Infections of the Neck in Children
December 1, 2013
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
Infections of the Neck in Children
Children frequently present with infections of the neck. A comprehensive understanding of both superficial and deep infections of the neck is essential for clinical practice. Accurate recognition and early definitive management can minimize the risk of subsequent complications.
— Ann M. Dietrich, MD, Editor
Executive Summary
- Viral pathogens cause acute lymphadenitis that is commonly bilateral, non-suppurative, and self-limited.
- Bacterial cervical adenitis, on the other hand, is more often unilateral, progressive if left untreated, and may result in abscess formation.
- Reflecting the increased prevalence of MRSA, a number of studies have demonstrated that approximately 30% of cervical abscesses are due to MRSA, with smaller proportions caused by methicillin-sensitive S. aureus (MSSA) and GAS.
- Cat scratch disease, caused by Bartonella henselae, occurs following a bite or scratch by an infected cat. Following an incubation period of days to weeks, a cervical lymph node may become inflamed. Systemic symptoms may or may not be present. The initial site of inoculation may be distant from the neck, so a careful history and skin examination is critical.
- Thyroglossal duct cysts are the most common cause of a midline neck mass and are typically located at the level of the thyroid cartilage. Approximately 20% become infected.
Introduction
Pediatric patients with infections of the various structures of the neck are often evaluated in the emergency department. The infections seen range from benign, self-limited viral processes to potentially life-threatening bacterial diseases. Emergency clinicians must be able to efficiently and accurately diagnose and treat these patients. This article will focus on the assessment, evaluation, and treatment of both superficial and deep neck space infections in emergency settings. While the most common causes of acute infection will be emphasized, a framework to help identify atypical infections and noninfectious processes will also be provided.
Superficial Neck Infections
Case 1. An 8-year-old girl presents to the emergency department with a two-day history of progressive left neck swelling, redness, and pain. She is generally well-appearing. Her temperature is 38.0°C. Overlying her left sternocleidomastoid muscle is visible erythema and edema; underlying this area is a 2 cm x 2 cm indurated and tender but nonfluctuant mass.
What evaluation is necessary? Imaging? Laboratory testing? What uncommon infections and noninfectious causes should be considered? Should an antibiotic be started and, if so, which one(s)? Does she require inpatient admission and/or surgical consultation?
Definitions and Epidemiology
Cervical lymphadenopathy, defined as enlarged lymph node(s) of the neck, is the most common cause of a palpable pediatric neck mass.1,2 Enlarged but non-inflamed lymph nodes are often reactive sequelae of a viral upper respiratory tract infection rather than a focal infectious process. In fact, nearly half of healthy children may have palpable cervical lymphadenopathy and no evidence of localized or systemic disease.3 A recent study of 282 pediatric patients who underwent evaluation for cervical adenopathy found that 64% of the cases were of unknown origin.4
Cervical lymphadenitis refers to enlarged, inflamed, and tender lymph nodes of the neck. Acute infectious processes are the most common cause in children. Most children with symptomatic lymphadenitis have either a viral or bacterial etiology of their symptoms, while smaller proportions of inflamed neck masses are due to congenital malformations or neoplasm.1 Focal superficial neck abscesses may occur as the suppurative sequelae of lymphadenitis, direct infiltration of soft tissue, or as a consequence of infected congenital malformations. Consequently, spreading cellulitis and spontaneous purulent drainage may also be seen. The rising incidence of methicillin-resistant S. aureus (MRSA) infected cervical abscesses has been demonstrated in a number of studies.5,6 More recently, a review of the 2009 Kids’ Inpatient Database found 3571 MRSA-infected abscesses of the head and neck requiring hospital admission.7
Anatomy
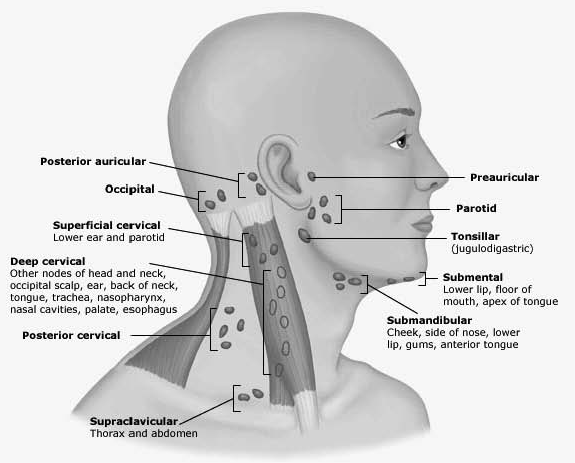
Defining the location of a neck mass helps formulate an initial differential diagnosis. (See Table 1.) Midline masses are more commonly congenital in origin, while lateral masses are more likely infectious. Determining whether a mass is unilateral or bilateral also helps guide whether bacterial or viral causes are more likely. Finally, understanding the lymphatic drainage of the head and neck may help identify the infectious origin of an inflamed or reactive lymph node. (See Figure 1.) For example, enlargement of the anterior cervical nodes is common during acute infectious processes of the mouth and pharynx, while posterior cervical and occipital adenopathy may be seen with scalp infections and dermatitis.
| Table 1.Anatomic Diagnosis of Neck Masses | |
|---|---|
| Location | Possible Cause |
|
Midline, above thyroid |
Thyroglossal duct cyst, dermoid, laryngocele |
|
Midline, near/overlying thyroid |
Thyromegaly, thyroid nodule |
|
Lateral, anterior to sternocleidomastoid |
Lymph node(s), branchial cleft cysts |
|
Lateral, posterior to sternocleidomastoid |
Lymph node(s), cystic hygroma |
|
Supraclavicular |
Lymph node(s), malignancy |
Pathophysiology and Etiology
Infectious lymphadenitis is the result of afferent lymphatic drainage of organisms that penetrate the oropharyngeal mucosa or skin of the head and neck into regional lymph nodes. Subsequently, an inflammatory infiltrate ensues within the affected node(s), resulting in either an acute or chronic infectious process.
Viral pathogens cause acute lymphadenitis that is commonly bilateral, non-suppurative, and self-limited. Bacterial cervical adenitis, on the other hand, is more often unilateral, progressive if left untreated, and may result in abscess formation. The most common bacterial pathogens associated with acute suppurative lymphadenitis are group A streptococcus (GAS) and S. aureus; anaerobic oral flora also may be isolated, particularly when there is associated periodontal disease.8 Reflecting the increased prevalence of MRSA, a number of studies have demonstrated that approximately 30% of cervical abscesses are due to MRSA, with smaller proportions caused by methicillin-sensitive S. aureus (MSSA) and GAS.9,10,11 This shift in microbiologic patterns is important for the emergency provider to consider when either initiating or changing antibiotic therapy in a child with cervical adenitis.
Clinical Features
History. A detailed history about the timing, associated symptoms, and preceding or intercurrent illness should be obtained for all children presenting with cervical lymphadenitis. An acutely inflamed neck mass is likely to be caused by common bacterial and viral pathogens, whereas subacute and chronic lesions tend to be associated with atypical infections, noninfectious inflammatory conditions, and neoplasms. Concurrent or preceding symptoms of upper respiratory tract infection, tonsillitis, pharyngitis, or otitis media are often found in the typical history of acute lymphadenitis. Asking about both cat and tuberculosis exposure, as well as ingestion of unpasteurized milk or uncooked meats, may uncover less common infectious diagnoses. A child with a previous history of a neck mass that has become acutely red, painful, and swollen may have a secondarily infected congenital lesion.
Physical Examination. Assessing vital signs helps to determine the degree of systemic toxicity and associated dehydration from an acute infection. A rapid assessment of the airway is important since some neck masses may cause external compression. The scalp, ears, mouth, and throat should be evaluated as potential primary sources of infection leading to cervical node inflammation. Neck range of motion and signs of meningeal irritation should be elicited.
Visually inspecting the neck will provide information about the degree of associated edema and erythema. Palpating areas of edema and erythema in the neck will identify underlying node mobility, tenderness, induration, and fluctuance. Complete palpation of the neck will reveal the presence of other enlarged or tender cervical nodes. Examination of the heart, lungs, abdomen, skin, and nervous system provides information about the degree of systemic toxicity from a focal neck infection and may uncover other findings suggestive of an illness that would result in an inflamed superficial cervical node (e.g., Kawasaki syndrome).
Differential Diagnosis
An initial differential diagnosis of superficial neck infections may be made by considering both the onset and duration of symptoms (acute, subacute, chronic) as well as the anatomic location of the neck mass. A probable diagnosis based on timing and location is provided in Table 2. In the majority of cases, an acutely inflamed neck mass represents bacterial or viral lymphadenitis. Less common causes of neck swelling should be considered for those masses that are subacute or chronic, in a midline location, or have a history suggestive of an atypical infection or systemic inflammatory process.
| Table 2.Differential Diagnosis of Pediatric Neck Infections | |
|---|---|
| Location/Timing | Possible Cause(s) |
|
Acute unilateral |
Bacterial (GAS, S. aureus, anaerobes) |
|
Acute bilateral |
Viral, less likely GAS |
|
Subacute/chronic unilateral |
Viral (EBV, CMV) |
|
Subacute/chronic bilateral |
NTM, tuberculosis, cat scratch, toxoplasmosis |

Less Common Infections. Several less common pathogens may cause cervical lymphadenopathy and/or lymphadenitis. Emergency medicine providers should be aware of their presenting signs and historical features, particularly when evaluating a child with a subacute or chronic neck mass.
Tuberculosis is a common cause of cervical adenitis in endemic areas of the world. Cervical node infection occurs via direct spread from the apical pleura or extension from paratracheal nodes. Concurrent symptoms suggestive of tuberculosis infection, as well as exposure risks, should prompt evaluation for this infection.
Nontuberculous mycobacteria (NTM) may cause a slowly growing, non-tender unilateral node. Overlying skin changes and a chronically draining sinus tract may result. This infection is most common in children younger than the age of 5 years and should be considered when a PPD test becomes positive in a child with a neck mass who has no signs or symptoms of active tuberculosis.
Cat scratch disease, caused by Bartonella henselae, occurs following a bite or scratch by an infected cat. Following an incubation period of days to weeks, a cervical lymph node may become inflamed. Systemic symptoms may or may not be present. The initial site of inoculation may be distant from the neck, so a careful history and skin examination is critical. Toxoplasma gondii may cause chronic tender lymphadenopathy after ingestion of poorly cooked meat. Cats are the definitive host for this organism, so asking about exposure to cats and their litter may lead to this diagnosis. HIV may cause chronic cervical lymphadenitis and should be considered in patients at risk for this infection.
Congenital Malformations. A number of congenital lesions may cause neck masses that may become secondarily infected. An understanding of their typical location in the neck will help inform treatment, imaging, and consultation. The two most common lesions are thyroglossal duct cysts and branchial cleft cysts. Thyroglossal duct cysts are the most common cause of a midline neck mass and are typically located at the level of the thyroid cartilage. Approximately 20% become infected.2 When infected, initial treatment includes antibiotics, followed by surgical excision.
Branchial cleft cysts, found in the lateral neck, are another common cause of congenital neck masses. (See Figure 2.) Second branchial cleft cysts are the most common and appear anterior to the sternocleidomastoid. First, third, and fourth branchial cleft cysts may occur, but much less frequently. Infection of these cysts is not uncommon and may be easily confused with cervical lymphadenitis. A history of a congenital mass in an area of acute inflammation or a recurrent inflammatory mass in the same lateral neck location is helpful. Surgical excision following a period of medical treatment with antibiotics is preferred when these cysts present with acute infection.
Diagnostic Testing
Laboratory Testing. While routine laboratory testing is often unnecessary and nondiagnostic in cases of acute lymphadenitis, elevations in white blood cell count and inflammatory markers may be seen. The presence of atypical lymphocytes on a CBC is suggestive of a viral infection, particularly in the patient with diffuse bilateral lymphadenitis, and may prompt confirmatory testing for Epstein-Barr virus (EBV). For the ill-appearing patient, blood cultures should be collected. If spontaneous purulent drainage is present from a fluctuant neck mass, culturing the purulent fluid will help identify the causative pathogen. When feasible, fine needle aspiration for Gram stain and culture of an infected node can be used to tailor antimicrobial therapy. If the presentation is atypical or if a chronic inflammatory neck mass is present, testing for Bartonella henselae, Toxoplasma gondii, and/or tuberculosis may be warranted; consultation with an infectious disease expert may be helpful in these cases. Some authors suggest placing a PPD in all pediatric patients presenting with an inflammatory superficial neck mass while monitoring response to antimicrobial treatment.1,12

Imaging. In most cases of acute lymphadenitis, the history and physical examination alone are sufficient for diagnosis. However, in cases in which a congenital malformation is suspected, the extent of a neck mass is unclear, or there is a question of whether surgical drainage is needed, imaging may be necessary. Ultrasound is the initial imaging modality of choice for superficial neck masses in children because it lacks radiation and has a high sensitivity for identifying the key structures of the neck. Ultrasonography can accurately identify both normal and inflamed lymph nodes, areas of fluid collection, vascularity of masses, and involvement of other structures of the neck.13,14 (See Figure 3.) In some instances, either when ultrasound is unavailable or non-diagnostic in atypical cases or when there is inadequate response to parenteral antibiotics, computed tomography (CT) or magnetic resonance imaging may be required. Plain films of the neck are not indicated unless a child has signs of airway compromise and rapid assessment is necessary.
Management
Antibiotics. Since children with diffuse bilateral cervical adenitis are likely to have a viral cause of their presentation, they may be treated expectantly without antibiotics, provided the child is generally well-appearing and there are no signs of associated secondary bacterial infection or superficial skin infection. Children with acute unilateral adenitis or bilateral adenitis with signs of bacterial infection should be treated with an antibiotic that is effective against both GAS and S. aureus. In areas where the prevalence of community-acquired MRSA is high or a child has not improved on an antibiotic that is ineffective against MRSA, either clindamycin (30 mg/kg/day divided every 6-8 hours) or trimethoprim-sulfamethoxazole (10 mg/kg/day of the TMP component divided every 12 hours) for 10-14 days would be an ideal choice. If MRSA is not a major concern, clindamycin is still an effective treatment, but cephalexin (25-100 mg/kg/day divided every 6-8 hours) or cefadroxil (30 mg/kg/day divided every 12 hours) may also be used.12 If GAS is strongly suspected based on concurrent streptococcal pharyngitis, amoxicillin (40 mg/kg/day divided every 12 hours) would be a reasonable alternative. If periodontal disease is present as the likely culprit of adenitis, clindamycin or amoxicillin clavulanate (40 mg/kg/day of the amoxicillin component every 12 hours) are suggested to treat oral flora. Finally, if a child requires parenteral antibiotics, clindamycin (40 mg/kg/day divided every 8 hours) is recommended because of the high incidence of MRSA in cultured aspirates from hospitalized patients; therapy may be subsequently tailored based on culture sensitivities or local MRSA resistance patterns. (See Table 3.)
| Table 3.Antibiotic Treatment for Adenitis | |
|---|---|
|
In areas of high MRSA prevalence or if a child has not improved on antibiotic ineffective against MRSA |
|
|
If MRSA is not a major concern |
|
|
If GAS is strongly suspected |
Amoxicillin 40 mg/kg/day divided every 12 hours |
|
If periodontal disease is likely culprit |
Clindamycin or amoxicillin clavulanate (40 mg/kg/day of amoxicillin component every 12 hours) |
|
If child requires parenteral antibiotics |
Clindamycin 40 mg/kg/day divided every 8 hours |
Supportive Care. Since most inflamed superficial neck infections tend to be painful and may cause fever, parents should be instructed on the appropriate use of an analgesic and antipyretic, such as ibuprofen or acetaminophen. Parents may also be instructed to apply warm compresses to areas of induration to help promote infectious accumulation and spontaneous drainage of purulent fluid.
Incision/Drainage. Emergency clinicians must identify those children who are in need of surgical drainage of a superficial neck infection, particularly when adenitis has transformed into a focal abscess. For simple superficial abscesses in older cooperative children, either needle aspiration or incision and drainage may be feasible within the emergency department. However, consultation with a surgeon or otolaryngologist is suggested in the following cases: uncooperative or very timid children who would require procedural sedation; potentially complex abscesses; secondarily infected congenital malformations; and concern about proximity of infection to vital neck structures. A recent retrospective study of 768 pediatric patients evaluated for a superficial neck infection at two tertiary care pediatric emergency departments found that patients without fluctuance on examination, prior antibiotic treatment, or previous emergency room visit had less than a 4% chance of requiring surgical incision and drainage.9 Based on these findings, it would be reasonable to seek routine consultation in cases in which fluctuance is found on examination or a child has failed to improve or worsened after a trial of appropriate antibiotics.
Disposition. Deciding whether a child requires admission for intravenous antibiotics rather than outpatient oral therapy is largely dependent on clinician judgment and the child’s appearance in the emergency department. A child who is generally well-appearing and has no systemic signs of illness may be safely discharged home with oral antibiotics and close outpatient follow-up. Emergency department return criteria should be clearly explained to a patient’s guardian(s) and may include increased size and spreading superficial erythema of the neck mass, increased pain and/or fever, signs of dehydration, and ill appearance. Children who are febrile and exhibiting signs of systemic toxicity, such as dehydration, vomiting, and altered mental status, as well as children requiring surgical drainage of a neck abscess should be admitted for intravenous antibiotics. Figure 4 outlines a management algorithm based on these recommendations.
Figure 4. Management Algorithm for Pediatric Cervical Lymphadenitis

Summary
Most children presenting to the emergency department with an acutely inflamed superficial neck mass have either a viral or bacterial infection of the cervical lymph nodes. Bacterial adenitis is likely in cases of acute unilateral disease, and GAS and S. aureus are the most common pathogens. Initiation of appropriate antibiotic therapy and surgical consultation when incision and drainage is required will often result in complete resolution of infectious symptoms without any complications. Emergency clinicians should be mindful that when a superficial neck mass is midline and/or chronic in nature, alternative diagnoses should be considered.
Deep Neck Space Infections
Case 2. A 4-year-old boy presents with a hoarse voice associated with neck pain and stiffness three days into an illness characterized by nasal congestion, throat pain, and cough. He is non-toxic but appears to be in pain. His temperature is 38.5° Celsius, and he is mildly tachycardic. He has no visible neck swelling or meningeal signs, but he resists passive extension and lateral rotation of his neck.
What clinical features suggest a deep neck space infection? What deep neck spaces/structures may be involved? What mode(s) of imaging should be considered? Is immediate surgical intervention necessary? What antibiotic(s) are most appropriate? What complications may occur?
Definition and Epidemiology
Several deep structures of the neck are prone to infection in children. While these infections are far less common than those in the superficial neck, the potential complications, including airway compromise, mediastinitis, CNS involvement, and sepsis, make them a major concern for emergency practitioners. Since a spectrum of disease severity and location of deep neck infections exist in children, true epidemiologic data about their incidence are difficult to describe.15 One study demonstrated that children requiring surgical intervention for deep neck space infections tend to be young (average age 4 years), male, and of white or black race. Age is also an important epidemiologic factor, as children younger than 10 years of age are more likely to develop a retropharyngeal or parapharyngeal abscess (mean age 5 years), whereas children and adolescents older than 10 years of age are more likely to present with a peritonsillar abscess.16
Anatomy
The deep neck is divided into three layers (superficial, middle, and deep) by fascial planes that extend upward into the cranium and downward into the mediastinum. Several critical structures are within these planes and help create important anatomic spaces. Specifically, the submandibular, parapharyngeal, masticator, buccal, parotid, peritonsillar, retropharyngeal, prevertebral, vascular, and danger spaces are all potential sites of infection. The most common spaces infected in children, in descending order of frequency, are the peritonsillar, retropharyngeal, submandibular, buccal, and parapharyngeal spaces.15
Etiology and Pathophysiology
Typically, the microbiologic profile of deep neck infections reflects the bacteria that cause infection of the teeth, tonsils, deep cervical nodes, middle ear, and sinuses.17 The deep neck spaces contain lymphoid tissue that may become seeded with bacteria arising from concurrent infection in these areas. An inflammatory response follows, leading to development of lymphadenitis and/or cellulitis. Necrosis and breakdown of infected nodes leads to abscess formation and possible spread of infectious material within and through fascial planes. The most common pathogens isolated include S. aureus, GAS, and oral anaerobes. In many cases, however, infectious isolates are polymicrobial.
Clinical Features
History. Determining which children have deep neck space infections based on history alone may be challenging. Many children will have a non-specific history of rhinorrhea, cough, fever, poor oral intake, dysphagia, and agitation. A history of voice change, drooling, trismus, and neck pain may be more specific but more difficult to obtain from a young child.18 Historical factors that may put a child at risk for developing a deep neck infection should be elicited, including premature birth, immunosuppression, previous history of severe infection or sepsis, and recent ear, nose, and throat infection. In general, a thorough history about symptom onset, duration, and associated signs of illness is important.
Physical Examination. Immediate assessment of the vital signs and airway patency is extremely important in a child with a suspected deep neck infection. The presence of excessive drooling, stridor, and respiratory accessory muscle use suggests imminent or progressive airway compromise. These findings should prompt urgent consideration of airway management in consultation with an anesthesiologist and/or otolaryngologist, given the potential difficulty and complications of endotracheal intubation. Fever is usually present, and dehydration secondary to odynophagia and poor oral intake is common.
The oropharynx should be examined for signs of concurrent infection. The dentition should be inspected for signs of disease that may lead to spreading infection. The tonsils should be examined for signs of edema, erythema, asymmetry, and fluctuance. A deviated uvula in association with an asymmetrically enlarged and exudative tonsil is seen with a peritonsillar abscess. Medial displacement of the lateral pharyngeal wall and tonsil suggests a parapharyngeal process. The neck should be visually inspected and palpated for associated lymphadenopathy, swelling, and induration. Of these findings, a large retrospective review found induration to be the most common presenting sign in 88% of patients.19 Neck range of motion is often limited due to pain and inflammation, and torticollis may be present. A complete examination of the patient is necessary to help determine the degree of associated systemic toxicity affecting other organ systems.
Diagnostic Testing
Laboratory Testing. Routine laboratory testing, such as metabolic panels, CBC, and inflammatory markers, is not diagnostic for deep neck infections. Patients with these infections are likely to have an elevation of white blood cell count, but may not have elevations in CRP.20 Surveillance monitoring of WBC during treatment may be helpful. When there is clinical concern for sepsis, blood cultures should be obtained.
Imaging. Lateral neck radiographs are easily accessible and may be a helpful screening tool for cases of suspected retropharyngeal abscess. With the neck properly extended, an increased width of the prevertebral soft tissues is quite sensitive and specific for detecting the presence of retropharyngeal edema. (See Figures 5 and 6.) The presence of air-fluid levels is even more suggestive of a suppurative process. If a parapharyngeal process is suspected or a high nasopharyngeal retropharyngeal abscess is possible, plain radiographs have limited utility.21
|
Figure 5. Lateral Radiograph Suggestive of Retropharyngeal Abscess 
|
Figure 6. Plain Film of Retropharyngeal Abscess 
|
CT is considered the gold standard for detecting inflammatory processes in the deep neck. (See Figure 7.) It provides critical information for the surgical consultant when incision and drainage is anticipated and when the expected response to appropriate parenteral antibiotics is lacking.
Figure 7. CT of Retropharyngeal Abscess

One study of 179 children evaluated for deep neck space infection found the duration of symptoms did not impact the rate of abscess identification, making it useful even early in the course of illness.20 Recent evidence, however, suggests that CT, while having a high positive predictive value, may be overly sensitive for detecting abscesses that may not require surgical drainage.22 Thus, the emergency provider should understand that not all documented cases of deep neck abscess require immediate surgical intervention. Consultation with an otolaryngologist will help guide conservative medical management and surgical staging.
Ultrasound is an intriguing modality given its availability, low cost, and lack of radiation. Some studies suggest ultrasound may actually be equal to, or even superior to, CT for detecting the presence of neck abscesses.23,24 However, reliable use of ultrasound is dependent on skilled sonographers and radiologists who feel comfortable diagnosing these infections in children using this imaging modality. Additionally, most surgical consultants will prefer more detailed anatomical information provided by CT before surgical intervention on the neck.
Management
Antibiotics. Initiating antibiotic therapy in the child with a confirmed or probable deep neck space infection is imperative. Many cases, particularly those with small abscesses or phlegmon, may ultimately be treated conservatively with antibiotics alone.25,26 Since the majority of cases are caused by GAS, S. aureus, or anaerobes, initial empiric therapy often includes either parenteral clindamycin or ampicillin-sulbactam.
Surgical Consultation. Con-sultation with an otolaryngologist is suggested for all cases of deep neck infection. Recent evidence suggests many cases may be treated solely with antibiotics and never require surgical intervention.27 One study of 187 pediatric patients with either retropharyngeal or parapharyngeal abscesses found that age younger than 51 months and CT evidence of an abscess greater than 2.2 cm in size was associated with a greater risk of conservative medical failure requiring surgical drainage.28 Based on most data, three distinct populations exist: children who improve with antibiotics alone, children who fail to respond to conservative management and require surgical intervention, and children who require immediate surgical drainage. Unless a child presents with signs of imminent airway compromise or mediastinitis necessitating immediate surgery, most cases are amenable to a trial of conservative medical therapy before proceeding to the operating room.
Disposition. While no consensus guidelines exist for the management of children with deep neck space infections, the potential for airway compromise, inability to tolerate fluids, and unclear course of illness in most children warrants admission for intravenous antibiotics and hydration, as well as cardiorespiratory monitoring. No studies have examined the effectiveness of outpatient oral antibiotics on outcomes for most deep neck space infections, so this approach is not recommended. The exception would be the well-appearing child with a peritonsillar abscess that has been effectively addressed in the emergency department with fine-needle aspiration. For these patients who are able to tolerate fluids, outpatient oral antibiotic therapy and pain control is appropriate.
Infectious Complications
Mediastinitis. Since the retropharyngeal space extends into the mediastinum, infection and extension within the space may lead to life-threatening mediastinal infection. This may be a rapidly progressive process and is a surgical emergency when detected. One study found this complication in 6.5% of pediatric patients with retropharyngeal abscess; younger age was the most significant contributing factor.29

Lemierre Syndrome. Deep neck infections may cause internal jugular vein thrombosis and subsequent septic embolization. Fusobacterium is the classic pathogen implicated, but others, including MRSA, have also been isolated.30 Aggressive medical therapy, sometimes including anti-coagulation, is necessary. This diagnosis should be considered in the ill-appearing patient, often adolescent, with localized neck pain, high fever, and respiratory distress due to septic emboli.31 (See Figure 8.)
Summary
Deep neck infections are relatively uncommon in children, but their potential consequences, if missed or left untreated, may be devastating. Emergency medicine providers should have a high level of suspicion for these infections in the child with a preceding or concurrent upper respiratory, dental, throat, or ear infection presenting with new or progressive symptoms of fever with neck pain and stiffness. A number of spaces within the deep neck structures may become secondarily infected with cellulitis, lymphadenitis, and abscess formation.
CT is the gold standard imaging modality for diagnosing deep neck space infections, but it may be overly sensitive in detecting phlegmon or abscesses that may not require surgical drainage.
Initiating antibiotic therapy promptly is important, while consulting an otolaryngologist regarding surgical treatment.
References
- Harper MB, Fleisher GR. Infectious disease emergencies. In: Fleisher GR, Ludwig S, et al, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia: Lipincott Williams & Wilkens; 2010: 905-907.
- Bly RA, Parikj SR. Neck masses and adenopathy. In: Schoem SR, Darrow DH, eds. Pediatric Otolaryngology. Elk Grove Village, IL: American Academy of Pediatrics; 2012:251-271.
- Larrson LO, Bentzon MW, Berg Kelly K, et al. Palpable lymph nodes of the neck in Swedish schoolchildren. Acta Paediatr 1994;83:1091-1094.
- Citak EC, Koku N, Demirci M, et al. A retrospective chart review of evaluation of the cervical lymphadenopathies in children. Auris Nasus Larynx 2011;38: 618-621.
- Naseri I, Jerris RC, Sobol SE. Nationwide trends in pediatric Staphylococcus aureus head and neck infections. Arch Otolaryngol Head Neck Surg 2009;135(1):14-16.
- Walker PC, Karnell LH, Ziebold C, et al. Changing microbiology of pediatric neck abscesses in Iowa 2000-2010. Laryngoscope 2013;123(1):249-252.
- McCormick ME, Chun RH, Lander L, et al. Socioeconomic implications of pediatric cervical methicillin-resistant Staphylococcus aureus infections. JAMA Otolaryngol Head Neck Surg 2013;139:124-128.
- Swanson DS. Etiology and clinical manifestations of cervical lymphadenitis in children. In: UpToDate, Torchia MM (Ed), UpToDate, Waltham, MA, 2013.
- Sauer MW, Sharma S, Hirsh DA, et al. Acute neck infections in children: Who is likely to undergo surgical drainage? Am J Emerg Med 2013;31:906-909.
- Neff L, Newland JG, Sykes KJ, et al. Microbioloigy and antimicrobial treatment of pediatric cervical lymphadenitis requiring surgical intervention. International Journal of Pediatric Otorhinolaryngology 2013;77:817-820.
- Duggal P, Naseri I, Sobol SE. The increased risk of community-acquired methicillin-resistant Staphylococcus aureus neck abscesses in young children. Laryngoscope 2011;121(1):51-55.
- Healy CM. Diagnostic approach to and initial management of cervical lymphadenitis in children. In: UpToDate, Torchia MM (Ed), UpToDate, Waltham, MA, 2013.
- Friedman ER, John SD. Imaging of pediatric neck masses. Radiol Clin North Am 2011;49:617-632.
- Rosenberg HK. Sonography of pediatric neck masses. Ultrasound Q 2009;25(3):111-127.
- Jaryszak EM, Choi SS. Deep neck space infections. In: Schoem SR, Darrow DH, eds. Pediatric Otolaryngology. Elk Grove Village, IL: American Academy of Pediatrics; 2012:273-299.
- Coticchia JM, Getnick GS, Yun RD, et al. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg 2004;130:201-207.
- Chow AW. Deep neck space infection. In: UpToDate, Thorner AR (Ed), UpToDate, Waltham, MA, 2013.
- Elden LM, Potsic WP. Otolaryngologic emergencies. In: Fleisher GR, Ludwig S, et al, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia: Lipincott Williams & Wilkens; 2010:1556-1558.
- Eftekharian A, Roozbahany NA, Vaezeafshar R, et al. Deep neck infections: a retrospective review of 112 cases. Eur Arch Otorhinolaryngol 2009;266(2): 273-277.
- Meyer AC, Kimbrough TG, Finkelstein M, et al. Symptom duration and CT findings in pediatric deep neck infection. Otolaryngol Head Neck Surg 2009;140(2):183-186.
- Uzomefuna V, Glynn F, Mackle T, et al. Atypical locations of retropharyngeal abscess: Beware of the normal lateral soft tissue neck X-ray. Int J Pediatr Otorhinolaryngol 2010;74(12): 1445-1448.
- Freling N, Roele E, Schaefer-prokop C, Fokkens W. Prediction of deep neck abscesses by contrast-enhanced computerized tomography in 76 clinically suspect consecutive patients. Laryngoscope 2009;119(9):1745-1752.
- Rozovsky K, Hiller N, Koplewitz BZ, Simanovsky N. Does CT have an additional diagnostic value over ultrasound in the evaluation of acute inflammatory neck masses in children? Eur Radiol 2010;20(2):484-490.
- Mallorie CN, Jones SD, Drage NA, et al. The reliability of high resolution ultrasound in the identification of pus collections in head and neck swellings. Int J Oral Maxillofac Surg 2012;41(2): 252-255.
- Grisaru-Soen G, Komisar O, Aizenstein O, et al. Retropharyngeal and parapharyngeal abscess in children — epidemiology, clinical features and treatment. Int J Pediatr Otorhinolaryngol 2010;74(9):1016-1020.
- Boscolo-rizzo P, Stellin M, Muzzi E, et al. Deep neck infections: A study of 365 cases highlighting recommendations for management and treatment. Eur Arch Otorhinolaryngol 2012;269(4): 1241-1249.
- Wong DK, Brown C, Mills N, Spielmann P, Neeff M. To drain or not to drain — management of pediatric deep neck abscesses: A case-control study. Int J Pediatr Otorhinolaryngol 2012;76: 1810-1813.
- Cheng J, Elden L. Children with deep space neck infections: Our experience with 178 children. Otolaryngol Head Neck Surg 2013;148:1037-1042.
- Baldassari CM, Howell R, Amorn M, et al. Complications in pediatric deep neck space abscesses. Otolaryngol Head Neck Surg 2011;144(4):592-595.
- Bentley TP, Brennan DF. Lemierre’s syndrome: Methicillin-resistant Staphylococcus aureus (MRSA) finds a new home. J Emerg Med 2009;37(2):131-134.
- Li HY, Grubb M, Panda M, Jones R. A sore throat — potentially life-threatening? J Gen Intern Med 2009;24(7):872-875.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
