Authors
Trahern (TW) Jones, MD, Assistant Professor, Division of Pediatric Infectious Diseases, Department of Pediatrics, University of Utah School of Medicine, Salt Lake City
Divyam Goel, B.S. Student, Philosophy of Science and Microbiology, College of Humanities, University of Utah, Salt Lake City
Peer Reviewer
Katherine Baranowski, MD, FAAP, FACEP, Chief, Division of Pediatric Emergency Medicine, Department of Emergency Medicine, New Jersey Medical School, Rutgers, The State University of New Jersey
Executive Summary
- In the United States, one to three deaths caused by rabies typically are observed annually, almost always due to acquisition of wildlife rabies domestically or importation of canine rabies from travel overseas.
- Rabies virus typically is transmitted through an infected animal’s saliva, where it is introduced to the victim through either a laceration or puncture to the skin or across intact mucous membranes.
- Bites from bats, while small, superficial, and sometimes inapparent, still may pose a serious risk and should be regarded as exposure events. Deeper bites, as well as bites to the hand, neck, head, and face, are known to lead to faster onset of symptomatic disease.
- Providers should err on the side of caution when attempting to discern the risk of rabies virus exposure when inquiring about animal behavior or symptoms. If any doubt arises, contact local infectious disease or public health authorities, and potentially provide postexposure prophylaxis (PEP) to those who have been exposed.
- Upon manifestation of symptomatic disease, patients may progress through several stages. The first stage is prodromal, with the second stage manifesting as acute neurological symptoms in which either the encephalitic (“furious”) or paralytic (“dumb”) form of rabies may be recognized. These symptomatic stages are inexorably and inevitably followed by coma and death.
- In the encephalitic form of rabies, patients experience confusion, agitation, and hyperactivity accompanied by signs of autonomic dysfunction, such as hypersalivation, excessive sweating, and piloerection. Patients may experience hydrophobia, a pathognomonic sign of encephalitic rabies.
- Following any potential exposure event, prompt wound care accompanied by PEP with passive immunization (i.e., infiltration of rabies immunoglobulin around the wound) and active immunization with rabies vaccine remain crucial steps in the prevention of rabies disease.
- Rabies PEP always should be provided in exposure events involving wild bats, skunks, racoons, coyotes, foxes, mongooses, and other mammalian carnivores, unless the animal can be demonstrated to be free of rabies by laboratory methods, or the geographic region is known to be rabies-free.
- Individual in rooms where a bat is discovered, unless they can reliably recall whether any contact was made with the animal, providers are best counseled to err on the side of providing PEP. Individuals who were deeply sleeping, medicated, or unable to communicate clearly and reliably about potential contact (e.g., infants or toddlers) should receive PEP unless immediate testing of the bat can exclude RABV infection.
- The CDC recommends that a dose of human rabies immunoglobulin should be given on the day of the exposure, if possible, infiltrated around the site of the wound. This should be accompanied by an appropriate dose of rabies vaccine on days 0, 3, 7, and 14.
Rabies is a rare, but devastating, disease. It is crucial for acute care providers to identify exposures and institute timely and appropriate treatment.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Case Study No. 1
A previously healthy 4-year-old boy presents to an emergency department with a dog bite to his left arm. The mother explains that the boy was playing with one of the family’s farm dogs and tried to pull the dog’s tail. The dog snapped at the child, drawing blood with one small laceration and a puncture wound on the child’s left arm, but otherwise immediately releasing its bite. The dog is now locked in a kennel and appears to be otherwise well. The dog is younger than 1 year of age, and the family has not had time to take it to the veterinarian’s office for routine care and immunizations. The farm is located in a rural area of Utah, and local authorities have warned of at least one rabid coyote identified this year. In addition to routine bite care, the mother asks what steps should be taken to protect this child from rabies.
Case Study No. 2
A mother and father present to your emergency department with their three children, ages 3, 7, and 12 years. The parents and children are all previously healthy. They explain that, one week ago, they stayed in a bungalow on a resort in Costa Rica. On the second night of their stay, they found a bat in the children’s room of the bungalow. The parents initially caught it with a bucket and their bare hands, and thereafter released it outside. They are unsure if the bat was present in the room while the children were sleeping. They cannot identify any specific bite marks; they had numerous small scratches and bug bites from going on hikes and undertaking various activities with their children during this trip. As they recounted the details of their trip to their pediatrician, they were informed that they should present to emergency care for potential rabies postexposure prophylaxis (PEP).
Introduction
Rabies virus (RABV) infections can pose a deadly danger to the children and adults who are exposed to them. While cases of human rabies remain rare, the outcomes of symptomatic disease are universally devastating. With numerous wild and peridomestic animal reservoirs throughout the world, such exposures remain a pertinent concern in daily life and travel, and knowledge of RABV postexposure prophylaxis measures is a valuable asset for any emergency provider.
In this review, the emergency provider will gain familiarity with RABV epidemiology, both from global and domestic perspectives. The pathophysiology of viral infection and progression to central nervous system (CNS) disease also will be explored. The symptoms and natural history of both animal and human rabies will be discussed, as well as the current state of treatment measures for symptomatic disease. Most importantly, prophylaxis measures for RABV infection will be established in detail, as well as pertinent considerations when deciding if animal exposures warrant administration of PEP. Lastly, preventative measures regarding animal rabies exposures will be discussed.
Epidemiology
Human RABV infections account for around 59,000 deaths globally each year, with some estimates more than doubling this figure.1 The majority of these deaths occur in India and Africa, where access to PEP is limited by lack of healthcare resources and underreporting of exposure events.1,2 Rabies disproportionately affects children internationally; an estimated 40% of people who are bitten by an animal suspected to be rabid are younger than 15 years of age.3 In the United States, one to three deaths typically are observed annually, almost always due to acquisition of wildlife rabies domestically or importation of canine rabies from travel overseas.4,5 Rabies virus is found in domestic and wild animal populations in nearly every region in the world, except for Australia, Antarctica, and certain islands, such as Hawai’i and Japan.1,2
Multiple unique RABV variants or strains have been described globally, the nomenclature of which usually is based on the reservoir in which the variant is found (e.g., the canine rabies virus variant, found in peridomestic dogs, also is known as CRVV).4,6 CRVV is the most common cause of human rabies worldwide, particularly in Africa and Asia.1 (See Figure 1.) In contrast, bat rabies variants and related lyssaviruses account for only a minority of human rabies cases globally.
Figure 1. Global Burden of Human Deaths per Year from Dog-Transmitted Rabies |
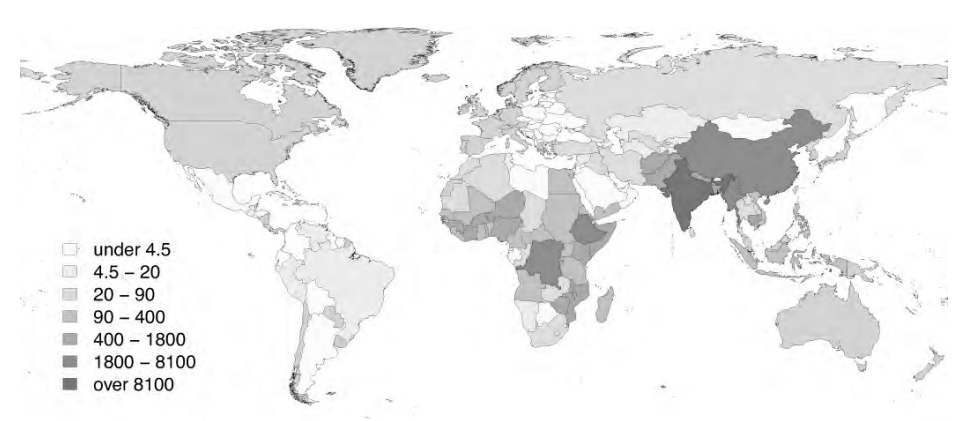 |
Used with permission from World Health Organization. WHO Expert Consultation on Rabies: Third Report. 2018. |
RABV is established in numerous animal species around the world. These include peridomestic dogs, coyotes, foxes, skunks, raccoons, monkeys, and bats, with occasional transmission to livestock, cats, and other terrestrial mammals.1, 7 While nearly all mammals are known to be susceptible to RABV infection in the laboratory, it should be noted that small rodents and lagomorphs (rabbits, hares, etc.) are almost never found to harbor wild RABV infections, and transmission events to humans have not been recorded from these species.8 Woodchucks (groundhogs) are an exception to this rule; RABV infections may be found among these rodents in areas where the raccoon rabies variant is endemic.
In the United States and Canada, widespread canine vaccination since 1947 has led to CRVV elimination, leaving wildlife variants as the only remaining zoonotic reservoir.4,6,7 Visit https://bit.ly/3tUdClq to see wildlife rabies surveillance figures from 1967-2017.
Because of this, overall U.S. cases of human rabies have dropped by tenfold during the course of the last century.4 Bat rabies variants now account for the majority of human rabies deaths in the United States and Canada, which typically totals fewer than six cases per year in both countries combined since the 1960s.9 Mexico remains the only location in North America with any documented canine rabies case in the 21st century, with five cases reported in 2015 in the southeast state of Chiapas.7
Transmission and Pathophysiology
Rabies virus consists of a bullet-shaped, enveloped virus carrying negative-sense single-stranded ribonucleic acid.2 Rabies virus typically is transmitted through an infected animal’s saliva, where it is introduced to the victim through either a laceration or puncture to the skin or across intact mucous membranes.10 Bites from rabid animals are known to carry higher risk of transmission than scratches, although both may constitute exposures.11 Bites from bats, while small, superficial, and sometimes inapparent, still may pose a serious risk and should be regarded as exposure events. Deeper bites, as well as bites to the hand, neck, head, and face, are known to lead to faster onset of symptomatic disease. Transmission does not occur through contact with urine, fecal matter, or blood. Airborne transmission from aerosolized, infected tissue is theoretically a concern in unique laboratory situations, but not for the vast majority of victims or healthcare scenarios. Aerosolization in cave environments because of the presence of thousands of infected bats also has been reported, although some have questioned if these exposure events were mediated by simple inapparent bites or exposure to rabid bat saliva.12 Human-to-human transmission has never been recorded, except through organ/tissue transplantation from infected donors to noninfected recipients.10 In healthcare scenarios, standard infection prevention precautions are indicated.13
Upon introduction through a break in the skin, RABV initially replicates in muscle cells, where it may or may not elicit an initial immune response.14 This initial infection may remain asymptomatic or even dormant from weeks to years, with the longest apparent dormancy reported in the literature being 19 years.15 However, incubation more commonly is reported to be within several weeks to three months, with 75% of patients developing symptoms within 90 days of the exposure event.
Following initial replication in muscle cells, RABV subsequently enters the peripheral nervous system through the neuromuscular junction, following the host’s motor neurons via retrograde axonal transport to the dorsal root ganglia, where it replicates again and travels into the CNS.14 (See Figure 2.) Prodromal symptoms will start to manifest at this point, often with localized neuropathic pain at the bite site, likely due to ganglioneuronitis.11 Upon reaching the host CNS, rapid replication and dissemination occurs, with viral spread to all major organs and salivary glands via anterograde axonal transport.
Figure 2. Rabies Pathophysiology in the Human Host |
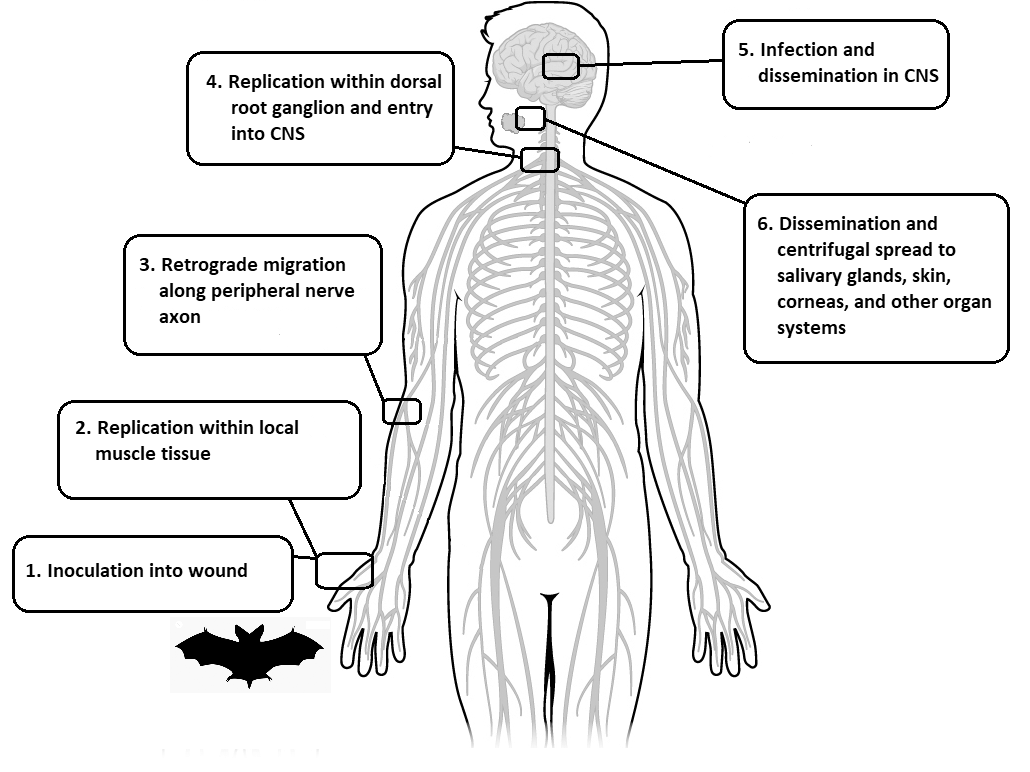 |
CNS: central nervous system |
Within the host CNS, RABV localizes preferentially in the brainstem, thalamus, basal ganglia, and spinal cord.11 Little is known about the mechanisms associated with RABV encephalitis that could explain the behavioral changes characteristic to rabies in the infected patient. Research suggests that the RABV G protein carries a region homologous to snake toxins, which has the ability to inhibit nicotinic acetylcholine receptors in the CNS.16 Excessive viral replication in the CNS, another characteristic of RABV, also may rapidly deplete host metabolic pools, forcing downregulation of host response gene expression and cellular death.11
Clinical Manifestations: Animal Rabies
Among animal reservoirs, clinical manifestations of RABV infection vary widely. While certain behaviors may be expected in domesticated dogs that have manifested symptomatic disease, these are not necessarily reliable indicators of infection and exposure in other species, and such signs and symptoms may be especially difficult to discern or may be unreliable in wildlife. Therefore, providers should err on the side of caution when attempting to discern the risk of rabies virus exposure when inquiring about animal behavior or symptoms. If any doubt arises, contact local infectious disease or public health authorities, and potentially provide PEP to those who have been exposed.
The World Health Organization (WHO) clinically defines a case of rabies based on the presence of any of the following signs or symptoms in a peridomestic or wild animal:1
- hypersalivation;
- paralysis;
- lethargy;
- unprovoked, abnormal aggression;
- abnormal vocalization;
- diurnal activity witnessed in a normally nocturnal species.
Additional signs and symptoms that may occur progressively over the course of several days include fever, vomiting, anorexia, cranial nerve dysfunction, dyspnea, dysphagia, ataxia, seizures, and self-mutilation, followed inevitably by coma and death.17 While domestic dogs may become more aggressive and bite without provocation, some sources report manifestations of “dumb” rabies are more common in dogs, which is associated with depression, withdrawal, paralysis of the neck, vocal cords, and hind legs, accompanied by dysphagia and hypersalivation.18 The animal typically dies within seven days of the first manifestation of symptoms. Importantly, rabies virus may be present in animal saliva two or three days before symptoms first appear. Therefore, when dogs, cats, or ferrets can be quarantined and observed following an unprovoked biting or mauling event, they should be monitored for at least 10 days for any signs or symptoms of RABV infection.13
This clinical definition of animal rabies provides the means to stratify the risk of rabies virus exposure in bite or contact situations. The WHO thus defines cases as “suspected,” “probable,” “confirmed,” or “not a case.”1 Specific criteria for these risk stratifications are found in Table 1.
Table 1. World Health Organization Risk Stratification System for Cases of Animal Rabies1 | |
| Stratification | Definition |
Suspected | A case that is compatible with a clinical case definition of animal rabies |
Probable | A suspected case with a reliable history of contact with a suspected, probable, or confirmed rabid animal, and/or an animal with suspected rabies that is killed, has died, or has disappeared within four to five days of illness being observed |
Confirmed | A suspected or probable case that is confirmed by laboratory diagnosis |
Not a case | A suspected or probable case that is ruled out by laboratory or epidemiological investigation (e.g., appropriate quarantine of eligible animals for at least 10 days) |
Clinical Manifestations: Human Rabies
Upon manifestation of symptomatic disease, patients may progress through several stages. The first stage is prodromal, with the second stage manifesting as acute neurological symptoms in which either the encephalitic (“furious”) or paralytic (“dumb”) form of rabies may be recognized.11,19 These symptomatic stages are inexorably and inevitably followed by coma and death.
The prodromal phase of rabies disease typically manifests following RABV transport from the site of initial infection and replication to the dorsal root ganglia.11,19 Among many nonspecific symptoms, such as fever, headache, malaise, nausea, and vomiting, the most commonly noted symptoms of the prodrome include paresthesias, neuropathic pain, and markedly severe itching at the site of the virus inoculation.18,20
The prodromal phase is followed by the acute neurological stage, which may manifest hours to days later.21 The majority of cases manifest as encephalitic rabies, while the remaining 20% to 33% generally manifest as paralytic rabies.20,22,23 The biological rationale for the clinical diversity between the two forms is widely speculated, but the strain of RABV and host immunization status do not seem to be factors predisposing victims to one form of rabies over another. In fact, a single dog is reported to have transmitted RABV infections that manifested as encephalitic rabies and paralytic rabies in two different patients.11
In the encephalitic form, patients experience confusion, agitation, and hyperactivity accompanied by signs of autonomic dysfunction, such as hypersalivation, excessive sweating, and piloerection.20,21 Patients may experience hydrophobia, a pathognomonic sign of encephalitic rabies, in which the victim may experience involuntary spasm of the pharynx with attempts to drink or swallow, evolving into generalized paroxysms with such attempts and resultant avoidance of drinking liquids, swallowing saliva, or even fear at the mention or sight of water.15 This also may evolve into aerophobia, in which a similar intolerance develops for deep inspiration or the sensation of moving air or breezes.
In paralytic rabies, paresthesia continues in the bitten limb or region and soon involves all limbs and muscles.15 Meningeal signs, such as headache and neck stiffness, may be prominent. Persistent fevers are common. Percussion myoedema may be observed, which consists of localized mounding or contraction of skeletal muscle when struck with a reflex hammer.15 Both the flaccid paralysis of all limbs and areflexia have led to frequent misdiagnosis as Guillain-Barré syndrome.20-22 Other incidents of misdiagnosis are well-documented. A study from France, which reviewed rabies cases over a 22-year period, described various incorrect diagnoses, including acute psychiatric illness, meningitis, anxiety, and depression, among others.24 Risk factors for incorrect or delayed diagnosis include the lack of a reported animal bite along with less common manifestations of rabies, such as the paralytic form.24,25
In both encephalitic and paralytic rabies, the evolution of symptoms culminates in coma and death. The average time from the initial onset of symptoms to death is 5.7 days for encephalitic rabies, and 11 days for paralytic rabies.21
Treatment
Historically, the prognosis of all individuals with symptomatic rabies is extremely grim, with mortality rates of 100% reported broadly in most scenarios, regardless of any attempted therapy.1 The vast majority of all patients with rabies die in poor, rural communities in Asia and Africa, where public health infrastructure is meager and access to PEP is limited. Therefore, the mainstay of all treatment, globally and historically, remains palliative care. The WHO advises providers to hold a frank conversation with families regarding the poor likelihood of survival or survival with severe neurological defects, discuss the risks vs. benefits of aggressive intensive care, and offer humane, deep sedation to avoid prolonging patient suffering.1
However, while rare, survival from symptomatic rabies has been reported in 15 known cases globally.1 All but one survivor had previously received one or more doses of rabies vaccine prior to the development of symptoms. Survival in this slim number of cases was thought to be mediated by late development of anti-RABV antibodies from immunization while intensive care was administered to sustain the patients’ vital functions. Only three patients among these 15 survivors did not experience severe neurological sequelae and managed to live independently after their disease.18
In 2004, physicians in Milwaukee, WI, offered to undertake aggressive therapy for an unimmunized adolescent with symptomatic rabies.28 This treatment protocol, later termed the “Milwaukee protocol,” included medical induction of coma with administration of ribavirin and amantadine antiviral therapy. The treating physicians did not provide rabies vaccine or rabies immunoglobulin, since the patient was found to have already produced an antibody response to natural RABV infection.26 Since then, the Milwaukee protocol has advised against administration of rabies immunoglobulin or vaccine to avoid interfering with natural production of rabies antibodies in cerebrospinal fluid.27 Following this therapy, the original patient was extubated after 27 days, discharged home after 76 days, and has since led an independent life.26 Since 2004, this protocol has been attempted with numerous patients, and up to 39 attempts have been published, although many other unsuccessful attempts likely have been unreported.28 Numerous failures of this therapy have been documented, and its rationale has been questioned.29 However, at this time, a recent search of the literature has attributed 11 cases of survival to the Milwaukee protocol, although the validity of rabies encephalitis diagnosis has been questioned in some of these cases.28,29 As of the writing of this article, the generalizability of the Milwaukee protocol remains uncertain.
For now, all cases of suspected symptomatic rabies virus infection in the United States warrant consultation with local public health authorities, infectious disease specialists, and neurologists, and engagement with the Centers for Disease Control and Prevention (CDC). The issue of palliative care vs. aggressive protocols (if available) must be discussed thoroughly with family members and expert consultation. Healthcare providers should exercise standard infection prevention precautions when encountering suspected cases of human rabies.13
Laboratory Diagnosis
A variety of techniques are available for antemortem and postmortem diagnosis of rabies in humans and animals. Any of the following methods should be employed in suspected cases with consultation of the appropriate public health and infectious disease specialists in the region where the case occurred.
Animals with suspected rabies following an exposure event with a human patient should be euthanized immediately.13 Following euthanization, RABV infections in animals can be diagnosed through direct fluorescent antibody (DFA) testing of brain tissue. Additional modalities to be performed on saliva and CNS specimens include enzyme-linked immunosorbent assays (ELISA), nucleic acid detection with reverse transcription polymerase chain reaction (RT-PCR), and virus isolation through cell cultures or direct inoculation in mouse models.1 Classic immunohistochemical methods for detecting intracytoplasmic inclusions (Negri bodies) are no longer used for routine diagnosis because of delays associated with these methods and their relative lack of sensitivity. In all cases, local public health authorities, veterinary specialists, and public health laboratories should be consulted regarding available modalities when requesting post-mortem diagnosis of rabies in animals.
Antemortem laboratory diagnosis in humans may be performed through several methods. DFA testing may be performed on hair follicles obtained from skin biopsies at the nape of the neck.1,13 The live virus may be isolated via cultures from saliva or CNS specimens. Anti-RABV antibodies may be detected from serum or cerebrospinal fluid. In addition, RT-PCR testing for RABV nucleic acid may be performed on saliva, skin, and CNS tissue. As with postmortem diagnosis, the relevant specialists always should be consulted when determining which laboratory diagnostic methods are employed, including public health authorities, infectious disease specialists, laboratorians, and the CDC.
Postexposure Prophylaxis
Following any potential exposure event, prompt wound care accompanied by PEP with passive immunization (i.e., infiltration of rabies immunoglobulin around the wound) and active immunization with rabies vaccine remain crucial steps in the prevention of rabies disease.30 Studies on the timing and administration of active immunization previously have estimated that > 1,000 persons received three- or four-dose rabies vaccine PEP annually in the United States following exposure events, and nearly 30% of these cases were exposed to truly rabid animals, but none developed rabies disease.30,31
In contrast, a multicenter study from India was able to demonstrate that in 192 rabies cases, all deaths were attributable to failure to seek timely PEP following animal bites.32 These data and similar studies support the conclusion that PEP may be the only means of averting the development of symptomatic rabies and eventual death in those with serious exposures.
Rabies PEP always should be provided in exposure events involving wild bats, skunks, racoons, coyotes, foxes, mongooses, and other mammalian carnivores, unless the animal can be demonstrated to be free of rabies by laboratory methods, or the geographic region is known to be rabies-free.13 (See Table 2.) In exposures involving dogs, cats, and ferrets, rabies PEP optionally may be withheld if the animal is healthy and available for a 10-day observation period. If the animal develops signs or symptoms consistent with rabies, or if the animal is confirmed by laboratory methods to be positive for rabies, PEP should be provided immediately to the patient. Exposure events involving dogs, cats, or ferrets who escape or are unavailable for observation require consultation with public health officials for advice.13 Bites involving livestock, rodents, rabbits, and hares are extremely unlikely to be considered as potential rabies exposures, but consultation with public health experts or infectious disease specialists may be warranted. Note that woodchucks (groundhogs) may be an exception to this rule regarding rodents.8
Table 2. Indications for Rabies Postexposure Prophylaxis Recommendations33 | ||
| Animal | Evaluation of Animal | PEP Recommendations for Bite Victims |
Dogs, cats, ferrets | Healthy and in custody for 10 days of observation | Prophylaxis only if animal develops signs/symptoms or rabies |
Rabid or suspected of having rabies (see Table 1) | Immediate rabies immunization and HRIG | |
Unknown or unavailable | Consult public health and infectious disease specialists | |
Bats, skunks, raccoons, coyotes, foxes, mongooses, and other wild mammalian carnivores; woodchucks (groundhogs) | Regarded as rabid unless endemic rabies is excluded geographically, or animal is proven negative by laboratory methods | Immediate rabies immunization and HRIG |
Livestock, rodents, lagomorphs (rabbits, hares)" | To be determined on a case-by-case basis | Consult public health and infectious disease specialists |
PEP: postexposure prophylaxis; HRIG: human rabies immunoglobulin | ||
Providers may sometimes encounter the relatively common scenario in which a dead or live bat was discovered in the room where an individual was sleeping. In general, bat exposures may be difficult to appreciate because of the inapparent and superficial nature of bat bites, scratches, or saliva exposure through licking.13,33 Therefore, unless the individual in the room with the discovered bat can reliably recall whether any contact was made with the animal, providers are best counseled to err on the side of providing PEP. Individuals who were deeply sleeping, medicated, or unable to communicate clearly and reliably about potential contact (e.g., infants or toddlers) should receive PEP unless immediate testing of the bat can exclude RABV infection.13
The underlying rationale behind PEP is to prevent RABV from entering the peripheral nervous system from its site of inoculation and replication.30 The CDC recommends that a dose of human rabies immunoglobulin (HRIG) should be given on the day of the exposure, if possible, infiltrated around the site of the wound. This should be accompanied by an appropriate dose of rabies vaccine on days 0, 3, 7, and 14.32 (See Table 3.)
Table 3. Rabies Postexposure Prophylaxis Recommendations36 | ||
| Rabies Vaccination Status | Intervention | Regimen |
Never previously vaccinated | HRIG | Administer 20 IU/kg in and around the bite wound. If a full dose cannot be given at the bite site, as much as possible should be infiltrated locally, and the remainder should be given IM in a separate site from any rabies vaccine. HRIG should never be given in the same syringe as the rabies vaccine. |
Rabies vaccine | HDCV or PCECV 1.0 mL IM administered on days 0, 3, 7, and 14 | |
Previously vaccinated | HRIG | Contraindicated |
Rabies vaccine | HDCV or PCECV 1.0 mL IM administered on days 0 and 3 | |
HRIG: human rabies immunoglobulin; HDCV: human diploid cell vaccine; PCECV: purified chick embryo cell vaccine; IM: intramuscular | ||
The timing of HRIG administration and the first vaccine dose ideally should be within 24 hours of exposure.13 A delay of several days may not adversely affect efficacy of this regimen, although it is not ideal. It is not clear how long of a window may be acceptable to administer this regimen, but longer delays than necessary may increase the risk of PEP failure. However, regardless of the time between exposure and therapy, PEP always should be considered and offered as long as the patient remains asymptomatic.13
Human rabies immunoglobulin provides immediate neutralizing antibodies against RABV while the patient’s body develops its own serologic response to vaccination.34 The CDC’s recommended dose for HRIG is 20 IU/kg body weight, which is applicable for all ages, including pediatric patients.13,35 No more than the recommended dose should be given because HRIG can suppress natural antibody production. The full dose should be carefully infiltrated into the wound and the area surrounding it; if this is not possible, any remaining amount of the HRIG should be injected intramuscularly at a site away from the site of vaccine administration.30 The CDC further suggests that HRIG should not be administered in the same syringe or site as the first vaccine dose, since it could neutralize the vaccine. HRIG should be administered on day 0, along with the first dose of rabies vaccine. If it was not given on day 0, HRIG may still be administered up to seven days following initiation of PEP.30 HRIG is effective, and also safe. A study of 30 pediatric patients with suspected or confirmed rabies exposure who were given HRIG found that, although treatment-related adverse effects were observed, they were mild in severity, and no serious adverse effects were recorded.34
Available rabies vaccine preparations include human diploid cell vaccine (HDCV) or purified chick embryo cell vaccine (PCECV).13 The first dose should be given the same day as the exposure, along with HRIG. The day of administration of the first dose is thereafter referred to as day 0. Subsequent doses should then be administered on days 3, 7, and 14.30 While intramuscular injection into the deltoid area is suggested for adults, the anterolateral aspect of the thigh is preferred for pediatric patients.13 All doses are 1.0 mL.
Serologic testing to measure the patient’s antibody response to vaccination should not be necessary, unless an altered vaccine schedule was given or an immunocompromising condition is identified. In these situations, serologic testing should be undertaken seven to 14 days following the final dose in the series. If insufficient antibody response is identified, or the patient is identified to have a known immunocompromising condition, a fifth dose of vaccine should be given on day 28.13,36
For previously immunized patients, the CDC recommends intramuscularly administered doses of vaccine on days 0 and 3.30 (See Table 3.) HRIG is contraindicated in these individuals because it may blunt the patient’s own neutralizing immune response. Patients are considered previously immunized if they have received appropriate regimens of HDCV, rabies vaccine adsorbed, PCECV, or a different vaccine and have a documented antibody response.30
Additional Considerations for Bite Wound Care
Aside from PEP against RABV infection as described earlier, providers also should be mindful of standards of care when addressing animal bites. Most animal bites are prone to polymicrobial infection caused by traumatic inoculation of bacteria from the offending animal’s oral flora and the skin of the patient.37 In general, all such wounds should undergo thorough cleansing with copious irrigation under moderate pressure, removal of foreign debris, and debridement of devitalized tissue. The Infectious Diseases Society of America recommends animal bite wounds should not undergo primary wound closure, except for facial wounds.38 Depending on the size and character of the wound, loose approximation may be considered. Structures in which mechanical function has been compromised (e.g., tendon laceration) should be assessed for potential repair. Depending on the size of the animal, patient characteristics, and site of the injury, imaging options may need to be considered to assess for deeper complications (e.g., computed tomography of the head in a young infant with scalp lacerations from a large dog bite).
Preemptive antimicrobial prophylaxis should be considered for a three- to five-day course in patients with immunocompromising conditions, asplenia, advanced liver disease, edema of the bite site, overall moderate to severe injuries, or potential puncture to periosteum or joint capsule structures.38 Gross wound contamination also should prompt consideration of antimicrobial prophylaxis. Antibiotic therapy should be selected for both aerobic and anaerobic coverage, such as amoxicillin-clavulanate. Patients with severe or unusual injuries may require input from an infectious disease specialist regarding antimicrobial prophylaxis (e.g., CNS injury).
The patient’s tetanus immunization status also should be assessed, and prophylaxis with tetanus immunization or tetanus immunoglobulin preparations should be provided as indicated. (See Table 4.)
Table 4. Tetanus Toxoid Recommendations | ||||
| Past Number of Doses of Previous Tetanus Toxoid-Containing Vaccines | Clean, Minor Wound | Any Other Wound, Including Those Contaminated by Dirt, Feces, as well as Frostbite, Crush Injuries, and Burns | ||
DTaP, Tdap, or Td | TIG* | DTaP, Tdap, or Td | TIG | |
Uknown or < 3 | Yes | No | Yes | Yes |
≥ 3 | No** | No | No*** | No |
DTaP: diphtheria, tetanus, and pertussis vaccine; Tdap: tetanus, diphtheria, and pertussis vaccine; Td: tetatnus and diphtheria vaccine; TIG: tetanus immune globulin *Patients with human immunodeficiency virus or severe immunocompromise with grossly contaminated wounds should receive TIG regardless of past immunization status. **Yes, if it has been 10 years or more since the patient’s last tetanus toxoid-containing vaccine. ***Yes, if it has been 5 years or more since the patient’s last tetanus toxoid-containing vaccine. Adapted from Centers for Disease Control and Prevention. Tetanus: For clinicians. Updated Jan. 23, 2020. https://www.cdc.gov/tetanus/clinicians.html | ||||
Preexposure Prophylaxis
Preexposure prophylaxis against RABV infection consists of active immunization prior to any exposure events according to the recommendations of the Advisory Committee on Immunization Practices.33 It should be emphasized that prior active immunization against rabies does not obviate the need for medical evaluation following any potential exposure event, nor does it eliminate the need for PEP in the form of booster doses of an appropriate RABV vaccine. However, it does eliminate the need for HRIG and reduces the number of booster doses of RABV vaccine required for PEP.33 (See Table 3.) Reactions to available rabies immunizations are relatively low in frequency, but remain an important consideration. Thus, preexposure prophylaxis is generally reserved for those in high-risk occupations or situations, including veterinarians, certain laboratory workers, those who handle animal populations at risk, and those traveling to areas where canine rabies is more common and PEP is not immediately accessible.
The primary regimen for active immunization against RABV consists of a three-dose series of appropriate rabies vaccine administered intramuscularly on days 0, 7, and 21 or 28.33 Subsequent booster doses are administered based on follow-up antibody titer determination, which should be measured every six months to two years based on the individual’s risk factors (e.g., continuous exposure to RABV in a laboratory setting, etc.). Traveling in rabies-endemic areas does not qualify as a risk factor that requires follow-up serologic testing.
Prevention
Domestic canine vaccination remains the most potent public health intervention for preventing human RABV deaths, as evidenced by the dramatic reduction in rabies cases in the United States and Canada following aggressive canine vaccination and elimination of canine rabies in these countries.6,7 Wildlife vaccination in various regions has proven to be partially successful.4 However, individuals must practice preventive habits themselves and avoid contact with wild animals that may harbor the disease.
Avoidance of animal rabies reservoirs remains an effective strategy, both domestically and during international travel. Note that imported cases of rabies from canine, bat, and other wildlife contact can reintroduce the disease into previously rabies-free regions or countries.39
Conclusions
RABV infections remain a major cause of death globally. Significant exposures to wildlife or peridomestic canine reservoirs that may harbor the virus warrant immediate medical attention. However, with proper PEP, patients may avert a dreaded outcome. From a public health perspective, rabies is a highly preventable disease, particularly when the elimination of canine rabies in the United States and Canada is taken into account. With reasonable attention to public health measures, veterinary interventions, and global health commitments, rabies deaths could be nearly eliminated internationally. For now, the emergency medicine provider should remain prepared to provide PEP and appropriate counseling for those who may find themselves at risk, either through travel or contact with local wildlife.
Case Study No. 1 Conclusion
The child’s wounds are cleaned thoroughly. The treating provider does not undertake primary closure, but keeps the small laceration approximated with an appropriate dressing. The child has previously received four doses of tetanus toxoid-containing vaccines, and thus tetanus prophylaxis is deemed unnecessary. The child is given a short course of amoxicillin-clavulanate prophylaxis for the bite wound.
Regarding the question of RABV exposure, the provider, public health authorities, and a local veterinarian determine that the dog is well and can remain in custody. The family thus agrees to an observation period of 10 days. At the end of this period, the dog has failed to demonstrate any symptoms suggestive of rabies infection, and the child forgoes any RABV PEP and remains well. The family rehomes the dog with an individual who does not have small children to avoid any further incidents.
Case Study No. 2 Conclusion
The entire family is ultimately deemed at risk for possible RABV exposure caused by exposure to a bat that is unavailable for necropsy as well as the consideration that they may have received undetected bite wounds. Each member of the family receives 20 IU/kg of HRIG, and additionally undertakes a four-dose series of RABV immunization on days 0, 3, 7, and 14. All family members were already up to date with tetanus toxoid-containing immunizations.
REFERENCES
- World Health Organization. WHO Expert Consultation on Rabies: Third Report. 2018.
- Singh R, Singh KP, Cherian S, et al. Rabies — epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: A comprehensive review. Vet Q 2017;37:212-251.
- World Health Organization. Rabies. Published May 17, 2021. https://www.who.int/news-room/fact-sheets/detail/rabies
- Pieracci EG, Pearson CM, Wallace RM, et al. Vital Signs: Trends in human rabies deaths and exposures — United States, 1938-2018. MMWR Morb Mortal Wkly Rep 2019;68:524-528.
- Centers for Disease Control and Prevention. Human rabies. Updated Sept. 22, 2021. https://www.cdc.gov/rabies/location/usa/surveillance/human_rabies.html
- Wallace RM, Cliquet F, Fehlner-Gardiner, et al. Role of oral rabies vaccines in the elimination of dog-mediated human rabies deaths. Emerg Infect Dis 2020;26:1-9.
- Fehlner-Gardiner C. Rabies control in North America – past, present and future. Rev Sci Tech 2018;37:421-437.
- Centers for Disease Control and Prevention. Rabies: Other wild animals. Updated Jan. 25, 2021. https://www.cdc.gov/rabies/exposure/animals/other.html
- De Serres G, Dallaire F, Côte M, Skowronski DM. Bat rabies in the United States and Canada from 1950 through 2007: Human cases with and without bat contact. Clin Infect Dis 2008;46:1329-1337.
- Centers for Disease Control and Prevention. Rabies exposure in healthcare settings. Updated Jan. 14, 2021. https://www.cdc.gov/rabies/specific_groups/hcp/exposure.html
- Hemachudha T, Laothamatas J, Rupprecht CE. Human rabies: A disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol 2002;1:101-109.
- Constantine DG. Rabies transmission by air in bat caves. Public Health Service Publication no. 1617. Washington;1967.
- American Academy of Pediatrics. Rabies. In: Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Red Book: 2021 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;2021:619-627.
- Mrak RE, Young L. Rabies encephalitis in humans: Pathology, pathogenesis and pathophysiology. J Neuropathol Exp Neurol 1994;53:1-10.
- Williams B, Rupprecht CE, Bleck TP. Rabies (rhabdoviruses). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020: 2127-2137.
- Hueffer K, Khatri S, Rideout S, et al. Rabies virus modifies host behaviour through a snake-toxin like region of its glycoprotein that inhibits neurotransmitter receptors in the CNS. Sci Rep 2017;7:12818.
- Centers for Disease Control and Prevention. Rabies: Clinical signs of rabies in animals. Updated April 22, 2021. https://www.cdc.gov/rabies/specific_groups/veterinarians/clinical_signs.html
- Warrell MJ, Warrell DA. Rabies and Related Viruses. In: Ryan ET, ed. Hunter's Tropical Medicine and Emerging Infectious Diseases. 10th ed. Elsevier;2020:382-420.
- Hemachudha T, Ugolini G, Wacharapluesadee S, et al. Human rabies: Neuropathogenesis, diagnosis, and management. Lancet Neurol 2013;12:498-513.
- Riccardi N, Giacomelli A, Antonello RM, et al. Rabies in Europe: An epidemiological and clinical update. Eur J Inter Med 2021;88:15-20.
- Hemachudha P, Hemachudha T. Rabies: Presentation, case management and therapy. J Neurol Sci 2021;424:117413.
- Mahadevan A, Suja MS, Mani RS, Shankar SK. Perspectives in diagnosis and treatment of rabies viral encephalitis: Insights from pathogenesis. Neurotherapeutics 2016;13:477-492.
- Jackson AC. Current and future approaches to the therapy of human rabies. Antiviral Res 2013;99:61-67.
- Carrara P, Parola P, Brouqui P, Gautret P. Imported human rabies cases worldwide, 1990-2012. PLoS Negl Trop Dis 2013;7:e2209.
- Tian Z, Chen Y, Yan W. Clinical features of rabies patients with abnormal sexual behaviors as the presenting manifestations: A case report and literature review. BMC Infect Dis 2019;19:679.
- Willoughby Jr., RE, Tieves, KS, Hoffman, GM, et al. Survival after treatment of rabies with induction of coma. N Engl J Med 2005;352:2508-2514.
- Medical College of Wisconsin. Milwaukee protocol, version 6. Updated November 2018. https://www.mcw.edu/-/media/MCW/Departments/Pediatrics/Infectious-Diseases/Milwaukee_protocol.pdf?la=en
- Ledesma LA, Lemos ERS, Horta MA. Comparing clinical protocols for the treatment of human rabies: The Milwaukee protocol and the Brazilian protocol (Recife). Rev Soc Bras Med Trop 2020;53:e20200352.
- Zeiler FA, Jackson AC. Critical appraisal of the Milwaukee protocol for rabies: This failed approach should be abandoned. Can J Neurol Sci 2016;43:44-51.
- Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2010;59:1-9. [Erratum in MMWR Recomm Rep 2010;59:493].
- Rupprecht CE, Briggs D, Brown C, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 2009;27:7141-7148.
- Ichhpujani RL, Mala C, Veena M, et al. Epidemiology of animal bites and rabies cases in India: A multicentric study. J Commun Dis 2008;40:27-36.
- Manning SE, Rupprecht CE, Fishbein D, et al; Advisory Committee on Immunization Practices Centers for Disease Control and Prevention. Human rabies prevention — United States, 2008: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008;57:1-28.
- Hobart-Porter N, Stein M, Toh N, et al. Safety and efficacy of rabies immunoglobulin in pediatric patients with suspected exposure. Hum Vaccin Immunother 2021;17:2090-2096.
- Centers for Disease Control and Prevention. Human rabies immune globulin. Updated April 22, 2011. https://www.cdc.gov/rabies/medical_care/hrig.html
- Kopel E, Oren G, Sidi Y, David D. Inadequate antibody response to rabies vaccine in immunocompromised patient. Emerg Infect Dis 2012;18:1493-1495.
- American Academy of Pediatrics. Bite wounds. In: Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, eds. Red Book: 2021 Report of the Committee on Infectious Diseases. American Academy of Pediatrics;2021:169-175.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:147-159.
- Lardon Z, Watier L, Brunet A, et al. Imported episodic rabies increases patient demand for and physician delivery of antirabies prophylaxis. PLoS Negl Trop Dis 2010;4:e723.
