Evaluation and Management of Sudden Vision Loss: Part II
September 15, 2014
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
Evaluation and Management of Sudden Vision Loss: Part II
This issue is the second part of our discussion of sudden vision loss, stressing conditions that present to the emergency department. You will note the utility of ocular ultrasound in diagnosing many of these conditions. This review was helpful to me as a practicing emergency physician.
— J. Stephan Stapczynski, MD, FACEP, Editor
Authors:
Medley Gatewood, MD, Assistant Professor, Division of Emergency Medicine, University of Washington Medical Center, Seattle.
Mitchell Kim, MD, Resident, Division of Emergency Medicine, University of Washington Medical Center, Seattle.
Peer Reviewer:
Jonathan Glauser, MD, Associate Professor, Emergency Medicine, Case Western Reserve University, Cleveland, OH.
Executive Summary
- Bedside ultrasound is a useful tool to evaluate many causes of sudden vision loss.
- Emergent ophthalmologic consultation is recommended for acute vision loss due to optic neuritis, giant cell arteritis, retinal detachment, and central retinal artery occlusion.
- Many treatments used for these conditions are of debatable overall benefit.
- Emergency physicians should follow the protocols and recommendations of their consultant.
Optic Neuritis
A 32-year-old woman presents with blurred vision for two days. She states she has trouble seeing out of her left eye. Her symptom is associated with left eye pain, which is exacerbated by eye movement. She feels like she has trouble distinguishing certain colors. She had similar symptoms last year, but they resolved spontaneously before she was able to seek medical attention. Her visual acuity is 20/100 OS and 20/20 OD. She has an afferent pupillary defect. The fundoscopic exam shows blurred optic disc margins. Tonometry and slit lamp exams are normal.
Etiology. Optic neuritis is an inflammatory, demyelinating condition of the optic nerve that typically causes unilateral vision loss. It is commonly idiopathic, but may be associated with an underlying disorder. (See Table 1.) It is most often associated with multiple sclerosis, and is the presenting symptom of this disease in 20% of cases. Infectious causes of optic neuritis include syphilis, tuberculosis, measles, mumps, varicella zoster virus (VZV), and Epstein-Barr virus (EBV). Optic neuritis predominantly affects white women between the ages of 20 and 40 who live in higher latitudes.1,2
Clinical Presentation. Patients typically present with subacute unilateral loss of vision that worsens over several days. Ocular discomfort that is exacerbated by movement is often present. Color vision (especially red) may be altered. On exam, patients may have relatively preserved vision, or may have no light perception at all. An afferent pupillary defect is almost always present. The fundoscopic exam is often normal because optic neuritis most commonly affects the retrobulbar optic nerve. When the optic disc is affected, it appears edematous and has blurred disc margins. This finding is known as papillitis.3 (See Figure 1.)

Note the blurred disc margins and edema of the optic nerve. Papilledema refers to bilateral papillitis caused by increased intracranial pressure.
Source: Wikimedia Commons. Credit: "The Eyes Have It" by Jonathan Trobe, MD.
Management. Once the diagnosis of optic neuritis is suspected based on clinical findings, ophthalmology and neurology consultations should be obtained in the emergency department (ED). Ocular ultrasound that shows an optic nerve sheath diameter greater than 5 mm is concerning for optic neuritis when the exam is equivocal. (See Figure 2.) Magnetic resonance imaging (MRI) is diagnostic for optic neuritis and can provide prognostic information to determine the likelihood of developing multiple sclerosis.2,3

For consistency, the optic nerve sheath diameter is measured 3 mm posterior to the optic disc.
Image courtesy of Teresa S. Wu, MD, FACEP
Since the 1950s, the primary treatment for optic neuritis has been IV corticosteroids, although the overall benefit remains debatable. The current treatment regimen is based on the Optic Neuritis Treatment Trial (ONTT) performed in 1992. The ONTT results showed that high-dose IV methylprednisolone improved the recovery of visual function, but did not affect long-term visual outcomes. This study also showed that IV methylprednisolone decreased the rate of developing multiple sclerosis over two years, but that there was no significant difference at five years. There is no role for oral corticosteroids in optic neuritis. The 2012 Cochrane review of corticosteroids for optic neuritis found no conclusive evidence to support the use of IV or PO corticosteroids in optic neuritis. Lastly, interferon therapy has shown promising results in patients with optic neuritis and white matter lesions on MRI. In either case, the treatment regimen for optic neuritis often includes admission and 1 g of methylprednisolone daily for 3 days, followed by 1 mg/kg PO prednisone for another 11 days. The decision to image and start treatment should be made with a neurologist and ophthalmologist.
Table 1: Summary of Painless Vision Loss
Disease |
Cause(s) |
Presentation |
Physical Findings |
ED Treatment/Management |
Disposition |
|
Optic neuritis |
Inflammatory, autoimmune demyelination of the optic nerve. Idiopathic vs. multiple sclerosis |
Subacute unilateral vision loss, alteration of color vision, eye discomfort |
|
IV methylprednisolone MRI to evaluate for multiple sclerosis |
Ophthalmology and neurology consultations in the ED Possible admission for IV steroids and work up |
|
Giant cell arteritis/ temporal arteritis |
Autoimmune, granulomatous vasculitis of the large vessels |
Sudden monocular vision loss in an older patient with a history of amaurosis fugax, jaw claudication, polymyalgia rheumatica, and headache |
|
Diagnosis based on clinical presentation and elevated ESR Treatment with PO or IV corticosteroids Consider low-dose aspirin |
Immediate ophthalmology evaluation Possible admission for IV steroids and work up If discharged, appropriate specialty follow-up for temporal artery biopsy |
|
Vitreous hemorrhage |
Vitreous detachment and rupture of retinal vessels, leading to hemorrhage |
Acute onset of vision loss or vision changes (flashing lights, floaters, cobwebs, etc.) |
|
Allow the blood to settle
Discontinuation of antiplatelet and/or anticoagulants if possible |
Ophthalmology evaluation in the ED or within 24 hours |
|
Retinal detachment |
Detachment of the neuroretina from the pigmented epithelial layer |
Acute onset of vision loss or vision changes (floaters, flashing lights, visual distortions) |
|
No specific ED management outside of diagnosis |
Immediate ophthalmology evaluation |
|
Central retinal artery occlusion |
Occlusion of the central retinal artery from a thrombus or embolus |
Sudden monocular vision loss in an older patient with cardiovascular risk factors |
|
General treatment involves dislodging the clot, improving retinal perfusion pressure, and vasodilating retinal vessels. This includes:
However, there is no evidence to support any specific treatment, and treatment should be guided by institutional protocols |
Immediate ophthalmology evaluation |
Acute Painless Vision Loss: Giant Cell Arteritis
A 72-year-old woman presents with sudden loss of vision. She states she suddenly and completely lost vision in her left eye while washing the dishes. She has had several episodes of transient vision loss during the past few months. She has also been having joint stiffness, fatigue, and jaw pain with prolonged chewing during the past year. She has normal visual acuity in her right eye and inability to detect any light in her left. Other than an afferent pupillary defect, the patient’s ophthalmologic exam is normal. She does have tenderness over her scalp. Laboratory tests show normocytic anemia and elevated inflammatory markers.
Etiology. Giant cell arteritis (GCA), also known as temporal arteritis, is the most common medium-large vessel vasculitis in Western nations. It primarily affects people older than the age of 50. It is more common in women and Caucasians. Affected vessels are typically the aorta, its major branches, and branches of the carotid arteries. Pathologically, there is granulomatous inflammation with giant cell infiltration of the arterial walls. It has many systemic manifestations in addition to the feared neuro-ophthalmologic complications. It is considered a medical emergency due to the potential for rapid and permanent loss of vision in both eyes.4,5
Clinical Presentation. Patients with GCA present with symptoms related to ischemia of the affected vessels and systemic involvement. Fevers, headache, weight loss, polymyalgia rheumatica, jaw claudication, CVA, and ischemic neuropathies are all extraocular manifestations of GCA. The vision loss seen in GCA is often due to the occlusion of the posterior ciliary arteries, leading to anterior ischemic optic neuropathy (AION, see subsequent section for details). Occasionally, the central retinal artery and ophthalmic arteries may become occluded. Patients typically have systemic symptoms of GCA prior to vision changes. Jaw claudication is considered a cardinal sign of impending vision loss. Patients may also have episodes of transient vision loss prior to an episode, causing persistent vision loss. Patients may be febrile and may have scalp tenderness. Aortic involvement may produce cardiac murmurs. Sensorimotor/cranial nerve deficits may be due to previous CVA or mononeuropathy. On ophthalmologic exam, the patient will have deficits in visual acuity and visual field exams. The fundoscopic exam will show a pale optic disc or foci of pallor of the retina during active ocular ischemia.5
Management. Fulfilling three of the five criteria set up by the American College of Rheumatologists in 1990 makes the diagnosis of GCA. The criteria are as follows:
• Age greater than 50;
• Recent onset headache;
• Swelling, tenderness to palpation, or decreased temporal artery pulsatility;
• Erythrocyte sedimentation rate (ESR) greater than 50 mm/hour;
• Temporal artery biopsy with evidence of granulomatous inflammation.
The ESR is a highly sensitive test for ruling out GCA, and should be ordered in all patients in whom this is a suspicion. It is important to note that 10-20% of patients with GCA will have normal ESR values. The ESR and CRP are important for monitoring the course of disease and response to therapy.5
Corticosteroids should be started immediately once GCA is suspected. In patients without vision loss, the typical dose of prednisone is 40-60 mg per day. Admission for intravenous methylprednisolone should be considered in all patients with vision loss in light of reports noting reversal of vision loss with this treatment. Lastly, low-dose aspirin should be started, as it has been shown to reduce the likelihood of vision loss and CVA. Immediate ophthalmologic consultation should be obtained regardless of disposition.1,4 Once there has been visual loss, only 15-34% of patients will have an improvement in visual acuity. Patients may continue to have deterioration of vision within the first week even after steroids are initiated.6
Vitreous Hemorrhage
A 78-year-old man with a history of poorly controlled diabetes mellitus presents with sudden onset of floaters seen in his left eye. He was gardening with his wife when he noticed this. He states that there is a reddish hue to his vision. His visual acuity is 20/60 OS and 20/30 OD. The rest of his exam is unremarkable except for difficulty visualizing the retina on fundoscopy.
Etiology. Vitreous hemorrhage occurs in seven per 100,000 people per year. It most commonly occurs in the setting of diabetic proliferative retinopathy, retinal detachment or tear, and ocular trauma. The mechanism in which this occurs is most frequently due to vitreous detachment and traction of a retinal vessel, leading to its breakage and subsequent hemorrhage into the posterior chamber. Retinal tears/detachments may occur with vitreous hemorrhage given this underlying mechanism.7 Less common causes of vitreous hemorrhage include retinal vein occlusion, sickle cell retinopathy, macular degeneration, subarachnoid hemorrhage (Terson’s syndrome), and retinal macroaneurysms.8
Clinical Presentation. Patients typically present with acute onset of vision changes. They may complain of having hazy vision, flashing lights, floaters, smoke, cobwebs, or shadows. They can have markedly decreased visual acuity in severe cases. A fundoscopic exam will reveal a partially or completely obscured retina.8,9
Management. Patients in whom vitreous hemorrhage is suspected but cannot be confirmed with physical exam should undergo ocular ultrasonography (if available) to confirm the diagnosis and to exclude other diagnoses such as retinal detachment. This can be performed by the emergency physician (EP) or through a formal radiology study. Acute vitreous hemorrhage appears as echogenic dots or linear densities in the posterior chamber on ultrasound. (See Figure 3.) Chronic vitreous hemorrhage appears as a thicker echogenic membrane as the blood settles. An ophthalmologist should be consulted in the ED when vitreous hemorrhage is diagnosed. If unavailable, follow up within 24 hours is prudent. Initial treatment includes elevating the head of the bed while sleeping and avoidance of strenuous activity to allow for the blood to settle. Anticoagulants and antiplatelet agents should be discontinued if this is not strongly contraindicated.1,9,10
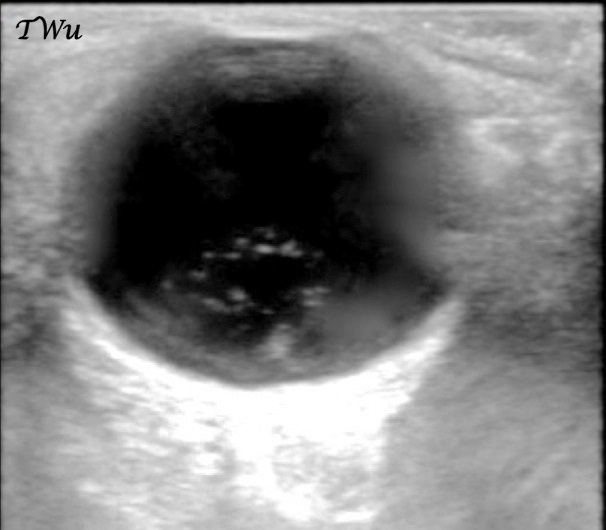
Image courtesy of Teresa S. Wu, MD, FACEP
Retinal Detachment
A 78-year-old woman with a history of diabetes, hypertension, and osteoarthritis presents with acute onset vision loss. She was chopping some carrots when she suddenly lost vision in her right eye ]as if a curtain came down.” She has a deficit in the temporal visual fields in her left eye. A fundoscopic exam shows a detached portion of her nasal retina. The rest of her exam is unremarkable.
Etiology. Retinal detachment occurs when the neural retina separates from the underlying pigmented epithelial layer of the retina. It is a relatively uncommon condition, with an incidence of 10 cases per 100,000. Retinal detachments are classified into three pathologic types:
• Exudative/serous: This occurs due to the accumulation of fluid or hemorrhage in the subretinal space. This typically happens from an underlying condition, such as hypertension or sarcoidosis.
• Tractional: Previous injury to the retina causes fibrotic tissue formation in the subretinal space. Centripetal forces on the retina can then cause retinal detachment.
• Rhegmatogenous: This is the most common type of retinal detachment. The pathogenesis begins with the shrinkage of the vitreous humor as a person ages. Over time, the vitreous can detach from the retina, which is known as posterior vitreous detachment. This process is present in roughly two-thirds of people older than the age of 70. In some patients, a retinal tear or hole forms as the vitreous pulls away from the retina. The retina detaches as the vitreous fluid enters the subretinal space through the retinal defect.
Patients with a history of severe myopia, ocular trauma, ocular surgery, and diabetic retinopathy have an increased risk of retinal detachment. Regardless of the pathologic process leading up to the retinal detachment, patients present with similar symptoms.9,11
Clinical Presentation. Patients experience acute onset unilateral photopsia (flashing lights), floaters, wavy distortion of objects, or vision loss that is often described as a curtain or veil coming down over their visual field. Central vision may be affected if the retina is detached at the macula. Patients will typically have decreased visual acuity and deficits in their visual field. Afferent pupillary defect is inconsistently seen. A detached retina can be seen on fundoscopic exam through a dilated pupil. The location of the retinal tear may be visible as well. A vitreous hemorrhage may be present. Ocular ultrasound shows an echogenic neuroretina that may be undulating within the vitreous humor of the posterior or lateral eye. (See Figure 4.) In contrast to choroidal detachments, this echogenic membrane moves with eye movement in retinal detachments.1,9,11

Image courtesy of Teresa S. Wu, MD, FACEP
Management. Patients with suspected retinal detachments with suboptimal direct fundoscopic exams should undergo bedside ocular ultrasound (if available). Visualizing retinal detachments using direct fundoscopy can be technically difficult, and may be missed if the entire retina cannot be visualized. Ocular ultrasound can be performed rapidly and accurately (97% sensitivity, 92% specificity in one prospective study) by the EP. It may be prudent to perform an ultrasound bilaterally if the patient reports binocular vision changes.12,13 If the diagnosis is either equivocal or confirmed, patients should be immediately referred to an ophthalmologist for further evaluation and possible surgical management.1,9
Central Retinal Artery Occlusion (CRAO)
A 64-year-old man with a history of atrial fibrillation presents with sudden, complete painless vision loss in his right eye. He was driving to the grocery store when it happened, and he took a detour to the emergency department. He denies any ocular trauma. On exam, the patient is noted to be in atrial fibrillation with a ventricular rate in the 90s-100s. He is unable to detect light in his right eye. Fundoscopic exam shows diffusely pale retina with a cherry-red macula. Slit lamp and tonometric exams are unremarkable.
Etiology. CRAO is a well-known clinical entity that was first described in the 1850s by Von Graefe. It occurs when there is ischemia to the retina due to an acute vaso-occlusive event in the central retinal artery. Its pathogenesis typically involves the formation of a platelet-fibrin thrombus or embolus. The most common causes of CRAO are the breakage and embolization of a plaque from ipsilateral carotid stenosis, and propagation of cardiogenic emboli. Giant cell arteritis is another entity known to cause arteritic CRAO (previously described). Early recognition and treatment are aimed at preventing another ischemic episode. Unfortunately, the vision loss associated with a complete occlusion of the retinal artery is often profound and irreversible. The source of the thrombus or embolus is atherosclerotic plaques seen in peripheral vascular disease. Therefore, risk factors for CRAO include tobacco use, hypertension, diabetes, dyslipidemias, coronary artery disease, and cerebrovascular disease. Systemic vasculitis (Wegener’s granulomatosis, polyarteritis nodosa), arterial vasospasm, migraine headache, sickle cell disease, myeloproliferative disorders, hypercoagulable disease (antiphospholipid syndrome, Factor V Leiden, protein C/S deficiency), and intravenous drug abuse are all entities known to cause CRAO, especially in younger patients without atherosclerotic disease.14-16
In some cases, a branch of the central retinal artery can become occluded, leading to acute vision deficits in the affected visual field. This is known as branch retinal artery occlusion (BRAO). The total incidence of BRAO and CRAO is estimated to be 1 per 1,000 outpatient ophthalmology visits. Roughly 15-30% of the population have a cilioretinal artery (a branch of the ophthalmic artery separate from the retinal artery), which provides additional blood supply to the neuroretina. The amount of retina that is supplied by this vessel varies among the population. However, these patients will have some visual sparing in the setting of a complete CRAO.14,17
Clinical Presentation. CRAO typically presents with a sudden, painless, monocular vision loss. If a cilioretinal artery is present, the patient may have some preservation of sight in the visual field corresponding to the distribution of that artery. These symptoms may be transient (amaurosis fugax), or may affect different parts of the visual field over time as the embolus moves along the vasculature. On exam, patients will have a pale retina with a cherry red spot of the macula. (See Figure 5.) The retina may be partially pale with BRAO or if the patient has a cilioretinal artery providing collateral circulation to the retina. Shiny cholesterol plaques, and ]boxcarring” of red blood cells (RBCs), may also be seen within vessels. (See Figure 6.) An afferent pupillary defect is usually present.16,18

Note the cherry red fovea surrounded by pale, ischemic retina.
Credit: Cogan Collection, NEI/NIH.

This image depicts "boxcarring" of the retinal arteries. This occurs due to RBC rouleaux formation.
Credit: Cogan Collection, NEI/NIH.
Management. CRAO is an ophthalmologic emergency, and the patient should be immediately evaluated by an ophthalmologist. Time is vision; retinal ischemia lasting greater than four hours can lead to significant, irreversible vision loss. Many different treatment modalities have been devised to treat this condition. The tenets of CRAO treatment follow the principles of treatment of other end organ ischemic disease. Emergent treatment primarily aims to dislodge the embolus, increase retinal perfusion pressure, and vasodilate the retinal vessels:16,19,20
Dislodging the embolus:
- Ocular massage by the physician or the patient.
Increasing retinal perfusion pressure:
- Anterior chamber paracentesis;
- Intravenous or oral acetazolamide (500 mg);
- Intravenous mannitol (0.5-2 g/kg);
- Pentoxifylline;
- Trabeculectomy;
Vasodilation of retinal vessels:
- Sublingual isosorbide dinitrate;
- Rebreathing expired CO2;
- Breathing mixture of 95% O2 and 5% CO2.
Other therapies that have been suggested include systemic or local thrombolysis, hemodilution, use of antiplatelet agents, and systemic steroids. More aggressive therapies include surgical embolectomy and laser embolysis. Hyperbaric oxygen therapy is recommended by the Undersea and Hyperbaric Medical Society in patients who present within 24 hours of vision loss. Despite the multiple different treatment options, there is no convincing evidence to support the use of any single treatment. Many clinicians use a combination of the above treatments. ED treatment decisions should be guided by institutional protocols and in conjunction with an ophthalmology and neurology consultant.1,19,20
Central Retinal Vein Occlusion (CRVO)
A 58-year-old woman with a history of hypertension presents after waking up with blurry vision. She states that the vision in her left eye has been worse over the past two to three weeks, but that it was acutely worse today when she woke up. She denies any pain. Her visual acuity is 20/200 OS and 20/30 OD. The central vision in her left eye is much worse than her peripheral vision. She has dilated retinal veins and multiple hemorrhages on fundoscopic exam. Her tonometric and slit lamp exams are unremarkable.
Etiology. First described in 1855, CRVO is a common retinal vascular lesion and cause of vision loss. Its prevalence was estimated to be 0.8 cases per 1,000 in an international epidemiology study, with increasing prevalence with age. The pathogenesis of CRVO involves stasis of the blood in the main vein of the retinal venous circulation, and subsequent thrombus formation. CRVO is broadly differentiated into the ischemic and nonischemic subtypes, depending on the extent of the clot, collateral circulation, and arterial perfusion of the retina. Further discussion of subtypes of retinal vein occlusion is outside the scope of this review. CRVO is associated with a higher incidence of arterial hypertension, peptic ulcer disease, cerebrovascular disease, coronary artery disease, obesity, dyslipidemias, glaucoma, hyperhomocysteinemia, diabetes mellitus, and thyroid disorders. Although half of all cases of CRVO occur in patients older than 65 years of age, CRVO has been reported in patients as young as age 14.16,21-23
Clinical Presentation. Vision loss due to retinal vein occlusion occurs once ischemia and edema of the macula occur. Patients with nonischemic CRVO (no frank thrombosis) may be diagnosed incidentally with the finding of mild retinal hemorrhages. Those who are symptomatic typically have blurriness in the central visual fields. Peripheral vision may be completely normal. Vision changes in nonischemic CRVO tend to be worse in the morning, with improvement over several hours. With ischemic CRVO, there is often profound vision loss, which is often noticed upon awakening. Amaurosis fugax may precede persistent vision loss in both ischemic and nonischemic cases. Final visual acuity is much worse with ischemic CRVO than nonischemic CRVO. The presenting visual acuity largely determines the long-term visual outcome. Eye discomfort may be present with prolonged, profound ischemia.
The classic fundoscopic finding in CRVO is the ]blood and thunder” retina that occurs due to hemorrhage from retinal veins. Cotton wool spots occur as yellow-white lesions indicative of retinal ischemia. (See Figure 7.) This hemorrhage may be diffuse and associated with optic nerve edema. (See Figure 8.) Papilledema is excluded by the presence of unilateral papillitis, and the presence of retinal hemorrhages distinguishes CRVO from optic neuritis.1,16,22

An acute occlusion of a retinal vein leads to hemorrhage as seen in the superior aspect of this image. Local retinal ischemia leads to "cotton wool spots" seen as bright yellow/white areas in the retina.
Source: Wikimedia Commons. Credit: http://www.biomedcentral.com/1471-2415/11/24.

This image shows diffuse retinal hemorrhage, and optic disc edema.
Credit: Cogan Collection, NEI/NIH.
Management. CRVO is a disease that typically self-resolves over the course of weeks to months. There are no proven methods of treatment for CRVO; rather, treatment is aimed at preventing future sequelae of this disease. Neurology and ophthalmology consults should be obtained in the emergency department, if available. Otherwise, patients may be referred to an ophthalmologist urgently (1-2 days). The consultants will confirm the diagnosis and differentiate between nonischemic and ischemic CRVO. This distinction is important in determining prognosis and predicting complications. There is no role for immediate ED treatment for CRVO.
Treatment for CRVO is aimed at reducing macular edema and retinal/anterior segment neovascularization. Medical treatment to restore the patency of the retinal vessels includes the use of anticoagulation, hemodilution, and thrombolytics. Hyperbaric oxygen has been used to reduce retinal ischemia. Intravitreal injections of corticosteroids and anti-VEGF (vascular endothelial growth factor) agents have also been used to reduce macular edema. Vision loss due to neovascularization may improve with retinal photocoagulation. Surgical approaches to CRVO include vitrectomy, radial optic neurotomy, chorioretinal venous anastomosis, and direct injection of thrombolytics into the retinal vein. Nonetheless, there are limited data to suggest any specific treatment improves visual outcomes in CRVO.1,16,24,25
Nonarteritic Ischemic Optic Neuropathy
A 54-year-old man presents with painless vision loss in his left eye. His symptoms have gradually worsened during the past three days. He also complains of altered color perception from his left eye. His visual acuity is 20/100 OS and 20/25 OD. He has an afferent pupillary defect and hyperemic optic disc edema on exam. Slit lamp and tonometric exams are unremarkable.
Etiology. Ischemic optic neuropathy (ION) is the most common cause of optic neuropathy in patients older than age 50. The pathophysiology principally involves the ischemia of the optic nerve. It is broadly differentiated into anterior ischemic optic neuropathy (AION) and posterior ischemic optic neuropathy (PION), depending on the appearance of the optic disc on fundoscopic exam and the portion of the optic nerve that is involved. In PION, the retrobulbar optic nerve is affected, and there is no optic disc edema on exam. AION accounts for 90% of all cases of ischemic optic neuropathy. ION is further divided into arteritic and nonarteritic ION. Arteritic ION is typically caused by giant cell arteritis (previously discussed). Therefore, the discussion of ION in this section will be limited to nonarteritic ION.26
AION. AION occurs most commonly in patients between the ages of 55 and 65. Its annual incidence is 2.3-10.2 cases per 100,000 patients older than the age of 50. It occurs more frequently in the Caucasian population, and there is no gender disparity. The ischemic injury occurs due to occlusion of the short posterior ciliary arteries that feed the optic head. It is associated with nocturnal hypotension, peripheral vascular disease (along with its risk factors, diabetes, hypertension, dyslipidemia, tobacco use), and hypercoagulable states. Medications such as interferon-alpha and sildenafil have been reported to precipitate AION. About 15% of patients will ultimately develop symptoms on the contralateral eye. Vision loss is less severe in nonarteritic AION when compared to arteritic AION.26,27
PION. PION presents as a different pathologic entity from AION due to the difference in vascular supply between the anterior and posterior optic nerves. It occurs due to vascular insufficiency of the pial capillary plexus that supplies the posterior optic nerve. There are nonarteritic, arteritic, and post-surgical causes of PION. Arteritic PION will not be further discussed. Patients with nonarteritic PION have similar vasculopathic risk factors as in AION. Post-surgical PION typically occurs after spinal surgery. It is often bilateral, affects younger patients, and is associated with a poor visual outcome.28
Clinical Presentation. Ischemic optic neuropathy presents with sudden, painless, monocular vision loss that worsens over the course of hours to weeks. Preceding ocular discomfort or vision changes are rare. There is an afferent pupillary defect in both AION and PION. The fundoscopic exam differentiates the two entities clinically. AION presents with optic disc edema that may be diffuse or segmental (usually superior portion) with prominent hyperemia. Splinter hemorrhages within the optic disc are seen in 70% of AION cases. In contrast, the optic disc is normal in acute PION. In both cases, the optic disc becomes pale one to two months after the onset of symptoms.26,28
Management: AION. Once the diagnosis of nonarteritic AION is suspected, two other disease entities must be excluded. It is important to exclude arteritic AION (principally giant cell arteritis), as this will require emergent evaluation by an ophthalmologist and the initiation of corticosteroid therapy. Secondly, especially in young patients, optic neuritis must be excluded. This may require neuroimaging with MRI. Neuroimaging is also useful if an intracranial mass is suspected.26,28
There is no established treatment for nonarteritic AION. Therefore, a nonemergent referral to an ophthalmologist is appropriate. Various medical therapies, including phenytoin, intraocular vasodilators, levodopa, norepinephrine, ASA, anticoagulation, and corticosteroids, have all been tried without definitive beneficial results. The Ischemic Optic Neuropathy Decompression Trial (IONDT) was a randomized, controlled trial conducted in 1995 to assess the efficacy of surgical optic nerve decompression for AION. This study was stopped early, as there was evidence of harm in the treatment group when compared to observation. There have not been further randomized, controlled studies to evaluate surgical intervention in AION. In addition, there is no role for prophylactic treatment to prevent AION in the contralateral eye. Current treatment is aimed at modification of risk factors.26,29,30
Management: PION. There is no established treatment for PION. Nonemergent referral to an ophthalmologist is appropriate. Efforts should be made to exclude arteritic PION and intracranial masses. Corticosteroids may be of benefit in nonarteritic, non-surgical PION, but there are no prospective data to support their routine use.26
Spontaneous Lens Dislocation
Clinical Vignette. A 49-year-old male presents with hazy vision out of his right eye. He states that his right eye was hit with the butt of a handgun about three months ago, and his vision has been hazy since. In a visual acuity test of his right eye, he is able to count fingers; the left eye acuity is 20/25. The right pupil is round and reactive to light. Depending on the position of his right eye, the edge of the lens and a cataract are visible through the pupil. The vitreous is clear and the fundus is normal. An ocular ultrasound was performed. (See Figure 9.)

A lens dislocated into the vitreous seen on ultrasound. The lens was partially attached to the ciliary body and a swinging motion was visible when the globe moved.
The lens is normally held in position behind the iris and in front of the vitreous body, suspended there by circumferentially attached small fibers — termed zonules — connecting the equator of the lens capsule and the ciliary body. Partial disruption of some zonules can lead to lens subluxation, in which a portion of the lens remains in the visual axis. If enough zonules are torn, the lens can be completely dislocated away from the visual axis. Trauma is the most common cause of lens subluxation and complete dislocation.31 Spontaneous lens dislocation can be seen in a variety of connective tissue disorders, such as Marfan syndrome, Ehlers-Donlos syndrome, and homocystinuria. Isolated ophthalmologic disorders associated with spontaneous lens dislocation include simple ectopic lentis, ectopia lentis et pupillae, retinitis pigmentosa, and uveitis.
Clinical symptoms include decreased or blurred vision. With a subluxated lens, the patient may note monocular diplopia. Patients may tolerate a partially dislocated lens for long periods of time before seeking medical attention. Physical findings of a dislocated lens include detection of the edge of the lens on ophthalmoscopy and movement of the lens with globe movement (termed phacodonesis).32 If the pupil is small, findings may not be visible. However, pharmacologic dilation of the pupil has a small potential risk if the lens is completely dislocated into the vitreous. Dislocation of the lens into the anterior chamber should be readily visible with close inspection.
Ocular ultrasound is useful, and may show an abnormal position of the lens, especially if it is completely dislocated into the vitreous. (See Figure 9.) Lens subluxation is also visible on CT scan. (See Figure 10.)

A subluxated lens due to blunt trauma seen on CT scan
A lens dislocated into the anterior chamber can produce corneal edema and blockage of aqueous fluid drainage (termed pupillary block), leading to increased intro-ocular pressure with the potential for retinal damage. If the lens capsule is disrupted, leakage of protein from the lens can produce inflammation, leading to uveitis and/or glaucoma.
Treatment depends on the extent of lens dislocation, presence of lens opacities (cataracts), location, and visual acuity.32,33 Small subluxations with a clear lens can be managed with refraction alone. A lens dislocated into the anterior chamber can sometimes be reduced by chemical dilation of the pupil and digital pressure on the cornea with the patient lying flat on his or her back; this maneuver may relocate the lens to its normal position behind the iris. Surgery is indicated if the lens is dislocated into the anterior chamber, producing increased intra-ocular pressure, and cannot be reduced by closed means. Surgery is also indicated if the visual acuity is significantly reduced or leakage of lens proteins is producing uveitis and/or glaucoma.
Conclusion
Acute vision loss is a common complaint encountered by emergency physicians. A prompt and thorough evaluation is important to diagnose and treat potentially vision-threatening diseases. The ocular exam includes measurement of visual acuity, visual fields, pupillary reactivity, slit lamp, fluorescein exam, tonometry, fundoscopy, and, more recently, ocular ultrasound. Although not all components are required in every case of vision loss, emergency physicians should become familiar with these modalities in case the need arises. Causes of vision loss are broadly categorized by whether ocular pain is present. Certain conditions, including bacterial keratitis, acute-angle closure glaucoma, giant cell arteritis, and CRAO require an immediate referral to an ophthalmologist, and should be rapidly ruled out in patients who present with vision loss.
References
- Walker RA, Adhikari S. Chapter 236. Eye Emergencies. In: Tintinalli JE, Stapczynski J, Ma O, Cline DM, Cydulka RK, Meckler GD, T. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 7e. New York, NY: McGraw-Hill; 2011. http://accessmedicine.mhmedical.com.offcampus.lib.washington.edu/content.aspx?bookid=348&Sectionid=40381722. Accessed June 20, 2014.
- Wayman D, Carmody KA. Optic neuritis diagnosed by bedside emergency physician- performed ultrasound: A case report. J Emerg Med 2014;1:1.
- Germann CA, Baumann MR, Hamzavi S. Ophthalmic diagnoses in the ED: Optic neuritis. Am J Emerg Med 2007;25:834.
- Borg FA, Salter VLJ, Dasgupta B. Neuro-ophthalmic complications in giant cell arteritis. Curr Allergy Asthma Rep 2008;4:323.
- Pacella F, Mazzeo F, Giorgi D, et al. Giant cell arteritis: The importance of immediate and appropriate diagnosis and treatment for better prognosis. Clin Ophthalmol 2012;6:909.
- Danesh-Meyer H, Savino PJ, Gamble GG. Poor prognosis of visual outcomes after visual loss from giant cell arteritis. Ophthalmology 2005;6:1098.
- Rabinowitz R, Yagev R, Shoham A, et al. Comparison between clinical and ultrasound findings in patients with vitreous hemorrhage. Eye 2004;18:253.
- Spraul CW, Grossniklaus HE. Vitreous hemorrhage. Surv Ophthalmol 1997;42:3.
- Addis VM, DeVore HK, Summerfield ME. Acute visual changes in the elderly. Clin Geriatr Med 2013;29:165.
- Frasure SE, Saul T, Lewiss RE. Bedside ultrasound diagnosis of vitreous hemorrhage and traumatic lens dislocation. Am J Emerg Med 2013;31:1002.e1.
- Gariano RF, Kim CH. Evaluation and management of suspected retinal detachment. Am Fam Physician 2004;69:1691.
- Shinar Z, Chan L, Orlinsky M. Use of ocular ultrasound for the evaluation of retinal detachment. J Emerg Med 2011;1:53.
- Palma J, Schott E. Acute, simultaneous, bilateral rhegmatogenous retinal detachment diagnosed with bedside emergency ultrasound. Am J Emerg Med 2013;31:466.e3.
- Beatty S, Au Eong KG. Acute occlusion of the retinal arteries: Current concepts and recent advances in diagnosis and management. J Accid Emerg Med 2000;17:324.
- Rudkin AK, Lee AW, Chen CS. Vascular risk factors for central retinal artery occlusion. Eye 2010;24:678.
- Vortmann M, Schneider JI. Acute monocular vision loss. Emerg Med Clin N Am 2008;26:73.
- Hayreh SS. Acute retinal arterial occlusive disorders. Prog Ret Eye Res 2011;30:359.
- Pokhrel PK, Loftus SA. Ocular emergencies. Am Fam Physician 2008;7:920.
- Fraser SG, Adams W. Interventions for acute non-arteritic central retinal artery occlusion (Review). Cochrane Database Syst Rev 2009;1:CD001989.
- Menzel-Severing J, Siekmann U, Weinberger A, et al. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol 2012;153:454.
- Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: Pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313.
- Hayreh SS. Retinal vein occlusion. Indian J Ophthalmol 1994;42:109.
- Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic disease associated with various types of retinal vein occlusion. Am J Ophthalmol 2001;131:61.
- Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol 2008;86:245.
- Central retinal vein occlusion. American Academy of Ophthalmology. 2014. http://www.aao.org/theeyeshaveit/non-trauma/vein-occlusion.cfm. Accessed July 17, 2014.
- Athappilly G, Pelak VS, Mandava N, et al. Ischemic optic neuropathy. Neurol Res 2008;30:794.
- Newman NJ, Scherer R, Langenberg P, et al. The fellow eye in NAION: Report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol 2002;134:317.
- Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol 2004;17:27.
- Dickersin K, Manheimer E, Li T. Surgery for nonarteritic anterior ischemic optic neuropathy. Cochrane Database Syst Rev 2012;1:CD001538.
- The Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA 1995;273:625.
- Gonzalez-Castano C, Castro J, Alvarez-Sanchez M. Subluxation of the lens: Etiology and results of treatment. Arch Soc Esp Oftalmol 2005;81980:471-478.
- Hoffman RS, Snyder ME, Devgan U, et al. Management of subluxated crystalline lens. J Cataract Refract Surg 2013;39:1904-1912.
- Salehi-Had H, Turalba A. Management of traumatic crystalline lens subluxation and dislocation. Int Ophthalmol Clin 2010;50:167-179.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
