is not sensitive or specific in such cases, and demargination may occur simply from the pain associated with renal colic. More suspicious findings for sepsis would include a predominance of neutrophils or elevated bands on the differential. If a patient presents with complete obstruction in the presence of a UTI, a full septic workup including blood and urine cultures should be obtained.
Because the opposite kidney compensates for the affected kidney in urinary obstruction, the blood urea nitrogen (BUN) and creatinine typically are not elevated unless a single kidney or bilateral obstruction is present.5 Electrolytes, BUN, and creatinine may aid in determining the etiology of disease in a patient (such as renal tubular acidosis), and may be required if a contrast study is needed to aid in the diagnosis.
Radiographic Evaluation
Patients with the first episode of nephrolithiasis should undergo radiographic evaluation to assess stone size and position, presence of hydronephrosis, obstruction, and renal function. Patients with recurrent nephrolithiasis may not require radiographic evaluation with every occurrence, and the emergency physician should judiciously order diagnostic tests based on clinical presentation, recent diagnostic evaluation, and likelihood of obstruction or hydronephrosis.
Plain Abdominal Radiograph (KUB). Because of its poor sensitivity, specificity, and inability to detect urinary obstruction or kidney dysfunction, the KUB is of limited value in the initial workup of acute flank pain due to kidney stones. In a recent retrospective study, abdominal radiography had a sensitivity of only 45% with a specificity of 77% in the acute workup of urinary tract stones.21 These results improved to 59% and 71% respectively when two of the authors retrospectively reviewed the films of patients with known stone disease.21 In patients undergoing conservative management for confirmed stone disease, the KUB serves as an excellent imaging tool for monitoring stone progression.
Intravenous Pyelogram (IVP) vs. Noncontrast Helical Computerized Tomography (NHCT). The IVP once was accepted as the test of choice for the initial evaluation of flank pain from suspected kidney stones. This has changed because of the increased sensitivity, specificity, speed of testing, and the ability to identify other etiologies of abdominal or flank pain with NHCT. NHCT also will identify most radiolucent kidney stones, the size of the stones, and eliminates exposure to IV contrast and its complications. A recent meta-analysis pooled data from four studies comparing NHCT with IVP and showed NHCT (sensitivity 94-100%, specificity 92-100%) to be unanimously more accurate than IVP (sensitivity 52-87%, specificity 92-94%) in the evaluation of kidney stones.22
IVP accurately characterizes the degree of ureteral obstruction, kidney function, and important anatomic anomalies in the collecting system, all of which may be helpful to the urologist for determination of definitive therapy. NHCT does not provide direct functional information, but often function and the degree of obstruction can be postulated using secondary signs of hydronephrosis such as perinephric stranding, ureteric dilatation, kidney size, and forniceal rupture. These secondary signs can also be used for diagnosis of kidney stones when the stone itself is not visible or an indefinite calcification is identified.23 The presence and severity of these secondary findings, however, play no role in determination of conservative versus interventional therapy.24 Boulay et al. claimed that stone size was the only parameter that correlated with the type of definitive therapy a patient would receive.24 This suggests NHCT alone is adequate for the diagnosis and treatment of kidney stones in patients with normal anatomy.
NHCT has some disadvantages, however. First, NHCT requires the patient be exposed to radiation doses approximately three times higher than that of IVP.25 This has especially high implications in patients with repeated bouts of flank pain due to higher stone burden or malingering. Interestingly, a recently published article suggests that there is no difference in the diagnostic accuracy for reduced dose NHCT using reduced tube current.26 One may consider other imaging modalities such as ultrasound or magnetic resonance imaging (MRI) in children or the pregnant patient.
Second, there has been controversy over the total cost of NHCT vs. IVP. Contrary to previous studies suggesting no difference in the charges (what the patient is billed) between NHCT and IVP, Li et al. claim that IVP is one quarter the price of NHCT.19 However, when considering the indirect costs such as length of stay, manpower utilization, and additional testing in cases with vague results, IVP ultimately may prove to be more expensive.27,28
Ultrasound. Previous studies have concluded that ultrasound has consistently low sensitivity for detection of urinary calculi (37-64%).29-33 Bedside ultrasound performed by the emergency physician to detect hydronephrosis also has had low sensitivity and specificity in prediction of kidney stones (72% and 73%, respectively).34 Ultrasound can be useful in select patients in whom radiation exposure should be avoided (pediatric and pregnant patients) despite its poor performance.31 Ultrasound yield also may vary depending on operator experience, body habitus, and presence of bowel gas and adjacent bone structures. These findings support the use of NHCT as the primary imaging tool for flank pain in the ED.
Magnetic Resonance Imaging (MRI). MRI, or more specifically, magnetic resonance urography (MRU) has been studied for evaluation of patients with acute ureteral obstruction. The major advantages of MRU are elimination of radiation exposure, enhanced identification of extrarenal causes of obstruction (such as neoplasm or hematoma), and the ability to accurately relay information about the structure and function of the urinary tract.
MRU alone, or in combination with targeted NHCT, has been proven effective in the workup of acute ureteral obstruction.35,36 A recent study revealed comparable sensitivity and specificity of MRU (93.8-100% and 100%, respectively) when compared with NHCT.37 Unfortunately, MRU is poor in identification of calcifications as they result in a signal void, hindering the direct identification of a ureteral stone. The presence of stones is assumed when a filling defect with signs of obstruction are present in the ureteral lumen. Such findings most frequently are due to the presence of a stone, but neoplasm or blood clots also may present this way.38 Because MRU does not directly characterize the stone, NHCT is more accurate in measuring stone size, the most important determinant of conservative versus interventional therapy.24,38 Other drawbacks to MRU are higher costs, longer testing time leading to delayed diagnoses, and the limited availability in most EDs. This limits use of MRU to patients more sensitive to radiation exposure.
Stone Analysis
For all patients with nephrolithiasis, stone analysis is indicated. A stone that has passed in the ED should be sent to the lab for classification. Patients who are discharged from the ED should be instructed to strain all urine and collect stones for analysis.
For patients with repeat episodes of nephrolithiasis, 24-hour urine collection is indicated. Although this is not typically initiated in the ED, it may be helpful to instruct patients about these likely diagnostic tests.
Differential Diagnosis
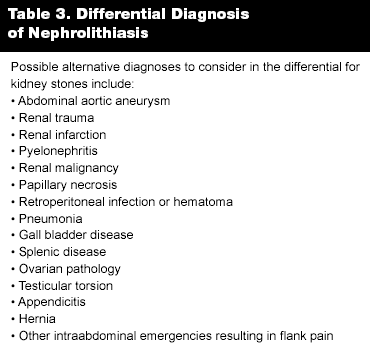
When considering the diagnosis of nephrolithiasis, the emergency physician should also consider alternative possible diagnoses. Perhaps the most life-threatening diagnosis to consider is abdominal aortic aneurysm, which may present with flank pain. Age, syncope, and hypotension may increase the likelihood of abdominal aortic aneurysm. Other diagnoses to consider include renal trauma, renal infarction, pyelonephritis, renal malignancy, papillary necrosis, retroperitoneal infection or hematoma, pneumonia, gall bladder disease, splenic disease, ovarian pathology, testicular torsion, appendicitis, hernia, and other intraabdominal emergencies resulting in flank pain. (See Table 3.)
Emergency Department Management
Analgesia. Prompt attention should be directed toward pain management in patients presenting with presumed ureteral colic. It is thought that pain from ureteral colic is due to ureteric muscle spasm provoked by the presence of a kidney stone. Further pain arises from obstruction caused by the stone with resulting distention of the ureters and renal pelvis. Prostaglandins then are released, stimulating stronger ureteric contractions and dilatation of the afferent arterioles leading to an increased glomerular filtration rate and even higher renal pelvis pressures. Understanding of this physiology has sparked studies for a multitude of analgesics for use in ureteral colic, including narcotics, non-steroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers, anticholinergics, and vasopressin analogues.
Nifedipine is a calcium channel blocker that inhibits human ureteric smooth muscle in vitro. Unfortunately, nifedipine was found to have no analgesic efficacy in the treatment of acute ureteral colic.39 Likewise, a recent study of the antispasmodic properties of sublingual hyoscyamine sulfate (an anticholinergic) in combination with ketorolac for use in renal colic found no difference in the level of pain relief as compared to ketorolac alone.40 Intranasal desmopressin, a vasopressin analogue, has been found somewhat effective alone or in combination with NSAIDs for the treatment of ureteral colic.41,42 These were very small studies, however, and further research is needed before more reliable conclusions can be drawn. Desmopressin is thought to work by decreasing diuresis, relaxing smooth muscle, and stimulating endorphin release centrally.42 Advantages to intranasal desmopressin include ease of administration and lack of clinically significant side effects.
Narcotics have long been the mainstay for achieving adequate analgesia in acute ureteral colic in the United States. However, NSAIDs have been used for decades in other countries for analgesia in ureteral colic with great success. Researchers pooled data from 19 studies and found the analgesic properties of NSAIDs to be equally as efficacious as opioids and other analgesics in ureteral colic.43 NSAIDs block synthesis of prostaglandins and their deleterious effects on the urinary tract during acute ureteral obstruction, as described above. Ketorolac is the only NSAID available for intravenous or intramuscular administration in the United States. Ketorolac recently has gained popularity for the treatment of ureteral colic despite a general lack of larger prospective blinded studies comparing its efficacy against opioids. Smaller studies have suggested significantly better analgesia using one 60-mg dose of ketorolac intravenously or intramuscularly alone versus meperidine alone.44,45 Despite the small size of these studies, results have been quite striking and suggest NSAIDs be used as a first line analgesic agent in the treatment of acute ureteral colic. Opioids may be used for rescue therapy in those with persistent pain, though combination therapy with ketorolac and meperidine appears no more efficacious than ketorolac alone.44
Antibiotics. Patients suspected to have an underlying urinary tract infection (UTI) with presence of ureteral obstruction require immediate intravenous antibiotics and emergent urology consultation. Initial choices should cover the typical Gram negative species, but also Enterobacteriaceae, P. aeruginosa, and enterococci. Appropriate antibiotics may include ampicillin plus gentamycin, piperacillin/tazobactam, ticarcillin/clavulanic acid, imipenem, meropenem, or a fluoroquinolone.46 There is obvious risk for developing severe sepsis and permanent renal damage when ureteral obstruction coincides with infection, so early antibiotic administration is essential.
Prevention of Nephrolithiasis
Diet and Hydration. Though aggressive fluid hydration has been used as a means of stone passage in the past, there is no evidence that this is of benefit while in the ED. Peristalsis of the ureter appears to be the driving mechanism behind stone passage. However, because recurrence of stone disease is common, increased long-term fluid intake should be recommended to all patients. The benefit of increased fluid intake has been well documented and effectively lowers the concentration of urine and resultant kidney stone formation.48 The patient should be advised to maintain sufficient fluid intake to produce two liters of urine per day.47 This is simple for the ED physician to recommend, but compliance typically is very poor.48
Low calcium diets historically have been recommended to patients with recurrent kidney stones. However, calcium restriction has been associated with reduced intestinal absorption, resulting in higher excretion of oxalate in the urine, associated with higher rates of stone formation.49-51 Currently, most experts recommend normal calcium intake.51-54 Many other dietary modifications have been recommended by some authors, including reduced intake of protein, sodium, and simple sugars.55,56 For patients with hyperoxaluria, reduced intake of foods high in oxalate is recommended (such as rhubarb, black tea, and spinach).
Maintenance of appropriate weight, or weight loss, if indicated, may be of benefit in prevention of recurrent nephrolithiasis.57
Preventive medications may be indicated for certain patients with nephrolithiasis. Thiazine and chlorthalidone may be effective in the prevention of nephrolithiasis for patients with recurrent calcium oxalate stone formation.58 Allopurinol may be indicated for patients with hyperuricosuria; however, therapeutic benefit may be limited to long-term use (more than 12 months). Potassium-magnesium citrate has been shown to reduce incidence or repeat nephrolithiasis in patients with calcium oxalate nephrolithiasis.49
Special Patient Populations
Pediatrics. Nephrolithiasis may occur in pediatric patients. Management is unchanged from that of adult patients. Appropriate diagnostic studies should be undertaken to assess stone size and position. To minimize radiation exposure, ultrasound or MRI may be preferred over NHCT. Stone composition should be determined for first-time stones, and 24-hour urine collection should be obtained for recurrent stones. Appropriate management includes pain control, hydration, and urologic follow-up. Complications such as infection, dehydration, intractable pain or vomiting, or anatomic abnormality may be indications for hospital admission and emergent urologic consultation.
Pregnancy. Nephrolithiais may occur during pregnancy, with an incidence of approximately one out of 1500 pregnancies, similar to the incidence for nonpregnant females.59 To minimize radiation exposure, the diagnostic test of choice is ultrasound. Alternative diagnostic studies may include MRI or limited intravenous urograms. The majority of pregnant patients (70-80%) with nephrolithiasis will pass stones spontaneously with supportive care and expectant management. If intervention is necessary, internal stents or percutaneous nephrostomy tubes may be appropriate.
Malingering. Unfortunately, there are certain patients who may attempt to feign symptoms of nephrolithiasis with the objective of obtaining pain medication. Some patients have gone to lengths, such as pricking a finger to introduce a drop of blood into the urine sample to mimic hematuria. If such behavior is suspected, obtaining a witnessed urine sample may be of value. Medical records should be referenced, and previous similar history should be sought. However, such past behavior does not definitively rule out nephrolithiasis, and all patients should be appropriately evaluated with discretionary diagnostic studies to confirm or refute the diagnosis of nephrolithiasis.
Disposition and Consultation
Most patients with nephrolithiasis can be appropriately discharged home with analgesia and instructions for follow-up. Oral NSAIDs should be continued at home, and oral opioids such as hydrocodone/acetaminophen or oxycodone/acetaminophen may be prescribed as needed for breakthrough pain.
As noted above, patients should be instructed to increase general fluid intake to ensure approximately two liters of urine output per day (or to maintain clear urine). Patients also should be instructed to strain their urine with strainers provided in the ED so the stone composition may be determined. The patient should have instructions to return to the ED immediately if he/she experiences uncontrolled pain, fever, chills, or vomiting. Follow-up with a urologist should be scheduled within 1-2 weeks.
Indications for admission include intractable pain or vomiting, UTI with obstruction, transplanted or single kidney with obstruction, or uncertainty about the diagnosis. The emergent nature of UTI in the face of obstruction has already been stressed, and many of these patients will require immediate surgical decompression to allow the antibiotics to better reach the affected site by means of improved urine excretion. UTIs with concomitant kidney stones and no obstruction may be safely discharged on an oral fluoroquinolone with urologic follow-up scheduled within 24 hours. (See Table 4 for Management Summary.) Because of the significant risk for renal failure in a patient with only one kidney and urinary obstruction, earlier surgical intervention may be required and a urologist should be involved early in the course. Finally, if no stone is positively identified and significant abdominal pain persists, the patient should be observed for improvement or deterioration, as with other abdominal pain of uncertain etiology.

References
1. Wershub LP. Urology: From Antiquity to the 20th Century. St. Louis, MO, Warren H. Green, 1970.
2. Saklayen MG. Medical management of nephrolithiasis. Med Clin North Am 1997;81:785-799.
3. Soucie JM, Coates RJ, McClellan W, et al. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol 1996;143:487-495.
4. Sutherland JW, Parks JH, Coe FL. Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab 1985;11: 267-269.
5. Manthey DE, Teichman J. Nephrolithiasis. Emerg Med Clin North Am 2001;19:633-654.
6. Hess B. Nutritional aspects of stone disease. Endocrinol Metab Clin N Am 2002;31:1017-1030.
7. Lieske JC, Toback FG. Interaction of urinary crystals with renal epithelial cells in the pathogenesis of nephrolithiasis. Semin Nephrol 1996;16:458-473.
8. Worcestor EM. Inhibitors of stone formation. Semin Nephrol 1996; 16:474-486.
9. Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: A guide for patient education. J Urol 1999;162:688-691.
10. Tanagho EA, McAninch JW, eds. Smith’s General Urology. New York, New York: The McGraw-Hill Companies, Inc.;2004.
11. Shokeir AA. Renal colic: New concepts related to pathophysiology, diagnosis, and treatment. Curr Opin Urol 2002;12:263-269.
12. Marx JA, et al, eds. Rosen’s Emergency Medicine, 5th edition. St. Louis, Missouri: Mosby Inc.; 2002.
13. Bushinsky DA. Nephrolithiasis. J Am Soc Nephrol 1998;9:917-924.
14. Cohen CD, Preminger GM. Struvite calculi. Semin Nephrol 1996; 16:425-434.
15. Halabe A, Sperling O. Uric acid nephrolithiasis. Miner Electrolyte Metab 1994;20:424-431.
16. Asplin JR. Uric acid stones. Semin Nephrol 1996;16:412-424.
17. Sakhaee K. Pathogenesis and medical management of cystinuria. Semin Nephrol 1996;16:435-447.
18. Dauon M, Jungers P. Drug-induced renal calculi. Drugs 2004; 245-275.
19. Li J, Kennedy D, Levine M, et al. Absent hematuria and expensive computerized tomography: Case characteristics of emergency urolithiasis. J Urol 2001;165:782-784.
20. Portis AJ, Sundaram CP. Diagnosis and initial management of kidney stones. Am Fam Physician 2001;63:1329-1338.
21. Levine JA, Neitlich J, Verga M, et al. Ureteral calculi in patients with flank pain: Correlation of plain radiography with unenhanced helical CT. Radiology 1997;204:27-31.
22. Worster A, Preyra I, Weaver B, et al. The accuracy of noncontrast helical computed tomography versus intravenous pyelography in the diagnosis of suspected acute urolithiasis: A meta-analysis. Ann Emerg Med 2002;40:280-286.
23. Smith RC, Verga M, Dalrymple N, et al. Acute ureteral obstruction: Value of secondary signs on helical unenhanced CT. AJR Am J Roentgenol 1996;167:1109-1113.
24. Boulay I, Holtz P, Foley WD, et al. Ureteral calculi: Diagnostic efficacy of helical CT and implications for treatment of patients. AJR Am J Roentgenol 1999;172:1485-1490.
25. Denton ER, Mackenzie A, Greenwell T, et al. Unenhanced helical CT for renal colic: Is the radiation dose justifieable? Clin Radiol 1999;54:444-447.
26. Heneghan JP, McGuire KA, Leder RA, et al. Helical CT for nephrolithiasis and ureterolithiasis: Comparison of conventional and reduced radiation-dose techniques. Radiology 2003;229:575-580.
27. Miller OF, Rineer SK, Reichard SR, et al. Prospective comparison of unenhanced spiral computed tomography and intravenous urogram in the evaluation of acute flank pain. Urology 1998;52: 982-987.
28. Pfister SA, Deckart A, Laschke S, et al. Unenhanced helical computed tomography vs. intravenous urography in patients with acute flank pain: Accuracy and economic impact in a randomized prospective trial. Eur Radiol 2003;13:2513-2520.
29. Sinclair D, Wilson S, Toi A, et al. The evaluation of suspected renal colic: Ultrasound scan versus excretory urography. Ann Emerg Med 1989;18:556-559.
30. Deyoe LA, Cronan JJ, Breslaw BH, et al. New techniques of ultrasound and color Doppler in the prospective evaluation of acute renal obstruction. Do they replace the intravenous urogram? Abdom Imaging 1995;20:58-63.
31. Aslaksen A, Gothlin JH. Ultrasonic diagnosis of ureteral calculi in patients with acute flank pain. Eur J Radiol 1990;11:87-90.
32. Sheafor DH, Hertzberg BS, Freed KS, et al. Nonenhanced helical CT and US in the emergency evaluation of patients with renal colic: Prospective comparison. Radiology 2000;217:792-797.
33. Fowler KAB, Locken JA, Duchesne JH, et al. US for detecting renal calculi with nonenhanced CT as a reference standard. Radiology 2002;222:109-113.
34. Rosen CL, Brown DF, Sagarin MJ, et al. Ultrasonography by emergency physicians in patients with suspected ureteral colic. J Emerg Med 1998;16:865-870.
35. Blandino A, Minutoli F, Scribano E, et al. Combined magnetic resonance urography and targeted helical CT in patients with renal colic: A new approach to reduce delivered dose. J Magn Reson Imaging 2004;20:264-271.
36. Rothpearl A, Frager D, Subramanian A, et al. MR urography: Technique and application. Radiology 1995;194:125-130.
37. Sudah MS, Vanninen RL, Partanen K, et al. Patients with acute flank pain: Comparison of MR urography with unenhanced helical CT. Radiology 2002;223:98-105.
38. Roy C, Saussine C, Guth S, et al. MR urography in the evaluation of urinary tract obstruction. Abdom Imaging 1998;23:27-34.
39. Caravati EM, Runge JW, Bossart PJ, et al. Nifedipine for the relief of renal colic: A double-blind, placebo-controlled clinical trial. Ann Emerg Med 1989;18:352-354.
40. Jones JB, Giles BK, Brizendine EJ, et al. Sublingual hyoscyamine sulfate in combination with ketorolac tromethamine for ureteral colic: A randomized, double-blind, controlled trial. Ann Emerg Med 2001;37:141-146.
41. El-Sherif AE, Salem M, Yahia H, et al. Treatment of renal colic by desmopressin intranasal spray and diclofenac sodium. J Urol 1995; 153:1395-1398.
42. Lopes T, Dias JS, Marcelino J, et al. An assessment of the clinical efficacy of intranasal desmopressin spray in the treatment of renal colic. BJU Int 2001;87:322-325.
43. Labrecque M, Dostaler LP, Rousselle R, et al. Efficacy of nonsteroidal anti-inflammatory drugs in the treatment of acute renal colic: A meta-analysis. Arch Intern Med 1994;154:1381-1387.
44. Cordell WH, Wright SW, Wolfson AB, et al. Comparison of intravenous ketorolac, meperidine, or both (balanced analgesia) for renal colic. Ann Emerg Med 1996;28:151-158.
45. Larkin GL, Peacock WF, Pearl SM, et al. Efficacy of ketorolac tromethamine versus meperidine in the ED treatment of acute renal colic. Am J Emerg Med 1999;17:6-10.
46. Gilbert DN, Moellering RC, Eliopoulos GM, et al, eds. The Sanford Guide to Antimicrobial Therapy, 34th edition. Hyde Park, Vermont: Antimicrobial Therapy Inc.; 2004.
47. Bushinsky DA. Nephrolithiasis. J Am Soc Nephrology 1998;9: 917-924.
48. Borghi L, Meschi T, Schianchi T, et al. Urine volume: Stone risk factor and preventive measure. Nephron 1999; 81(suppl):31-37.
49. Pak CYC. Medical prevention of renal stone disease. Nephron 1999;81:60-65.
50. Goldfarb DS, Coe FL. Prevention of recurrent nephrolithiasis. Am Fam Physician 1999;60:2269-2276.
51. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346:77-84.
52. Curhan GC, Willett WC, Rimm EB, et al. Prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993;328:833-838.
53. Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 1997;126:497-504.
54. Hess B. Nutritional aspects of stone disease. Endocrinol Metab Clin N Am 2002;31:1017-1030.
55. Reynolds TM. ACP Best practice No. 181: Chemical pathology clinical investigation and management of nephrolithiasis. J Clin Pathol 2005;58:134-140.
56. Pearle MS. Prevention of nephrolithiasis. Curr Opin Nephrol Hypertension 2001;10:203-208.
57. Meschl T, Schianchl T, Ridolo E, et al. Body weight, diet and water intake in preventing stone disease. Urol Int 2004;72 (suppl):29-33.
58. Pearle MS, Roehrbom CG, Pak CY. Meta-analysis of randomized trials for medical prevention of calcium oxalate nephrolithiasis. J Endourol 1999;13:679-685.
59. McAleer SJ, Loughlin KR. Nephrolithiasis and pregnancy. Curr Opin Urol 2004;14:123-127.
There have been multiple advances in evaluation and management of kidney stones in recent years. This article will provide the emergency physician with an understanding essential for timely diagnosis, management, and disposition of kidney stones in the ED.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
