Modalities for Non-Invasive Assessment of Coronary Artery Disease
Authors: S. Benjamin Bartsch, MD, Kettering Medical Center, Clinical Instructor, Wright State University, Dayton, OH; Ryan F. Biegler, MD, Kettering Medical Center, Clinical Instructor, Wright State University, Dayton, OH; Lyndetta Schwartz, MD, FACP, Senior Associate Program Director, Internal Medicine, Kettering Medical Center, Assistant Clinical Professor, Wright State University, Dayton, OH; and Brian P. Schwartz, MD, FACP, FACC, FSCAI, Assistant Clinical Professor of Medicine, Wright State University, President Southwest Cardiology, Dayton, OH; Director of Cardiovascular Teaching Service, Kettering Medical Center, Kettering, OH.
Peer Reviewer: Brian Olshansky, MD, Professor of Medicine, University of Iowa Hospitals, Iowa City, IA.
Primary care physicians (PCPs) are often at the forefront of diagnosing coronary artery disease. Occasionally, it's the legendary patient comment as the doctor has his hand on the door about to leave the office visit, "Doc, what about this chest pain?" Whether it's new onset or established disease, the PCP has an arsenal of diagnostic tests available. The array of tests, however, can be quite daunting in identifying the right test to order, especially in light of sensitivity and specificity, not to mention cost. This issue provides the PCP with a comprehensive survey of the diagnostic tests available, a guide to selection, an estimate of cost, and limitations of each test. Because of the complexity and expense of some of these tests, it is always helpful to coordinate with the patient's cardiologist when appropriate to avoid duplication, unnecessary testing, and to determine when to proceed directly to coronary angiography.
The Editor
Introduction
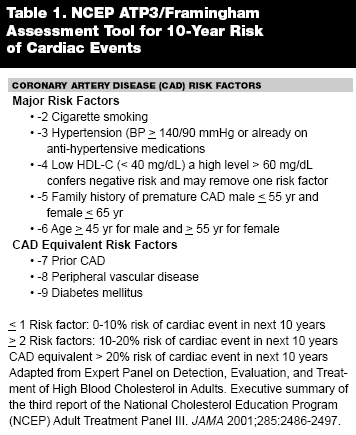
Atherosclerosis, including coronary artery disease (CAD), remains the major cause of death and premature disability in the United States. Although mortality from CAD has fallen substantially over the past three decades, it remains the leading cause of death in adults. In a study of 7733 participants in the Framingham Heart Study who were initially free of CAD, the lifetime risk of CAD for individuals at age 40 was 49% in men and 32% in women. The lifetime risk was also appreciable in those free of CAD at age 70: 35% in men and 24% in women.1 The Framingham data further showed that beyond age 65, CAD still predominates, with coronary events comprising 33-65% of atherosclerosis cardiovascular events in men and 28-58% in women. The Framingham Heart Study also provided rigorous support for the concept of specific identifiable risk factors correlated with cardiovascular risk. Similar observational studies performed in the United States and abroad provided independent support for the concept of "risk factors" for cardiovascular disease. Based on the National Cholesterol Education Project Adult Treatment Panel III (ATP III) and Framingham data, assessment tools have been established to stratify individuals based on these risk factors.2 (See Table 1.) Patients can be classified as low, intermediate, and high risk with a 10-year event rate of less than 10%, 10-20%, and greater than 20%, respectively. Intermediate and high risk groups need aggressive lifestyle intervention and risk factor modification. Further evaluation is necessary if individuals develop symptoms of CAD in order to determine outcomes and need for additional therapies. However there is also considerable interest in the diagnosis of CAD when patients are still asymptomatic. An increasing number of physicians are screening for asymptomatic CAD, and many participants in wellness programs also are requesting screening for themselves, believing that there are legitimate screening methods for early detection of CAD that are necessary prior to beginning an exercise program. Critical questions remain, however, regarding the appropriateness of screening and the optimal screening test.3 Until recently, angiography has been the major approach to assess patients for the presence of CAD; however, cardiac catheterization is a less than ideal screening tool since it is expensive, invasive, and it exposes patients to risk of complications such as reactions to contrast media, nephrotoxicity, access site bleeding, and vascular injury.4,5 For these reasons, approximately 40 million noninvasive tests are performed annually to assess CAD, incurring an annual Medicare reimbursement of $372-749 million.6
 |
In determining the best modality for screening or evaluating CAD, the evidence supporting the role of assessment of ischemia versus coronary anatomy must be considered. Data from the Coronary Artery Surgery Study (CASS) registry have shown that even in the setting of anatomic multivessel CAD, a survival benefit from revascularization compared with medical therapy only occurred in the setting of ECG evidence of ischemia, poor exercise tolerance, or both.7 Catheterization evidence, however, suggests that survival is a function of the anatomic extent of CAD such as multivessel disease, severity of stenosis, and location of stenosis, particularly if involving the left anterior descending artery (LAD). Therefore, the presence of severe CAD is a surrogate of the extent of jeopardized myocardium.8,9 In selecting an ideal diagnostic approach, anatomy remains an important determinant regarding the suitability of revascularization and may help with determining the role of medical therapy, but a pathophysiological approach continues to play an important role in decisions about medical therapy versus revascularization.
This review discusses currently available modalities used to assess and risk stratify patients with known or suspected coronary artery disease and provides a rationale for proper selection of non-invasive testing. Noninvasive imaging augments ECG evaluation by providing additional information about myocardial perfusion, left ventricular function, and coronary anatomy. This review will discuss various well established techniques including exercise stress testing, stress echocardiography, myocardial perfusion imaging (MPI), multi-slice CT (MSCT), positron emission tomography (PET), cardiac MRI (CMR), and combination MSCT/PET scanners.
Exercise Electrocardiography
Exercise electrocardiography (ETT), one of the least costly tests, has evolved into a modality of considerable importance in the evaluation of patients with known or suspected CAD, and is one of the most commonly used forms of noninvasive cardiac testing. The ETT study is designed to increase myocardial oxygen demand to assess the presence of inducible ischemia based on changes on the 12 lead ECG. An adequate test requires patients to reach 85% of their maximum predicted heart rate (220 - age). Inability to reach this threshold provides sub-optimal or equivocal results.
This test is best suited for patients who are able to exercise without limitations, have no prior evidence of coronary artery disease, and no underlying ECG abnormalities. Ideally, the individual has intermediate risk factors and needs further evaluation and risk stratification.
Exercise stress testing produces ST segment changes in patients with hemodynamically significant CAD and thus suggests the presence of ischemia. Test results can be reported in multiple fashions, but the most common and simplest report would be: normal (no inducible ischemia) or abnormal (ischemic changes). When used alone there is a high false-positive rate. The predictive value of an abnormal screening exercise test is determined by the presence or absence of risk factors for CAD. In addition, treadmill scores have been devised to estimate patient prognosis according to test results. The most popular validated treadmill score comes from Duke University based on data from 2758 consecutive patients with chest pain. This score, known as the Duke Treadmill Score (DTS), is based on three clinical variables: the exercise duration, maximal ST segment deviation, and presence of symptoms. The DTS has been shown to stratify prognosis accurately for both inpatient and outpatient symptomatic ischemic heart disease populations. Shaw and colleagues further demonstrated that this non-invasive risk index provided both diagnostic accuracy and prognostic risk estimates. The equation for calculating the DTS is DTS = exercise time-(5 x ST deviation)-(4 x exercise angina), with 0 = none,1 = nonlimiting, and 2 = exercise-limiting. The score typically ranges from –25 to +15. Low risk is a score greater than or equal to +5, moderate risk –10 to +4 and high risk less than or equal to –11.10,11 This allows individuals to be placed into low, intermediate, or high-risk groups. Those with a low Duke treadmill score had a less than 3% chance of multi-vessel disease or cardiac event in the next 5 years. On the other hand, those in the high-risk group had a 75% chance of multi-vessel disease and a 35% chance of cardiac event in the next 5 years.10,11
Other significant prognostic information can be obtained based on how much exercise capacity or workload a patient can perform, as expressed in Metabolic Equivalents (METs). Individuals who can achieve a higher exercise capacity perform better than their counterparts who do not exercise as long.12,13 Other prognostic information suggesting a higher risk is seen in patients with hypotensive or hypertensive response to exercise.10,11
Limitations to ETT occur most notably in women, where there is up to a 50% false-positive rate when used alone. The addition of a myocardial perfusion imaging or wall motion study improves the sensitivity and specificity of this test (discussed later).14 Other conditions including left ventricular hypertrophy, prior MI, bundle branch block, and other baseline ST-T wave abnormalities may also make the test nondiagnostic. Beta blockers can affect heart rate and may interfere with the ability to reach 85% of maximum predicted heart rate and should be avoided for 24 hours prior to the test unless contraindicated.15
Contraindications to exercise testing are active unstable angina, recent myocardial infarction, severe arrhythmias, hypertensive crisis, severe aortic stenosis, and other outflow tract obstruction such as hypertrophic obstructive cardiomyopathy.
Stress Echocardiography
Stress echocardiography provides visualization of the myocardium under physiologic or pharmacologic stress to assess for ischemia demonstrated by regional wall motion abnormalities. As in the case of ETT, a maximum predicted heart rate of 85% must be achieved requiring that the images be obtained during or immediately following exercise. Even a small delay as short as 90 seconds can alter test results. Either method is acceptable since the sensitivity and specificity of each of these methods are similar.16
The best candidates for this form of testing have no prior known CAD, are at moderate risk, and must be able to exercise to 85% of his/her maximum predicted heart rate. They should also be non-obese and have a small chest diameter since the distance from the chest wall to the heart can affect image quality. The true utility of this test is to observe for stress-induced ischemic changes in patients who are unable to exercise in a conventional fashion or who have other contraindications to standard ETT, such as left ventricular hypertrophy or other ST-T wave abnormalities that might produce equivocal results of an ETT.
Nishioka and colleagues found a 95% sensitivity for detection of stress-induced regional wall motion abnormalities in patients with or without prior MI, and the specificity for true ischemia was 84%, although in patients with prior MI this was cut in half to 42%.17
Another benefit can be noted in patients who are able to use the treadmill for their stress echocardiogram. By using the echo report of normal, single vessel, or multi-vessel disease based on regional wall motion abnormalities and linked with their treadmill score, important mortality predictions can be achieved. Those with low-risk Duke scores and no regional wall motion abnormalities had the best long-term mortality compared to all other groups.18 (See Table 2.)
 |
Image quality is paramount and factors that affect this such as obesity, COPD, or delayed image acquisition can produce equivocal results or uninterpretable images. Since patients with left bundle branch block or severe dysynchrony of the ventricles have abnormal wall motion, the test can be falsely interpreted as ischemic regional wall motion abnormalities. The same contraindications to other forms of stress testing apply here, such as active angina, MI, hypertensive crisis, or syncope, to name a few.19
Myocardial Perfusion Imaging (MPI)
Myocardial perfusion imaging (MPI) uses a radionucleotide such as thallium-201 or technetium 99 injected at the time of maximal exercise or after pharmacologically induced coronary vasodilatation with dipyridamole or adenosine. Exercise images are compared to rest images. Coronary artery disease is detected by reversible defects that represent inducible ischemia under myocardial stress. Perfusion defects imply the presence of significant CAD but do not demonstrate the degree or extent of coronary plaque. Perfusion defects yield information regarding the physiology of the CAD and not the anatomy. Technetium 99 has a sensitivity and specificity of 84% for detection of inducible ischemia; thallium has similar sensitivity but a 20% lower specificity.20 Technetium 99 also delivers lower levels of radiation and hence is widely accepted as the radioisotope of choice.
Patients who need risk stratification as well as identification of flow limiting lesions are ideal candidates for MPI. Virtually any patient is a candidate for this test since either physiologic stress or vasodilatation is used for assessment of inducible ischemic changes. Since exercise yields more information including interpretable ECG changes and is less expensive than pharmacologic agents, it should be used whenever possible. The physiologic stress of exercise or a pharmacologic agent can be used to induce work on the heart, and there does not appear to be any appreciable difference in sensitivity, specificity, or accuracy between the two.21,22 Dipyridamole, adenosine, and dobutamine have all been used effectively as alternatives to exercise. Dobutamine may have the advantage of achieving target heart rate and thus may have the added benefit of producing ST segment ECG changes in patients with ischemia.21,23
When exercise stress testing and nuclear perfusion imaging are combined, it is important to make sure the patient reaches at least 85% of his maximum predicted heart rate. A study by Iskandrian et al showed that patients who did not reach 85% of their maximum predicted heart rate had a much lower rate of detection of flow limiting lesions in patients with proven coronary artery disease.24 This effect was also shown in a small study of patients who underwent exercise MPI until they had symptom limiting exercise, and all reached 85% of their maximum heart rate. Two days later, the same patients underwent the same testing but stopped at 70% of their maximum heart rate. When the two studies were compared, the area of inducible ischemia was much smaller or even absent when patients stopped at 70% of their maximum predicted heart rate.25 These two studies demonstrate clearly the need for reaching 85% of maximum predicted heart rate.
Myocardial perfusion studies help with the diagnosis of coronary artery disease; aid in identifying those who are at low, moderate, and high risk for coronary events; and yield information about the degree of myocardium at risk. In a study of 4649 patients followed for 7 years who were at intermediate risk based on the Duke treadmill score and had normal or near-normal MPI studies, there was a 1% risk for cardiac mortality and a 3% risk for myocardial infarction.26 In patients with any perfusion defect, however, there is a significantly higher rate of cardiac events in the following year at a rate of 8.6% compared to 1.4% of patients with a normal study.25,27,28
It has also been shown that post-stress left ventricular dysfunction can have a prognostic value. In patients with an inducible perfusion defect, a left ventricular ejection fraction of > 45% was associated with a yearly mortality of < 1%. Those patients with left ventricular ejection fraction of < 45%, however, had a yearly mortality rate of 6.6%.13
A disadvantage of MPI is the exposure to ionizing radiation where doses range from 6-18 millisievert (mSv) depending on the agents used. This can be even higher with Thallium 201. Some women and obese individuals have a large amount of attenuation artifact that can be misinterpreted as false positives leading to additional invasive testing.29-34 Patients should avoid caffeine and theophylline for 24 hours before the use of adenosine or dipyridamole, although one cup of coffee before the test will not affect the results and should not delay testing.35 If a patient cannot stop theophylline, the preferred agent is dobutamine.
The major contraindications to adenosine and dipyridamole testing agents include sick sinus syndrome, high degree atria-ventricular block, hypotension, concurrent use of dipyridamole agents, and reactive airway disease.19
Multi-Slice Computer Axial Tomography (MSCT)
Imaging of the coronary anatomy by multi-slice computed tomography demonstrates the presence and extent of plaque in the coronary arteries, thus giving an anatomical picture similar to angiography. The entire study takes about 10 minutes from the time the patient is placed on the MSCT scanner table until the imaging is complete.36 To obtain good quality images, the patient should be in normal sinus rhythm with a heart rate under 70 beats per minute. Most patients are given a beta-blocker to ensure that their heart rate is at a desirable level. Images are obtained as an iodinated contrast bolus passes through the coronary arteries.
Patients most suited for this procedure are those with intermediate risk based on a Duke treadmill score, non-diagnostic test results, contraindication to other modalities, and those who prefer a noninvasive test. This test is best used to rule out coronary artery disease since there is a very high negative predictive value when using MSCT. It can also be used to further evaluate patients who have suspected false-positive stress tests, or those with a low or intermediate pretest probability for coronary artery disease.37,38 Patients with known disease who will need intervention or those with an existing stent are likely to need additional testing or intervention despite results of the MSCT and therefore are not the best candidates for this procedure.
Additional information available using CT includes the Agatston calcium score, which, while controversial, is used for prediction of coronary events and further evaluation of CAD.39 Patients with a score of 100 or less did not correlate with significant disease and are low risk. Scores over 400 are highly correlated with extensive plaque and would warrant further testing or aggressive risk modification. Scores between 100-400 are not useful to predict disease and are considered equivocal. However there is a 9.6% increase in relative risk for coronary events in patients with a score greater than 100.40
Some have argued that the Framingham risk tool provides similar information at a fraction of the cost and without exposure to ionizing radiation and thus do not recommend routine screening or assessment of CAD by coronary calcium score. It has been used effectively, however, in patients with multiple risk factors to predict benefit from the most aggressive medical therapies.
The best use of MSCT is to rule out the presence of disease. Patients who are at risk for multivessel disease or large areas of myocardial ischemia and have anginal symptoms should have their coronary anatomy visualized. This method allows anatomical evaluation without exposure to potential complications of invasive testing. In addition to the presence or absence of significant stenosis, MSCT suggests the extent of the plaque burden that may not be recognized by angiography.
Due to positive remodeling of the artery and compensatory vasodilatation (Glagov phenomenon), significant stenosis of the total diameter of the artery can occur before it becomes flow limiting. This effect allows the lumen to appear relatively unchanged when viewed by conventional angiography despite significant disease. Therefore conventional cardiac angiography is often referred to as "lumenography."41,42 The limitation of conventional angiography is eclipsed by MSCT, which allows the reviewing physician to determine the percent of stenosis to the lumen as well as the plaque burden to the entire artery. The plaques are labeled as hard or soft, allowing greater appreciation for unstable plaque burden.36
This information can be very important as soft plaque or extensive non-occlusive plaque may lead the clinician to prescribe a more aggressive medical regimen and motivate the patient to make lifestyle changes.
With 16 slice scanners there is a greater then 90% sensitivity and specificity for detection of significant coronary artery disease when segment stenosis is greater than 50%. These results are improved by using a 64-slice MSCT. There are convincing data to support this method of non-invasive evaluation for detection of CAD by arterial segment, and compared to conventional coronary angiography, sensitivity and specificity are very well maintained. There is also a high positive and negative predictive value. However when the artery is assessed as a whole, the sensitivity and specificity decrease, and there is a decrease in positive and negative predictive values.43 (See Tables 3 and 4.)
 |
 |
Radiation exposure is a prime safety concern. Many of these tests result in exposure to ionizing radiation. Diagnostic tests should be chosen that have the highest yield of information while minimizing the exposure. As a reference, the average individual is exposed to approximately 3 millisieverts (mSv) from naturally occurring sources per year. Since different body tissues require different energies to penetrate them, the radiation doses vary according to body size and mass. For example, women on average will receive higher doses of radiation due to breast tissue, and patients who are obese will require larger doses of radiation to penetrate through the fatty tissue.29 (See Figure 1.) The amount of radiation needed to penetrate body tissue should be considered when ordering a test. If the test is not going to change clinical management of the patient, then the risk of exposure to radiation is not justified. The utility of MSCT as a screening tool in asymptomatic patients has not been established and therefore MSCT should not be used routinely for screening of asymptomatic patients who are otherwise low risk for CAD.
 |
Patients likely to have significant stenosis and an interventional procedure should not have MSCT but should receive conventional coronary angiography to avoid excessive ionizing radiation and IV contrast.38,44
The contraindications for MSCT relate to the IV contrast. There are limitations to current MSCT, the most important of which is patients with known extensive CAD as heavy calcification may obscure the resolution and lead to artifacts or a non-diagnostic test. Similarly, patients with a prior stent are likely to exhibit artifact in the segment around the stent that may lead to non-diagnostic test results. Currently it is not possible to evaluate for in-stent stenosis. Also those with a very heavy plaque burden have lower specificity for vessel stenosis due to artifact.36,44
 |
Cardiac Magnetic Resonance Imaging (CMR)
Cardiac magnetic resonance imaging (CMR) utilizes a standard MRI machine that is equipped with specific additions of hardware and software. Advances in cardiac gating and new software designed to allow free breathing have improved image quality of CMR by minimizing motion artifact, allowing assessment of the entire cardiac arterial tree with minimal motion artifact.37
Patients who are the best candidates for this procedure are similar to those who benefit from MSCT where there are equivocal tests or contraindications to other procedures. Obvious advantages of CMR over conventional angiography include no ionizing radiation and no need for radiopaque contrast with the associated side effects of nephrotoxicity and anaphylactoid reaction. Gadolinium has been used as an alternative contrast medium that is superior to iodinated contrast in CMR, however several recent case reports show the potential for skin fibrosis.45 The major advantage CMR has over MSCT is the ability to detect subendocardial perfusion defects and micro-vessel ischemia.
The strength of this modality is clearly its negative predictive value. When CMR is utilized for the evaluation of 3 vessel disease or strictly left main disease, there is a sensitivity of 100%, specificity of 85%, PPV of 54%, and NPV of 100%. Just as with MSCT, there is decreasing sensitivity and specificity when vessels are viewed as a whole instead of in segments.46 When CMR was used for detection of CAD, Plein et al showed that myocardial perfusion reserve index (the change in signal intensity-time profiles of stress and rest myocardial perfusion by MRI) showed a sensitivity of 88% and a specificity 82%.47 Other studies have shown similar results with sensitivity 87-90% and specificity of 88-90% for detection of CAD.48,49
The technique of late enhancement has been used to determine myocardial viability and micro-vessel ischemia, which is a distinct advantage over MSCT or any other modality discussed so far. After injection of gadolinium-based contrast agent normal myocardium will enhance and then be washed out, leaving areas of ischemia appearing darker. As the contrast is washed out and achieves steady state, the areas of under-perfused tissues or damaged tissue with edema will retain contrast longer allowing these areas to appear "brighter" than the normal areas. This phenomenon is called late enhancement.50 The late enhancement technique yields results comparable to PET scan techniques, which are considered the gold standard for functional myocardial studies.
This technique is very sensitive to show subendocardial damage and can differentiate between hibernating versus scarred myocardium. A recent study by Holstrum et al.43 showed that despite normal left ventricular function, some patients with late enhancements were seen to have small subendocardial infarctions. Techniques utilizing parallel acquisition methods, such as sensitivity encoding, have decreased data collection times for cardiac imaging which enables non-uniform signal intensity correction by receiver coils thus allowing for a semi-quantitative and quantitative analysis of perfusion defects in the subendocardium.51
As with other studies, CMR also is imperfect. Patients with implanted metallic devices or pacemakers and those who are claustrophobic are not candidates for this procedure. This study is significantly longer than MSCT (30-60 minutes), and many patients find being in the supine position for this amount of time uncomfortable. The most important drawback is that there are not enough data at present in the form of large multi-center clinical outcomes. This lack of data leads to less standardized protocol and interpretation.52
Comparison of MSCT and CMR
When comparing MSCT and CMR it can be difficult to differentiate between the utility of the two modalities. The true power of these tests is their ability to rule out coronary artery disease. CMR, however, has the added benefit of being able to assess for subendocardial infarctions and areas of perfusion defects.
A recent meta-analysis by Schuijf and colleagues has shown a higher sensitivity and specificity for MSCT than for CMR for noninvasive coronary angiography. This may be partially due to the larger body of evidence that is already done for MSCT compared to CMR.45,53 Since both CMR and MSCT utilize cardiac gating methods, a patient who is not in sinus rhythm will likely have a non-diagnostic result.
Patient safety is also a concern that is paramount. Since there is no ionizing radiation with MRI, it has an advantage over both MSCT and conventional angiography. There are other factors that will influence the use of these tests such as patient preference, cost, and reimbursement. One study comparing MSCT, MRI, and conventional angiography by Dewey et al. showed that patients preferred the shorter time and less invasive nature of MSCT.38 With the rapid advances in hardware, software, and other technology in all areas of cardiac imaging, it will be very exciting and interesting to see what new developments are over the horizon.
Positron Emission Tomography (PET)
Patients with known CAD or with exercise limiting angina are may benefit from PET testing as it has the distinct advantage of assessing perfusion defects and viability of myocardium. This test is useful when equivocal results are reported with other modalities. There are few contraindications to PET scanning. Virtually all patients are candidates for this test, and it is being used as an alternative to standard perfusion testing as it gives better resolution and fewer problems with artifact. However PET does not give direct visualization of arteries but shows perfusion defects similar to MPI. PET does not have the limitation of soft-tissue attenuation and can be used to measure fractional flow reserve.
Recent work shows that PET has 90% sensitivity for detection of lesions greater than 50% with a specificity of 89%.8 The distinct advantage of PET is the ability to measure perfusion and viability of myocardium.54 Therefore, in one test the physician could determine what vessels are diseased with flow limiting lesions and whether the myocardium below the lesion is viable. This differentiates hibernating versus scarred myocardium when perfusion defects are compared at rest and at stress, and then appropriate revascularization therapy can be offered to patients who will truly benefit from it.
Combination PET/CT scanners are becoming more readily available and have the distinct advantage of offering the best of MSCT and PET scan in one. This allows high quality images of the coronary tree by MSCT and the perfusion and viability data of PET. This is essentially a "one stop shop" for assessing coronary artery disease.
Unfortunately this technology is very expensive and only available at limited facilities. As with all newer technologies, over time it will become more widely available for use and will likely replace the use of adenosine perfusion testing where available.
Conclusion
The most appropriate test for a patient is one that yields high quality information, has low risk, and will add further diagnostic or prognostic data. All patients should initially be risk stratified using a Noninvasive Prognostic Index such as Framingham or other risk assessment tool.55,56
Initial evaluation of symptomatic patients should be done by an exercise treadmill test as this is easy to perform, inexpensive, easily assesses for CAD, and can be used to predict mortality. If additional information is needed or for patients with high likelihood of false-positive results (women), a myocardial perfusion study (SPECT or PET) can be added to increase sensitivity and specificity or in the event of equivocal test results. The option of stress echocardiogram is a viable alternative and can be a substitute for perfusion imaging.57
In patients with high risk, equivocal stress test results or when anatomy of the coronaries is necessary, MSCT is an ideal test. This can definitively rule out coronary disease or make the presence and size of lesions known.
In patients with known disease, or with symptoms that need evaluation for subendocardial perfusion defects or the viability of myocardium, CMR or PET is an excellent choice. Currently these modalities are not recommended for routine screening of low risk patients.19,35 Combination scanners using PET/CT will likely become the diagnostic test of choice as newer protocols limit the radiation exposure and this combination provides both physiologic and anatomic results.
Regardless of what testing modalities are chosen. All patients need aggressive risk reduction and optimization of medical therapies.
References
1. Lloyd-Jones DM, Larson MG, Beiser A. Lifetime risk of developing coronary heart disease. Lancet 1999;353:89.
2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III. JAMA 2001;285:2486-2497.
3. West RR, Ellis GR, Brooks N. Complications of diagnostic cardiac catheterization: Results from a confidential enquiry into cardiac catheter complications. Heart 2005 Nov 24.
4. Duffin DC, Muhlestein JB, Allisson SB, et al. Femoral arterial puncture management after percutaneous coronary procedures: A comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invasive Cardiol 2001;13:354-362.
5. Meyerson SL, Feldman T, Desai TR, et al. Angiographic access site complications in the era of arterial closure devices. Vasc Endovascular Surg 2002;36:137-144.
6. Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus panel report. J Cardiovasc Magn Reson 2004;6:727-765.
7. Weiner DA, Ryan TJ, McCabe CH, et al. The role of exercise testing in identifying patients with improved survival after coronary artery bypass surgery. J Am Coll Cardiol 1986;8:741-748.
8. Di Carli, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation 2007;115:1464-1480.
9. Califf RM, Phillips HR, Hindman MC, et al. Prognostic value of a coronary artery jeopardy score. J Am Coll Cardiol 1985;5:1055-1063.
10. Mark DB, Hlatky MA, Harrell FE Jr, et al. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med 1987;106:793-800.
11. Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation 1998;98:1622-1630.
12. Roger VL, Jacobson SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing. Circulation 1998;98:2836-2841.
13. Sharir T, Germano G, Kavanagh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation 1999;100:1035.
14. Henneman MM, Schuijf JD, van der Wall EE, et al. Non-invasive anatomical and functional imaging for the detection of coronary artery disease. Br Med Bull 2006;79-80:187-202.
15. Shehata AR, Gillam LD, Mascitelli VA, et al. Impact of acute propranolol administration on dobutamine induced myocardial ischemia as evaluated by myocardial perfusion imaging and echocardiography. Am J Cardiol 1997;80:268.
16. Flachskampf FA, Hoffmann R, vom Dahl J, et al. Functional assessment of PTCA results by stress echocardiography: When and how to test. Eur Heart J 1995; Oct 16 Suppl J: 31-34.
17. Nishioka T, Mitani H, Uehata A, et al. Utility and limitation of treadmill exercise echocardiography for detecting significant coronary stenosis in infarct-related arteries in patients with healed myocardial infarction. Am J Cardiol 2002;89:159.
18. Marwick TH, Case C, Vasey C, et al. Prediction of mortality by exercise echocardiography: A strategy for combination with the Duke treadmill score. Circulation 2001;103:2566.
19. Ha JW, Juracan EM, Jahoney DW, et al. Hypertensive response to exercise: a potential cause for new wall motion abnormality in the absence of coronary artery disease. J Am Coll Cardiol 2002;39:323.
20. Taillefer R, Gordon D, Udelson J, et al. Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG gated-SPECT) in detection coronary artery disease in women. J Am Coll Cardiol 1997;29:69.
21. Dagianti A, Penco M, Agati L, et al. Stress echocardiography: Comparison of exercise, dipyridamole and dobutamine in detecting and predicting the extent of coronary artery disease. J Am Coll Cardiol 1995;26:18-25.
22. Mahmarian JJ, Verani MS. Exercise thallium-201 perfusion scintigraphy in the assessment of coronary artery disease. Am J Cardiol 1991;67:2D.
23. Kim C, Kwok YS, Heagerty P, et al. Pharmacologic stress testing for coronary disease diagnosis: A meta-analysis. Am Heart J 2001;142:934.
24. Iskandrian AS, Heo J, Kong B, et al. Effect of exercise level on the ability of thallium-201 tomographic imaging in detecting coronary artery disease. J Am Coll Cardiol 1989;14:1477.
25. Heller GV, Ahmed I, Tilikemeier PL, et al. Influence of exercise intensity on the presence, distribution, size of thallium-201 defects. Am Heart J 1992;123:909.
26. Gibbons RJ, Hodge DO, Berman DS, et al. Long-term outcome of patients with intermediate-risk exercise electrocardiograms that do not have myocardial perfusion defects on radionuclide imaging. Circulation 1999;100:2140.
27. Heller GV, Herman SD, Travin MI, et al. Independent prognostic value of intravenous dipyridamole with technetium-99m sestamibi tomographic imaging in predicting cardiac events and cardiac related hospital admissions. J Am Coll Cardiol 1995;26:1202.
28. Geleijnse ML, Elhendy A, van Domburg RT, et al. Cardiac imaging for risk stratification with dobutamine-atropine stress testing in patients with chest pain. Circulation 1997, 96, 137-147.
29. Hunold P, Vogt FM, Schmermund A, et al. Radiation exposure during cardiac CT: Effective doses at multi-detector row CT and electron-beam CT. Radiology 2003;226:145-152.
30. Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation 2003;107:917-922.
31. Crean A, Dutka D, Coulden R. Cardiac imaging using nuclear medicine and positron emission tomography. Radiol Clin North Am 2004;42:619-634.
32. Rao DV, Shepstone BJ, Wilkins HB, et al. Kinetics and dosimetry of thallium-201 in human testes. J Nucl Med 1995;36:607-609.
33. Setting up a myocardial perfusion scintigraphy service: Clinical and business aspects. Prepared jointly by the British Cardiac Society, the British Nuclear Cardiology Society, and the British Nuclear Medicine Society. Heart 2005;91(suppl IV):iv6-iv14.
34. Conti CR. One-stop cardiovascular diagnostic imaging (and radiation dose). Clin Cardiol 2005;28:450-453.
35. Zoghbi GJ, Htay T, Aqel R, et al. Effect of caffeine on ischemia detection by adenosine single photon emission computed tomography perfusion imaging. J Am Coll Cardiol 2006;47:2296.
36. Ropers D. Multi-slice computed tomography for detection of coronary artery disease. J Interv Cardiol 2006;19:574-582.
37. Escolar E, Weigold G, Fuisz A, et al. New image techniques for diagnosing coronary artery disease. CMAJ 2006;174:487-495.
38. Dewey M, Teige F, Schnapauff D, et al. Noninvasive detection of coronary artery stenosis with multislice computed tomography or magnetic resonance imaging. Ann Int Med 2006;145:407-415, and appendix w123-w126.
39. Berman DS, Rory H, Shaw LJ, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Assessment of patients with suspected coronary artery disease. J Nucl Med 2006;47:74-82.
40. Berman DS, Hachamovitch R, Shaw LJ, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Noninvasive risk stratification and a conceptual framework for the selection of noninvasive imaging tests in patients with known or suspected coronary artery disease. J Nucl Med 2006;47:1107-1118.
41. Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371-1375.
42. Topol E, Nissen S. Our preoccupation with coronary luminology: The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1996.
43. Miia H, Paula V, Helena H, et al. Noninvasive analysis of coronary artery disease with combination MDCT and functional MRI. Acad Radiol 2006;13:177-185.
44. Musto C, Simon P, Nicol E, et al. 64-multislice computed tomography in consecutive patients with suspected or proven coronary artery disease: Initial single center experience. Int J Cardiol 2007;114.
45. Khurana A, Runge VM, Narayanan M, et al. Nephrogenic systemic fibrosis: A review of 6 cases temporally related to gadodiamide injection. Invest Radiol 2007;42:139-145.
46. Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for detection of coronary stenosis. N Engl J Med 2001;345:1863-1869.
47. Plein S, Radjenovic A, Ridgway JP, et al. Coronary artery disease: Myocardial perfusion MR imaging with sensitivity encoding verses conventional angiography. Radiology 2005;235:423-430.
48. Al-saadi N, Nagel E, Gross M. Non-invasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation 2000;101:1379-1383.
49. Schwitter J, Nanz D, Kneifel S. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: A comparison with positron emission tomography and coronary angiography. Circulation 2001;103:2230-2235.
50. Barkhausen J, Hunold P, Waltering K. MRI in coronary artery disease. Eur Radiol 2004;14:2155-2162.
51. Pruessmann KP, Weiger M, Scheidegger MB, et al. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med 1999;42:952-962.
52. Cuocolo A, Acampa W, Imbriaco M. The many ways to myocardial perfusion imaging. QJ Nucl Med Mol Imaging 2005;49:4-18.
53. Schuijf JD, Bax JJ, Shaw LJ, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multi-slice computed tomography for non-invasive coronary angiography. Am Heart Journal 2006;151:404-411.
54. Yoshinaga K, Katoh C, Noriyasu K, et al. Reduction of coronary flow reserve in areas with and without ischemia on stress perfusion imaging in patients with coronary artery disease: A study using oxygen 15–labeled water PET. J Nucl Cardiol ;10:275-283.
55. Califf RM, Armstrong PW, Carver JR, et al. 27th Bethesda Conference: Matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 5. Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol 1996;27:1007-1019.
56. Mark DB, Shaw L, Harrell FE Jr, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med 1991;325:849-853.
57. Elhendy A, van Dombury RT, Bax JJ, et al. Accuracy of dobutamine technetium 99m sestamibi SPECT imaging for the diagnosis of single-vessel coronary artery disease comparison with echocardiography. Am Heart J 2000;139(2 Pt 1):224-230.
Whether it's new onset or established disease, the PCP has an arsenal of diagnostic tests available. The array of tests, however, can be quite daunting in identifying the right test to order, especially in light of sensitivity and specificity, not to mention cost. This issue provides the PCP with a comprehensive survey of the diagnostic tests available, a guide to selection, an estimate of cost, and limitations of each test.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
