Pediatric Tuberculosis
December 1, 2021
AUTHORS
Joseph U. Becker, MD, Clinical Associate Professor, Department of Emergency Medicine, Stanford University, Palo Alto, CA
Joseph Kalanzi, MD, Graduate Fellow, Department of Anaesthesia and Emergency Medicine, Makerere University College of Health Sciences, Mulago Hill, Kampala, Uganda
PEER REVIEWER
Katherine Baranowski, MD, FAAP, FACEP, Chief, Division of Pediatric Emergency Medicine, Department of Emergency Medicine, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark
Executive Summary
- The risk of progressing from tuberculosis (TB) infection to disease is multifactorial, but age at the time of exposure is of particular importance, with younger children and infants having an increased risk of early disease progression.
- Given the existence of latent Mycobacterium tuberculosis (MTB) infection and the nonspecific and wide range of symptomology possible in MTB infection, the diagnosis of MTB may be challenging. Only 35% of cases of MTB are diagnosed accurately, and less than 15% of pediatric cases are sputum smear positive, with only 30% to 40% of all cases confirmed by culture.
- Pulmonary symptoms, such as a cough that persists longer than two weeks, should prompt consideration of MTB in regions of high prevalence. Persistent radiographic findings in symptomatic or asymptomatic children likewise should prompt consideration and diagnostic testing for MTB, particularly if the imaging findings persist after treatment with antibiotics.
- Many cases of pediatric tuberculosis are discovered via the diagnosis of MTB in a close or household contact, which then prompts immuno-stimulatory testing of children in the household and detection of immunologic exposure.
- The diagnosis of TB in pediatric populations is challenging on several fronts. No biomarkers have been clinically validated for use in the diagnosis of pediatric TB. Furthermore, pulmonary TB is paucibacillary in children, yielding sputum acid-fast bacterium positivity less than 50% of the time.
- Clinicians typically rely on the combination of clinical history and findings consistent with TB in conjunction with (but not solely reliant on) laboratory diagnostic testing and a history of exposure to an individual (typically a family member) who is positive for TB to make the diagnosis.
- According to the World Health Organization (WHO), GeneXpert is the recommended initial diagnostic test in children suspected of having multidrug-resistant TB or human immunodeficiency virus-associated TB. Centers for Disease Control and Prevention (CDC) guidelines state that GeneXpert does not replace the need for acid-fast bacilli and culture along with growth-based sensitivity testing for the diagnosis of resistance.
- The treatment of MTB is focused on both curing the individual patient and minimizing the transmission of MTB to others.
- In the absence of evidence for active MTB, the CDC and WHO have established recommended treatment courses for latent MTB infection. Children are more likely to progress to active MTB than adults, so prompt commencement of therapy is critical.
Mycobacterium tuberculosis (MTB) is a significant challenge to the health of the world’s children. Barriers exist at multiple levels of the care system for MTB. Early recognition and involvement of MTB specialists is critical to facilitate the best outcome for pediatric patients. The authors provide a thorough review of the current standards for care of these challenging patients.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Mycobacterium tuberculosis (MTB) is an acid-fast bacterium (AFB) and a major contributor to mortality globally. It causes a wide range of clinical syndromes and is one of the top 10 causes of death, second only to human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) as the leading cause of death from a single infectious agent.1 Globally, an estimated 10 million people fell ill with tuberculosis (TB) in 2019, with children accounting for 12% of these cases. Approximately 70 million children currently are infected with M. tuberculosis globally, and an estimated 25,000 children develop multidrug-resistant MTB (MDR MTB) every year.1-3
Epidemiology
MTB is spread via the respiratory route, commonly by close contacts sharing the same living space. Coughing, speaking, or even breathing can generate airborne bacteria that can float in the air for hours. These bacteria then are inhaled by others close by. Patients with nonpulmonary MTB are unlikely to transmit the infection via the respiratory route. Upon exposure to MTB, most children can immunologically eradicate the organism without developing symptoms.
Latent MTB infection is a clinical state in which MTB can elude the host immunologic response and establish a smoldering, longer-term infection. In latent MTB, children exhibit no symptoms or signs of disease, but, if tested with antibody or immunostimulation tests, demonstrate evidence of immunological sensitization.
Only 5% to 10% of patients with latent MTB will go on to develop active MTB, but risk factors, such as malnutrition, immunocompromise, or other chronic illnesses, increase the risk of progression. It is estimated that 13 million people in the United States have latent tuberculosis infection and that approximately 80% of the active MTB cases in the United States arise from prior, latent MTB.4-6
With that in mind, identification and treatment of latent MTB cases are central pillars of MTB control in the United States.4 Although latent MTB may progress to active MTB, in some cases, the organism overcomes the host’s immunologic defenses and progresses to active TB soon after exposure. Children and patients with malnutrition or immunodeficiencies are at an increased risk of progressing to active MTB. Active MTB in children usually is pulmonary, as is most MTB globally. However, disseminated disease and MTB meningitis also commonly are diagnosed in children. Each year, approximately 1 million children develop active TB, with nearly 25% eventually succumbing.7,8 Active MTB disease is a clinical state characterized by symptoms, signs, radiological evidence, and, in some children, microbiological isolation of M. tuberculosis.7
The risk of progressing from TB infection to disease is multifactorial, but age at the time of exposure is of particular importance, with younger children and infants having an increased risk of early disease progression. This risk drops off some in childhood, but then rises again in adolescence.6 In 2019, the global case fatality rate for MTB was 14%. About 85% of people who develop TB disease can be treated successfully with a six-month drug regimen.1
Adolescence is characterized by a dramatic increase in the incidence of active MTB. An estimated 1.8 million adolescents and young adults around the world develop tuberculosis disease each year.9 Among adolescents with TB disease, 10% to 20% have pleural MTB, 10% to 20% have extrathoracic MTB, and approximately 25% have pulmonary disease.9,10
Given the existence of latent MTB infection and the nonspecific and wide range of symptomology possible in MTB infection, the diagnosis of MTB may be challenging. Only 35% of cases of MTB are diagnosed accurately, and less than 15% of pediatric cases are sputum smear positive, with only 30% to 40% of all cases confirmed by culture.11,12 Drug-resistant MTB continues to be a significant global public health threat. In 2019, close to half a million people developed rifampicin-resistant TB globally. Seventy-eight percent of these infections were MDR MTB, typically with added resistance to isoniazid (INH).1,13 Challenges in isolating MTB in children mean that MDR infections rarely are detected when initiating treatment, and only treatment failure allows the determination of drug resistance.13
Pathophysiology
MTB typically affects the lungs (pulmonary TB) but frequently also involves other extrapulmonary sites. Intrathoracic tuberculosis is defined by involvement of either the lung parenchyma (infiltrates, cavities, miliary disease), the pleural space, or intrathoracic (hilar, mediastinal) lymph nodes.14 In pulmonary MTB, the bacteria enter the body via inhalation and colonize terminal alveoli.15 The presence of MTB activates the host immune response, stimulating the migration of macrophages and lymphocytes to the infection site. These immune cells commence granuloma formation as a component of the inflammatory response. (See Figure 1.) However, it is within these granulomas that MTB can persist in a latent stage for months to decades. Changes in host immune status can cause latent infection to become active.16 The balance between bacterial pathogenicity and the strength of the host immune system determines the clinical presentation of TB disease. This balance is influenced by several factors, including the initial infectious dose of MTB, the virulence of the pathogen, and overall host health and the presence of comorbid diseases (e.g., HIV/AIDS or diabetes).11
Figure 1. Pulmonary Tuberculosis |
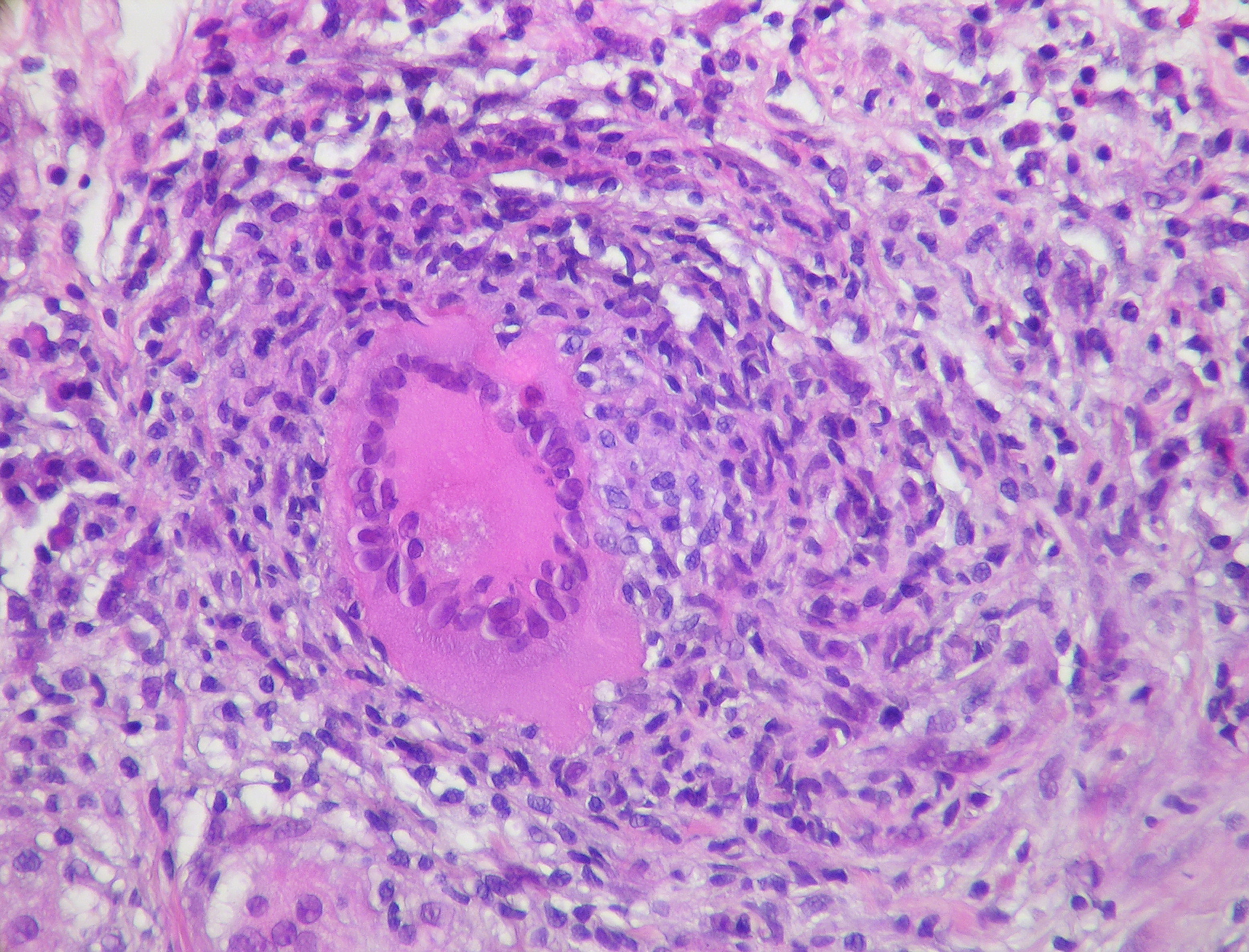 |
Source: Rosen Y. Pulmonary tuberculosis — non-necrotizing granuloma. Published Dec. 20, 2011. https://commons.wikimedia.org/wiki/File:Pulmonary_tuberculosis_-_Non-necrotizing_granuloma_(6545183785).jpg |
The incomplete maturation of the immune system in children is a contributing factor in disease manifestation and progression.14 The developing immune system of children can result in a varied response depending on the stage of disease manifestation, which consequently leads to increased risk of active TB.15 Pediatric TB more commonly presents as a disseminated disease, making symptoms more atypical and nonspecific and rendering it more difficult to detect via traditional sputum-based diagnostics.17,18
Drug-Resistant TB
Drug-resistant MTB remains a major public health concern in many countries. MDR MTB is defined by microbiological or molecular evidence of resistance to, at a minimum, INH and rifampin (RIF), the two most potent first-line anti-TB drugs. MTB that is resistant to RIF alone is called RR MTB. A patient is diagnosed with extensively drug-resistant TB (XDR TB) if the TB isolate is resistant to INH and RIF, any fluoroquinolone, and at least one of three injectable second-line drugs (amikacin, kanamycin, or capreomycin).13 Individuals may contract drug-resistant MTB via transmission from another individual (known as primary infection), or they may develop it via incorrect, inadequate, or intermittent anti-MTB drug dosing, leading to the development of resistance.13 In 2016, an estimated 490,000 people were diagnosed with MDR MTB. Forty-seven percent of these cases occurred in China, India, and the Russian Federation. Of these, an estimated 6.2% are thought to be XDR MTB.13,19
Evaluation
Given the wide range of symptoms associated with MTB infection, it is imperative that pediatricians keep MTB in the differential diagnosis for a variety of patient complaints and presentations. The evaluation of a child for MTB focuses on the history, with emphasis on the duration of symptoms. Pulmonary symptoms, such as a cough that persists longer than two weeks, should prompt consideration of MTB in regions of high prevalence. Persistent radiographic findings in symptomatic or asymptomatic children likewise should prompt consideration and diagnostic testing for MTB, particularly if the imaging findings persist after treatment with antibiotics.
Given the higher rate of disseminated MTB in children, many cases may present with nonpulmonary symptoms, including nonspecific complaints, such as weight loss, persistent fever, or malaise. Many cases of pediatric tuberculosis are discovered via the diagnosis of MTB in a close or household contact, which then prompts immuno-stimulatory testing of the children in the household and detection of immunologic exposure. Once a child has had proven immunologic exposure to MTB, examination and diagnostic testing, including sampling for culture and AFB staining, as well as radiography, should be targeted to symptoms and clinical findings.
Diagnostic Evaluation
The diagnosis of TB in pediatric populations is challenging on several fronts. No biomarkers have been clinically validated for use in the diagnosis of pediatric TB.11 Furthermore, pulmonary TB is paucibacillary in children, yielding sputum AFB positivity less than 50% of the time.
The diagnosis of extrapulmonary TB is even more challenging, with detection of the organism in as little as 30% of extrapulmonary samples, depending on the body tissue or fluid and patient condition.18,20 As such, TB commonly is diagnosed clinically in children, while a laboratory diagnosis is never achieved. This is not to suggest that laboratory testing should not be performed in children, as the low yield of detection requires testing of any suspected extrapulmonary site (e.g., cerebrospinal fluid) along with the use of both tuberculin skin testing (TST) and interferon gamma release assay (IGRA) to increase the sensitivity of the diagnosis.13
Clinicians typically rely on the combination of clinical history and findings consistent with TB in conjunction with (but not solely reliant on) laboratory diagnostic testing and a history of exposure to an individual (typically a family member) who is positive for TB to make the diagnosis. Although radiographic findings may be variable in children, a suggestive symptom pattern, such as chronic or persistent cough, in conjunction with atypical thoracic X-ray findings, also will frequently be present. Any child who is under suspicion for TB infection also should have an HIV test performed. The determination of drug resistance in pediatric populations frequently is based on clinical treatment failure or upon the diagnosis of drug resistance in a close household contact or family member.
Globally, sputum smear with AFB staining is the most common diagnostic test performed for MTB diagnosis. Sputum may be obtained via expectoration in older children, via gastric aspiration in infants, and via induction or bronchoalveolar lavage at any age.21 Induction typically involves the inhalation of bronchodilators or saline followed by suctioning. Other tissues and body fluids commonly assessed for MTB include stool, urine, ascitic fluid, pleural fluid, and cerebrospinal fluid, as well as skeletal and soft tissue samples. It is important to consider lumbar puncture in any child under consideration for MTB, given the higher frequency of disseminated and meningeal MTB in children.22
Tuberculin Skin Test
The World Health Organization (WHO) currently recommends the TST for diagnosing latent TB infection in adult and pediatric populations. The TST also will be positive in active TB, and so cannot differentiate between the two.1 The tuberculin purified protein derivative (PPD) is injected intradermally, and the diameter of the inflammatory response on the skin is measured at a usual time frame of 48 to 72 hours. A diameter of greater than 10 mm within two days is considered positive for TB exposure.17 (See Figure 2.)
Figure 2. Positive Tuberculin Skin Test |
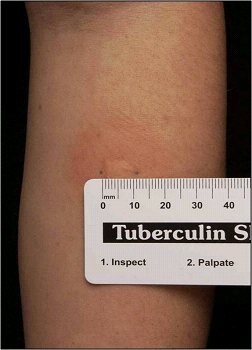 |
Source: Centers for Disease Control and Prevention. Mantoux tuberculin skin test. https://www.cdc.gov/tb/webcourses/tb101/page385.html |
Despite approval for use in children, the TST suffers from lower sensitivity in this population and poor specificity in individuals exposed to nontuberculous mycobacteria or prior bacillus Calmette-Guérin (BCG) vaccination.18 False-negative tests are relatively common and can occur in early infancy, in the setting of immunodeficiency, and after certain viral infections or vaccinations with live viruses, such as measles, mumps, rubella, oral polio, oral typhoid, varicella, or yellow fever. In the past, when dealing with cases of suspected or confirmed immunodeficiency, a positive control (usually Candida or measles protein antigens) was co-administered with the PPD to assess for anergy or lack of a generalized immune response. However, given the multiple problems with this approach, it is no longer recommended.23 Thus, while a positive test may support the diagnosis of TB, a negative test cannot be used to rule out disease and must be used in conjunction with history of exposure, symptoms, and the results of other diagnostic tests.17,18,20,24
Interferon Gamma Release Assay
The IGRA is based on the quantitative measurement, in whole blood, of the interferon gamma released upon activation of innate immune receptors when exposed to MTB antigens. Available IGRAs include QuantiFERON-TB Gold (QFT-G), QuantiFERON-TB Gold in-tube (QFT-G-IT), and T-SPOT.TB.9 The sensitivity and specificity of IGRA is superior to the TST, and IGRA can differentiate between BCG vaccination and MTB exposure, as well as other nontuberculous mycobacteria.19,21,25,26
Like with the TST, there is some decline in sensitivity with immunosuppressed patients because the mechanism of the test is dependent on immune response. A positive control test usually is administered as part of the IGRA to assess for the presence of overall immune function. IGRAs are recommended for use in children 2 years of age and older.
The use of both the TST and IGRA together increases the sensitivity for TB diagnosis.18,21,24,27-29 Both the TST and the IGRA are limited in that they are not useful in distinguishing between latent and active MTB, nor are they helpful in predicting who will progress to active MTB.20
AFB Microscopy
For decades, the traditional approach to diagnosing pulmonary MTB has relied on acid-fast staining of sputum. Acid-fast staining, also known as Ziehl-Neelsen staining, takes advantage of the higher concentration of mycolic acids in the cell walls of mycobacteria, causing them to stain red compared to the green/blue staining of non-acid-fast bacilli.30 (See Figure 3.) The sensitivity of sputum smear AFB microscopy is variable, and some studies have shown it to be quite low depending on the technique used to generate the sample (expectoration, sputum induction, or gastric aspiration) and the experience and capability of the microscopist.18,21
Figure 3. Acid-Fast Bacilli Stained in Smear |
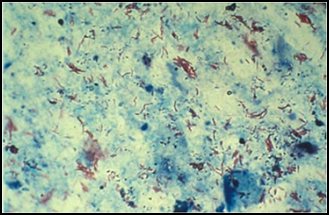 |
Source: Centers for Disease Control and Prevention. Laboratory examination: Acid-fast bacilli (AFB) smears. https://www.cdc.gov/tb/webcourses/Course/chapter4/4_diagnosis_of_tb_ |
A minimum of three respiratory samples (including gastric aspirates) spaced out in eight- to 24-hour intervals (with at least one specimen being an early morning collection) should be sent for AFB smear microscopy and culture.21,24 Pay attention to infection control during sputum production and collection, since this potentially is an aerosol-generating procedure.
In general, 5,000 to 10,000 bacilli per milliliter of specimen must be present to allow the detection of AFB in stained smears of any source. In contrast, 10 to 100 bacilli are needed to yield a positive culture.24
MTB Culture
Despite the development of newer diagnostic techniques, bacteriologic culture remains the gold standard for MTB diagnosis. However, because of the slow growth rate of MTB, culture is time-consuming, with results commonly requiring more than three to four weeks.20 The determination of culture growth-proven resistance typically requires additional weeks.
The ability to perform MTB culture and resistance testing requires sophisticated and costly laboratory resources, which are not present at scale in many of the regions where MTB is most prevalent. The paucibacillary and disseminated nature of pediatric MTB results in reduced sensitivity in children, generally only 7% to 40%.20
Drug-Susceptibility Testing
Once an MTB culture becomes positive, the isolate should be tested for the ability to grow in the presence of first-line anti-TB drugs, e.g., INH, RIF, ethambutol, and pyrazinamide. Culture- or growth-based testing for resistance is known as phenotypic drug-susceptibility testing. Growth-based or phenotypic testing can take days to weeks, given the slow rate of growth of MTB. Growth-based testing should be combined with molecular drug-susceptibility testing to inform clinical decision-making regarding antitubercular therapeutics.
Patients who experience treatment failure can have repeat culture and growth-based susceptibility testing performed to assess for resistance that might develop during treatment.8,17,18,21,24
In contrast to phenotyping, genotyping uses polymerase chain reaction (PCR) to analyze MTB deoxyribonucleic acid (DNA) to identify specific DNA sequences. These sequences can be used epidemiologically to identify chains of transmission within a community and antituberculosis drug resistance. Genotyping also can be used to determine whether a person who develops MTB during or after treatment has a reemergence of prior disease or a new infection.31
GeneXpert MTB/Resistance to RIF Nucleic Acid Amplification Assay
GeneXpert MTB/resistance to RIF nucleic acid amplification assay detects DNA sequences specific to MTB using PCR. The test also is capable of diagnosing RIF resistance. Because RIF resistance commonly co-occurs with INH resistance, GeneXpert also may offer early detection of MDR.32
GeneXpert can provide results within two hours of sample collection, compared to an average of 12 to 20 days for culture and RIF resistance identification, respectively. GeneXpert is an automated test, so no significant technical training is required.32 Studies of the GeneXpert assay on induced sputum samples have yielded a sensitivity of 59% and a specificity of 99%. The performance of a second GeneXpert test changed these to 99% and 79%, respectively.33 The performance of GeneXpert in sputum smear-negative samples is variable, with some studies demonstrating a sensitivity as low as 33% and others as high as 83.9%.33,34 The sensitivity and specificity of GeneXpert for identifying RIF resistance has been found to be 90% and 98%, respectively. 35 Data on the performance of GeneXpert on nonsputum samples is promising. A study of GeneXpert MTB/RIF performed on various tissue/body fluid samples revealed the highest sensitivity for gastric aspirate samples, at 73%, followed by sputum (65%) and stool (62%), and a specificity across all sample sources of 98%.35
According to the WHO, GeneXpert is the recommended initial diagnostic test in children suspected of having multidrug-resistant TB or HIV-associated TB.19 Centers for Disease Control and Prevention (CDC) guidelines state that GeneXpert does not replace the need for acid-fast bacilli and culture along with growth-based sensitivity testing for the diagnosis of resistance.32 Experience with GeneXpert in extrapulmonary MTB is promising, with a study from South Africa suggesting a sensitivity of 59% and a specificity of 92% in comparison to culture.36
An updated and improved version of the GeneXpert MTB/RIF assay termed Gene Xpert MTB/RIF Ultra was first studied in 2017 and in general has shown improved sensitivity, particularly in paucibacillary cases, and similar specificity compared to GeneXpert MTB/RIF across several studies.37
Lipoarabinomannan
Detection of the biomarker lipoarabinomannan (LAM) in urine is a highly promising strategy for pediatric TB diagnosis because of the nonreliance on sputum as the diagnostic sample. LAM is a component of the mycobacterial cell wall and, in conjunction with other testing modalities, may assist in the diagnosis of MTB. The lateral flow urine LAM (LF-LAM) assay (DetermineTM TB LAM Ag by Abbott) or LAM enzyme-linked immunoassay is recommended to detect active TB in severe HIV-positive patients.
The urine LAM study also may be useful in predicting outcomes in HIV-positive children. In a study in Malawi, HIV-positive children with a positive urine LAM had a 3.7-fold higher mortality than those with negative LAM testing.38
X-Ray and Computed Tomography
The anterior-posterior/lateral chest X-ray series is a vital tool in the diagnosis of pediatric MTB. MTB may take on several different patterns on chest radiography.20 The findings most commonly associated with adult pulmonary MTB, e.g., upper lobe cavitary lesions, are less common in children and typically are only seen in adolescents. Radiographic abnormalities in children may be minimal despite often prolonged symptoms, with a greater likelihood of lymphadenopathy more easily diagnosed on the lateral film.18,21
HIV disease and immunodeficiency can lead to atypical or less-pronounced chest X-ray findings. The “primary complex” is the most common X-ray finding in pulmonary MTB. The primary complex consists of a discrete parenchymal lesion, usually in the mid to lower lung fields, with an enlarged hilar lymph node.39
Computed tomography (CT) can assist in providing a means of further investigating mild or atypical chest X-ray findings or identifying lymphadenopathy not notable on physical examination. Brain CT or magnetic resonance imaging may be useful in identifying leptomeningeal enhancement, vasculitis, or hydrocephalus, which are consistent with MTB meningitis.
Ultrasound
Ultrasound may be used to investigate the clinical findings or symptoms of extrapulmonary MTB. In populations with a significant prevalence of MTB, the detection of ascitic, pleural or pericardial fluid may carry a high pretest probability of extrapulmonary MTB. The focused assessment with sonography in HIV (FASH) exam is a rapid ultrasound assessment of the pleural, pericardial, and peritoneal cavities, as well as the lymph nodes, liver, and spleen (for splenic MTB microabscesses).40-42 (See Figure 4.) Ultrasound may be more available than molecular- or culture-based laboratory diagnostics, radiography, or CT in many resource-limited settings where MTB is highly prevalent. FASH has been useful in augmenting point-of-care clinical decision-making in patients with HIV and symptoms or clinical findings concerning for MTB in multiple studies in sub-Saharan Africa. Further research is needed to better describe the sensitivity and specificity of the FASH assessment, as well as individual FASH ultrasound findings, and to establish the role of FASH in justifying anti-MTB treatment in patients lacking a microscopic, molecular, or culture diagnosis of MTB.40-42
Figure 4. Pericardial Effusion |
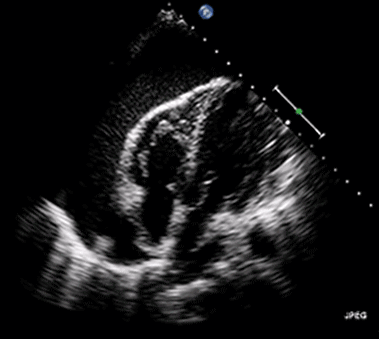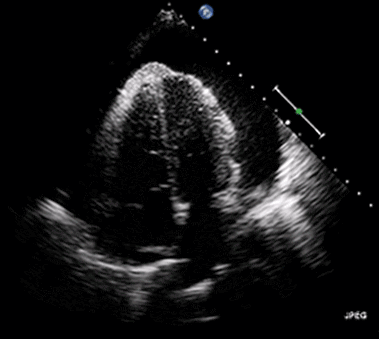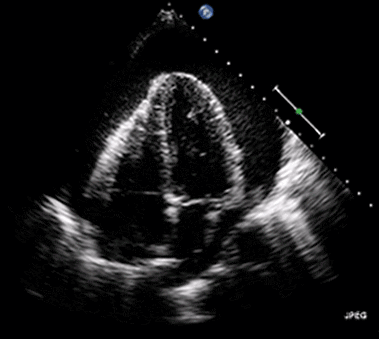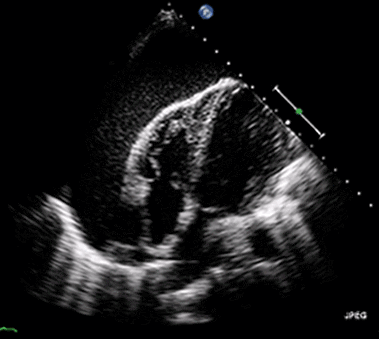 |
Source: Jer5150. Pericardial effusion with tamponade (cropped). Published May 26, 2012. https://commons.wikimedia.org/w/index.php?curid=52252592 |
Treatment
The treatment of MTB is focused on both curing the individual patient and minimizing the transmission of MTB to others. Treatment protocols for MTB in children are similar to those in adults. Treatment regiments can be long, and adherence can be challenging, particularly in children. Directly observed therapy (DOT), wherein a patient is directly observed taking anti-MTB medications at a clinic or by a visiting healthcare worker, is an important tool for assuring adherence to anti-MTB drug regimens. It is recommended by the WHO and mandated by some jurisdictions, particularly in cases where resistance has been confirmed or is suspected.43 Because DOT can be resource-consuming, expensive, inconvenient for patients, and not available in all regions, multiple studies have examined the utility of self-administered therapy (SAT) along with electronic reminders, such as text messages and video or phone check-ins with health professionals at specified intervals. Along with DOT or assisted SAT, appropriate case management is crucial in identifying household contacts and others possibly exposed to MTB by an identified patient.43
Latent MTB Infection
Patients who test positive via TST or IGRA for prior exposure to MTB initially should be evaluated for the presence of active MTB. In the absence of evidence for active MTB, the CDC and WHO have established recommended treatment courses for latent MTB infection. Children are more likely to progress to active MTB than adults, so prompt commencement of therapy is critical. In all cases, an infectious diseases specialist or a pediatric tuberculosis expert should be consulted.44,45 The CDC recommends children older than 2 years of age be treated with once-weekly INH-RIF for a duration of 12 weeks, with DOT when possible. Alternative treatments for latent TB infection in children include four months of daily RIF or nine months of daily INH. (See Table 1.) Clinicians should try to simplify and shorten MTB treatment as adherence improves.44,45
Table 1. Treatment Regimens for Latent Mycobacterium tuberculosis Infection in Children | ||
Agent |
Duration |
Frequency |
Isoniazid and rifapentine |
Three months |
Once per week |
Rifampin |
Four months |
Daily |
Isoniazid and rifampin |
Three months |
Daily |
Isoniazid (6H/9H) |
Six to nine months |
Daily/twice weekly |
Adapted from Centers for Disease Control and Prevention. Latent tuberculosis infection treatment regimens. https://www.cdc.gov/tb/topic/treatment/pdf/LTBITreatmentRegimens.pdf | ||
MTB Disease
Attention to appropriate dosing for pediatric cases is critical to assure that appropriate serum levels of antituberculosis medications are attained.44,45 The currently recommended treatment for cases of drug-susceptible TB disease is a six-month regimen of four first-line drugs: INH, RIF, ethambutol, and pyrazinamide. (See Table 2.) The WHO emphasizes using DOT to assure adherence to these long and challenging regimens.43
Table 2. Medication Groups for Tuberculosis Patients | ||
Drug Group |
Class of Drugs |
Drug |
Group A |
Fluoroquinolones |
Levofloxacin |
Moxifloxacin | ||
Gatifloxacin | ||
Group B |
Second-line injectables |
Kanamycin |
Amikacin | ||
Capreomycin | ||
Group C |
Other second-line agents |
Ethionamide/prothionamide |
Cycloserine/rerizidone | ||
Linezolid | ||
Clofazimine | ||
Groups D1, D2, and D3 |
Add-on agents |
D1: Pyrazinamide, ethambutol, high-dose isoniazid |
D2: Bedaquiline, delamanid | ||
D3: P-aminosalicyklic acid, imipenem-cilastatin, meropenem, amoxicillin-clavulanate, thioacetazone | ||
Multidrug-resistant regimens should consist of at least five drugs with likely efficacy, including four second-line drugs and pyrazinamide. For the four second-line drugs, one should be from Group A, one from Group B, and at least two from Group C. If four agents cannot be included from groups A to C, groups D2 and D3 should be used to complete the regimen. Agents from Group D1 can be included if there is reason to believe they would be beneficial (after resistance testing). Reprinted with permission from World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update (October 2016 revision). https://apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf | ||
Per CDC guidelines, regimens for treating MTB disease begin with a two-month intensive phase where all four first-line antituberculosis drugs are used, followed by a continuation phase lasting either four or seven months in which the treatment regimen is simplified to two drugs. The seven-month continuation phase should be used in patients with cavitary disease on chest radiography or who have a positive culture at two months (after completion of the intensive phase). While multiple dosing regimens exist for both the intensive and continuation phases, in general, the regimen with the shortest duration and fewest overall doses should be selected unless resistance patterns or the specifics of the clinical case dictate a particular regimen.46 The WHO last updated their guidelines for the treatment of drug-susceptible MTB in 2017, and another update is underway.47
Clinicians should monitor for adverse effects and signs of toxicity from the terapy. Many patients on anti-MTB therapy experience a range of associated symptoms, ranging from mildly inconvenient to severely disabling. These symptoms can present a challenge to adherence. Common symptoms associated with anti-MTB regimens include nausea, vomiting, abdominal pain, headaches, fatigue, and anorexia. Many anti-MTB medications adversely interact with other medications, including medications used in the treatment of HIV.48
Several frontline anti-MTB drugs have been associated with transient and mild liver injury, which generally does not require cessation of therapy but may require ongoing laboratory monitoring. INH therapy in the setting of alcohol use may induce nausea, vomiting, and hepatotoxicity. The principal toxicity associated with INH therapy is vitamin B6 deficiency and resultant peripheral neuropathy.
In severe cases, seizures can occur. They typically are seen in cases of severe INH overdose and frequently are recalcitrant to antiepileptic therapy. A history of treatment with INH should be elicited from all patients presenting in status epilepticus without a prior history of seizure. Prompt treatment with vitamin B6 (pyridoxine) along with appropriate supportive care is critical. Peripheral neuropathy is seen more commonly in adults. Routine supplementation with pyridoxine in children receiving INH is not recommended, but it should be considered in children at risk for malnutrition or those with HIV, diabetes, or renal failure. The most serious and commonly recognized side effect of ethambutol is optic neuritis and resultant visual disturbances, but this is quite rare and reversible with cessation of therapy. Many frontline anti-MTB medicines have been associated with severe reactions, including Stevens-Johnson syndrome and toxic epidermal necrolysis, although these reactions typically are rare.49
Drug Resistance
Because many cases of pediatric MTB never achieve a laboratory diagnosis, individualized treatment decisions should be based on prior therapy exposure, initial clinical response to therapy, and the resistance profiles of the isolates of close contacts or family members. Repeat cultures of the relevant biologic samples should be performed at intervals, and positivity past three months of therapy or culture positivity after initial negative cultures should prompt drug resistance testing.50,51
Although the WHO and CDC have released guidelines for treating drug-resistant MTB, data regarding the efficacy of many regimens are lacking, and new studies may support changes in specific resistance circumstances and treatment regimens for MDR MTB. Medication studies in children lack significant published experience, causing uncertainty regarding effectiveness, dosing, and toxicity, as well as an overall lack of pediatric formulations.
In general, treatment regimens for MDR and XDR MTB are individualized, and consultation with an expert in pediatric tuberculosis is recommended.50,52 The treatment of drug-resistant MTB requires a course of second-line drugs lasting from nine to 20 months, supported by counselling and monitoring for adverse events. Second-line drugs have less favorable side-effect profiles and are more difficult to take, particularly for the prolonged courses necessary, and, thus, toxicity occurs more often. The following are considerations taken from CDC and WHO guidelines regarding the treatment of drug-resistant MTB in children.50,52
- Avoid regimens using injectable agents whenever possible.
- In RIF-susceptible/INH-resistant MTB, treatment with RIF, ethambutol, and pyrazinamide with levofloxacin is recommended for a duration of six months. Long-term use of fluoroquinolones in children has not been approved by the U.S. Food and Drug Administration. However, most experts agree that fluoroquinolones should be considered for children with drug-resistant MTB.
- A shorter all-oral bedaquiline-containing regimen of nine to 12 months’ duration is recommended in eligible patients with MDR/RR-MTB who have not been exposed to treatment with second-line MTB medicines used in this regimen for more than one month and in whom resistance to fluoroquinolones has been excluded.
- Aside from the previously mentioned specific shorter treatment courses, the treatment of drug-resistant MTB requires a course of treatment of at least nine months and up to 20 months. However, in cases of persistent symptoms or culture positivity treatment, treatment may last even longer.
- In general, resistance to one medication within a class renders the rest of the drugs of that class ineffective. However, testing should be pursued, since this may not always be the case.
- Response to treatment must be obtained by repeated sputum cultures (monthly, if possible).
- In MDR/RR MTB patients on longer regimens, all three Group A agents and at least one Group B agent should be included to ensure that treatment starts with at least four TB agents likely to be effective and that at least three agents are included for the rest of treatment if bedaquiline is stopped. If only one or two Group A agents are used, both Group B agents should be included. If the regimen cannot be composed with agents from Groups A and B alone, Group C agents are added to complete it.
- A treatment regimen lasting six to nine months composed of bedaquiline, pretomanid, and linezolid may be used under research conditions in MDR MTB that is resistant to fluoroquinolones in patients who have had less than two weeks total prior exposure to bedaquiline and linezolid.
- Treatment for XDR MTB is highly individualized, and using nonstandard antimicrobials may be attempted. Expert consultation is necessary.
HIV Coinfection
Globally, TB is one of the most common causes of death among people living with HIV. Patients with latent MTB and HIV are more likely than those without HIV to develop active disease. Antiretroviral (ARV) therapy is recommended for all patients with HIV and tuberculosis, particularly those with drug resistance, requiring second-line antituberculosis drugs irrespective of CD4 cell count as early as possible (within the first eight weeks) following initiation of antituberculosis treatment.52 In patients with untreated HIV and MTB coinfection who experience severe immunodeficiency, ARVs are critical to restoring immune function.
However, because ARVs interrupt viral reproduction and CD4 cell counts rebound, many patients experience inflammation related to the immune attack of previously unaddressed infections. This inflammation is termed the immune reconstitution inflammatory syndrome (IRIS) and usually is treated with symptomatic therapy. TB-related IRIS is relatively common in HIV patients receiving ARVs, with incidences of 10% to 30%. Commencement of anti-MTB therapy prior to the ARVs may blunt the severity of IRIS associated with MTB. ARVs should not be ceased in response to the symptoms of IRS if possible.51,53
Vaccination
The BCG vaccine is the only one licensed for prevention of TB.1 BCG is a live, attenuated vaccine that provides partial protection against severe manifestations of TB disease during the first years of life.4 There is evidence of a 90% protective effect of initial BCG vaccination against disseminated TB in infants, and moderate protection (60% to 75%) against pulmonary disease in children and young adolescents.11 BCG is not routinely administered in the United States, but circumstances do exist in which BCG administration might be indicated:
- a child who tests negative for MTB via TST and IGRA testing but is continuously exposed to incompletely treated MTB-positive adults and is unable to take preventive MTB medicines;
- a child who is continuously exposed to adults with incompletely treated MTB with INH and/or RIF resistance;
- healthcare workers with substantial and chronic exposure to patients with TB, particularly in settings where resistance is common.54
Conclusion
MTB represents a significant challenge to the health of the world’s children. Significant barriers exist at multiple levels of the care system for MTB. These include the low sensitivity of sputum smear microscopy and the lack of viable standard diagnostic testing in children as well as difficulties and the high resource and time burdens associated with culture and phenotypic drug-resistance testing. As a result, there likely is significant global underreporting of pediatric MTB.
Courses of treatment, even for latent MTB and nonresistant infections, are long, and regimen adherence may suffer because of adverse effects and drug toxicity, which are common. Lastly, the continued development of drug resistance represents a substantial further challenge, with longer, more toxic regimens being necessary, and the threat of untreatable MTB infection being a real concern.
In response to these challenges, global priorities for further research and focus include the development of improved diagnostics capable of rapid, sensitive, and specific diagnosis of MTB and drug resistance. Further investment in the development of anti-MTB medications is critical to reduce the duration, toxicity, and complexity of current treatment regimens and to improve adherence and cure rates.
REFERENCES
- World Health Organization. Global tuberculosis report 2020. Published Oct. 14, 2020. https://www.who.int/publications/i/item/9789240013131
- Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: A mathematical modelling study. Lancet Glob Health 2014;2:e453-e459.
- Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: A mathematical modelling study. Lancet Infect Dis 2016;16:1193-1201.
- Centers for Disease Control and Prevention. CDC fact sheet: TB in the United States. Published September 2018. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/tb-in-the-us-a-snapshot.pdf
- World Health Organization, UNICEF. Ending tuberculosis in children fact sheet. https://www.who.int/tb/challenges/childhood_tb_informationsheet.pdf?ua=1
- Marais BJ, Gie RP, Schaaf HS, et al. The clinical epidemiology of childhood pulmonary tuberculosis: A critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:278-285.
- Whittaker E, López-Varela E, Broderick C, Seddon JA. Examining the complex relationship between tuberculosis and other infectious diseases in children. Front Pediatr 2019;7:233.
- Gilpin C, Korobitsyn A, Migliori GB, et al. The World Health Organization standards for tuberculosis care and management. Eur Respir J 2018;51:1800098.
- Snow KJ, Sismanidis C, Denholm J, et al. The incidence of tuberculosis among adolescents and young adults: A global estimate. Eur Respir J 2018;51:1702352.
- Chiang SS, Dolynska M, Rybak NR, et al. Clinical manifestations and epidemiology of adolescent tuberculosis in Ukraine. ERJ Open Res 2020;6:00308-2020.
- Jakhar S, Bitzer AA, Stromberg LR, Mukundan H. Pediatric tuberculosis: The impact of “omics” on diagnostics development. Int J Mol Sci 2020;21:6979.
- Nhu NTQ, Ha DTM, Anh ND, et al. Evaluation of Xpert MTB/RIF and MODS assay for the diagnosis of pediatric tuberculosis. BMC Infectious Dis 2013;13:31.
- Centers for Disease Control and Prevention. Drug-resistant TB. Updated Jan 17, 2017. https://www.cdc.gov/TB/Topic/DRTB/
- Snow KJ, Cruz AT, Seddon JA, et al. Adolescent tuberculosis. Lancet Child Adolesc Health 2020;4:68-79.
- Starke JR. Transmission of Mycobacteriumtuberculosis to and from children and adolescents. Semin Pediatr Infect Dis 2001;12:115-123.
- Flynn JL, Chan J. Tuberculosis: Latency and reactivation. Infect Immun 2001;69:4195-4201.
- Chiappini E, Vecchio AL, Garazzino S, et al. Recommendations for the diagnosis of pediatric tuberculosis. Eur J Clin Microbiol Infect Dis 2016;35:1-18.
- Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. American Academy of Pediatrics; 2018.
- World Health Organization. Multidrug-resistant tuberculosis (MDR-TB). Published Jan. 16, 2018. https://www.who.int/news-room/q-a-detail/tuberculosis-multidrug-resistant-tuberculosis-(mdr-tb)
- Thomas TA. Tuberculosis in children. Pediatr Clin North Am 2017;64:893-909.
- Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017;64:e1-e33.
- Aulakh R, Chopra S. Pediatric tubercular meningitis: A review. J Pediatr Neurosci 2018;13:373-382.
- Centers for Disease Control and Prevention. Anergy skin testing and preventive therapy for HIV-infected persons: Revised recommendations. MMWR Recomm Rep 1997;46(RR-15):1-10.
- Centers for Disease Control and Prevention. Core Curriculum on Tuberculosis. Chapter 3: Testing for tuberculosis infection and disease. https://www.cdc.gov/tb/education/corecurr/pdf/chapter3.pdf.
- Starke JR. Interferon-gamma release assays for the diagnosis of tuberculosis infection in children. J Pediatr 2012;161:581-582.
- Chiappini E, Bonsignori F, Mazzantini R, et al. Interferon-gamma release assay sensitivity in children younger than 5 years is insufficient to replace the use of tuberculin skin test in western countries. Pediatr Infect Dis J 2014;33:1291-1293.
- Wang MS, Wang JL, Wang XF. The performance of interferon-gamma release assay in nontuberculous mycobacterial diseases: A retrospective study in China. BMC Pulm Med 2016;16:163.
- Ahmed A, Fend PI, Gaensbauer JT, et al; the Tuberculosis Epidemiologic Studies Consortium. Interferon-gamma release assays in children <15 years of age. Pediatrics 2020;145:e20191930.
- Velasco-Arnaiz E, Soriano-Arandes A, Latorre I, et al. Performance of tuberculin skin tests and interferon-gamma release assays in children younger than 5 years. Pediatr Infect Dis J 2018;37:1235-1241.
- Science Direct. Acid-fast staining. https://www.sciencedirect.com/topics/medicine-and-dentistry/acid-fast
- Weinrick B. Genotyping of Mycobacterium tuberculosis rifampin resistance-associated mutations by use of data from Xpert MTB/RIF Ultra enables large-scale tuberculosis molecular epidemiology studies. J Clin Microbiol 2019;58:e01504-e01519.
- Centers for Disease Control and Prevention. A new tool to diagnose tuberculosis: The Xpert MTB/RIF assay. Updated May 4, 2016. https://www.cdc.gov/tb/publications/factsheets/testing/xpert_mtb-rif.htm
- Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: A descriptive study. Lancet Infect Dis 2011;11:819-824.
- Reechaipichitkul W, Phetsuriyawong A, Chaimanee P, Ananta P. Diagnostic test of sputum GeneXpert MTB/RIF for smear negative pulmonary tuberculosis. Southeast Asian J Trop Med Public Health 2016;47:457-466.
- Shah W. To determine diagnostic accuracy of GeneXpert and sputum Ziehl-Neelsen staining taking sputum culture as gold standard. Eur Respir J 2016; doi: 10.1183/13993003.congress-2016.PA2779.
- Scott LE, Beylis N, Nicol M, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: Establishing a laboratory testing algorithm for South Africa. J Clin Microbiol 2014;52:1818-1823.
- Kay AW, González Fernández L, Takwoingi Y, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev 2020;8:CD013359.
- LaCourse SM, Chester FM, Matoga M, et al. Implementation and operational research: Implementation of routine counselor-initiated opt-out HIV testing on the adult medical ward at Kamuzu Central Hospital, Lilongwe, Malawi. J Acquir Immune Defic Syndr 2015;69:e31-e35.
- Veedu PT, Bhalla AS, Vishnubhatla S, et al. Pediatric vs. adult pulmonary tuberculosis: A retrospective computed tomography study. World J Clin Pediatr 2013;2:70-76.
- Bobbio F, Di Gennaro F, Marotta C, et al. Focused ultrasound to diagnose HIV-associated tuberculosis (FASH) in the extremely resource-limited setting of South Sudan: A cross-sectional study. BMJ Open 2019;9:e027179.
- Heller T, Wallrauch C, Goblirsch S, Brunetti E. Focused assessment with sonography for HIV-associated tuberculosis (FASH): A short protocol and a pictorial review. Crit Ultrasound J 2012;4:21.
- Kahn D, Pool K-L, Phiri L, et al. Diagnostic utility and impact on clinical decision making of focused assessment with sonography for HIV-associated tuberculosis in Malawi: A prospective cohort study. Glob Health Sci Pract 2020;8:28-37.
- Frieden TR, Sbarbaro JA. Promoting adherence to treatment for tuberculosis: The importance of direct observation. Bull World Health Organ 2007;85:407-409.
- Centers for Disease Control and Prevention. Treatment regimens for latent TB infection. Updated Feb. 13, 2020. https://www.cdc.gov/tb/topic/treatment/ltbi.htm
- World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. https://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf
- Centers for Disease Control and Prevention. Treatment for TB disease. Updated April 5, 2016. https://www.cdc.gov/tb/topic/treatment/tbdisease.htm
- World Health Organization. Update of the WHO guidance on the treatment of drug susceptible tuberculosis. Published April 16, 2021. https://www.who.int/news/item/16-04-2021-update-of-the-who-guidance-on-the-treatment-of-drug-susceptible-tuberculosis
- Lei S, Gu R, Ma X. Clinical perspectives of isoniazid-induced liver injury. Liver Res 2021;5:45-52.
- Shin H-J, Chang J-S, Kim M-S, et al. Hypersensitivity reactions to multiple anti-tuberculosis drugs. PLoS One 2021;16:e0246291.
- Nahid P, Mase SR, Migliori GB, et al. Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med 2019;200:e93-e142.
- Murthy AR, Marulappa R, Hegde U, et al. Treatment guidelines and prognosis of immune reconstitution inflammatory syndrome patients: A review. J Int Oral Health 2015;7:92-95.
- [No authors listed]. WHO Consolidated Guidelines on Tuberculosis. Module 4: Treatment — Drug-Resistant Tuberculosis Treatment. Geneva; 2020.
- Breton G, Bourgarit A, Pavy S, et al; Paradox-TB Study Group. Treatment for tuberculosis-associated immune reconstitution inflammatory syndrome in 34 HIV-infected patients. Int J Tuberc Lung Dis 2012;16:1365-1370.
- Centers for Disease Control and Prevention. Tuberculosis vaccines. Updated March 15, 2016. https://www.cdc.gov/tb/topic/basics/vaccines.htm
Mycobacterium tuberculosis (MTB) is a significant challenge to children's health. Barriers exist at multiple levels of the care system for MTB. Early recognition and involvement of MTB specialists is critical to facilitate the best outcome for pediatric patients. The authors provide a thorough review of the current standards for care of these challenging patients.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
