Subarachnoid hemorrhage: Misdiagnosed and overlooked
By Charles E. Rawlings, MD, JD, Rawlings Neurosurgical Consulting, Winston-Salem, NC; Stephanie Rifkinson-Mann, MD, JD, Neurosurgical Consultant, Mount Kisco, NY; Attorney, New York City.
Editor’s note: Headache is a common chief complaint encountered by emergency physicians (EPs). It may be a symptom of benign diseases such as migraine headaches or a common virus. Unfortunately, it also may represent other, more life-threatening illnesses, including subarachnoid hemorrhage. Delineating which patients need radiologic imaging, spinal fluid testing, and even angiography is part of the challenge encountered by the EP. This issue will outline strategies for reducing risk in the headache patient. Specifically, diagnosis and management of subarachniod hemorrhage will be detailed.
Introduction
An otherwise healthy patient presents to the emergency department (ED) with the worst headache of her life. A brain computerized tomographic (CT) scan reveals a subarachnoid hemorrhage (SAH), and an arteriogram reveals an accessible Circle of Willis aneurysm. The patient undergoes successful intracranial surgery for clipping of her aneurysm and is discharged several weeks later in good condition.
Another patient presents to the ED with complaints of an unusually severe migraine headache. She is treated and released without investigative studies. The same patient returns the following day with similar complaints. Again, she is treated and released. She is found the next day comatose and is declared brain-dead the following day. An autopsy reveals a ruptured basilar tip aneurysm with significant vasospasm.
A third patient presents to the ED with complaints of a migraine headache unlike any other she has experienced. Her headache is accompanied by neck pain and stiffness and began the day after physical exercise. She exhibits no other findings except mild neck stiffness. She consequently is diagnosed with a migraine headache and a cervical strain. She is treated symptomatically and released with no further investigative studies. The following day, she returns to the ED with a more significant headache and some photophobia. Again, she is treated symptomatically and released. She continues with significant headaches over the next week and presents again to the ED the following weekend. While in the ED, she becomes comatose, seizes, and is declared brain-dead. An autopsy reveals a posterior communicating artery aneurysm.
The above scenarios illustrate routine encounters in the ED between patients and physicians involving the failure to diagnose and the failure to treat SAH. In the first two scenarios, no medical negligence occurred since each patient’s ultimate outcome was not affected directly by their initial management in the ED. In the third case, the medical negligence should be obvious.
The most common causes of ED malpractice arise from misdiagnosis, the failure to diagnose, failure to admit, improper or incorrect treatment, and lack of consultation with appropriate specialists.1 Most failure-to-diagnose cases include the failure to diagnose appendicitis, myocardial infarction, meningitis, ectopic pregnancy, and fractures.1 While not among those entities most commonly misdiagnosed in the ED, patients with SAH present with a common ED complaint — headaches — and if not properly diagnosed and treated, the condition carries high rates of morbidity and mortality.2,3 As a result, a patient who presents to the ED complaining of a headache must be approached in a thorough and careful manner to exclude devastating illnesses and potentially curable conditions such as SAH due to aneurysmal rupture.
SAH has multiple known causes, including trauma, hypertension, arteriovenous malformations, brain tumors, endocarditis, anticoagulants, infections, as well as unknown processes.3 The most common atraumatic cause of SAH is a ruptured "berry" aneurysm (so named because of its morphology) arising from an intracerebral vessel in the region of the Circle of Willis. (See Table 1.)4,5 Aneurysmal SAH is fairly common, accounting for 5-10% of all strokes. It is the cause of death in about 0.5% of the population.6-8 About 2% of the population harbors an aneurysm, and about 1% of the population will experience an aneurysmal SAH. The annual incidence of SAH ranges from nine to 28 per 100,000 population.9 In the U.S. population of 300 million, about 6 million people harbor aneurysms, and 3 million will suffer a SAH.3 The majority of these patients, if diagnosed early, can be treated successfully with surgical clipping of the aneurysm or endovascular techniques to obliterate it. The following cases will illustrate instances of failure to diagnose intracranial aneurysms and will present pointers for and pitfalls of managing aneurysmal SAH in the ED.

Case #1. Luoma v. Kaiser, et al. 10
In Luoma, the husband and minor children of the deceased brought a wrongful-death suit based upon the failure to diagnose and treat the decedent’s aneurysmal SAH. The defendants included the hospital, the treating EP, and the consulting neurologist. In 1989, Mrs. Luoma, a 37-year-old meat wrapper, was diagnosed with mild migraine headaches that were infrequent and controlled by nonprescription medications. In 1996, Mrs. Luoma also was diagnosed as hypertensive and was prescribed atenolol. She had no other significant medical history.
On May 4, 1996, Mrs. Luoma presented to a Kaiser ED, complaining of the worst headache she’d ever had. Immediately prior to presenting to the ED, she had experienced leg weakness, visual difficulty in her left eye, and hearing dysfunction; however, her neurological complaints had cleared by the time she presented to the ED. In the ED, Mrs. Luoma was told to continue taking her atenolol, which she had missed for two days. No investigative procedures were performed, and she was discharged home with directions to follow up with her family doctor in a week, and only if her headaches persisted. The following day, Mrs. Luoma stayed in bed with a severe headache.
Two days later, Mrs. Luoma presented to a different Kaiser ED and underwent a brain CT scan and a lumbar puncture (LP). The brain CT scan was read as negative, but the cerebrospinal fluid (CSF) from the LP revealed xanthochromia (i.e., blood breakdown products indicative of SAH). Both the ED physician and the consulting neurologist failed to appreciate Mrs. Luoma’s symptoms and CSF findings. Mrs. Luoma was discharged home and was told she had "nothing to worry about."
Mrs. Luoma continued to experience severe headaches and presented to the second Kaiser ED again the following day. A repeat LP again revealed xanthochromic CSF. The same neurologist was re-consulted. He advised discharging the patient and discouraged a neurosurgical consultation. Another staff physician then was consulted, and as a result, Mrs. Luoma finally was admitted three days after her initial presentation. She underwent a cerebral arteriogram that revealed an anterior communicating artery aneurysm, but she suffered an aneurysmal re-bleed following the study. Despite successful clipping of her aneurysm, she died seven days after her initial presentation to the ED.
At trial, the defendants admitted liability and admitted that they breached the applicable standard of care regarding the failure to diagnose and treat Mrs. Luoma’s SAH. However, they argued that their breach was not the direct cause of Mrs. Luoma’s death, but rather that her death was due to the aneurysmal re-bleed following an arteriogram, which would not have been avoided by earlier diagnosis or treatment. Ultimately, the jury found for the plaintiffs, basing its decision upon testimony given by several medical experts to the contrary, and awarded the plaintiffs more than $700,000.
Discussion
Luoma illustrates several important points regarding the failure to diagnose SAH in an ED setting. The first point involves patients who present with the worst headache of their lives regardless of medical or headache history. Approximately 90% of patients with aneurysms present with SAH, the cardinal diagnostic feature of which is a very severe headache of sudden onset.11 As a result, patients presenting to the ED or to any medical professional with the worst headache of their lives, or with headaches that are substantially different from any of their prior headaches, must be assumed to be suffering from a SAH until proven otherwise. In patients with suspected SAH, CT scanning should be performed immediately. With modern CT scanners, more than 90% of patients with a SAH will demonstrate evidence of blood in the cerebral subarachnoid spaces. In patients with a negative CT, an LP generally will reveal an elevated number of red blood cells or xanthochromia, indicating recent SAH. Once a diagnosis of SAH is made, the patient must be admitted immediately, neurosurgical care instituted, and appropriate angiographic studies obtained quickly to localize and define the surgical anatomy of the actual aneurysm.3-5,12
As a reminder to physicians who are in the position to diagnose a patient with SAH during its initial presentation, more than a third of these patients do not demonstrate localizing signs such as hemiparesis, aphasia, or ptosis.13 Generalized signs of meningeal irritation, such as meningismus (i.e., stiff neck) and coma, are present in more than 50% of patients with SAH.13 Based on what may seem to be a relatively benign clinical presentation for SAH in a significant number of patients, the ED physician should suspect a ruptured aneurysm in any individual with a new, changed, or severe headache and immediately should obtain an unenhanced brain CT scan.
In Mrs. Luoma’s case, her headache was characterized as the worst headache she’d ever had and differed significantly from her past mild migraines. Such a headache, even in a patient with a known migraine history, should have been evaluated immediately with a CT scan and, if negative, an LP. Failing to investigate Mrs. Luoma’s headache by CT scan or LP was a clear breach of the applicable standard of care. When Mrs. Luoma finally underwent LP, which revealed xanthochromic fluid, the standard of care required that she be admitted with a working diagnosis of SAH. Failure to do so again breached the applicable standard of care.
Another issue presented in Luoma regards aneurysmal rupture following cerebral angiography. The risk of aneurysmal re-rupture does not present a contraindication to angiographic study of patients suspected of having suffered SAH. While angiography may be associated with an increased incidence of aneurysm re-bleed, this is more likely to occur if the angiogram is performed more than three days after the original bleed.14,15 Most aneurysmal re-bleeds occur within the first seven to 14 days after initial hemorrhage, especially after the third day following aneurysmal rupture.16 This time period coincides directly with the most likely time period that arteriographic investigation of the patient is performed. Those aneurysms that do rupture following arteriography tend to be in patients who are in extremely poor clinical condition, as indicated by their Hunt and Hess grade, which is helpful in determining surgical risk; the higher the grade, the greater the risk for death or poor clinical outcome.17 Aneurysmal rupture also can occur with cross-compression of the carotid artery in the neck, done during the angiographic study for better visualization of the cerebral vasculature.14 In Mrs. Luoma’s case, the timing of her angiography coincided with the period during which the risk of aneurysmal re-bleed is greatest, within seven to 14 days of her initial bleed. It is far less likely that Mrs. Luoma’s re-hemorrhage was related to cross-compression of her carotid artery; and, as such, it probably was completely unrelated to her arteriographic procedure. Had Mrs. Luoma undergone an angiogram on the day she first presented to the ED rather than on the seventh day after her SAH, and then undergone appropriate neurosurgical treatment, she most likely would not have re-bled following the angiographic study since it would have been done prior to the period of time during which she would have been at the highest risk for another SAH.
Finally, a third important issue raised by this case is that SAH patients who are clinically in good condition (e.g., a favorable Hunt and Hess grade), and who are treated neurosurgically in a timely manner, generally experience good clinical outcomes. (See Table 2.)

The Hunt and Hess scale estimates prognosis based upon the severity of the Hunt and Hess grade which, in turn, is based upon the severity of the patient’s symptoms at the time of presentation. (See Table 3.)17 Based upon that scale, the prognosis for a SAH patient declines dramatically as the grade increases. Grade I represents a patient with ptosis or no localizing neurological signs beyond meningismus and photophobia, while a Grade V patient is moribund.
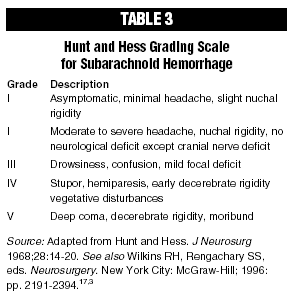
In the International Cooperative Study of the Timing of Aneurysm Surgery involving more than 3,500 patients (hereinafter, the "International Cooperative Study"), of those patients who presented with an anterior communicating artery aneurysm and who were classified as Hunt and Hess grades I or II, 84% had good outcomes following surgery, while only 47% of those patients presenting with Hunt and Hess grades III, IV, or V did well after aneurysmectomy (i.e., surgical obliteration of the aneurysm). 3-5 The most important factors predicting patient prognosis are level of consciousness at the patient’s time of presentation and associated hemiparesis or aphasia.4,5 In that same study, the correlation between Hunt and Hess grade and prognosis was established for most patients with anterior circulation aneurysms, including anterior communicating, middle cerebral, and posterior communicating artery aneurysms. In general, 75% or more of patients with commonly located, nongiant aneurysms presenting in good neurological condition should have a good prognosis for recovery.
Mrs. Luoma, in particular, presented repeatedly to the ED with severe headache unaccompanied by a decrease in consciousness, hemiparesis, aphasia, dysphasia, or any other localizing neurological sign. Given that clinical presentation, she would have been classified as a Hunt and Hess grade I or II had she been evaluated properly. Given her good Hunt and Hess score, had her SAH been diagnosed timely, and had she undergone appropriate neurosurgical intervention, she would have had a greater than 75% chance of having a good outcome.
Case #2. Cohn v. Frost 18
In Cohn, the husband and minor children of the deceased brought a wrongful death suit based upon the failure to diagnose and treat the decedent’s SAH. The plaintiffs alleged that the defendants negligently relied upon the findings of a brain CT scan performed eight days following the onset of the decedent’s severe headaches, and alleged that the defendants negligently failed to perform LP following a normal brain CT scan. The defendants included the decedent’s family physician and Dr. Frost, a neurologist.
On Oct. 10, 1984, Mrs. Cohn, a 39-year-old office manager with a history of mild menstrual migraine headaches and no other significant medical history, developed unusually severe headaches. Her family physician evaluated her and recommended chiropractic treatment for what he termed a "military neck." Eight days later, the physician obtained a brain CT scan, which was read as normal. He did not perform LP or any other test. Mrs. Cohn’s severe headaches continued, and her physician subsequently admitted her to the local hospital with a diagnosis of possible anorexia nervosa accompanied by migraine headaches. Two days following her hospital admission, Dr. Frost, a neurologist who diagnosed her with viral meningitis, evaluated Mrs. Cohn. Dr. Frost did not repeat her brain CT scan and did not perform LP to sample her CSF. Mrs. Cohn was discharged home a few days later with no further treatment. Three weeks after her initial presentation, Mrs. Cohn collapsed as a result of a massive SAH while at a relative’s home and died the same day. Study results confirmed that she had suffered a ruptured right, middle cerebral artery aneurysm.
At trial, the plaintiffs alleged that the neurologist was negligent based upon his failure to diagnose Mrs. Cohn’s SAH; that he was negligent in failing to perform LP in light of her severe headaches and her normal brain CT scan; and that he was negligent for having relied for his diagnosis only upon the brain CT scan that had been obtained eight days after Mrs. Cohn originally complained of a headache. The plaintiffs’ experts testified that a brain CT scan performed more than a week after initial presentation of hemorrhage would be diagnostic in only 50% of cases, and that LP therefore would have been indicated to diagnose the SAH in Mrs. Cohn’s case. The defense agreed that, had Mrs. Cohn’s aneurysm been diagnosed prior to her death, timely and efficacious neurosurgical treatment most likely would have been successful.
However, the defense argued that Mrs. Cohn’s headaches were consistent with migraine or menstrual headaches and claimed that LP was not necessary based upon Mrs. Cohn’s symptoms and the neurologist’s diagnosis of viral meningitis. The jury agreed with the plaintiff’s defense and awarded $9.8 million. Only the neurologist — and not the family physician — was found to have been negligent.
Discussion
Cohn illustrates several important principles regarding the failure to diagnose SAH. First, the case reinforces the principle that any headache said to be the worst headache ever or any headache that is unusual or that fails to conform to a prior diagnosis, must be investigated aggressively by brain CT scanning, and possibly with LP if indicated clinically. This point was illustrated previously in Luoma.
The second principle illustrated by the Cohn case concerns the limits of brain CT scanning and supplementation of its diagnostic capabilities by LP. As has been emphasized repeatedly, the diagnostic procedure of choice (and the easiest to obtain) in the work-up of a patient with a severe headache is a noncontrast, brain CT scan. Although most radiology departments have implemented their own protocols, techniques of CT scanning can affect the diagnosis of SAH. Thin tomographic axial images or "cuts" are more efficacious than thick, axial cuts (e.g., 3 mm in thickness as opposed to 10 mm) when investigating the base of the brain and associated CSF cisterns.12,19 The increased density of blood adjacent to bone also may impede the recognition of small amounts of blood in the subarachnoid spaces, while artifact associated with patient movement also may impede diagnosis. More significantly, the ability of CT scanning to detect subarachnoid blood decreases over time, due to lysis of blood cells and the continued wash-out and dilution of CSF.3,12,20
The International Cooperative Study demonstrated the decreasing efficacy of CT scanning in the diagnosis of SAH. The study showed that 92% of CT scans were positive on the day of the actual hemorrhage; 86% were positive 24 hours later; and only 58% were positive five days later.4,5,16 A similar study showed that only 50% of the scans were positive one week following the initial SAH.12,21 More recent studies evaluating the efficacy of more modern, third-generation, CT scanners also have shown a decrease in the rate of diagnosis as time elapses from the moment of the initial bleeding. These studies show that 100% of the scans were positive within 12 hours of the bleed, while 93% were positive 24 hours later.22 Based upon the decreasing sensitivity of CT scanning with increased time elapsing from the moment of bleeding, LP is indicated to help establish the diagnosis of SAH.23 Most authors maintain that LP must be performed for a patient presenting with clinical signs of SAH and with a brain CT scan that is technically inadequate or that is read as negative or questionable.24-27
The use of LP particularly is important in a patient who presents some time after initial headache, when CT scanning has reduced diagnostic sensitivity significantly. As noted previously, following SAH, erythrocyte wash-out occurs quickly, thereby diluting the CSF, although elevated erythrocyte numbers may persist for weeks. Red blood cells begin to lyse in 12-24 hours with a concomitant rise in CSF xanthochromia. It is this xanthochromia that LP detects. One study showed that 100% of SAH patients were found to have xanthochromic CSF upon LP between 12 hours and two weeks following their initial hemorrhage, suggesting that xanthochromic CSF would be present even when CT scanning is said to be negative for SAH.28
In Mrs. Cohn’s case, the brain CT scan was performed more than a week following her initial symptoms. Based upon the above studies, her CT scan, more likely than not, would have failed to reveal her SAH. As a result, she should have undergone LP, which would have revealed xanthochromic CSF indicative of earlier SAH. As should be clear, reliance solely upon a CT scan performed more than a week following the onset of symptoms and the failure to perform a spinal tap to supplement such a diagnostic work-up, constitutes a breach of the applicable standard of care.
The third principle illustrated by Cohn concerns the concept of sentinel bleeds occurring prior to the actual, massive rupturing of a patient’s intracerebral aneurysm. Many patients experience some type of warning symptom prior to a massive aneurysmal rupture. Most studies reveal that 20-59% of SAH patients will experience pre-rupture warning signs or symptoms that are related either to aneurysmal expansion (e.g., new onset of a third nerve palsy), or to small leaks from the aneurysm itself manifested as a fairly severe headache, known as a "sentinel headache" due to a sentinel bleed.12,3
Most studies of aneurysm patients indicate that the most common pre-rupture warning symptom is the sentinel headache. Completed prior to the larger International Cooperative Study, results from another cooperative study involving more than 2,500 aneurysm patients revealed that in those patients with pre-rupture symptoms, 48% suffered headaches.29 Results from a different study also reported that the most common symptom forewarning SAH was headache, noting that patients with missed warning signs had a significantly higher mortality rate than those whose aneurysms had been diagnosed early.30
In another study of patients with single intracranial aneurysms, headaches were the most common pre-rupture warning symptom, with warning signs and symptoms occurring more frequently in younger patients.31 This same report observed an interval of several weeks elapsing between the warning headache and massive SAH. Finally, another study of sentinel headaches reported that these headaches occurred in 40% of SAH patients, but that the sentinel bleeds were diagnosed by brain CT scan in only 45% of these individuals, while the sentinel bleed was demonstrated by LP in 100% of the cases.32
In Cohn, Mrs. Cohn obviously suffered from a sentinel headache that predated her massive aneurysmal rupture by several weeks. Her sentinel bleed occurred within the time frame that most sentinel bleeds occur, causing headaches. A CT scan performed more than a week after her initial presentation was read as normal. Such negative CT readings, as noted previously, are not unexpected. Mrs. Cohn did not undergo the one test that would have revealed her sentinel bleed — a spinal tap, which is known to demonstrate SAH or associated xanthochromia in almost 100% of cases. Mrs. Cohn died unnecessarily due to her second, massive aneurysmal rupture. Her case illustrates that patients with sentinel bleeds who go undiagnosed have a significantly elevated mortality rate.
Case #3. Brillant v. Royal, et al. 33
Brillant v. Dr. Lorenza Royal and Sterling Medical Associates, et al. is a medical malpractice case brought by the patient and her husband against Dr. Royal and Sterling Medical Associates in which the plaintiffs appealed a lower (trial) court’s earlier decision dismissing the case against the defendants. The plaintiffs contended that Dr. Royal failed to diagnose Mrs. Brillant’s cerebral aneurysm and SAH by not performing a CT scan of the brain and/or LP, and by not consulting a neurologist; they also contended these failures prevented an accurate diagnosis of her condition, thereby preventing timely medical care, and proximately caused her subsequent paralysis. In addition, the plaintiff contended that Sterling Medical Associates was vicariously liable for Dr. Royal’s negligence because he was an employee or an agent of the medical group at the time the incident occurred. The trial court directed a verdict for Sterling on the grounds that there was no evidence that Dr. Royal was anything other than an independent contractor and directed a verdict for Dr. Royal on the grounds that the Brillants had not presented substantial evidence that Dr. Royal’s actions were the direct cause of Mrs. Brillant’s injuries. The court found the plaintiffs’ exclusive remedy was against the United States under the Federal Torts Claim Act (FTCA)34 because Dr. Royal was working at an Army hospital at the time of the incident.
While the Brillants were visiting friends in Georgia, Mrs. Brillant experienced the sudden onset of an extremely severe headache. When the headache would not subside, the Brillants returned home to Alabama that night; and the following morning, Mrs. Brillant went to the ED at Lyster Army Community Hospital. Dr. Royal, the physician on call in the ED, documented the sudden onset of Mrs. Brillant’s headache, neck soreness on range of motion and palpation, and that her "pain was worse than her migraines" that she had suffered for years. Dr. Royal found no deficits on his neurological examination and diagnosed a "spastic muscular headache," prescribed an analgesic and a muscle relaxant, and instructed Mrs. Brillant to "return as needed."
Eight days later, Mrs. Brillant returned to the Lyster Army Community Hospital for further treatment for her headache, which though persistent, was not quite as severe. A different physician — who was unaware of her prior ED visit — examined her. He did a routine medical examination, prescribed an analgesic and a muscle relaxant, and recommended physical therapy.
Seventeen days after the onset of Mrs. Brillant’s headache, she suffered a massive SAH due to cerebral aneurysmal rupture. She was taken to the ED at a different hospital where the ED physician obtained a CT scan of the brain, confirming the SAH. She was transferred to a tertiary care hospital with neurosurgical services, where a cerebral aneurysm was diagnosed. Eighteen days after the plaintiff’s initial presentation to the ED with severe headache, she underwent successful clipping of an aneurysm, but three days later, a CT scan performed in conjunction with new onset of left hemiparesis revealed right frontotemporal edema, apparently due to vasospasm and ischemia. Subsequent rehabilitation had not corrected the hemiparesis, which was permanent.
Discussion
The medical question in Brillant was whether, in general, timely diagnosis and treatment of an aneurysm after a warning leak would have prevented vasospasm and subsequent neurological deficits, and in particular, whether they would probably have done so in Mrs. Brillant’s case. The crucial issue the court chose to study was whether an excess amount of blood in the brain would be more likely to irritate the cerebral blood vessels and cause vasospasm than a small amount of blood, such as that occurring after a "warning leak" or a sentinel bleed.
At trial, defendant Dr. Royal admitted that, in retrospect, Mrs. Brillant probably had a leaking aneurysm when he saw her; however, he argued that his failure to diagnose her aneurysm was not the proximate or direct cause of her hemiparesis. He argued that her paralysis was due to vasospasm and subsequent stroke following her surgery, which would not have been avoided by earlier diagnosis. The court found that there was sufficient evidence presented at trial to indicate that there was more blood in the subarachnoid space after aneurysmal rupture than after a warning leak and that such an excess amount of blood would be more likely to cause vascular irritation, vasospasm, and ischemia, resulting in neurologic deficits, such as Mrs. Brillant’s hemiparesis. The court also noted that there was substantial evidence to indicate that a person with a warning leak had a better prognosis for full recovery since that individual would be more likely to have a small amount of blood in the subarachnoid space and less vascular irritation.
Essentially, a patient with a good grade neurologically (i.e., alert, in general good health and without large amounts of blood in the brain) is a better candidate for neurosurgery than a person with a poor grade neurologically (i.e., obtunded with a significant neurological deficit or unconscious or comatose with large amounts of blood in the brain). Although the defendant’s argument that earlier diagnosis would not have altered the patient’s outcome because her injuries were due to vasospasm, there was evidence that the plaintiff was a good candidate for surgery when she presented with her first symptom of severe headache, if her aneurysm had been diagnosed in a timely fashion. Since there was a delay of more than two weeks between Mrs. Brillant’s original presentation and her subsequent aneurysmal rupture, the court observed that there might have been more than a mere possibility that Dr. Royal’s alleged negligence in failing to diagnose Mrs. Brillant’s aneurysm ultimately caused her injuries, and held that the case be remanded for a new trial on this key question.
As to the issue of Sterling Medical Associates’ liability, the court found that the FTCA provides immunity to a physician only if he or she is serving in active military duty or is a civilian employee of the Armed Forces of the United States, and that Dr. Royal did not come under its provisions. In examining Dr. Royal’s contract with the U.S. government, the court found that the contract specified that Dr. Royal would perform as an independent contractor at the Army hospital and not as an employee of the government. The court noted that doctors practicing medicine at military hospitals as independent contractors bear personal liability for their negligent acts just as doctors practicing in the private sector bear their own liability,35 and directed a verdict for Sterling, but not for Dr. Royal.
Case #4. Engolia v. Allain, et al. 36
In Frank Engolia and Priscilla Engolia v. Dr. Joseph Allain, et al.,36 Mr. and Mrs. Engolia appealed a jury verdict in favor of the defendant physician, Dr. Allain, in a medical malpractice suit for the wrongful death of their daughter who died following SAH due to aneurysmal rupture.
Carmella Engolia developed sudden onset of severe neck pain while exercising at home. She was taken to the ED and was evaluated by the defendant, Dr. Allain, who was the ED physician on duty that night. Ms. Engolia was found to have exquisite tenderness of the neck, shoulder, and upper back, and after x-rays were performed, the defendant concluded that Ms. Engolia had suffered a musculoskeletal injury for which she was given prescriptions for analgesics and a muscle relaxant. She was discharged home with instructions to follow up with her family physician.
Ms. Engolia improved during the next several days, although on the fifth day after her initial presentation to the ED, she complained that she did not feel well again. She was evaluated by her family physician the next day, and a CT of the brain scan was ordered — a scan that Mrs. Engolia said was not done. Six days later, Ms. Engolia still had not improved. Her family physician requested that she return for a follow-up examination and a CT scan, which were scheduled for the following day. That following morning, Ms. Engolia was found dead in her bedroom. An autopsy revealed that she had died from an aneurysmal rupture.
Ms. Engolia’s parents then sued Dr. Allain for medical malpractice. However, the jury found in favor of the defendant. The plaintiffs subsequently appealed, claiming that the standard of care Dr. Allain provided to their daughter fell below the acceptable minimum standard of care, or alternatively that the standard itself was negligent, and in addition, that the trial court erred in the instructions it gave the jury.
Discussion
The question the court had to consider was whether the plaintiffs’ request for a new trial —based upon their contention that the verdict was contrary to the evidence presented — was directed at the accuracy of the jury’s factual determinations. At trial, the ED nurse who initially had seen Ms. Engolia testified that Ms. Engolia presented only with a history of neck pain radiating down her spine.
The nurse testified that Ms. Engolia explained that she had been exercising when the pain began and that she denied feeling nauseated. Dr. Allain, the defendant, also testified that Ms. Engolia presented with a history of severe neck pain only, which began while she was exercising. He also did not get any history of headache or other symptoms. However, both Ms. Engolia’s mother and boyfriend testified that Ms. Engolia had a severe headache and had vomited in addition to experiencing severe neck pain while exercising, and that this history should have been elicited by the defendant.
Given the disparate accounts of what happened, the question before the jury was whom to believe, the defendant or the plaintiffs.
The court found that the jury was presented with two permissible views of the facts that were in contention. Under the fact scenario presented by the plaintiffs, the defendant committed malpractice; under the fact scenario presented by the defendant, he did not, although he incorrectly diagnosed Ms. Engolia’s condition. The jury had been instructed that "[I]t is not malpractice to make a mistake in diagnosis. Making a diagnosis is an act of professional judgment and an incorrect diagnosis is not necessarily an act of negligence." The court, in considering the plaintiffs’ contention that the trial court erred in its charge to the jury, found that the jury instructions were a correct statement of law and that they adequately informed the jury with respect to making a diagnosis.36 The court found that since there was a material issue of fact, not requiring expert opinion, there was no error in the instructions given to the jury at the first trial.
The plaintiffs also contended that the jury was not entitled to hold the defendant to a lesser standard of care than what the plaintiffs maintained was the standard applicable in this case, based upon medical expert testimony, or alternatively, that the standard that was being used was, in itself, negligent. In considering this question, the court noted that all of the expert witnesses testified that, without the classic headache and symptoms, a diagnosis of SAH would be extremely difficult for any physician to make. The court observed that there was no dispute as to the actual standard of care itself, but rather whether the defendant had met the standard of care in treating Ms. Engolia. The court maintained that the question as to whether the defendant’s treatment was below the standard of care depended solely upon the findings of fact by the jury, and noted that the jury’s verdict should not be set aside if it was supported by any fair interpretation of the evidence. In considering both of these issues, the court ultimately decided that the view the jury chose was a permissible, reasonable interpretation of the facts based upon the evidence presented at trial and ultimately affirmed the lower court’s order, finding in favor of the doctor.
The medical issue in this case, setting aside the jury’s interpretation of the facts and the court’s ultimate decision in favor of the defendant, rests solely upon whether the defendant took an adequate medical history. For any patient presenting to the ED, with sudden onset of excruciating headache, neck pain or back pain, with or without vomiting, whether it is associated with exertion, one must consider SAH and the resulting menin-geal irritation that accompanies such bleeding as a cause of the patient’s symptoms. As such, it is incumbent upon any physician or health care professional to take an exhaustive history, and to not rely simply upon a patient’s recollection of the events preceding the medical evaluation. In cases where the diagnosis is not clear based upon the history and the medical examination, a simple unenhanced brain CT and/or LP, if the CT is not diagnostic of SAH, is indicated.
Summary
As seen in these cases, SAH in a patient easily may be overlooked or misdiagnosed in an ED setting. The failure to promptly recognize, diagnose, and initiate appropriate neurosurgical treatment can have severe consequences not only with regard to patients and their ultimate outcome, but also with regard to the ED or family physician who first evaluates a patient with SAH.
The standard of care requires that any patient with sudden onset of the worst headache of his or her life be evaluated promptly with a brain CT scan. Moreover, any patient with a history of headaches who presents with an unusually severe headache or one that is unlike any of their previous headaches must undergo a brain CT scan.
Even though the brain CT scan will be positive in more than 90% of patients presenting with a SAH, approximately 10% of the patients with SAH will have a negative brain CT scan. In those patients, the standard of care requires that LP be performed to rule out a bleed. Failure to perform a brain CT scan or LP in such scenarios falls below the standard of care. Defending a case by claiming that earlier diagnosis and treatment would have not affected the patient’s final outcome is no longer a valid defense in this modern era of neurosurgery.
Of those patients with nongiant, anterior circulation aneurysms (approximately 85-90% of all patients with intracerebral aneurysms) who present as Hunt and Hess grades I or II — and who undergo timely neurosurgical treatment — about 90% will have a good to excellent outcome. Those patients who are misdiagnosed most commonly with something other than aneurysmal SAH are those patients who are most likely to experience a good outcome following timely neurosurgical treatment. As a result, clinicians seeing patients with headaches must be acutely aware of patients presenting with severe or uncharacteristic headaches, and must have a low threshold for working them up appropriately to rule out SAH.
Endnotes
1. Louisell DW, Williams H. ED Malpractice. In: Medical Malpractice Litigation Guide: Types of Liability and Malpractice. Newark, NJ: Matthew Bender; 2003. Web: www. lexisnexis.com/bender.
2. Eskesen V, Rosenorn J, Schmidt K. The influence of unruptured intracranial aneurysms on life expectancy in relation to their size at the time of detection and to age. Br J Neurosurg 1988;2:379-384.
3. Wilkins RH, Rengachary SS, eds. Neurosurgery. New York City: McGraw-Hill; 1996:2191-2394.
4. Kassell NF, Torner JC, Haley EC Jr., et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990;73:18-36.
5. Kassell NF, Torner JC, Jane JA, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 2: Surgical results. J Neurosurg 1990;73:37-47.
6. Bannerman RM, Ingall GB, Graf CJ. The familial occurrence of intracranial aneurysms. Neurology 1970;20:283-292.
7. Jellinger K. Pathology and aetiology of intracranial aneu-rysms. In: Pia HW, Langmaid C, Zierski J, eds. Cerebral Aneurysms. Advances in Diagnosis and Therapy. New York City: Springer; 1979, 5-19.
8. Pakarinen S. Incidence, aetiology, and prognosis of primary subarachnoid haemorrhage; a study based on 589 cases diagnosed in a defined urban population during a defined period. Acta Neurol Scand 1967;29(suppl):1-128.
9. Inagawa T, Ishikawa S, Aoki H, et al. Aneurysmal subarachnoid hemorrhage in Izumo City and Shimane Prefecture of Japan. Incidence. Stroke 1988;19:170-175.
10. Gary Luoma and Nicholas Luoma, Marissa Luoma and Joseph Luoma, minors v. Kaiser Foundation Hospitals; Kaiser Foundation Health Plan, Inc.; The Permanente Medical Group Inc.; M. Lozano, M.D.; and Joshua Nelson, M.D., No. 42-18-03, Superior Court of Contra Costa County, Walnut Creek, CA (Jan. 8, 1998).
11. Fox JL. Intracranial Aneurysms. New York City: Springer-Verlag; 1983.
12. Edlow JA, Coplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. NEJM 2000;342:29-36.
13. Sarner M, Rose FC. Clinical presentation of ruptured intracranial aneurysm. J Neurol Neurosurg Psychiatr 1967; 30:67-70.
14. Koenig GH, Marshall WH Jr, Poole GJ, et al. Rupture of intracranial aneurysms during cerebral angiography: Report of 10 cases and review of the literature. Neurosurgery 1979; 5:314-324.
15. Weir B. Aneurysms Affecting the Nervous System. Baltimore: Williams & Wilkins; 1987.
16. Kassell NF, Torner JC. Aneurysmal rebleeding: A preliminary report from the Cooperative Aneurysm Study. Neurosurgery 1983;13:479-481.
17. Hunt WE, Hess RM, Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14-20.
18. Cohn v. Frost, No. 85 L 11905, Cook County Circuit Court, Chicago (July 11, 1991).
19. Latchaw RE, Silva P, Falcone SF. The role of CT following aneurysmal rupture. Neuroimaging Clin N Am 1997; 7:693-708.
20. Ferro J, Lopes J, Melo T, et al. Investigation into the causes of delayed diagnosis of subarachnoid hemorrhage. Cerebrovasc Dis 1991;1:160-164.
21. van Gijn J, van Dongen KJ. The time course of aneurysmal haemorrhage on computed tomograms. Neuroradiology 1982;23:153-156.
22. Sames TA, Storrow AB, Finkelstein JA, et al. Sensitivity of new-generation computer tomography in subarachnoid hemorrhage. Acad Emerg Med 1996;3:16-20.
23. Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: Lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med 1996; 3:827-831.
24. Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 1994;25:2315-2328.
25. Vermuelen M. Subarachnoid haemorrhage: Diagnosis and treatment. J Neurol 1996;243:496-501.
26. Vermuelen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatr 1990;53:365-372.
27. van der Wee N, Rinkel GJ, Hasan D, et al. Detection of subarachnoid haemorrhage on early CT: Is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatr 1995;58:357-359.
28. Vermuelen M, Hasan D, Blijenberg BG, et al. Xantho-chromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatr 1989;52:826-828.
29. Sahs AL, Perret GE, Locksley HB, et al, eds. Intracranial Aneurysms and Subarachnoid Hemorrhage: A Cooperative Study. Philadelphia: Lippincott; 1969.
30. Bassi P, Bandera R, Loiero M, et al. Warning signs in subarachnoid hemorrhage: A cooperative study. Acta Neurol Scand 1991;84:277-281.
31. Okawara S. Warning signs prior to rupture of an intracranial aneurysm. J Neurosurg 1973;38:575-580.
32. LeBlanc R. The minor leak preceding subarachnoid hemorrhage. J Neurosurg 1987;66:35-39.
33. Helen B. Brillant and Eugene G. Brillant v. Dr. Lorenza Royal and Sterling Medical Associates; Dr. Lorenza Royal and Sterling Medical Associates v. Helen B. Brillant and Eugene G. Brillant, No. 582 So. 2d 512 (1991).
34. The Federal Torts Claim Act (FTCA), passed in 1983, provides that "the remedy against the United States . . . for damages for personal injury, including death, caused by the negligent or wrongful act or omission of any physician . . . of the armed forces . . . while acting within the scope of his duties or employment therein or therefore shall hereafter be exclusive of any other civil action by reason of the same subject matter against such physician . . . whose act or omission gave rise to such action or proceeding." 10 U.S.C. § 1089 (1983).
35. The court cited two cases as precedent: Lilly v. Fieldstone, 876 F.2d 857 (10th Cir. 1989); Norton v. Murphy, 661 F.2d 882 (10th Cir. 1981).
36. The court cited several cases on point, including Dupuy v. Rodriguez, 620 So. 2d 397 (La. App. 1st Cir. 1993); Sparacello v. Andres, 501 So. 2d 269 (La. App. 1st Cir. 1985); Mitchell v. Fire & Cas. Ins. Co., 540 So. 2d 352 (La. App. First Cir.), writ denied, 541 So. 2d 1390 (La. 1989).
Headache is a common chief complaint encountered by emergency physicians (EPs). It may be a symptom of benign diseases such as migraine headaches or a common virus. Unfortunately, it also may represent other, more life-threatening illnesses, including subarachnoid hemorrhage. Delineating which patients need radiologic imaging, spinal fluid testing, and even angiography is part of the challenge encountered by the EP. This issue will outline strategies for reducing risk in the headache patient. Specifically, diagnosis and management of subarachniod hemorrhage will be detailed.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
