Drug Criteria & Outcomes: Palonosetron (Aloxi) Formulary Evaluation
Drug Criteria & Outcomes: Palonosetron (Aloxi) Formulary Evaluation
Part 1 of 2: Mechanism of Action, Pharmacokinetics, Indications, Dosage, and Administration
By Brian Watkins,
PharmD Candidate
Harrison School of Pharmacy
Auburn (AL) University
Palonosetron, developed by MGI Pharma and its partner Helsinn Healthcare SA, is a recently approved selective antagonist of serotonin subtype 3 receptors (5-HT3).
Palonosetron’s affinity for 5-HT3 receptors gives it utility as an antiemetic and antinauseant. Other "setrons" include ondansetron (Zofran), granisetron (Kytril), and dolasetron (Anzemet).
Mechanism of action
Palonosetron, as well as other setrons, inhibits emesis by blocking serotonin receptors both peripherally on vagal afferents and centrally in the chemoreceptor trigger zone (CTZ) of the area postrema.
Pharmacokinetics
Absorption: Limited studies have demonstrated that following intravenous (IV) dosing, maximal plasma concentrations and area under the concentration-time curve are dose-proportional over the dose range of 0.3-90 ug/kg.
Distribution: Palonosetron is 62% bound to plasma proteins and has a large volume of distribution equal to 8.3 +/- 2.5 L/kg.
Metabolism: Fifty percent of the palonosetron dose is metabolized via the CYP450 system. In vitro studies implicate CYP2D6 to be the primary enzyme involved in palonosetron’s metabolism; however, CYP3A and CYP1A2 may play minor roles. The major metabolites produced are N-oxide-palonosetron and 6-S-hydroxypalonosetron, each of which are virtually devoid of activity.
Elimination: After a single intravenous carbon-14 labeled dose of palonosetron (10 ug/kg), approximately 80% of the dose was recovered within 144 hours in the urine. Approximately 40% of that dose is recovered as parent drug. Total body clearance of palonosetron is 160 +/- 35 mL/hr/kg and renal clearance was 66.5 +/- 18.2 mL/hr/kg.
The mean terminal elimination half-life is approximately 40 hours.
Kinetically, the major difference between palonosetron and other setrons is a mean terminal elimination half-life equal to 40 hours (see Table 1). Another major difference that exists between the setrons is their relative selectivity for different isoenzymes of the CYP450 system.
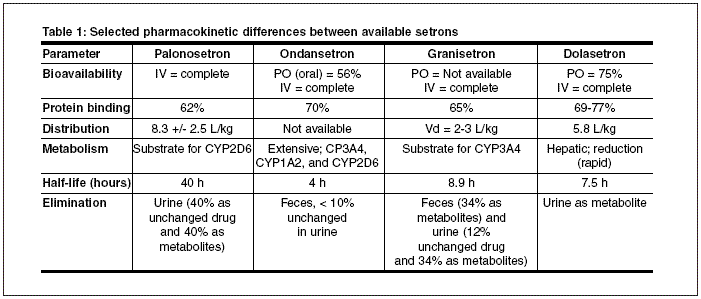
Ondansetron and granisetron are both substrates for CYP3A4, the isoenzyme responsible for the metabolism of approximately 50% of marketed drugs.
Finally, ondansetron is eliminated primarily in feces while granisetron displays a more balanced elimination profile. Palonosetron and dolasetron are eliminated primarily in urine. Only small differences exist between the available agents with respect to bioavailability, protein binding, and distribution.
FDA-labeled indications
FDA-approved indications for palonosetron includes (see Table 2 for other setrons):
- Prevention of acute nausea and vomiting associated with initial and repeat courses of moderately and highly emetogenic cancer chemotherapy; and
- Prevention of delayed nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.

Dosage and administration
The recommended dosage of palonosetron is 0.25 mg administered as a single dose approximately 30 minutes prior to the start of chemotherapy (see Table 3). Any repeat dosing within seven days has not been evaluated and thus cannot be recommended. No dose adjustments are recommended for renal or hepatic impairment.

Palonosetron is administered intravenously over 30 seconds (see Table 4). Palonosetron should not be mixed with other drugs; therefore, the infusion line should be flushed before and after with normal saline.

Clinical trials
To date, four clinical trials have evaluated the safety and efficacy of palonosetron in preventing acute and delayed chemotherapy-induced nausea and vomiting (CINV); however, only one of the four clinical trials has been published. Two Phase III trials compared single, fixed intravenous doses of palonosetron with either single-dose intravenous ondansetron or dolasetron for the prevention of nausea and vomiting induced by moderately emetogenic chemotherapy.
Also, one Phase II dose-ranging study and a randomized, controlled Phase III trial have been conducted to evaluate palonosetron’s safety and efficacy in preventing nausea and vomiting induced by highly emetogenic chemotherapy.
A discussion of clinical trial data, adverse effects, and cost analysis will appear in the April issue of Drug Formulary Review's Drug Criteria & Outcomes.
Palonosetron, developed by MGI Pharma and its partner Helsinn Healthcare SA, is a recently approved selective antagonist of serotonin subtype 3 receptors (5-HT3).Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
