Mesenteric Ischemia
Mesenteric Ischemia
Authors: Jeffrey A. Manko, MD, Assistant Professor of Emergency Medicine, Assistant Residency Director, NYU/Bellevue Medical Center, New York; Phillip D. Levy, MD, Department of Emergency Medicine, NYU/Bellevue Medical Center, New York.
Peer Reviewer: Lilly C. Lee, MD, SM, Associate Residency Director, Beth Israel Medical Center-Harvard Affiliated Emergency Medicine Residency; Instructor in Medicine, Harvard Medical School, Boston, MA.
As the population continues to age and the life expectancy continues to rise, emergency departments (EDs) will be dedicating more time and resources to treating geriatric (> 65 years) patients. Because diagnoses in this population can be obscured and often delayed, this will require emergency medicine physicians to sharpen their clinical acumen. These delays result in increased morbidity and mortality.1,2
Mesenteric ischemia is a diagnosis that must be considered in all geriatric patients presenting to the ED with abdominal pain. The diagnosis often is one of the toughest to make clinically due to a lack of objective physical findings. However, it is imperative for all physicians to consider mesenteric ischemia in their differential and attempt to make an early, accurate diagnosis.
Acute mesenteric ischemia can result from several etiologies. The most common etiology is occlusive (~ 80%) because of mesenteric emboli or thrombi.3 Non-occlusive mesenteric ischemia (NOMI) accounts for about 20% of acute mesenteric ischemia and occurs in patients with low-flow states.4,5 Chronic mesenteric ischemia usually occurs in patients with concurrent diseases and other vasculopathies.
The diagnosis must be contemplated and aggressively investigated to reduce the morbidity and mortality often associated with this disease; delays in treatment and surgical intervention account for the staggering 70-90% mortality.5 Unfortunately, these numbers have not improved in the last decade, and with the aging population, this diagnosis is certain to become more prevalent.
This article will review mesenteric ischemia—its etiology and risk factors, and the general approach to a patient suspected of having this diagnosis. Pertinent historical and physical findings, diagnostic studies, and treatment modalities all will be discussed.
— The Editor
Epidemiology
The incidence of mesenteric ischemia is on the rise, and an aging population is responsible for the majority of the increase. Mesenteric ischemia is reported to be the cause of approximately 1.0% of all acute abdomen admissions and 0.1% of all hospital admissions.6 The geriatric population includes patients with cardiovascular comorbidities and increased intensive care unit admissions, which contributes to more diagnoses of mesenteric ischemia in this age group. Outcome often is determined by the underlying etiology (acute vs chronic, occlusive vs nonocclusive) and the timeliness of diagnosis and treatment. Unfortunately, the prognosis still remains dismal. However, all is not totally bleak as researchers, emergency medicine physicians, radiologists, and surgeons continue to work together to improve time to diagnosis and treatment.
The geriatric population is the fastest growing segment of the population. With the life expectancy of both genders increasing by about five years over the past 25 years, the population older than age 85 will grow by three to four times its present number between 1990 and 2010.7,8 This will have a tremendous effect on the prevalence of diseases that generally affect the geriatric population, such as mesenteric ischemia.
Timeliness of the diagnosis and initiation of treatment is the best predictor of outcome for patients diagnosed with mesenteric ischemia. This becomes increasingly challenging, as geriatric patients often are unable or do not provide thorough histories and/or reliable physical examinations. Therefore when caring for this special population, the onus is on the practitioner to maintain a high index of suspicion for lethal entities such as mesenteric ischemia.
Anatomy
To understand the basics of mesenteric ischemia, the clinician must know the anatomy involved. The entire gut is supplied by three major branches coming off the abdominal aorta: the celiac artery (CA), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA).
The CA exits perpendicular from the ventral abdominal aorta at the level of T12. It has a large diameter and a short length and is responsible for supplying blood to the foregut, (distal esophagus to second portion of the duodenum). The splenic, left gastric, and common hepatic arteries branch off the CA. The gastroduodenal artery, which branches off the common hepatic artery, provides important collateral circulation between the celiac and superior mesenteric arteries via the pancreaticoduodenal arteries. The CAs excellent collateral circulation, coupled with its length (short and wide), accounts for the scarcity of mesenteric ischemia at the level of the foregut.
The SMA arises off the abdominal aorta at the level L1 at a 45° angle and supplies the midgut (latter duodenum through large bowel up to the splenic flexure). The inferior pancreaticoduodenal, middle colic, right colic, ileocolic, jejunal, and ileal arteries branch off the SMA. The SMA has important anastomoses with the IMA as well. These include the marginal artery of Drummond and the Arc of Riolan. These collaterals often increase if vascular insufficiency occurs over time.
The inferior mesenteric artery arises at L3 before the bifurcation of the aorta into the iliacs. The IMA supplies the hindgut, beginning with the transverse colon and extending to the rectum. Its branches include the left colic artery, sigmoidal arteries, and hemorrhoidal arteries, and it ends as the superior rectal artery. The IMA anastomoses with the SMA (as above), and with lumbar branches off the aorta and internal iliacs.
The venous system closely mirrors the arterial system. The inferior mesenteric vein drains into the splenic vein, joining the superior mesenteric vein to form the portal vein. This is how the midgut and hindgut are drained before entering the inferior vena cava.
Etiology
Mesenteric ischemia often is divided into acute vs. chronic and occlusive vs. nonocclusive. (See Table 1.) Acute presentations are much more common than chronic ones, with arterial occlusion accounting for approximately 65% and venous occlusion about 15% of mesenteric ischemia cases. NOMI represents about 20% of the mesenteric ischemia cases. SMA emboli account for the largest percentage of acute etiologies in mesenteric ischemia (~ 50%).3
|
Table 1. Classification of Mesenteric Ischemia |
SMA emboli are most frequently the result of cardiac disease. The origin of the emboli is from the left atrium, and is caused by dysrhythmias (most often atrial fibrillation) or ventricular/septal thrombi as a result of myocardial infarction.9,10 Valvular thrombi also may contribute to SMA emboli, but this etiology has diminished with the declining incidence of rheumatic fever. The emboli most frequently lodge just distal to where the middle colic artery leaves the SMA. This is where the artery begins to taper and explains why the proximal small bowel often is spared during embolic events.
SMA thrombi often occur in patients with chronic vascular disease. Patients who develop SMA thrombi often have long-standing significant atherosclerosis. The acute event occurs when a plaque breaks off and a thrombus forms. The thrombus usually occurs at the origin of the SMA, and this may distinguish it from an embolic event. The evolution of an SMA thrombus and its gradual onset often allow time for collateral circulation to develop, thereby decreasing the extent of ischemia.
Mesenteric venous thrombosis (MVT) is the least frequent etiology of mesenteric ischemia and often results from underlying medical conditions. MVT may result from hypercoagulable states such as protein deficiencies, malignancies, sepsis, or liver disease that results in portal hypertension or disturbances in portal blood flow. Mortality is much lower (38% vs 82%) in MVT patients compared to the other etiologies of mesenteric ischemia.11
NOMI is believed to be the result of low cardiac output states, most commonly congestive heart failure (CHF). NOMI commonly has been associated with patients in intensive care units suffering from sepsis, dehydration, or other causes of hypotension. Some drugs, such as cocaine, digitalis, ergotamines, and vasopressors, have been linked to NOMI.12,13 Elderly patients account for a large percentage of intensive care unit admissions and it remains important to aggressively treat these patients for hypotension and prevent NOMI.
Risk Factors
In trying to assess patients for mesenteric ischemia, it is very important to consider risk factors and comorbidities. (See Table 2.) Generally, acute mesenteric ischemia has been linked to cardiac disease. Entities that predispose patients to thrombus formation and embolic phenomena, such as arrhythmias, recent myocardial infarction, and valvular disease, account for the vast majority of acute mesenteric ischemia.14,15 Atrial fibrillation and recent MI are the most common cardiac risk factors.16
|
Table 2. Risk-Enhancing Conditions |
|||||
| CARDIOVASCULAR DISEASE |
PHARMACOTHERAPY |
||||
Amitryptiline
Cocaine
Digitalis
Diuretics
Dextroamphetamine
Ergot alkaloids
Estrogen/oral contraceptives
Methamphetamine
Pitressin
Propranolol
Vasopressors
ABDOMINAL DISEASE
Bowel obstruction
Cholangitis
Crohn’s disease
Diverticulitis
Intussusception
GI bleeding
Peritonitis
Portal hypertension
Parasitic infection
Trauma
Volvulus
CHF and low cardiac output states are contributing factors for NOMI. Sepsis, dehydration, shock, and inadequate fluid resuscitation may all be contributing factors leading to NOMI.
Chronic mesenteric ischemia usually is associated with coronary artery disease, peripheral vascular disease, hypertension, diabetes, and tobacco use. In fact, it has been reported that 70-96% of patients with mesenteric ischemia used a significant amount of tobacco.17,18
Other risk factors include drugs (e.g., cocaine), hypercoagulable states, and malignancy. Digitalis also is thought to be an independent risk factor in NOMI; however, it is difficult to separate that from underlying cardiac disease.
History
The classic triad often associated with mesenteric ischemia is severe abdominal pain, gut emptying, and cardiac disease.19 The pain often is unbearable and out of proportion to the physical examination. In the setting of an embolic event, the patient may be able to pinpoint the exact time when the pain began. The pain commonly is followed by gastrointestinal symptoms, with nausea and anorexia occurring 80% of the time, vomiting 60%, and diarrhea 50%.4 Cardiac disease is present in the majority of patients, with 25-33% reporting prior embolic events.20,21
Patients presenting with chronic mesenteric ischemia will have varied histories from those with acute mesenteric ischemia. The patient often complains of postprandial abdominal pain (referred to as intestinal angina), weight loss, and gastrointestinal symptoms.18,22,23 The pain is reproducible; it often leads to small, frequent meals (small-meal syndrome). Large meals tend to exacerbate the pain. Risk factors for chronic mesenteric ischemia include atherosclerosis, diabetes, and tobacco.
It is critical to understand that this is a disease of the geriatric population, and obtaining an accurate history often is challenging. Patients may present with an altered mental status of either acute or chronic etiology that may delay timely diagnosis and treatment. Often, elderly patients may suffer from dementia, and speaking with family members and caregivers may be the only way to elucidate events leading up to the current ED presentation.
It is important to find out what medications the patient is taking, as well as to obtain the patient’s complete medical history. Patients with prior embolic events and cardiac disease are at particular risk. Patients taking diuretics, digoxin, and anticoagulants should be suspected of being at risk for mesenteric ischemia.
Physical Exam
The physical exam of the patient with mesenteric ischemia requires a great deal more than just examining the abdomen. The early presentation of mesenteric ischemia may not yield many positive findings. It is important to do a comprehensive exam, because the history may not provide all the necessary details needed to make an early diagnosis.
Early in the process, vital signs are frequently normal, though there may be a slight tachycardia or low-grade fever. The elderly patient may not mount a febrile response, so not having a fever does not preclude the possibility of having ischemia. In fact, as geriatric patients become septic, they may become hypothermic rather than hyperthermic.
The most sensitive vital sign is heart rate. Often, the patient will become tachycardic due to any combination of pain, dehydration, and fever. The clinician needs to know if the patient is taking beta blockers, which may blunt this response.
As the patient’s condition worsens, the respiratory rate will increase. Tachypnea is a response to metabolic acidosis generated by lactate buildup from ongoing ischemia/infarction.
As the patient becomes more critically ill, the blood pressure drops. The patient who presents tachypneic and hypotensive is in need of emergent treatment because the mortality already is extremely high. By this time, the diagnosis appears more apparent.
The abdominal exam often can be misleading. Often the pain is "out of proportion" to the initial exam. There may be no focal area of tenderness, rather only some non-specific diffuse discomfort. Peritoneal signs are a late finding and often are not initially present. Bowel sounds may be normal or hyperactive. Occult blood on rectal exam also is a late finding and occurs due to ischemia of the mucosa, which leads to sloughing.
Other areas of the exam may be the most helpful in providing clues to the diagnosis of mesenteric ischemia. The cardiac exam may be helpful in picking up murmurs or arrhythmias, suggesting that a patient may be at risk for emboli. An in-depth neurologic exam may uncover prior strokes as a manifestation of embolic disease. Peripheral edema or pulmonary rales may help to diagnose CHF, placing the patient at risk for NOMI. Evidence of deep vein thrombosis or malignancy can predispose the patient to mesenteric ischemia.
Many elderly patients have altered pain perception, and this often leads to delayed presentations with higher morbidity and mortality. The clinician must maintain a high index of suspicion for mesenteric ischemia in geriatric patients with risk factors and abdominal pain, and must look for subtle clinical findings that can help expedite diagnosis and treatment. Although the overall mortality of mesenteric infarction generally is accepted to be around 70%, one study reported a survival rate of 90% in patients with mesenteric ischemia who underwent angiography before peritonitis developed.5
Diagnosis
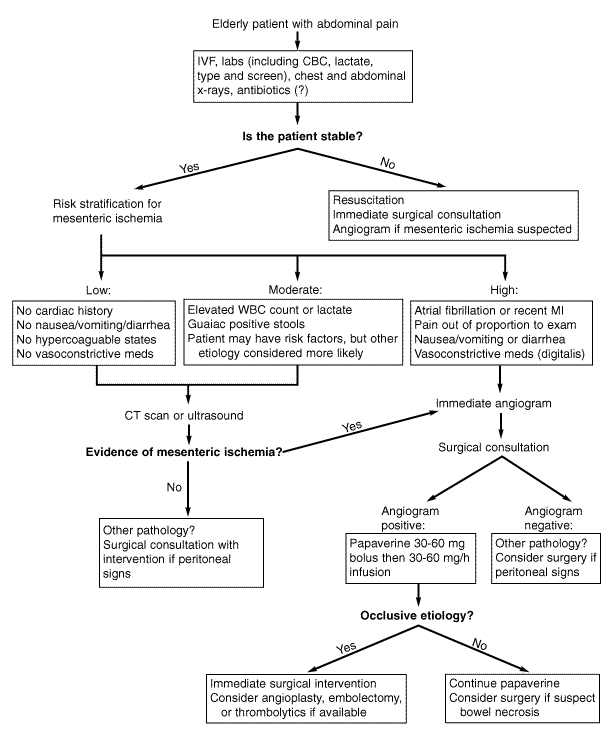
The high mortality associated with mesenteric ischemia, coupled with the diagnostic ambiguity of history and physical examination, has led to an ongoing search for reliable indices of the disorder.24 As it stands now, the most important prognostic factor regarding outcome is the time interval between the onset of symptoms and the initiation of intervention.25,26 Limiting the delay in diagnosis to 12 hours or less has been shown to correlate directly with increased survival.25 It has been reported that mortality rates as low as 10% can be achieved if intervention occurs before signs of peritonitis.5 Historically, angiography has been the mainstay of non-surgical evaluation and has allowed clinicians to begin treatment more rapidly. 24,26-29 Recently, much progress has been made in the analysis of suspected cases through the use of biochemical assays and noninvasive radiographic technology.16,24,30,31 The ability to diagnose intestinal ischemia in a more directed fashion enables clinicians to distribute prompt surgical and pharmacological therapy to the appropriate patients. The following is a concise description of currently available diagnostic modalities, as well as other options that may be utilized in the near future. (See Figure 1.)
|
Figure 1. Proposed Approach to Suspected Mesenteric Ischemia |
|
|
Laboratory Analysis. Leukocytosis. A hemogram is a routine part of the work up of patients with acute abdominal complaints. While the finding of marked leukocytosis is not considered diagnostic for any particular intra-abdominal illness, its presence should alert the treating clinician and raise the index of suspicion for surgical pathology.16,24,31 A recent study of 46 patients with confirmed intestinal ischemia found that 90% had a leukocyte count greater than 15,050/mL.31 Others have noted similar results.16 Based on these data, the assumption that leukocytosis in excess of 15,000/mL is highly suggestive of mesenteric ischemia may be accurate. It is important to remember, though, that the absence of an elevated white blood cell count does not exclude the diagnosis.
Serum Lactate. The initial evaluation of serum lactate in animal models showed promise for its utility as a marker of intestinal ischemia.30,32 Serum lactate was noted to be elevated within one hour of induced ischemia, and remained elevated for 3-4 hours.30,32 Subsequent analysis in human subjects found that more than 90% of patients diagnosed with mesenteric ischemia had elevated serum lactate levels, and that significant lactate elevation at diagnosis was disproportionately associated with patient death.31,12 Furthermore, lactate levels themselves have been found to correlate with the degree and extent of ischemia noted at surgery. Compilation of recent data has yielded a sensitivity approaching 100% and a specificity near 40% for serum lactate in the diagnosis of mesenteric ischemia.33,34 It is generally felt that despite a low specificity, serum lactate is the best measurable indicator of mesenteric ischemia currently available.12,24,26,30-34
Serum Inorganic Phosphate. Serum inorganic phosphate initially was thought to be useful as an early indicator of mesenteric ischemia, as it is released into the blood by sloughing intestinal mucosa.35 Preliminary animal studies revealed elevated levels after induced ischemia.30,35 Reassessment of utility in humans was less encouraging, though, as the sensitivity and specificity in an ED analysis was only 26% and 85%, respectively.36 Serum inorganic phosphate should be considered of limited diagnostic value in the evaluation of mesenteric ischemia.36
Intestinal Fatty Acid Binding Protein. Intestinal fatty acid binding protein (IFABP) is a cytoplasmic protein produced by mature enterocytes.37-39 It is released into the blood stream early in the course of intestinal injury and subsequently eliminated by the kidneys.37-39 Both a serum and a urine assay have been developed, neither of which is currently available for general use. While IFABP is too short-lived in the serum to be of diagnostic value, preliminary studies using the urine assay have been encouraging.37-39 It appears that IFABP may serve as a viable diagnostic marker in the near future.
Intramucosal pH. Several researchers have reported the potential for monitoring gastric or jejunal intramucosal pH (pHi) as a parameter to follow intestinal perfusion and detect early mesenteric ischemia.40-42 The most feasible application of this technology appears to be indirect analysis utilizing gastric tonometry.42 The initial results show promise, but widespread adaptation seems more likely in the intensive care unit setting rather than the ED.
Other Serum Markers. D(-) lactate, a by-product of bacterial metabolism, has been found in elevated quantities in the serum of animal models with experimentally induced intestinal ischemia.43,44 It is thought that in the ischemic segment of bowel, bacterial overgrowth leads to production of D(-) lactate in quantities sufficient to allow detection with modern assays.43,44 Human studies need to be performed before determining applicability.
Creatinine phosphokinase, lactate dehydrogenase, aspartate transferase, and alkaline phosphatase all have been found to be elevated with mesenteric ischemia, but none are considered to be sensitive or specific indicators of the disorder.45
Ammonia, serum malondialdehyde, cytosolic beta-glucosidase, and hexosaminidase are other markers released with ischemic intestinal injury and purported to have diagnostic potential based on limited studies.46-48 Again, larger human trials are needed before further consideration.
Radiographic Analysis
Angiography. Angiography has been the gold standard in the diagnosis of mesenteric ischemia for more than two decades.27-29 (See Table 3.) It allows definition of the ischemic segment, identification of the etiology (occlusive vs nonocclusive), evaluation of collateral circulation, and treatment through intra-arterial infusion therapy.27-29A significant decrease in mortality has resulted from the judicious use of angiography, and as a result, clinicians are forced to accept a large number of negative studies.27,29,49 Although generally considered safe, adverse events have been noted. Approximately 5% will develop local hematomas, whereas 2% will develop more severe complications, such as hypotension or arrhythmias.50 The risk of death is low, however, occurring 0.3% of the time and usually secondary to rupture of an aneurysm.50 Acute tubular necrosis related to contrast dye loads, vascular injury from guide wire threading, and persistent hemorrhage from the puncture site have been reported as well. Despite a high sensitivity and specificity for diagnosing mesenteric ischemia, angiography is limited to pathology of vascular origin; it is unable to assist with other intra-abdominal diagnoses. Additionally, patients with low flow states and those on vasopressors may have false-positive findings.51 One final concern with reliance solely on angiography is the unavailability of this technology in smaller hospital centers.52 Therefore, it is the opinion of these authors that the current approach should be to assess stable, low-to-moderately suspected cases first with a noninvasive radiographic study (i.e., CT or possibly ultrasound), and confirm positives with an angiogram.49,52-54 If a patient is unstable or strongly believed to have occlusive mesenteric ischemia, angiography should be performed emergently.27,51,55
|
Table 3. Radiographic Diagnosis of |
||
| Modality |
Sensitivity |
Specificity |
| Angiography |
100% |
99% |
| Computed tomography |
83% |
93% |
| Doppler ultrasonography |
100% |
87%* |
| Magnetic resonance angiography |
100% |
95% |
|
* For SMA occlusion greater than 70% |
||
Plain Film X-Rays. Plain film abdominal x-rays often are used early in the evaluation of patients with severe abdominal pain of uncertain etiology.56,57 Plain films are considered most helpful in ruling out other causes of illness, with the findings indicative of intestinal ischemia occurring with advanced bowel injury. Plain film abnormalities may include "thumb-printing" caused by submucosal edema or hemorrhage, bowel dilatation, intramucosal pneumotosis, or pneumobilia.52,57 Although limited in value, most authors continue to recommend plain abdominal x-rays in the early work-up of suspected mesenteric ischemia.
Computed Tomography. Computed tomography (CT) scanning is a vital diagnostic tool in the modern assessment of abdominal disorders. It has emerged as an important modality for both identifying mesenteric ischemia and elucidating the underlying cause.52,56,58,59 CT scanning has been reported to have a sensitivity approaching 83%, with a specificity near 93% for the diagnosis of intestinal ischemia.60,61 Bowel wall thickening with or without a target sign, lack of bowel wall enhancement with intravenous contrast, presence of arterial or venous thrombosis, parietal pneumotosis, or intraportal gas and blurring of mesenteric fat are the CT abnormalities found with the greatest consistency in cases of confirmed mesenteric ischemia.52,56,58,60 (See Table 4.)
|
Table 4. CT Scan Findings Indicative |
| "Target sign" |
| Bowel wall thickening |
| Absence of bowel wall intravenous contrast |
| Presence of arterial or venous thrombus/embolus |
| Parietal pneumotosis |
| Pneumobilia |
| Blurring of mesenteric adipose tissue |
Based on the general availability, high reliability and the presence of widely accepted diagnostic findings, CT has become the method of choice in the initial evaluation of stable patients with suspected mesenteric infarction.52,58-60 (See Figures 2a and 2b.)
|
Figures 2a and 2b. Ischemic Bowel as Seen |
|
|
|
2a. Note prominent target sign in several loops of bowel. |
|
|
|
2b. Note bowel wall with intramural gas (parietal pneumotosis). |
|
Both figures reproduced with permission from: Spates M, Schwartz DT, Savitt D, et al. Abdominal Imaging. In: Emergency Radiology. Schwartz DT, Reisdorff EJ, eds. New York, NY: McGraw-Hill Companies; 2000:535. |
Ultrasonography. Early studies of ultrasonography showed that the technology was good at identifying the presence of intraluminal echogenic material, as well as vascular engorgement in cases of superior mesenteric vein thrombosis, but unreliable in detecting arterial narrowing.54,62 The development of Doppler ultrasound improved the reliability of conventional sonogram, and enhanced the feasibility of its future use in identifying patients with SMA or CA occlusion.54,63 Further work using color flow duplex sonography of both the CA and SMA found the peak systolic velocity (PSV) and end diastolic velocity (EDV) were excellent criteria to detect the presence of significant stenosis.53,64,65 Using these parameters, researchers were able to show a high degree of accuracy for identification of SMA and CA stenosis using duplex sonograms.53 Most recently, data from a prospective evaluation were reported, revealing a sensitivity of 100%, and a specificity of 87% and 98%, respectively, for identifying CA and SMA occlusion greater than 70% with Doppler ultrasonography.49 It is justifiable to conclude that, when performed by trained personnel, duplex sonography can be useful as an initial screening method to help detect mesenteric vascular stenosis49,53 and determine those patients who truly require angiography.54
Magnetic Resonance Imaging/Angiography. Due to its limited invasiveness and excellent degree of resolution, magnetic resonance imaging (MRI) has obvious potential in the diagnosis of mesenteric ischemia.66 Gadolinium-enhanced magnetic resonance angiography (MRA) allows for expansion of this applicability through the precise delineation of the mesenteric vasculature.67,68 Results of a recent evaluation show a sensitivity of 100% and a specificity of 95% when MRA was compared to conventional angiography and surgery for the diagnosis of bowel ischemia.67 Despite encouraging data and the quality of its imaging, magnetic resonance has yet to be fully endorsed, as it is still considered an emerging technology.
Basic Electrical Rhythm. The basic electrical rhythm (BER) is the underlying slow wave electrical activity of the gastrointestinal tract.69,70 The BER is detectable by measuring the magnetic fields generated using transabdominal superconducting quantum interference devices (SQUIDs).69,70 Definable aberrations in baseline BER have been noted within 30 minutes of induced ischemia in animal studies.69,70 Although a long way off until clinical application, future research is certainly warranted, as the potential for earlier diagnosis of mesenteric ischemia seems possible.
Treatment
As with any critically ill patient, early treatment should be focused on general resuscitative management. Volume status needs to be addressed and may require invasive monitoring. Respiratory and cardiovascular systems must be managed appropriately, but careful attention should be paid to vasoconstrictive medications that may exacerbate the patient’s condition. Digitalis, in particular, should be avoided. Alpha-adrenergic agents such as norepinephrine and dopamine must be used with extreme caution, and generally are reserved for cases of severe hypotension as their effects on the splanchnic circulation could exacerbate ongoing intestinal ischemia. Nasogastric tube decompression should be considered, and all patients must be kept NPO. It is important to give broad-spectrum antibiotics with good anaerobic coverage early, as septic complications often arise. While these basic therapeutic interventions are being initiated, concurrent surgical consultation must be obtained in order to expedite definitive therapy to optimize patient survival.
Surgery. Surgical interventions for mesenteric ischemia include bowel resection with anastamosis, revascularization with grafting, or laparotomy to assess intestinal viability. Controversy exists, as grossly ischemic appearing segments may revitalize rapidly once blood flow is restored. "Second look" procedures often are performed 24-48 hours after initial surgical treatment to reassess tissue viability. Operative decisions may be made based on the results of the analyses mentioned above. An important role for the emergency physician is to obtain early surgical consultation and facilitate the availability of blood for the operating room by obtaining a type and screen for the blood bank.
Vascular Intervention. Angiography already has been discussed for its role in diagnosing mesenteric ischemia. It is considered one of the fundamental treatment options as well. In fact, all patients who are diagnosed with mesenteric ischemia by another modality must undergo angiography prior to surgery. As an adjunct, angiography allows the evaluation of possible alternative, more conservative courses of action. For example, embolectomy for acute arterial occlusions has been practiced in the United States since 1951, with avoidance of surgery and reversal of intestinal ischemia repeatedly reported.71,72 More recently, success has been achieved utilizing percutaneous angioplasty.73-75 Results of angioplasty have been especially good, with excellent long-term clinical improvement and persistent vessel patency rates near 88%.73,74 Further advances may be possible with the addition of intra-arterial stent placement, although few centers are well trained enough for full-scale application of this technology.75
Pharmacotherapy
In cases of angiographically confirmed mesenteric ischemia, direct intra-arterial vasodilator infusion has proven beneficial in reducing vasospasm and effective in restoring blood flow.27,51,76-78 Clinically successful use of tolazoline, phenoxybenzamine, and verapamil have been reported, but papaverine has long stood as the agent of choice.51,76-78 Papaverine has been shown to reduce mortality in both occlusive and nonocclusive cases, and should be given early in the course of treatment.27,51 Papaverine is administered as a bolus of 30-60 mg intra-arterially, followed by a continuous infusion of 30-60 mg/h until surgical intervention or symptom resolution.79
Although not widely accepted in the therapeutic regimen, intra-arterial thrombolysis with streptokinase, urokinase, or tissue plasminogen activator (t-PA) can be considered as an alternative when treating thromboembolic causes of bowel infarction.80-85 Anecdotal reports have shown success using each of these, but guidelines have not been established. Systemic venous infusion of thrombolytic agents for mesenteric ischemia has yet to be evaluated and, thus, cannot be recommended at this time.
Much of the current pharmaceutical research is focused on the prevention of reperfusion-related morbidity. Ischemia causes the formation of oxygen free radicals, with resultant injury to intestinal wall, allowing the systemic release of bacterial toxins. Return of blood flow carries these toxins to distant organs. These toxins are thought to be responsible for pulmonary complications and distant organ system failure often found in advanced stages of mesenteric ischemia. It has been hypothesized that by diminishing this cycle, drastic reductions in protracted complications will result. Medications that may prevent changes in intestinal wall permeability are listed in Table 5. Studies to date are promising, but implementation is still far off.86-99
|
Table 5. Medications Reported to Prevent |
|
| Verapamil | Superoxide dismutase |
| 3-aminobenzamide | Ornithine alpha-ketoglutarate |
| Insulin-like growth factor-1 | Lisifylline |
| Ginko-bilboa extract | Hyperbaric oxygen |
| L-arginine | Alpha-1 acid glycoprotein |
| Octerotide | Iloprost |
| Ro 20-1724 | ATP-MgCL2 |
| Indomethicin | Allopurinol |
Conclusion
Mesenteric ischemia continues to be a diagnosis that makes emergency medicine physicians feel uneasy. It is extremely difficult to diagnose early, and delays in treatment often result in fatal outcomes. There are no bedside tests currently available to assist in making the diagnosis with certainty. The most accurate test, angiography, is not without its risks; therefore, clinicians may be reluctant to order the test when the diagnosis is ambiguous.
Overall, despite being a difficult task, the diagnosis can be made in a timely fashion with appropriate treatment and improved outcomes. The most important aspect is to always suspect mesenteric ischemia, especially in geriatric patients presenting with abdominal pain.
The classic presentation involves the triad of abdominal pain, gastric emptying, and cardiac disease. Be wary of patients with underlying cardiac arrhythmias, recent myocardial infarctions, or other risk factors for thromboembolic phenomena. The pain usually is present but may be overlooked because of a normal abdominal exam on physical examination. The histories often are distorted due to confounding issues such as dementia. A high index of suspicion must be maintained when entertaining the diagnosis of mesenteric ischemia.
Once the diagnosis has been determined to be mesenteric ischemia, prompt consultation with a vascular surgeon and interventional radiologist are essential for expeditious treatment. The sooner the patient gets diagnosed and treated, the greater the likelihood of survival.
The future may be a little brighter as researchers and physicians continue to work toward achieving more accurate and timely methods for diagnosis. Until the time when a bedside assay can definitively diagnose mesenteric ischemia, it is up to the astute emergency medicine clinician to contemplate the diagnosis and aggressively manage the patient when indicated. The geriatric population is growing and so, too, will be the incidence of mesenteric ischemia presenting to EDs across the country.
References
1. Fenyo G. Acute abdominal disease in the elderly: Experience from two series in Stockholm. Am J Surg 1982;143:751-754.
2. Bender JS. Approach to the acute abdomen. Med Clin North Am 1989;73:1413-1422.
3. Stoney RJ, Cunningham CG. Acute mesenteric ischemia. Surgery 1993;114:489.
4. Pierce GE, Brockenbrough EC. The spectrum of mesenteric infarction. Am J Surg 1970;119:233-239.
5. Boley SJ, Sprayregan S, Siegelman SS. Initial results from an aggressive roentgenological and surgical approach to mesenteric ischemia. Surgery 1977;82:848-855.
6. Klein HM, Lensing R, Klosterhalfen B. Diagnostic imaging of mesenteric infarction. Radiology 1995;197:79.
7. Sanders AB. Care of the elderly in the emergency department: Where do we stand? Ann Emerg Med 1992;21:792-795.
8. US Bureau of the Census. Statistical Abstract of the United States: 1990. 110th ed. Washington, DC: US Government Printing Office; 1990.
9. Ottinger LW, Austen WG. A study of 136 patients with mesenteric infarction. Surg Gynecol Obstet 1967;124:251-261.
10. Sachs SM, Morton JH, Schwartz SI. Acute mesenteric ischemia. Surgery 1982;92:646-653.
11. Clavien PA, Durig M, Harder F. Venous mesenteric infarction: A particular entity. Br J Surg 1988;75:252.
12. Newman TS, Magnuson TH, Ahrendt SA, et al. The changing face of mesenteric infarction. Am Surg 1998;64:611-616.
13. Sai Sudhakar CB, Al-Hakeem M, MacArthur JD. Mesenteric ischemia secondary to cocaine abuse: Case reports and literature review. Am J Gastroenterol 1997;92:1053.
14. McKinsey JF, Gewertz BL. Acute mesenteric ischemia. Surg Clin North Am 1997;77:307.
15. Boley SJ. Early diagnosis of acute mesenteric ischemia. Hospital Practice (Off Ed) 1981;16:63.
16. Potts FE, Vokov LE. Utility of fever and leukocytosis in acute surgical abdomens in octogenarians and beyond. J Gerontol. Series A, Biological Sciences and Medical Sciences. 1999;54:
M55-M58.
17. Gentile AT, Moneta GL, Taylor LM. Isolated bypass to the SMA for intestinal ischemia. Arch Surg 1994;129:926.
18. Johnston KW, Lindsay TF, Walker PM. Mesenteric arterial bypass grafts: Early and late results and suggested surgical approach for chronic and acute mesenteric ischemia. Surgery 1995;118:1.
19. Rogers DM, Thompson JE, Garrett WV. Mesenteric vascular problems: A 26-year experience. Ann Surg 1982;195:554.
20. Kazmers A. Operative management of acute mesenteric ischemia. Ann Vasc Surg 1998;12:187.
21. Moore WM, Hollier LH. Mesenteric artery occlusive disease. Cardiol Clin 1991;9:535.
22. Hollier LH, Bernatz PE, Pairolero PC. Surgical management of chronic intestinal ischemia: A reappraisal. Surgery 1981;90:940.
23. McAfee MK, Cherry KJ, Naessens JM. Influence of complete revascularization on chronic mesenteric ischemia. Arch Surg 1992;164:220.
24. Ruotolo RA, Evans SR. Mesenteric ischemia in the elderly. Clin Geriatr Med 1999;15:527-557.
25. Deehan DJ, Heys SD, Brittenden J, et al. Mesenteric ischemia: Prognostic factors and influence of delay upon outcome. J Royal Coll Surgeons Edinburgh 1995;40:112-115.
26. Ritz JP, Runkel N, Berger G, et al. Prognostic factors in mesenteric ischemia (German). Zentrablatt fur Chirurgie 1997;122:332-338.
27. Kaleya RN, Boley SJ. Acute mesenteric ischemia: An aggressive diagnostic and therapeutic approach. Can J Surg 1992;35:613-623.
28. Carrasco D, Gordo R, Olaso V, et al. The value of emergency angiography in the diagnosis and prognosis of acute mesenteric ischemia. Am J Gastroenterol 1978;69:295-301.
29. Clark RA, Gallant TE. Acute mesenteric ischemia: Angiographic spectrum. Am J Roentgenol 1984;142:555-562.
30. Liao XP, She YX, Shi CR, et al. Changes in body fluid markers in intestinal ischemia. J Pediatr Surg 1995;30:1412-1415.
31. Meyer T, Klein P, Schweiger H, et al. How can the prognosis of acute mesenteric artery ischemia be improved? Results of a retrospective analysis (German). Zentrablatt fur Chirurgie 1998;123:230-234.
32. Jonas J, Schwarz S, Alebrahim-Dehkordy A. Behavior of the lactate level in occlusion and reperfusion of the right superior mesenteric artery. An animal experiment study (German). Langenbecks Archiv fur Chirurgie. 1996;381:1-6.
33. Lange H, Jackel R. Usefulness of plasma lactate concentration in the diagnosis of acute abdominal disease. Eur J Surg 1994;160:381-384.
34. Lange H, Toivola. Warning signals in acute abdominal disorders. Lactate is the best marker of mesenteric ischemia (Swedish). Lakartidningen 1997;94:1893-1896.
35. Taylor BM, Jamieson WG, Durland D. Preinfarction diagnosis of acute mesenteric ischemia by a simple measurement of inorganic phosphate in body fluids. Can J Surg 1979;22:40-45.
36. Leo PJ, Simonian HG. The role of serum phosphate level and acute ischemic bowel disease. Am J Emerg Med 1996;14:377-379.
37. Lieberman JM, Sacchettini J, Marks C, et al. Human intestinal fatty acid binding protein: Report of an assay with studies in normal volunteers and intestinal ischemia. Surgery 1997;121:335-342.
38. Lieberman JM, Marks WH, Cohn S, et al. Organ failure, infection and the systemic inflammatory response are associated with elevated levels of urinary intestinal fatty acid binding protein: A study of 100 consecutive patients in a surgical intensive care unit. J Trauma 1998;45:900-906.
39. Fernandez WG, Lieberman JM, Levy PD, et al. Absence of urinary intestinal fatty acid binding protein is unlikely in the presence of mesenteric ischemia. Abstract # 057. Annual Meeting of the Society of Academic Emergency Medicine, 2000.
40. Pargger H, Staender S, Studer W, et al. Occlusive mesenteric ischemia and its effects on jejunal intramucosal pH, mesenteric oxygen consumption and oxygen tensions from surfaces of the jejunum in anesthetized pigs. Intensive Care Med 1997;23:91-99.
41. Robbins MR, Smith RS, Helmer SD. Serial pHi measurement as a predictor of mortality, organ failure and hospital stay in surgical patients. Am Surg 1999;65:715-719.
42. Ruettimann U, Urwyler A, Von Flue M, et al. Gastric intramucosal pH as a monitor of gut perfusion after thrombosis of the superior mesenteric vein. Acta Anaesthesiologica Scandinavica 1999;43:780-783.
43. Gunel E, Caglayan O, Caglayan F. Serun D(-) lactate as a predictor of intestinal ischemia-reperfusion injury. Pediatr Surg Int 1998;14:59-61.
44. Murray MJ, Barbose JJ, Cobb CF. Serum D(-)lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res 1993;54:507-509.
45. Kurland B, Brandt LJ, Delany HM. Diagnostic tests for intsetinal ischemia. Surg Clin North Am 1992;72:85-104.
46. Aydin M, Guler O, Ugras S, et al. Blood and tissue findings in the diagnosis of mesenteric ischemia: An experimental study. Clin Chem Lab Med 1998;36:93-98.
47. Morris S, Hays W, Enomoto M, et al. Serum cytosolic beta-glucosidase elevation and early ischemic injury to guinea pig small intestine. Surgery 1999;125:202-210.
48. Watari M, Murakami H, Orihashi K, et al. Ammonia determination as an early indicator of experimental superior mesenteric artery occlusion. Hiroshima J Med Sci 1997;46:159-167.
49. Lim HK, Lee WJ, Kim SH, et al. Splanchnic arterial stenosis or occlusion: Diagnosis at Doppler US. Radiology 1999;211:405-410.
50. Hessel SJ, Adams DF, Abrams LH. Complications of angiography. Radiology 1981;138:273-281.
51. Boley SJ, Feinstein FR, Sammartano R, et al. New concepts in the management of emboli of the superior mesenteric artery. Surg Obstet Gynecol 1981;153:561-569.
52. Salzano A, De Rosa A, Carbone M, et al. Computerized tomography features of intestinal infarction: 56 surgically treated patients of which 5 with reversible mesenteric ischemia (Italian). Radiologia Medica 1999;97:246-250.
53. Zwolak RM. Can duplex ultrasound replace arteriography in screening for mesenteric ischemia? Semin Vasc Surg 1999;12:252-260.
54. Hartnell GG, Gibson RN. Doppler ultrasound in the diagnosis of intestinal ischemia. Gastrointestinal Radiol 1987;12:285-288.
55. Eker A, Malzac B, Teboul J, et al. Mesenteric ischemia after coronary artery bypass grafting: Should continuous intra-arterial perfusion with papaverine be regarded as treatment? Eur J Cardio-Thoracic Surg 1999;15:218-220.
56. Clark RA. Computed tomography of bowel infarction. J Comput Assist Tomogr 1987;11:757-762.
57. Yamada K, Saeki M, Yamaguchi T, et al. Acute mesenteric ischemia. CT and plain radiographic analysis of 26 cases. Clin Imaging 1998;22:34-41.
58. Rha SE, Ha HK, Lee SH, et al. CT and MRI imaging findings of bowel ischemia from various primary causes. Radiographics 2000;20:29-42.
59. Ha HK, Rha SE, Kim AY, et al. CT and MRI diagnoses of intestinal ischemia. Semin Ultrasound CT MR 2000;21:40-55.
60. Taourel PG, Deneuville M, Pradel JA, et al. Acute mesenteric ischemia: Diagnosis with contrast enhanced CT. Radiology 1996;199:632-636.
61. Balthazar EJ, Liebskind ME, Macari M. Intestinal ischemia in patients in whom small bowel obstruction is suspected: Evaluation of accuracy, limitations, and clinical implications of CT in diagnosis. Radiology 1997;205:519-522.
62. Franquet T, Bescos JM, Reparaz B. Noninvasive methods in the diagnosis of isolated superior mesenteric vein thrombosis: US and CT. Gastrointestinal Radiol 1989;14:321-325.
63. Danse EM, Van Beers BE, Goffette P, et al. Acute intestinal ischemia due to occlusion of the superior mesenteric artery: Detection with Doppler sonography. Ultrasound Med Biol 1996;15:323-326.
64. Volteas N, Labropoulos N, Leon M, et al. Detection of superior mesenteric and coeliac artery stenosis with colour flow Duplex imaging. Eur J of Vasc Surg 1993;7:616-620.
65. Erden A, Yurdakul M, Cumhur T. Marked increase in flow velocities during sleep expiration deep expiration: A duplex Doppler sign of celiac artery compression syndrome. Cardiovasc Intervent Radiol 1999;22:331-332.
66. Wilkerson DK, Mezrich R, Drake C, et al. Magnetic resonance imaging of acute occlusive intestinal ischemia. J Vasc Surg 1990;11:567-571.
67. Meaney JF, Prince MR, Nostrant TT, et al. Gadolinium enhanced MR angiography of visceral arteries in patients with suspected chronic mesenteric ischemia. J Magn Reson Imaging 1997;7:171-176.
68. Baden JG, Racy DJ, Grist TM. Contrast enhanced three dimensional magnetic resonance angiography of the mesenteric vasculature. J Magn Reson Imaging 1999;10:369-375.
69. Seidel SA, Bradshaw LA, Ladipo JK, et al. Noninvasive detection of ischemic bowel. J Vasc Surg 1999;30:309-319.
70. Seidel SA, Hegde SS, Bradshaw LA, et al. Intestinal tachyarrhythmias during small bowel ischemia. Am J Physiol 1999;277:G993-G999.
71. Zechini F, Reale R, Sorgi G, et al. Mesenteric artery embolectomy: A case report. Intestinal Surg 1980;65:275-277.
72. Lobo-Martinez E, Merono Carvajosa E, Sacco O, et al. Embolectomy in mesenteric ischemia (Spanish). Revist Espanola de Enfermedades Digestivas 1993;83:351-354.
73. Golden DA, Ring EJ, McLean GK, et al. Percutaneous transluminal angioplasty in the treatment of abdominal angina. Am J Roentgenol 1982;139:247-249.
74. Maspes F, Mazzetti di Pietralata G, Gandini R, et al. Percutaneous transluminal angioplasty in the treatment of chronic mesenteric ischemia: Results and 3 years of follow-up in 23 patients. Abdom Imag 1998;23:358-363.
75. Nyman U, Ivancev K, Lindh M, et al. Endovascular treatment of chronic mesenteric ischemia: Report of five cases. Cardiovasc Intervent Radiol 1998;21:305-313.
76. Athansoulis CA, Wittenberg J, Bernstein R, et al. Vasodilatory drugs in the management of nonocclusive bowel ischemia. Gastroenterol 1975;68:146-150.
77. MacCannell KL. Comparison of an intravenous selective mesenteric vasodilator with intraarterial papaverine in experimental nonocclusive mesenteric ischemia. Gastroenterol 1986;9:79-83.
78. Dunn MM, McFall TA, Rigano WD, et al. Adjunctive vasodilator therapy in the treatment of murine ischemia. Am J Surg 1993;165:697-699.
79. Brandt LJ, Boley SL. Ischemic and vascular lesions of the bowel. In: Sleisenger MH, Fordtran JS, eds. Gastrointestinal Disease: Pathophysiology, Diagnosis and Management. 5th ed. Philadelphia: WB Saunders; 1993:1927-1961.
80. Hillers TK, Ginsberg JS, Pahju A, et al. Intra-arterial low dose streptokinase for superior mesenteric artery embolus. Can Med Assoc J 1990;142:1087-1088.
81. Rivitz SM, Geller SC, Hahn C, et al. Treatment of acute mesenteric venous thrombosis with tranjugular intramesenteric urokinase infusion. J Vasc Intervent Radiol 1995;6:219-223.
82. Train JS, Ross H, Weiss JD, et al. Mesenteric venous thrombosis: Successful treatment by intraarterial lytic therapy. J Vasc Intervent Radiol 1998;9:461-464.
83. Kwauk ST, Bartlett JH, Hayes P, et al. Intra-arterial fibrinolytic treatment for mesenteric arterial embolus: A case report. Can J Surg 1996;39:163-166.
84. Rosen MP, Sheiman R. Transhepatic mechanical thrombectomy followed by infusion of TPA into the superior mesenteric artery to treat acute mesenteric vein thrombosis. J Vasc Intervent Radiol 2000;11:195-198.
85. Ludwig DJ, Hauptmann E, Rosoff L Jr, et al. Mesenteric and portal vein thrombosis in a young patient with protein S deficiency treated with urokinase via the superior mesenteric artery. J Vasc Surg 1999;30:551-554.
86. Kimura M, Kataoka M, Kuwabara Y, et al. Beneficial effects of verapamil on intestinal ischemia and reperfusion injury: Pretreatment versus post ischemic treatment. Eur Surg Res 1998;30:191-197.
87. Williams JP, Weiser MR, Pechet TT, et al. Alpha1-acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. Am J Physiol 1997;273:G1031-G1035.
88. Anderson SH, Dalton HR. Use of octreotide in the treatment of mesenteric angina. Am J Gastroenterol 1997;92:1222-1223.
89. Manasia A, Kang H, Hannon E, et al. Effects of the stable prostacyclin analogue iloprost on mesenteric blood flow in porcine endotoxic shock. Crit Care Med 1997;25:1222-1227.
90. Carcillo JA, Herzer WA, Mi Z, et al. Treatment with the type IV phosphodiesterase inhibitor Ro 20-1724, protects renal and mesenteric blood flow in endotoxemic rats treated with norepinephrine. J Pharmacol Exper Therapeut 1996;279:1197-1204.
91. Kreienberg PB, Darling RC 3rd, Shah DM, et al. ATP-MgCL2 reduces intestinal permeability during mesenteric ischemia. J Surg Res 1996;66:69-74.
92. Sare M, Bozkurt S, Onuk E, et al. The effects of indomethacin, NDGA, allopurinol and superoxide dismutase on prostaglandin E2 and leukotriene C4 levels after mesenteric ischemia-reperfusion injury. Prostaglandins Leukot Essent Fatty Acids 1996;55:379-383.
93. Cuzzocres S, Zingarelli B, Costantino G, et al. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br J Pharmacol 1997;121:1065-1074.
94. Duranton B, Schleiffer R, Gosse F, et al. Preventive administration of ortnithine alpha-ketoglutarate improves intestinal mucosal repair after transient ischemia in rats. Crit Care Med 1998;26:120-125.
95. Haglind E, Malmlof K, Fan J, et al. Insulin-like growth factor-I and growth hormone administration in intestinal ischemia shock in the rat. Shock 1998;10:62-68.
96. Wattanasirichagoon S, Menconi MJ, Delude RL, et al. Lisophylline ameliorates intestinal mucosal barrier dysfunction caused by ischemia and ischemia/reperfusion injury. Shock 1999;11:269-275.
97. Onen A, Deveci E, Inaloz SS, et al. Histopathological assessment of the prophylactic effect of ginko-bilboa extract on intestinal ischemia-reperfusion injury. Acta Gastroenterologica Belgica 1999;62:386-389.
98. Tjarnstrom J, Wikstrom T, Bagge U, et al. Effects of hyperbaric oxygen treatment on neutrophil activation and pulmonary sequestration in intestinal ischemia-reperfusion on rats. Eur Surg Res 1999;31:147-154.
99. Ward DT, Lawson SA, Gallagher CM, et al. Sustained nitric oxide production via l-arginine administration ameliorates effects of intestinal ischemia-reperfusion. J Surg Res 2000;89:13-19.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.