AUTHORS
Amar Mittapalli, MD, Department of Emergency Medicine, Kendall Regional Medical Center, Miami, FL
Ma Lovely Batasin, DO, Department of Emergency Medicine, Kendall Regional Medical Center, Miami, FL
Andrew Allen, MD, Department of Emergency Medicine, Kendall Regional Medical Center, Miami, FL
Murtaza Akhter, MD, Department of Emergency Medicine, Kendall Regional Medical Center, Miami, FL; University of Arizona College of Medicine–Phoenix
PEER REVIEWER
Larry B. Mellick, MD, MS, FAAP, FACEP, Professor of Emergency Medicine and Pediatrics, Vice Chairman for Academic Affairs, Division Chief of Pediatric Emergency Medicine, Department of Emergency Medicine, University of South Alabama, Mobile
EXECUTIVE SUMMARY
- Migraine is a clinical diagnosis.
- The common symptoms of migraine are unilateral location, pulsating or throbbing quality, moderate to severe intensity, nausea, phonophobia, and photophobia.
- Migraines are more common in women, and the prevalence peaks in the fourth and fifth decades of life.
- Treatment of acute migraine is guided by the severity of the symptoms and knowledge of prior successful treatment.
- The agent with the highest treatment response rate for acute migraine is prochlorperazine.
- The addition of diphenhydramine is useful to reduce the akathisia side effect of prochlorperazine or metoclopramide.
- Administer dexamethasone 10 mg intravenously prior to discharge in patients with a history of recurrent migraines.
Headaches are a common complaint encountered by emergency physicians (EP). There were 4 million emergency department (ED) visits in the United States in 2016 for headaches, with migraines composing the majority of this group.1 Migraines have a significant impact on patient health as a chronic ailment and society as a cause of short- and long-term disability. They occur in different cultures, ethnic groups, socioeconomic classes, and regions. Migraines tend to emerge in adolescence, with increased prevalence up to age 40 years and a decline thereafter. They have a notable familial component as well.
The impact of migraine in the United States was assessed using an online survey between 2016 and 2017, finding that of about 95,000 adults who reported episodes of headaches, 15.8% met criteria for migraine and had at least one headache day per month over the preceding three-month period.2 Almost three-quarters of these migraine patients were women (73%), and compared to men, women were younger, reported more headache days per month, described more severe headaches, and were more likely to seek out medical consultation for their headache.
Migraine symptoms are characterized by headaches with typical features of unilateral location, pulsating quality, moderate to severe pain intensity, and identifiable aggravating factors, all while not secondary to another disorder. Migraines warrant attention because episodes tend to be debilitating for patients and, with a duration of four to 72 hours, impair daily life.3
When a patient with a self-identified migraine presents to the ED, the EP is tasked with sorting through the history to ensure that the diagnosis is correct, to reasonably exclude other causes of an acute headache, initiate treatment, assess the response, and make an appropriate disposition for the patient, with referral to primary care or specialists as needed. This article will focus on the acute treatment of migraines in the ED.
Pathophysiology
There are several proposed mechanisms for acute migraine headaches; no one process can explain the cause of such headaches in all patients. Thus, migraine can be viewed as a syndrome, a constellation of specific symptoms and signs that can be produced by multiple processes. The mechanisms currently proposed for migraines include cortical spreading depression, activation of the trigeminovascular system, neuronal sensitization, serotonin receptor activation, and calcitonin gene-related peptide (CGRP) expression.
The generation of headache pain in migraines, particularly those associated with aura, is thought to be explained by a cortical spreading depression of the cerebral cortex. This occurs by neuronal depolarization, including glial cells, that are self-propagating in nature. This event has been thought to produce the aura of migraine, since the area affected generally perceives the depolarization as perceptions or sensations such as visual or auditory, and with the release of inflammatory mediators that irritates the meninges, generating headache.4
With the initiation of the cortical spreading depression and release of inflammatory markers, the trigeminal system also may be activated such that neuronal activation of this system causes nociception of the afferent neurons, further contributing to the pain in a specific distribution over the head. In contrast to this, a cortical spreading depression of the cerebellum may manifest with migraine without aura, since this portion of the brain is not perceived consciously. Furthermore, trigeminal afferent neuronal activation, along with trigeminal ganglion and upper cervical nerve roots, and the release of vasoactive neuropeptides, such as substance P, CGRP, and neurokinin A, produce neurogenic inflammation and vasodilation. The release of these substances has been thought to be key for migraine pain generation and intensity, since elevated levels have been associated with migraine.5 Inhibition of these vasoactive peptides is being explored as new avenues for migraine treatment.
Continued release of inflammatory mediators on a chronic basis also leads to sensitization. Over time, neurons involved in migraine become increasingly responsive to the mediators, with decreased activation thresholds and intensified responses.6 This phenomenon may play a role in the symptoms experienced by patients when repeated stimuli provoke more intense pain. Examples include pain with certain movements such as bending, increased exertion, coughing, as well as hyperalgesia and allodynia.
Serotonin also has been suggested as a possible mediator of migraines and a treatment target for modulation. The exact mechanism is unclear, but it is thought to be from potential vasodilation of cranial vasculature. Medications such as tricyclic antidepressants (TCAs), which possess serotonergic activity, are used prophylactically to reduce migraine attacks, but this property may not fully explain the effectiveness of TCAs, since other serotonergic medications are ineffective. The possibility of deficient serotonin has been suggested to produce increased activation of the trigeminovascular system and augment the cortical spreading depression.7
CGRP is an amino acid neuropeptide expressed by trigeminal ganglia neurons that also has been linked to migraines and is a focus for new treatments. CGRP acts as a vasodilator of head vasculature and has been seen to increase during acute migraine episodes of patients. CGRP antagonists have been developed to modulate and reduce the effect of circulating CGRP and, thus, reduce the frequency of migraines.8
A genetic basis also has been identified for the predisposition for migraines. The presence of a family history helps to narrow the diagnosis when presented with a headache patient with features of a migraine. The risk of migraines has been found to be three times higher in patients with family members who also experience migraines.9 Despite a genetic predisposition, no single form of inheritance has been found to explain this pattern. The genetic predisposition is likely a mix of genetic associations that affect membrane channels, receptors, and enzymes that are integral to the pathophysiology of the syndrome. Migraine pathophysiology and genetics remain an active research area, and much is needed to further characterize the processes to guide management and treatment of migraine patients.
Symptoms
Migraine episodes follow four phases or stages, which are characteristic of the process and help to clinically diagnose patients.10 (See Figure 1.) These phases — prodrome, aura, headache, and postdrome — can unfold over several hours and possibly days.
Figure 1. Migraine Stages |
 |
The prodrome typically lasts fewer than 12 hours, but may appear up to two days prior to headache onset.11 Typical and common symptoms experienced at this time include fatigue, mood change, and gastrointestinal changes. Approximately 77% of all migraine patients describe a prodrome of symptoms prior to their migraine. Roughly 45% of these patients have prodromal symptoms for less than one hour prior to migraine onset, 13.6% of patients have them one to two hours prior, 15% have them two to four hours prior, 13.1% have them four to 12 hours prior, and 13.2% have them more than 12 hours prior.11 However, these prodromal symptoms should not be confused with auras.
Migraines also may include the aura phase, which is the experience of focal neurologic symptoms. The aura may precede the headache portion or occur simultaneously with it. The neurological symptoms fall into positive and negative categories and are completely reversible with resolution of the migraine episode.12 Positive symptoms include visual, auditory, somatosensory, and motor changes. Negative symptoms also may be present in the form of loss of functions, such as decreased or loss of vision, hearing, or mobility of one body part. Of all these potential aura symptoms, visual auras are more common.
The stage that usually brings patients to the ED is the headache portion of the migraine episode. Usually, the pain is unilateral and has a pulsatile or throbbing quality. Patients may experience nausea and occasionally vomiting, as well as photophobia, phonophobia, and, to lesser extent, allodynia.13 The headache portion of migraines may last several hours to days if left untreated, and patients generally seek to minimize symptoms by resting in a quiet and dark room. The postdrome is characterized by resolution of constant pain, with feelings of exhaustion and intermittent pain with movement in the area where the headache was experienced.
Causes/Triggers
In a retrospective study performed in 1,750 patients, 75% of those studied were found to have an identifiable trigger. Common triggers, listed from most to least common, were stress, hormonal swings in women, not eating, weather changes, sleep disturbances, perfume or smells, neck pain, lights, alcohol, smoke, sleeping late, heat, food, exercise, and sexual activity.14
Poor sleep quality has been well documented as a cause for migraines. A study performed in Atlanta that attempted to assess the relationship between sleep parameters and migraine headaches found that in 1,283 migraine patients assessed, more than half indicated they had difficulty initiating and maintaining sleep. Approximately 71% of these patients described having migraine headaches that woke them from sleep. This study showed that 85% of the patients chose to sleep because of their headache, and 75% of the overall study group were forced to sleep because of their headache.15 People with chronic migraine were noted to have shorter hours of sleep when compared to people with episodic migraine. Those with chronic migraine who slept fewer hours were found to have more frequent and severe headaches when compared to those with episodic migraine; chronic migraine patients also were found to have more morning headaches.16,17
Diagnosis/Evaluation
The diagnosis of migraine is mainly clinically based on good history taking, proper physical exam, and fulfillment of diagnostic criteria. With migraine headaches, the most common clinical features include nausea, photophobia, phonophobia, and worsening with physical exertion.
The International Classification of Headache Disorders specifies diagnostic criteria for migraine with and without auras. Criteria for migraine without auras must include at least five headache attacks that fulfill the following: duration from four to 72 hours and either untreated or unsuccessfully treated; nausea, vomiting, or both, and/or photophobia or phonophobia; and association with at least two of the following: unilateral quality, pulsatile, moderate or severe intensity, or worsened by or resulting in avoidance of physical activity.12
For migraine patients with auras, criteria include at least two headache attacks with one or more of the following reversible aura symptoms: visual, sensory, speech/language, motor, brainstem, or retinal; at least three of the six characteristics: at least one aura symptom spanning five or more minutes, two or more symptoms occurring back to back, individual aura symptoms lasting at least five to 60 minutes, at least one aura symptom is unilateral and/or positive, and the aura is either accompanied or followed by a headache within 60 minutes.12
Migraine is a clinical diagnosis, and neuroimaging is not necessary to make the diagnosis and initiate treatment if the clinical criteria are present. The American Academy of Neurology suggests that neuroimaging be considered if patients present with unexplained findings on neurologic examination and/or if headaches do not fulfill the strict definition of migraine or other primary headache disorders.18 History and physical examination features — “red flags” — that include the following may warrant further neuroimaging: first or worst headache; change in pattern, severity, or frequency; new or unexplained neurologic signs or symptoms; unresponsiveness to treatment; new onset after age 50 years; new onset in patients with cancer or human immunodeficiency virus; and signs and symptoms, such as fever, stiff neck, papilledema, or change in mental status or personality.19,20
Imaging for patient reassurance often is performed in clinical practice. Patients should be informed that incidental findings may arise should imaging be performed; patients also should be provided with guidance regarding how these findings should be managed.
The choice of imaging and the need for contrast depend on the clinical setting and indication. A noncontrast head computed tomography (CT) should be sufficient in patients who require neuroimaging. In the ED, CT has several advantages over magnetic resonance imaging (MRI). Most hospital EDs have 24-hour CT availability and this modality takes but a few minutes to perform, which is helpful in busy departments. Most life-threatening causes of headache are easily detected on CT imaging (higher sensitivity for hemorrhage when compared to MRI), and CT is safer than MRI for patients who are unstable and require monitoring and/or life support. Newer techniques for gathering CT imaging minimize the radiation exposure for most adult and pediatric patients.
CT angiography should be considered for patients with suspected arterial lesions (obstructive lesions or aneurysms). CT venography is performed for suspected cerebral venous thrombosis.
The advantage of MRI is that it provides better discrimination between tissue densities, better detection of posterior fossa lesions, and no exposure to ionizing radiation.21 MR angiography and MR venography should be considered when arterial lesions or venous thrombosis is suspected, respectively.21 Because of availability and technical issues, MRI generally is reserved for nonemergent elective study.
The American College of Radiology Appropriateness Criteria provide general guidance when pursuing imaging for scenarios of patients presenting with headache. When deciding which imaging modality is best, discussion with the radiologist is recommended.19 (See Figure 2.)
Figure 2. Red Flags for Neuroimaging of Headache Patients |
 |
CT: computed tomography; MRI: magnetic resonance imaging; MRA: magnetic resonance angiography; MRV: magnetic resonance venography |
Acute Treatments
Prochlorperazine
This dopamine receptor antagonist is effective in the acute management of migraine headache pain. Multiple controlled trials performed have shown that intravenous (IV) prochlorperazine is more effective in the treatment of acute migraine headache compared to IV metoclopramide or intramuscular (IM) sumatriptan, as well as more effective than IV hydromorphone. In particular, a randomized controlled trial from an emergency department in New York City showed that the combination of 10 mg IV prochlorperazine plus 25 mg IV diphenhydramine was more effective than 1 mg IV hydromorphone in sustaining headache relief without the need for rescue medication.22 Another double-blind, randomized controlled trial performed in Virginia demonstrated the combination of 10 mg IV prochlorperazine plus 25 mg IV diphenhydramine was more effective than IM sumatriptan in the reduction of pain due to migraine headache.23 A committee from the Canadian Headache Society performed a systematic review and, based on a high level of evidence, the use of prochlorperazine is recommended in the treatment of migraine pain in the emergency setting.24
Metoclopramide
Metoclopramide, another dopamine receptor antagonist, is effective in the treatment of acute headache pain as well as controlling nausea and vomiting. A meta-analysis of randomized controlled studies comparing IV metoclopramide to placebo found metoclopramide was more effective in providing pain relief for acute migraines; the number needed to treat to allow one patient to achieve significant reduction in pain from migraine headache was four.25 Although effective in migraine headache relief, metoclopramide by itself is not as effective as either chlorpromazine or prochlorperazine by itself.26
Systematic reviews and meta-analyses have shown that IV metoclopramide combined with IV diphenhydramine is as effective as IV prochlorperazine, triptans, and nonsteroidal anti-inflammatory drugs (NSAIDs).26
Chlorpromazine
With similar properties to metoclopramide and prochlorperazine, chlorpromazine also is a dopamine receptor antagonist. When compared to placebo, IV chlorpromazine produces significant improvement in pain, nausea, photophobia, and phonophobia at 60 minutes post medication administration. The benefits of IV chlorpromazine extended to both migraines with and without auras. The number needed to treat to allow one patient to achieve significant improvement at 60 minutes was two patients.27
Droperidol
Droperidol is suspected to antagonize the actions of glutamic acid, but the true mechanism of action in migraine pain remains unknown. In a randomized, double-blind, placebo-controlled, dose-ranged multicenter study with more than 300 patients, it was found that IM droperidol in dosages of 2.75 mg, 5.5 mg, and 8.25 mg had significant improvement in symptoms associated with migraine headaches when compared to placebo. These symptoms included nausea, vomiting, photophobia, and phonophobia. More patients treated with droperidol achieved pain relief (85%) at two hours compared to placebo (57%). No patients were observed to have QT prolongation.28 Systematic reviews of multiple controlled trials found that droperidol and prochlorperazine were at least equal if not superior in efficacy when compared to other treatment regimens; however, both were noted to have more side effects (especially akathisia).28
Haloperidol
Haloperidol’s mechanism of action is to inhibit the effects of dopamine and to increase the rate of dopamine turnover. A double-blinded, placebo-controlled study from Finland showed that 5 mg of IV haloperidol in 500 mL of normal saline was effective for migraine headache relief when compared to placebo (500 mL of normal saline alone). The most common side effects noted were sedation and akathisia.29 Another study from Virginia showed that haloperidol 5 mg IV is as safe and effective as metoclopramide 10 mg IV for the treatment of acute migraine headache without the need for rescue medication.30
Ketorolac
An antipyretic and analgesic, ketorolac functions by competitively blocking cyclooxgenase (COX) and inhibiting prostaglandin synthesis. An extensive systematic review of multiple databases performed by the University of Alberta showed that parenteral ketorolac was more effective in significant pain control for migraine headaches when compared to intranasal sumatriptan. However, when compared to metoclopramide, parenteral ketorolac was not as effective.31
Triptans
These medications work as serotonin 1a/1b agonists and were specifically developed for the treatment of acute migraine headaches. Triptans work by inhibiting the release of vasoactive peptides, promoting vasoconstriction, and blocking pain pathways within the brainstem. By inhibiting transmission within the trigeminal nucleus caudalis, these medications can block afferent input to second order neurons.32
Sumatriptan in the subcutaneous, oral, and IM routes of administration has been shown to be efficacious as an abortive therapy for acute migraine headaches. Oral sumatriptan is available in 25 mg, 50 mg, and 100 mg forms. When given orally, 50 mg and 100 mg were both more effective than a 25-mg dose, but the 50-mg dose of sumatriptan was associated with a lower incidence of adverse events compared to the 100-mg dose. With that said, all three doses were more effective at relieving nausea, photophobia and/or phonophobia, and headache pain than placebo.33
Subcutaneous doses of sumatriptan are available in 3-mg, 4-mg, and 6-mg formulations. A randomized, double-blinded crossover study showed that a 3-mg dose of subcutaneous sumatriptan was noninferior in time of onset and relief of migraine headaches when compared to a 6-mg dose.34 Subcutaneous sumatriptan at a dose of 6 mg was more effective than 100 mg oral sumatriptan in migraine headache and symptom relief, with a faster onset of action.35 The parenteral formulation is more expensive than the oral form.36 The recommended dosing for subcutaneous sumatriptan is 6 mg per dose and at total of 12 mg per 24 hours.
A systematic review from Italy showed that there were no adverse cardiovascular effects associated with the use of triptans at recommended doses for migraine relief, albeit only four studies met inclusion criteria.37 This systematic review found that intense consumption of triptans was associated with cardiovascular and cerebral ischemic insult.37 Thus, it is recommended that triptans be limited to no more than 10 days of use per month.
Limited evidence recommends the avoidance of triptans in patients with confirmed hemiplegic migraine, basilar migraine, ischemic stroke, heart disease, Prinzmetal’s angina, uncontrolled hypertension, and pregnancy.38 A relative contraindication exists for the use triptans in patients on monoamine oxidase inhibitors because of the risk of serotonin syndrome. Triptans also should be avoided in patients who recently took ergotamine preparations or other triptan derivatives within 24 hours. There is a theoretical risk of developing serotonin syndrome when consuming triptans and SSRI/serotonin norepinephrine reuptake inhibitor medications in unison, so shared decision-making is very important when choosing this therapy.39
Lasmiditan
Lasmiditan is a selective serotonin 1F receptor agonist without vasoconstrictor activity that can be used safely in patients who have relative contraindications to triptans because of cardiovascular disease or risk factors. A randomized trial of 1,856 episodic migraine patients showed that more patients were pain-free at two hours with oral lasmiditan 200 mg (32%) when compared to placebo (15%).40 The initial dosing of lasmiditan is 50 mg or 100 mg (50 mg was noninferior in efficacy compared to 100 mg). The dose may be increased to 100 mg or 200 mg as needed with subsequent attacks, but no more than one dose should be taken within 24 hours. The most common side effect associated with lasmiditan is dizziness. Other side effects noted were paresthesia, somnolence, fatigue, and nausea.41
Valproate
Although mainly used as an anticonvulsant, this gamma-aminobutyric acid (GABA) agonist and voltage-gated sodium channel blocking agent has been used as a prophylactic agent for migraines. Migraine prevention was found at doses ranging from 500 mg to 1,500 mg daily when compared to placebo when assessed by patients experiencing a greater than 50% reduction in migraine frequency.42 Doses lower than 1,000 mg were found to achieve adequate migraine prevention; higher doses (> 1,500 mg daily) were associated with the potential for more adverse side effects, such as hair loss, nausea and vomiting, somnolence, tremor, dizziness, and weight gain. Valproate is contraindicated in pregnancy because of teratogenicity. Children with prenatal exposure to valproate have been noted to have lower intelligence quotient scores; therefore, women of childbearing age should not be started on valproate for migraine prevention.43
Magnesium
Magnesium is the second most common cation found in the human body. It is an important cofactor used in approximately 350 enzymes, particularly those requiring adenosine triphosphate (ATP). Magnesium balance is regulated by renal and gastrointestinal absorption. Magnesium has been suggested as a treatment for migraines because of its N-methyl-D-aspartate (NMDA) receptor blockade properties, which has been identified as an important contributor to pain transmission.44 A systematic review of IV magnesium sulfate for the treatment of nontraumatic headaches found that pain intensity was improved vs. comparators at 60-120 minutes, but with conflicting evidence for complete pain relief, 50% reduction in headache intensity, or prevention of recurrence within 24 hours.45
Ergots
Ergotamine and dihydroergotamine are both medications that bind to 5HT 1b/d receptors just as triptans do. Dihydroergotamine is a weaker arterial vasoconstrictor and more potent venoconstrictor than ergotamine and also has fewer side effects. Dihydroergotamine is available in IV, IM, intranasal, and subcutaneous routes of administration. It often is given with an antiemetic when administered intravenously.
Dihydroergotamine administered with metoclopramide was found to be more effective in migraine headache relief when compared to dihydroergotamine alone. It is unclear if the co-administration of dihydroergotamine with metoclopramide provided any more symptomatic relief when compared to metoclopramide alone.46
Ergots should be avoided in patients with a prior medical history of coronary artery disease, peripheral vascular disease, hypertension, and hepatic or renal disease. Ergots have been associated with an increased risk of cerebrovascular, cardiovascular, and peripheral ischemic complications. Ergots should not be used in patients with migraines with prolonged aura because of the risk of reduction of cerebral blood flow.47
Addition of Dexamethasone
The addition of parenteral dexamethasone (10 mg to 24 mg) to standard migraine therapy is used to reduce the rate of early recurrence (within 24-72 hours) of headaches. A meta-analysis of seven randomized trials found that parenteral dexamethasone was significantly more effective than placebo for reducing the recurrence of migraine within 24-72 hours after treatment.48 Oral prednisone given concomitantly with standard migraine therapy was not found to be effective for prevention of migraine headache recurrence.49
Peripheral Nerve Blocks
Peripheral nerve blocks increasingly are used in the ED, especially with ultrasound guidance. The blocks are useful for the pain associated with bedside procedures and as an effective alternative for pain control and relief for acute illness and injury. Contraindications to nerve blocks include local anesthetic allergy, adjacent infection of skin, and deformed anatomy likely from trauma.50
For migraines, occipital and sphenopalatine ganglion blocks are viable options to alleviate migraine headache if other first-line agents fail to deliver adequate pain reduction. The occipital nerve block targets the greater occipital nerve with adjacent infiltration of soft tissue with local anesthetic. Another approach is a sphenopalatine ganglion nerve block, performed by soaking a cotton applicator with local anesthetic, inserting it into the nasal cavity along the superior border of the middle turbinate, and leaving it for 10 minutes.51
One study demonstrated a 50% reduction of pain in 55% of the lidocaine arm compared to 6% of the saline control arm for the utility of the sphenopalatine block.62 Similarly, greater occipital nerve blocks show improved pain reduction and headache control, reducing need for further medication administration.52 A meta-analysis of nine studies found that pain scores were significantly lower at 15 and 30 minutes in patients with primary headache disorders treated with peripheral nerve blocks compared to placebo.53 Because of clinical heterogeneity, no conclusions could be drawn comparing peripheral nerve blocks with other active therapy or other secondary outcomes.
Peripheral nerve blocks are useful adjuncts and alternatives if other medication contraindications exist or if patient preference limits standard therapy. These blocks use medications readily available in the ED and should be considered for refractory migraine.54
CGRP Antagonists
Injectable CGRP monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) for the prevention of migraine headaches and oral CGRP antagonists (ubrogepant and rimegepant) for the treatment of migraine headaches have been approved recently by the Food and Drug Administration (FDA). The anti-CGRP monocloncal antibodies and the CGRP receptor antagonists’ mechanism of action is likely related to CGRP’s role in noxious stimuli of the trigeminal system.55
Atogepant, a small molecule CGRP receptor antagonist, is an effective preventative agent, reducing migraine days by approximately 50% after three months of treatment. Atogepant is dosed at 10 mg to 60 mg daily by mouth.56 Increased adverse events occur at higher doses, although increased benefits, such as decreased monthly headaches and > 50% reduction of migraine days, were observed.56
Rimegepant was first approved as an acute treatment of migraines but also now is approved as prophylactic treatment. The rimegepant dose for acute treatment is 75 mg available as a fast-acting orally disintegrating tablet, with termination of pain or the most bothersome symptom within an average time of two hours.57 At a dose of 75 mg taken every other day, rimegepant becomes an effective preventive treatment, reducing moderate to severe monthly migraine days by 50%.58
Ubrogepant 50 mg to 100 mg PO is effective for the acute treatment of migraine. The dose can be repeated after two hours if needed. The maximum daily dose is 200 mg.
Common reactions for the gepants were nausea and urinary tract infections. Mild elevations in liver function tests were observed, but none were greater than three times the upper limit of normal and seemed to resolve without intervention or modification of treatment.
Propofol
Small case series and individual case reports have described rapid relief of chronic headache and refractory migraine with subanesthetic doses of propofol. Theories regarding the mechanism by which propofol treats migraine include modulation of inhibitory GABA receptors to induce anxiolysis, sedation, and anesthesia, and action on NMDA similar to ketamine.
There is no consensus dose for the use of propofol in refractory migraine. The administration protocol and total dose administered vary by article. With careful cardiorespiratory monitoring, doses of 10 mg via IV bolus given every five minutes until a therapeutic response is seen was an effective treatment in eight patients with refractory migraine.59 The total dose of propofol varied between 20 mg and 60 mg, with time to headache relief of five to 30 minutes. Other studies have used larger individual doses (20 mg to 30 mg), resulting in greater total doses (averaging 110 mg) to achieve relief.60
Beta-Blockers
The mechanism of action of beta-blockers still is unclear but it appears that their therapeutic effect is mainly related to inhibitory effects on beta-1 receptors.61 The only two FDA-approved beta-blockers for migraine prevention are propranolol (80 mg/day to 240 mg/day) and timolol (20 mg/day to 30 mg/day). Metoprolol (200 mg/day) is an off-label migraine prevention treatment commonly used. Nadolol and atenolol have fewer studies to support their use but are reported to have probable efficacy. Beta-blocker use in migraine treatment should be targeted depending on patient clinical features, such as hypertension or anxiety.
Acute Treatment Protocol
A proposed treatment protocol is displayed in Figure 3. The severity of symptoms is used to guide the intensity of treatment. In patients with recurrent episodes, it is useful to ask patients or review the medical record to see if any specific treatment given in prior ED visits was effective, since it is likely to be effective with the current episode.
Figure 3. Acute Migraine Treatment in the Emergency Department |
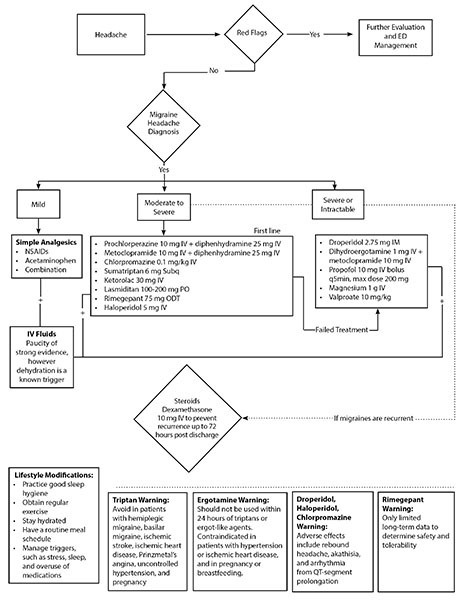 |
Neuromodulation
Given the complexity of migraines, nonpharmacologic interventions have arisen as a means to serve patients with this debilitating process. These include electrical current or magnetism to act on the central and peripheral nervous system to exert abortive effects. Patients may seek out these means as adjuncts or preferences to current pharmacologic therapy, or because of an inability to tolerate medications or contraindications to certain medications.62
Transcutaneous Supraorbital Nerve Stimulation
This treatment method includes the use of electrodes placed on the skin to electrically stimulate nerves at or adjacent to electrode placement. In a double-blind, randomized, sham-controlled study of transcutaneous supraorbital nerve stimulation, visual analogue pain scale scores decreased 59% with treatment compared to a 30% decrease with placebo.63 There were no serious adverse effects, suggesting this method to be a safe and effective alternative or adjunct for patients with migraine.63
Remote Electrical Neuromodulation
Results similar to transcutaneous supraorbital nerve stimulation have been noted with the use of nonpainful quick impulse electrical stimulation of an extremity.64 Pain was reduced by active treatment with moderate to near complete reduction of pain, most notably when given earlier in the migraine episodes. This therapy can be used easily in EDs, and it is inexpensive with a low risk for adverse events.64
Transcranial Magnetic Stimulation
Another noninvasive option is the use of transcranial magnetic stimulation as second-line treatment for migraine patients.65 Patients in the acute phase of attack with migraine were shown to have pain freedom at two hours post-treatment vs. placebo, with continued resolution of pain at one to two days after. As with electrical stimulation, there were no adverse effects with device use. This appears to be a good option for those patients with contraindications to medical therapy. However, there is theoretical concern of this method lowering the seizure threshold and, thus, it should be avoided in patients with epilepsy.65
Noninvasive Vagus Nerve Stimulation
Noninvasive vagus nerve stimulation is a novel method similar to those mentioned earlier and it potentially can be used as an adjunct for acute-onset migraines. In a study of patients with acute migraine, subjects were instructed to self-administer the electrical impulse of the nerve stimulator within 20 minutes of migraine attack to each side of the neck and continue administration if not pain free.66 Results indicated improved pain control, with pain-free results at 30 and 60 minutes. However, there was no significant difference at 120 minutes when compared to the sham arm. Adverse effects included application site discomfort and nasopharyngitis, which potentially may limit use if used frequently by migraine patients.66
Special Populations
Children
Migraines are common in children, with a prevalance that increases with age: 1% to 3% in children 3 to 7 years of age; 4% to 11% in those 7 to 11 years of age; and 8% to 23% in adolescents 15 years of age and older.67 Migraines in children can occur with and without aura, and the duration tends to be shorter in younger children compared to adolescents. Diagnosis can be challenging and is made clinically with a careful history looking at distinguishing features, such as associated symptoms, timing, and duration, and thorough neurological examination.
Simple analgesics, such as acetaminophen, ibuprofen, and naproxen, are used as initial treatment. Less commonly used are triptans or a combination of triptan and naproxen if no response is achieved. Triptans are available as a rapid oral disintegrating tablet or as a nasal spray. Preventive treatments in children are best achieved with trigger controls and lifestyle changes, such as improving sleep hygiene and addressing comorbidities, such as depression and obesity. Another preventive measure found to be effective is the use of cognitive behavioral therapy in combination with a triptan.68
Pregnancy
Most pregnant patients who present with primary headaches have a history of them before gestation. Some may have improvement in their migraines during pregnancy, and a very small percentage may develop migraines for the first time during pregnancy. Most recurrences occur during the first month postpartum and may be prevented with breast-feeding.69
Diagnosis and evaluation in pregnant women is similar to that in nonpregnant women. It is important to carefully evaluate the history and physical exam to exclude preeclampsia and other serious underlying disorders, such as cerebral venous thrombosis. Stroke in younger women also is more common during pregnancy, occurring most often in the third trimester and six to 12 weeks postpartum.70,71
Treatment of migraine in pregnancy should be tailored to the patient’s gestational age, previous treatment successes, and avoidance of medications known to be teratogenic or harmful to the fetus. Acetaminophen is considered the first-line of treatment. Second-line and refractory medications generally are used for moderate to severe migraines when benefits will outweigh the risks. Low-dose aspirin and NSAIDs are acceptable in the second trimester before 20 weeks; however, limited evidence shows this can be associated with congenital adverse effects.72
Triptans were contraindicated initially, but now they have been shown to have no major congenital defects. However, their use may affect the child’s neurodevelopment and cause behavioral problems.72,73 Opioids, such as tramadol and codeine, may be considered if nonopioid medications bring no relief and the headaches become debilitating. However, chronic use is associated with a higher risk for cardiac defects and there is the potential for maternal addiction and neonatal withdrawal.72
Metoclopramide generally is accepted to be without significant fetal side effects and may be better if used in combination with diphenhydramine, since studies have shown this to be better than opioids at treating acute migraines.72,73 Medications including low-dose steroids (prednisone or methylprednisolone), magnesium sulfate, and botulinum toxin may be considered in severe refractory chronic migraines.72 Prophylactic therapy rarely is indicated; however, metoprolol and propranolol can be used with a prelabor taper by the third trimester to avoid hypoglycemia, bradycardia, and hypotension in the neonate.74,75 Similar to in nonpregnant patients, an attempt should be made to limit the number of medications and to use the lowest possible dose to achieve relief.
Prognosis
The long-term prognosis of migraine is highly variable. However, in the longest and largest studies, most migraines eventually resolve (complete remission), and only some progress or persist.76 Progression of migraines from acute to chronic (transformed migraines) can be linked to modifiable factors (sleep hygiene, caffeine intake, weight, frequent use of pain relievers, stress, anxiety, etc.) and nonmodifiable factors (race, sex, socioeconomic level, etc.). The mechanism of progression is complex and thought to be caused by repetitive external triggers or generated from changes in the neurovascular structure of the brain, such as activation of the trigeminal neurons and development of cutaneous allodynia.77,78
Continued identification of modifiable factors, mechanism of progression, prevention, and treatment is needed to improve the overall prognosis of migraine headaches.
REFERENCES
- Burch R, Rizzoli P, Loder E. The prevalence and impact of migraine and severe headache in the United States: Updated age, sex, and socioeconomic-specific estimates from government health surveys. Headache 2021;61:60-68.
- Lipton RB, Munjal S, Alam A, et al. Migraine in America Symptoms and Treatment (MAST) Study: Baseline study methods, treatment patterns, and gender differences. Headache 2018;58:1408-1426.
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:954-976.
- Hadjikhani N, Sanchez Dl Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 2001;98:4687-4692.
- Sarchielli P, Alberti A, Floridi A, Gallai V. Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology 2001;57:132-134.
- Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996;384:560-564.
- Hamel E. Serotonin and migraine: Biology and clinical implications. Cephalalgia 2007;27:1293-1300.
- Charles A, Pozo-Rosich P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019;394:1765-1774.
- Merikangas KR, Risch NJ, Merikangas JR, et al. Migraine and depression: Association and familial transmission. J Psychiatr Res 1988;22:119-129.
- Charles A. The evolution of a migraine attack — a review of recent evidence. Headache 2013;53:413-419.
- Kelman L. The premonitory symptoms (prodrome): A tertiary care study of 893 migraineurs. Headache 2004;44:865-872.
- [No authors listed]. Headache Class-ification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1-211.
- Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia 2006;26:548-553.
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394-402.
- Kelman L, Rains JC. Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache 2005;45:904-910.
- Cevoli S, Giannini G, Favoni V, et al. Migraine and sleep disorders. Neurol Sci 2012;33(Suppl 1):S43-S46.
- Freedom T, Evans RW. Headache and sleep. Headache 2013;53:1358-1366.
- Silberstein SD. Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;55:754-762.
- Expert Panel on Neurologic Imaging; Whitehead MT, Cardenas AM, Corey AS, et al. ACR Appropriateness Criteria® Headache. J Am Coll Radiol 2019;16:S364-S377.
- Evans RW. Diagnostic testing for migraine and other primary headaches. Neurol Clin 2009;27:393-415.
- Kent DL, Haynor DR, Longstreth WT Jr, Larson EB. The clinical efficacy of magnetic resonance imaging in neuroimaging. Ann Intern Med 1994;120:856-871.
- Friedman BW, Irizarry E, Solorzano C, et al. Randomized study of IV prochlorperazine plus diphenhydramine vs IV hydromorphone for migraine. Neurology 2017;89:2075-2082.
- Kostic MA, Gutierrez FJ, Rieg TS, et al. A prospective, randomized trial of intravenous prochlorperazine versus subcutaneous sumatriptan in acute migraine therapy in the emergency department. Ann Emerg Med 2010;56:1-6.
- Orr SL, Aubé M, Becker WJ, et al. Canadian Headache Society systematic review and recommendations on the treatment of migraine pain in emergency settings. Cephalalgia 2015;35:271-284.
- Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 2: Neuroleptics, antihistamines, and others. Headache 2012;52:292-306.
- Colman I, Brown MD, Innes GD, et al. Parenteral metoclopramide for acute migraine: Meta-analysis of randomised controlled trials. BMJ 2004;329:1369-1373.
- Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: A randomized controlled trial. J Emerg Med 2002;23:141-148.
- Silberstein SD, Young WB, Mendizabal JE, et al. Acute migraine treatment with droperidol: A randomized, double-blind, placebo-controlled trial. Neurology 2003;60:315-321.
- Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: A randomized, double-blind, placebo-controlled study. Headache 2006;46:781-787.
- Gaffigan ME, Bruner DI, Wason C, et al. A randomized controlled trial of intravenous haloperidol vs. intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med 2015;49:326-334.
- Taggart E, Doran S, Kokotillo A, et al. Ketorolac in the treatment of acute migraine: A systematic review. Headache 2013;53:277-287.
- Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: A comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259-1287.
- Pfaffenrath V, Cunin G, Sjonell G, Prendergast S. Efficacy and safety of sumatriptan tablets (25 mg, 50 mg, and 100 mg) in the acute treatment of migraine: Defining the optimum doses of oral sumatriptan. Headache 1998;38:184-190.
- Cady RK, Munjal S, Cady RJ, et al. Randomized, double-blind, crossover study comparing DFN-11 injection (3 mg subcutaneous sumatriptan) with 6 mg subcutaneous sumatriptan for the treatment of rapidly-escalating attacks of episodic migraine. J Headache Pain 2017;18:17.
- Tfelt-Hansen P. Efficacy and adverse events of subcutaneous, oral, and intranasal sumatriptan used for migraine treatment: A systematic review based on number needed to treat. Cephalalgia 1998;18:532-538.
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults — overview of Cochrane reviews. Cochrane Database Syst Rev 2014;2014:CD009108.
- Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: Systematic review of observational studies. Cephalalgia 2015;35:118-131.
- Jamieson DG. The safety of triptans in the treatment of patients with migraine. Am J Med 2002;112:135-140.
- Orlova Y, Rizzoli P, Loder E. Association of coprescription of triptan antimigraine drugs and selective serotonin reuptake inhibitor or selective norepinephrine reuptake inhibitor antidepressants with serotonin syndrome. JAMA Neurol 2018;75:566-572.
- Kuca B, Silberstein SD, Wietecha L, et al. Lasmiditan is an effective acute treatment for migraine: A phase 3 randomized study. Neurology 2018;91:e2222-e2232.
- Tepper SJ, Krege JH, Lombard L, et al. Characterization of dizziness after lasmiditan usage: Findings from the SAMURAI and SPARTAN acute migraine treatment randomized trials. Headache 2019;59:1052-1062.
- Pringsheim T, Davenport WJ, Becker WJ. Prophylaxis of migraine headache. CMAJ 2010;182:E269-E276.
- Harden C, Thomas SV, Tomson T, eds. Epilepsy in Women. John Wiley & Sons; 2013.
- Hoffmann J, Charles A. Glutamate and its receptors as therapeutic targets for migraine. Neurotherapeutics 2018;15:361-370.
- Miller AC, Pfeffer BK, Lawson MR, et al. Intravenous magnesium sulfate to treat acute headaches in the emergency department: A systematic review. Headache 2019;59:1674-1686.
- Bell R, Montoya D, Shuaib A, Lee MA. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med 1990;19:1079-1082.
- Wammes-van der Heijden EA, Rahimtoola H, Leufkens HGM, et al. Risk of ischemic complications related to the intensity of triptan and ergotamine use. Neurology 2006;67:1128-1134.
- Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: Meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008;336:1359-1361.
- Kelly A-M, Kerr D, Clooney M. Impact of oral dexamethasone versus placebo after ED treatment of migraine with phenothiazines on the rate of recurrent headache: A randomised controlled trial. Emerg Med J 2008;25:26-29.
- Maizels M, Scott B, Cohen W, Chen W. Intranasal lidocaine for treatment of migraine: A randomized, double-blind, controlled trial. JAMA 1996;276:319-321.
- Nair AS, Rayani BK. Sphenopalatine ganglion block for relieving postdural puncture headache: Technique and mechanism of action of block with a narrative review of efficacy. Korean J Pain 2017;30:93-97.
- Friedman BW, Mohamed S, Robbins MS, et al. A randomized, sham-controlled trial of bilateral greater occipital nerve blocks with bupivacaine for acute migraine patients refractory to standard emergency department treatment with metoclopramide. Headache 2018;58:1427-1434.
- Patel D, Yadav K, Talijaard, et al. Effectiveness of peripheral nerve blocks for the treatment of primary headache disorders: A systematic review and meta-analysis. Ann Emerg Med 2022;79:251.
- Binfalah M, Alghawi E, Shosha E, et al. Sphenopalatine ganglion block for the treatment of acute migraine headache. Pain Res Treat 2018;2516953.
- Chiang C-C, Schwedt TJ. Calcitonin gene-related peptide (CGRP)-targeted therapies as preventive and acute treatments for migraine—The monoclonal antibodies and gepants. Prog Brain Res 2020;143-170.
- Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: A double-blind, randomised phase 2b/3 trial. Lancet Neurol 2020;19:727-737.
- Gao B, Yang Y, Wang Z, et al. Efficacy and safety of rimegepant for the acute treatment of migraine: Evidence from randomized controlled trials. Front Pharmacol 2020;10:1577.
- Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: A phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021;397:51-60.
- Soleimanpour H, Taheraghdam A, Ghafouri RR, et al. Improvement of refractory migraine headache by propofol: Case series. Int J Emerg Med 2012;5:19.
- Dhir A. Propofol in the treatment of refractory migraine headaches. Expert Rev Neurother 2016;16:1007-1011.
- Sprenger T, Viana M, Tassorelli C. Current prophylactic medications for migraine and their potential mechanisms of action. Neurotherapeutics 2018;15:313-323.
- American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019;59:1-18.
- Chou DE, Yugrakh MS, Winegarner D, et al. Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia 2019;39:3-14.
- Yarnitsky D, Volokh L, Ironi A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology 2017;88:1250-1255.
- Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: A randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol 2010;9:373-380.
- Tassorelli C, Grazzi L, de Tommaso M, et al. Noninvasive vagus nerve stimulation as acute therapy for migraine: The randomized PRESTO study. Neurology 2018;91:e364-e373.
- Gelfand AA, Goadsby PJ. Treatment of pediatric migraine in the emergency room. Pediatr Neurol 2012;47:233-241.
- Oskoui M, Pringsheim T, Holler-Managan Y, et al. Practice guideline update summary: Acute treatment of migraine in children and adolescents: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2019;93:487-499.
- Hoshiyama E, Tatsumoto M, Iwanami H, et al. Postpartum migraines: A long-term prospective study. Intern Med 2012;51:3119-3123.
- Chang BP, Wira C, Miller J, et al. Neurology Concepts: Young women and ischemic stroke-evaluation and management in the emergency department. Acad Emerg Med 2018;25:54-64.
- Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med 1996;335:768-774.
- Negro A, Delaruelle Z, Ivanova TA, et al. Headache and pregnancy: A systematic review. J Headache Pain 2017;18:106.
- Saldanha IJ, Roth JL, Chen KK, et al. Management of primary headaches in pregnancy. Agency for Healthcare Research and Quality; 2020 Nov. 12. Report No.: 20(21)-EHC026. https://effectivehealthcare.ahrq.gov/products/headaches-pregnancy/research
- Pfaffenrath V, Rehm M. Migraine in pregnancy: What are the safest treatment options? Drug Saf 1998;19:383-388.
- Lee M-J, Guinn D, Hickenbottom S. Headache during pregnancy and postpartum. UpToDate. Updated Jan. 24, 2022. https://www.uptodate.com/contents/headache-during-pregnancy-and-postpartum/print?search=propranolol+migraine&topicRef=3345&source=related_link
- Manack A, Buse DC, Serrano D, et al. Rates, predictors, and consequences of remission from chronic migraine to episodic migraine. Neurology 2011;76:711-718.
- Bigal ME, Lipton RB. The prognosis of migraine. Curr Opin Neurol 2008;21:301-308.
- Mungoven TJ, Henderson LA, Meylakh N. Chronic migraine pathophysiology and treatment: A review of current perspectives. Front Pain Res 2021; Aug. 25.
When a patient with a self-identified migraine presents to the emergency department, the emergency physician is tasked with sorting through the history to ensure that the diagnosis is correct, to reasonably exclude other causes of an acute headache, initiate treatment, assess the response, and make an appropriate disposition for the patient, with referral to primary care or specialists as needed. This article will focus on the acute treatment of migraines in the emergency department.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
