Adjuvant Radiation Determined for Intermediate Risk Endometrial Cancer! Or has it?
Adjuvant Radiation Determined for Intermediate Risk Endometrial Cancer! Or has it?
Special Report by Robert L. Coleman, MD
The strength of a clinician’s recommendation for patient care is greatest in data generated through carefully conducted experimental studies. In many cases, this quality of data is generated from randomized clinical trials where a novel intervention is compared against some standard of care. When properly designed and conducted, positive or negative results will generally provide convincing evidence to support a change in that standard or refute one, particularly if the outcomes are independently confirmed. The US Preventative Services Task Force recognized the confusion of interpreting clinical studies generated a rating system which categorizes both the quality of evidence into 1 of 3 levels and the strength of recommendation into 5 levels.1 In general, the best quality data (Level 1) and strongest recommendations (Level A) are assigned to data generated through randomized, controlled trials. In the gynecologic cancer business, therapeutic intervention trials, for example, have methodically, albeit slowly, refined the care of affected women, in many cases to the improvement of survival or the reduction of toxicity. With the "muscle" of an international cooperative group research network and expanded collaboration, confirmatory trials have been increasingly contemporaneously launched or provided important data in a timely fashion to help solidify important issues in the care of our patients.
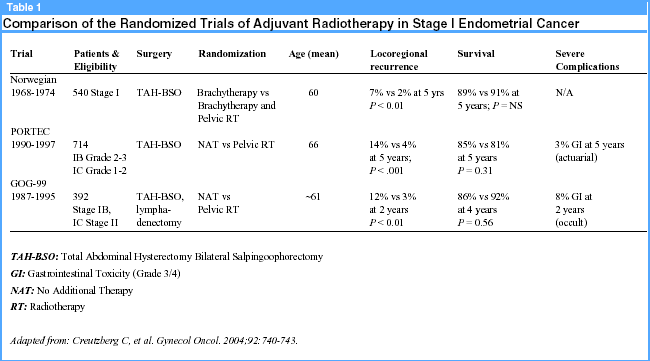
And so it is in the care of women with early, but intermediate risk, endometrial cancer. This disease site will account for approximately 40,320 new cases this year and be responsible for about 7090 cancer-related deaths.2 Fortunately, many new cases of this disease are early stage, low grade and cured with surgery. However, an important and sizeable fraction of patients are diagnosed either at an older age, with some element of myometrial invasion, or with a higher grade or atypical histology that increases the risk for recurrence. These features we have known for many years and have been well documented through careful surgical staging studies reported more than 20 years ago.3-6 In addition, 3 randomized controlled clinical trials involving more than 1600 patients and spanning almost 4 decades have been completed (see Table 1, below).7-9 So it may come as somewhat a surprise that we still don’t have a clear answer to the question, "What’s the best treatment for women with intermediate risk uterine cancer?"
The latest and likely most anticipated results were recently published and represented the efforts of 40 member Gynecologic Oncology Group institutions and 8 years of accrual.7 In this trial, "intermediate risk" was defined as any grade non-clear cell or papillary serous adenocarcinoma with any degree of myometrial invasion (FIGO Stage IB, IC), confined to the uterus (FIGO Stage IIA & B [occult]) with negative lymph nodes and cytology. The source for this broad entry criteria was historical and rooted in a prior GOG trial (GOG #33) where patients included in this cohort would have a risk for disease recurrence of 20 to 25% at 5 years with nearly all recurrences occurring within the first 2 years.4 Surgical staging was required for entry and while adequacy was left to the discretion of the primary surgeon, it included hysterectomy, bilateral salpingo-oophorectomy and nodal sampling from key nodal basins unless enlarged nodes were identified. In the latter case, these were to be biopsied, but if negative, the patient could be enrolled. Eligible patients were randomized to either standard pelvic radiation after surgery or no additional treatment. The primary end point was a variable termed recurrence-free interval (RFI) which was defined as "the time from study entry to clinical, histologic or radiographic evidence of disease recurrence." This is different from the traditional progression-free interval (which was also evaluated in the trial) in that patients who died of intercurrent disease were censored in survival statistics. Ordinarily, these events would be captured as "events." Over the long accrual period, 448 patients were randomized, of which, 392 (88% of total) were included in the final analysis (202 to surgery alone, 190 to surgery + radiation). In regard to the primary endpoint, disease recurrence was reduced by 58% (hazard ratio [HR], 0.42; 90% confidence interval [CI], 0.25- 0.73; P = 0.007). At 2 years, the recurrence rate was 12% vs 3% in the adjuvant radiation cohort. In fact, the 2 women with vaginal recurrences randomized to radiation actually never received the therapy but are included in the intent-to-treat analysis. Most of this difference was in isolated local vaginal recurrences where the risk at 2 years was significantly reduced from 7.4% to 1.6%. So far, so good, right?
Well, yes and no. While a reduction in cancer recurrence is always a good thing, unfortunately, the reduction didn’t translate into a survival benefit (HR, 0.86; 90% CI, 0.57-1.29). This is largely the result of effective salvage radiation in cases of local vault recurrence. Four-year survival estimates were 86% for surgery and 92% for surgery and radiation. And, as anticipated, intercurrent disease was a significant contributor to mortality with nearly one-half of the patients dying from causes not related to their primary cancer. In addition, the study grossly overestimated recurrence risks. It was determined that the current sample size could detect with 80% power a 58% decrease in recurrence and a 56% decrease in death with its initial recurrence estimates. However, recurrence was far less than anticipated. This prompted a post hoc creation of a high intermediate risk group (HIR) and a low intermediate risk group (LIR). Criteria used for this new determination are listed in Table 2, below. Under the new allocation, 132 patients (one-third of total) were determined HIR (70: surgery alone, 62: surgery + radiation) and had a 2-year recurrence of 27%; 260 were LIR with a 2-year recurrence of 6%. Analysis of the primary end point, RFI, again demonstrated a significant reduction of recurrence (HR, 0.42; 90% CI, 0.21-0.83) for those treated adjuvantly with radiation, but only among the HIR cohort. However, even with this secondary cohort allocation, survival was not significantly different with radiation (HR, 0.73; 90% CI, 0.43-1.26), although the authors state the benefit is somewhat lower.

The purported benefits of radiation do come at a premium though. In this trial, the combination of surgical staging and postoperative pelvic radiation produced more frequent and higher-grade gastrointestinal toxicity with the only 2 treatment-related deaths occurring in the radiation arm from intestinal injury. In summary, radiation given in adjuvant-to-surgical staging produces lower isolated local recurrences, without a clear improvement in survival and with more toxicity compared to surgery alone in patients with early stage endometrial cancer.
The results seem cogent, so what’s the confusion? Three critical elements continue to keep the controversy vibrant. First, surgical staging, while the mantra of contemporary care in the United States, is not universally accepted among investigators and, even among its advocates, comprises a range of procedural intents. Results from the large PORTEC trial concluded that adjuvant pelvic radiation produced a similar reduction in isolated vaginal recurrences among a slightly differently defined intermediate risk cohort compared with surgery alone.8 Patients in this trial did not undergo any formal surgical staging and as such had approximately one-third the bowel complications as the GOG trial (see Table 1, above). These authors opined (in a subsequent editorial) that surgical staging added little more than toxicity and should be avoided in many patients with early endometrial cancer.10 In their view, in the absence of surgical staging procedures, radiation was associated with a better therapeutic ratio and should be administered in all such patients. On the other hand, surgical staging when performed as a complete lymphadenectomy identifies with precision patients who are at risk for pelvic and distant recurrence, and as such, call into question the merits of pelvic radiation if the at-risk nodal tissues are resected. Indeed, several advocates of therapeutic lymphadenectomy have reported rare pelvic recurrences in these patients not treated with radiation.11-13 As isolated local failures are frequently salvaged, these authors opine that surgical staging, if complete, can reduce toxicity and cost from radiation without affecting survival—as similarly presented in the GOG trial. Although there is no consensus on the issue of surgical staging between the two camps, trials are underway to evaluate whether radiation can be modified (PORTEC-II) and what information is gained by formal surgical staging in stage I endometrial cancer (MRC-ASTEC).10
The second element obstructing a consensus on the issue of adjuvant therapy is whether vaginal brachytherapy can be substituted for pelvic radiotherapy, regardless of whether surgical staging is done. In the only other randomized trial of clinically staged, intermediate-risk patients, loco-regional recurrences were significantly higher in women treated with adjuvant vaginal brachytherapy compared to adjuvant pelvic radiation and vaginal brachytherapy.9 Survival, again, was not adversely impacted but staging data were not collected and presumably, some of these recurrences may have represented patients with occult stage IIIC disease. A comparative trial with carefully selected, but clinically defined intermediate-risk patients is underway in the Netherlands. A similar trial among formally staged patients has also been advocated.14
The third, and arguably most critical, element fueling the ongoing debate is the inconsistent definition of intermediate risk. As outlined above, the GOG intended to identify a risk cohort where recurrence would be expected in approximately 25% of accrued patients. However, by their definition, both a 45 year-old woman with 10% invasion of a grade I tumor and a 75 year-old woman with 95% invasion and cervical extension of a grade III tumor could have been equally enrolled. Clearly, these represent different risk groups. In the PORTEC trial, all stage IC grade III tumors were excluded, as were stage II patients. In some respects, while the recurrence rates and survival estimates are similar between these 2 recent trials, they represent different cohorts and lack sufficient power to evaluate important subgroups. The authors of the PORTEC trial have recently reported the outcomes of this latter excluded stage I cohort who were registered in the trial but were treated with pelvic radiation.15 Ninety-nine of 104 such patients were followed for a median 83 months. In comparison to other randomized patients in the original trial, this patient cohort was characterized by significantly higher loco-regional relapse rates (14% vs 3%), shorter 5-year survival (58% vs 83%; HR, 5.5; P < 0.0001), and more frequent distant recurrence (32% vs 8%). Grade III was the most important prognostic factor to cancer-specific death by multivariate analysis. Effective therapeutic strategies in this cohort will need to address both loco-regional and distant failure. These data, thus, highlight the importance of case selection in constructing randomized protocols for intermediate-risk patients.
Fortunately, the quest for truth is embraced heartily in every generation. To this end, randomized trials of not only surgery but also radiation and chemotherapy are being planned and conducted in the worldwide gynecologic oncology theater. Eventually, these issues should be ironed out, but I for one am not sure if I should breathe a sigh of relief or hold my breath!
References
1. Force. USPST: Guide to Clinical Preventive Services. Baltimore, MD: Williams & Wilkins, 1989.
2. Jemal A, et al. CA Cancer J Clin. 2004; 54:8-29.
3. Boronow RC, et al. Obstet Gynecol. 1984;63:825-832.
4. Creasman WT, et al. Cancer. 1987;60:2035-2041.
5. DiSaia PJ, et al. Am J Obstet Gynecol. 1985;151: 1009-1115.
6. Morrow CP, et al. Gynecol Oncol. 1991;40:55-65.
7. Keys HM, et al. Gynecol Oncol. 2004;92:744-751.
8. Creutzberg CL, et al. Lancet. 2000;355:1404-1411.
9. Aalders J, et al. Obstet Gynecol. 1980;56:419-427.
10. Creutzberg CL, et al. Gynecol Oncol. 2004;92:740-743.
11. Berclaz G, et al. Int J Gynecol Cancer. 1999;9:322-328.
12. Larson DM, et al. Obstet Gynecol. 1998;91:355-359.
13. Fanning J. Gynecol Oncol. 2001;82:371-374.
14. Berman ML. Gynecol Oncol. 2004;92:737-739.
15. Creutzberg CL, et al. J Clin Oncol. 2004;22:1234-1241.
The strength of a clinicians recommendation for patient care is greatest in data generated through carefully conducted experimental studies. In many cases, this quality of data is generated from randomized clinical trials where a novel intervention is compared against some standard of care. When properly designed and conducted, positive or negative results will generally provide convincing evidence to support a change in that standard or refute one, particularly if the outcomes are independently confirmed.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.