Pediatric Facial Lacerations in the Emergency Department
October 1, 2021
Related Articles
AUTHORS
Travis R. Wieland, MD, Clinical Instructor, Global Health Fellow, Department of Emergency Medicine, University of Wisconsin, Madison
Daniel Migliaccio, MD, Clinical Assistant Professor, Ultrasound Fellowship Director, Department of Emergency Medicine, University of North Carolina, Chapel Hill
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Clinical Assistant Professor of Emergency Medicine, Mt. Sinai Medical School and Bon Secours Hospital, NY
EXECUTIVE SUMMARY
- Multiple factors determine scar structure, including both static and dynamic skin tension, laceration mechanism, tissue trauma, infection, location, repair technique, and patient factors, such as medications and diseases. It should be noted that some of these factors are within the physician’s control while others are not, and expectations should be clearly delineated for the patient’s parents. The patient and parents also should understand that all lacerations will leave a scar.
- Because of the dynamic process of remodeling, the physician also must explain to the patient and parents that the final appearance of the scar will not be known for at least six to 12 months from the time of injury, since there is a poor correlation between scarring at the time of suture removal and at subsequent appraisals several months later.
- Although some topical anesthetic agents, such as eutectic mixture of local anesthetics or liposomal lidocaine, are indicated only for use on intact skin, other agents, such as lidocaine 4%, epinephrine 0.1%, tetracaine 0.5%, can be used on open wounds. These agents should not be used on mucosa or in patients younger than 3 months of age.
- Local infiltration of anesthetic can be used. Lidocaine is used most commonly, with 1% or 2% concentrations and with or without epinephrine. Lidocaine solution itself is somewhat acidic and can cause pain with injection. A 1:9 ratio solution can be made of sodium bicarbonate to lidocaine, respectively, which can help to buffer this acidity. Ensuring that the lidocaine is at a warmer temperature by placing it in a blanket warmer or submerging the bottle into warm water also will reduce pain with infiltration.
- Anxiolysis often is used in combination with topical anesthetics for pediatric laceration repair and can be very useful as an adjuvant to local anesthetics or nerve blocks. Benzodiazepines are the most common, but nitrous oxide is another option, if available. Intranasal midazolam (trade name Versed) with an anxiolytic dose of 0.3 to 0.5 mg/kg is an excellent choice, since the intranasal route of administration avoids the use of needles, and midazolam is a short-acting benzodiazepine that begins to take effect around 10 minutes after administration.
- Level B recommendations exist to not defer sedation based on last oral intake and to use capnography for ventilation and apnea monitoring. Both ketamine and propofol have Level A recommendations for use in procedural sedation in the emergency department. Postsedation, patients should return to their presedation Aldrete scores.
- The wound should be cleansed thoroughly through irrigation using either tap water or saline, since wound infection rates with either are equivalent. Additionally, tap water is far more cost effective than sterile saline. High-pressure irrigation with a syringe (35 cc syringe with a 19-gauge catheter in the study) has been shown to reduce wound bacterial count and subsequent infection rates.
- A recent review of antiseptics recommends polyhexanide for acute, contaminated wounds and povidone-iodine for bite, stab, or puncture wounds with less contamination.
- A meta-analysis did not demonstrate statistically significant differences in cosmesis, infection rate, dehiscence, or scar hypertrophy between absorbable and nonabsorbable suture materials.
- Criteria for tissue adhesive use include low tension, well-approximated, and hemostatic lacerations that are located in areas where runoff can be contained.
- Lacerations surrounding the eye that require consultation include violation of the tear duct apparatus, medial palpebral ligament, levator palpebrae, or tarsal plate.
- Ear injuries with auricular avulsion should be deferred to consultant management, along with lacerations with exposed cartilage that the emergency physician does not feel confident in repairing.
Pediatric facial lacerations are common, and every emergency medicine physician needs to be familiar with the approach to pediatric facial and scalp lacerations, child-friendly methods for repair, and different options for analgesia.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
A sound understanding of the basic principles surrounding pediatric facial and scalp laceration repair is essential for any emergency physician. Lacerations in general consistently have been one of the more common reasons for emergency department (ED) visits, particularly in pediatric patients.1 Facial and scalp lacerations make up approximately 60% of pediatric lacerations and present unique challenges, given their cosmetic importance, as well as child-specific considerations, including the need to simultaneously manage the patient and address the parents’ concerns.2 Additionally, facial injuries comprise around 25% of injuries recorded in the National Trauma Data Bank.3 Both the location and mechanism of injury will vary according to the age of the child.4 A solid foundation in each of these elements will equip the emergency physician to manage pediatric facial and scalp lacerations competently and efficiently.
Initial Evaluation and Special Considerations
There are several special considerations to take into account when evaluating a child with a facial or scalp laceration. Some of these will apply to the evaluation of any pediatric patient, while others are more specific to the types of injuries under discussion. Given the traumatic mechanism, initial evaluation particularly should focus on airway, breathing, and circulation (ABCs), and any immediate interventions to stabilize the patient should be performed.
As with the evaluation of any chief complaint, obtain a complete history from the parents and the patient, if old enough. Include timing, mechanism, loss of consciousness (particularly with head lacerations), vaccination status, medical and surgical history, allergies, and any current medications. It is important to know if the patient is taking steroids or has diabetes, for example, to determine whether delayed wound healing is a concern or if the patient is at increased risk of wound infection. If the patient has other injuries that require more immediate attention or meets criteria for imaging by the Pediatric Emergency Care Applied Research Network (PECARN) Pediatric Head Trauma Algorithm or other similar tool, then these should receive first priority before the laceration repair is performed. Information outlining the PECARN criteria is readily available from many online and print sources and will not be covered in detail here, but elements of significance in moving through the algorithm include the age of the patient (younger than 2 years or older than/equal to 2 years), any mental status changes, loss of consciousness, vomiting, the presence of severe headache, and the severity of the mechanism of trauma.
Ascertaining vaccination status is important in any pediatric patient encounter, but it is particularly important to establish for lacerations because of the risk of tetanus. Wounds that appeared to be minor on initial presentation to the ED have been responsible for more than 30% of documented tetanus cases.5 According to the Centers for Disease Control and Prevention (CDC), the standard schedule for childhood vaccination is diphtheria, tetanus, and pertussis (DTaP) doses — or diphtheria and tetanus doses in pediatric patients who cannot receive vaccines that contain acellular pertussis — at ages 2 months, 4 months, 6 months, 15-18 months, and 4-6 years of age for a total of five doses.6,7 Meanwhile, Tdap (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis) is subsequently given around 12 years of age.7 The most up-to-date tetanus vaccination recommendations are available from the CDC, and physicians should ensure their understanding of vaccination best practices is current.
It also is particularly important in pediatric patients to screen for nonaccidental trauma as the etiology of the child’s injury — ensure that the history provided and the child’s developmental capabilities match with the injury.5 If a history is provided that a head laceration was sustained after a 5-month-old fell while running, for example, the physician should be highly suspicious for nonaccidental trauma because of the discrepancy between a report of a running fall and the expected developmental stage of a 5-month-old. Examine the patient for other injuries that could point to abuse, such as cigarette burns or bruises at various stages of healing. If the index of suspicion is high for nonaccidental trauma, involve social work and child protective services for further investigation into the matter. Recall that healthcare providers are mandated reporters, and a failure to alert the appropriate authorities could have professional repercussions.
Once the security of the ABCs has been established, a history has been obtained, and the patient has been evaluated for other injuries, the physical examination should focus on the evaluation of the laceration itself. If there is significant bleeding, a dressing should be provided so the child or parent can apply firm pressure to the area and better hemostatic control can be obtained prior to a more thorough assessment. Careful examination must be emphasized, since children have particularly strong and rapid vasoconstriction, even of arterial injuries.8 Many commercial types of hemostatic dressings are available, but none have been shown to be superior to each other.9 It should be noted that these dressings may not be as effective in coagulopathic patients.10 If brisk bleeding persists despite sustained pressure with a dressing, a figure-of-eight stitch can be placed at the affected vessel. Weight-based dosing of analgesics, such as acetaminophen, should be provided to the patient. Younger children in particular should be held by their parents as much as possible during the physical examination to reduce their anxiety and fear.
If the parent does not appear to be coping with the situation at any step during the process, even with reassurance from the physician, more effective and efficient care may be provided by having the parent wait outside the room during the repair itself. The acquisition of a second patient if, for example, a parent has a vasovagal episode while seeing the child’s laceration repaired, is to be avoided assiduously. Another parental concern may pertain to having a plastic surgeon perform the repair instead of the emergency physician. It is important to be mindful that local requirements and regulations about patient/parental choice will vary, as will the availability of a plastic surgeon to consult on a patient in the ED. Physician should be fully aware of the practice patterns and policies in their practice settings to best inform parents of their specialist options, if desired.
Evaluating the wound should include the mechanism of injury, its location, length, depth, shape (linear, lunate, irregular, etc.), and level of contamination, as well as an assessment of neurovascular and motor function, if applicable. Each of these elements should be noted, since they will guide management decisions. For wounds on the scalp, the physician should evaluate carefully for violations of the galea aponeurotica, since galeal involvement necessitates further repair considerations, given the risk of subgaleal hematoma.11
It also is worth mentioning that there are long-held beliefs in a laceration repair “golden window,” after which time a laceration should not be repaired out of concern for increased infection risk. Although recommended time ranges for this golden period were between three and 24 hours, depending on the source, a reappraisal of studies on delayed closure and infection risk did not demonstrate any statistically significant increase in infection risk for delayed closure of simple lacerations. Therefore, this should not be used as a contraindication to repair of the laceration, assuming the physician believes that the laceration warrants it.12
One of the first questions parents ask (even before the physician evaluates the laceration in some cases) is about scarring. Understandably, many parents are concerned about scarring, and familiarity with the variables that can affect scar visibility are essential to address the inevitable questions that will arise. These cosmetic concerns usually are minimal for scalp lacerations, but they can cause a great deal of parental anxiety for facial lacerations. Multiple factors determine scar structure, including both static and dynamic skin tension, laceration mechanism and tissue trauma, infection, location, repair technique, and patient factors, such as medications and diseases. It should be noted that some of these factors are within the physician’s control while others are not, and expectations should be clearly delineated for the patient’s parents. The patient and parents also should understand that all lacerations will leave a scar.
Before further discussion of the factors determining scar structure, it will be useful to have a brief discussion of normal wound healing by primary intention. The phases of normal wound healing are hemostasis, inflammation, angiogenesis, granulation tissue formation, and connective tissue remodeling.13 Immediately following the initial tissue insult, activation of the coagulation cascade leads to clot formation at the wound site, leading to hemostasis and providing the initial architecture for neutrophils and other cells that will migrate to the area based on signaling from cytokines, chemokines, and growth factors.
During the inflammatory phase, the site is characterized by the hallmark characteristics of inflammation: warmth (calor), redness (rubor), swelling (tumor), and pain (dolor) as neutrophils and other inflammatory mediators migrate to the area. Following this initial inflammation, angiogenesis, or the formation of new blood vessels, starts to occur. Vascular endothelial growth factor (VEGF) causes increased vascular permeability with subsequent endothelial cell migration, periendothelial cell recruitment, and proliferation suppression via several mechanisms, including growth factors (VEGF-A, fibroblast growth factors, angiopoietins, etc.), notch signaling (ensuring appropriate new vessel spacing for tissue perfusion), and extracellular matrix proteins and enzymes, such as metalloproteinases.
Granulation tissue formation occurs by fibroblast migration, proliferation, and extracellular matrix protein deposition and is heavily influenced by the presence of transforming growth factor-beta (TGF-beta). Collagen synthesis by fibroblasts begins around three to five days after the initial injury and continues for many weeks thereafter. The granulation tissue gradually transforms into scar tissue consisting of inactive fibroblasts, collagen, and other matrix tissue components with contraction secondary to the presence of actin filaments in certain fibroblasts (dubbed myofibroblasts) within the matrix.
The remodeling phase comprises a balance between extracellular matrix-digesting metalloproteinases with fibrogenics, such as TGF-beta, and the activity of tissue inhibitors of metalloproteinases. This tissue strength is only at about 10% of the prewound skin at one week post injury but gradually increases to 70% to 80% over subsequent months.13 Because of this dynamic process of remodeling, the physician must also explain to the patient and parents that the final appearance of the scar will not be known for at least six to 12 months from the time of injury, since there is a poor correlation between scarring at the time of suture removal and at subsequent appraisals several months later.14
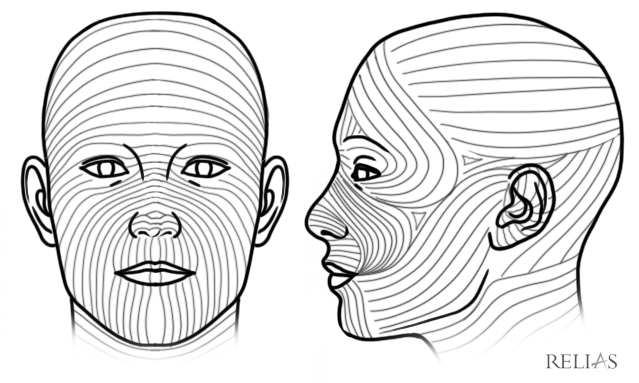
If the traumatic mechanism involves a crush injury, the tissue surrounding the wound is more likely to be devitalized and, thus, to have a less favorable cosmetic result.15 Skin tension’s role in scarification will depend on the width, location, and orientation of the laceration.5 Both static and dynamic tension play a role, and these forces are delineated by Langer’s lines and Kraissl’s lines, respectively, which map both types of tension. If a laceration is parallel to these lines, the scar is likely to be narrower and less visible than with lacerations that are perpendicular to the lines.16 (See Figure 1.) Retraction of the laceration in margins ≥ 5 mm is likely to leave a wider and more visible scar because of higher skin tension.16 As a corollary of this, the physician should not aggressively debride too much tissue, since this can increase the distance between the wound margins and, thus, will increase the static tension under which the newly approximated tissue will be held. Although the physician cannot control the width of the wound, the apposition should be as complete as possible, since incomplete apposition has been associated with worse cosmetic outcomes.17
Figure 1. Langer's Lines |
Patient factors, such as current medications and comorbidities, also will affect wound healing. Patients who take steroids, have diabetes, or are malnourished likely will have suboptimal wound healing. Steroids inhibit production of TGF-beta, and malnutrition can affect healing via such mechanisms as impaired collagen formation through vitamin C deficiency, for example.13 Wound healing in people with diabetes is impaired by their increased risk of infection and vascular pathology, among other factors, which impede the regular biochemical and immunologic processes involved in healing.13
To summarize the initial approach to pediatric facial lacerations, a careful history should be taken, keeping in mind vaccination status as well as the possibility of nonaccidental trauma. Other injuries should be assessed for, and any impairment of airway, breathing, or circulation should be attended to prior to repair planning. The wound itself should be assessed, keeping in mind the factors that may affect scarification or wound healing in a particular patient. If there is concern for the presence of a foreign body, radiographs or ultrasound may be obtained in addition to thorough examination and exploration of wound. (For particulars, see Weinberger LN, Chen EH, Mills AM. Is screening radiography necessary to detect retained foreign bodies in adequately explored superficial glass-caused wounds? Ann Emerg Med 2008;51:666-667.) After this, it is time to consider one’s approach to the repair.
Analgesia and Sedation
There are many options for anesthetics. The choice will depend on the child’s age and anxiety level, the laceration’s location, and clinician discretion. These elements become even more relevant when nerve blocks are considered. Overall, the options for anesthetics are topical agents, local infiltration, or nerve blocks. These may be used in combination with anxiolysis, conscious sedation, or, rarely, general anesthesia.
Topical Agents
Topical agents often are excellent for anesthesia in children and offer several benefits. The use of a commercially available topical agent is commonplace in pediatric EDs and can reduce pediatric patient anxiety because it avoids the use of needles. The parents of the patient also can be enlisted to help with placement of the solution, further helping to reduce patient anxiety. It is much easier to achieve anesthesia with topical preparations for scalp lacerations in particular, given that the scant subcutaneous tissue present on the scalp does not always readily lend itself well to local infiltration of anesthetic.
Although some topical anesthetic agents, such as eutectic mixture of local anesthetics or liposomal lidocaine, are indicated only for use on intact skin, other agents, such as lidocaine 4%, epinephrine 0.1%, tetracaine 0.5% (LET), can be used on open wounds.18 These agents should not be used on mucosa or in patients younger than 3 months of age. The LET should be applied by calculating the dose to a maximum of 0.2 cc/kg, placing half the quantity into the wound itself while saturating a piece of cotton with the other half and securing this in place with a transparent dressing, leaving it in place for 30-45 minutes.18 After this time has passed, the dressing can be removed, and the area can be tested for anesthesia. Since LET contains epinephrine, the presence of blanching can be taken as a sign that the area should be anesthetized. A small amount of local anesthetic may be necessary, but ensure the total anesthetic delivered does not exceed the calculated maximum dose.
Mobile apps, such as Safe Local, can be useful in calculating dosages. Keeping track of anesthetic dose is extremely important, whether topical, local, or regional anesthesia is chosen, to prevent toxicity.
Local Infiltration
If topical agents do not achieve sufficient anesthesia at the site, or if the child is old enough to tolerate it, local infiltration of anesthetic can be used. Lidocaine is used most commonly, with 1% or 2% concentrations and with or without epinephrine. Lidocaine solution itself is somewhat acidic and can cause pain with injection. A 1:9 ratio solution can be made of sodium bicarbonate to lidocaine, respectively, which can help to buffer this acidity. Ensuring that the lidocaine is at a warmer temperature by placing it in a blanket warmer or submerging the bottle into warm water also will reduce pain with infiltration.19 If the patient has a lidocaine (amide) allergy, diphenhydramine or procaine (ester) can be used for local infiltration, although diphenhydramine causes more discomfort on injection than other agents and has a shorter duration of anesthetic action.5 If diphenhydramine is chosen, use a dilution ratio of 1:4 of diphenhydramine 5% to normal saline to avoid the complication of local tissue destruction that has been reported with undiluted use.19
When infiltrating the area, it is important to use the minimum quantity of the chosen agent necessary to achieve adequate anesthesia, not only from the perspective of the anesthetic toxicity, but also from a procedural perspective, since the infiltration will distort the edges of the wound and may make the most cosmetically advantageous approximation of the wound margins more challenging. Care should be taken to administer the anesthetic through the inner portion of the wound edge to avoid disruption of surrounding intact skin. If the wound is heavily contaminated, the intact skin should be used so as to avoid further contamination of the actual laceration.19 Begin at the apex of the laceration, then advance the needle down the entire length of the wound edge, if possible. Aspirate with the plunger to ensure that there is no blood return, then proceed to inject the anesthetic while slowly retracting the needle. Repeat this process on the contralateral wound margin, then wait for several minutes before checking for anesthesia. As with topical agents, if lidocaine with epinephrine is used, blanching of the skin beside the wound is a good indicator of anesthetic placement.
Nerve Blocks
The third option to achieve procedural anesthesia is a nerve block. Nerve blocks may be more challenging in young children, and physicians may wish to avoid blocks that are administered intraorally, depending on the age of the patient and provider discretion regarding the overall level of anxiety of the patient and parents. Despite this caveat, nerve blocks have the advantage of accomplishing complete anesthesia of the area involved without distortion of the wound margins or the risk of further wound contamination and are an elegant solution to the problem of anesthesia for the carefully selected patient. Blocks that will be addressed here include supraorbital, supratrochlear, infraorbital, mental, and auricular.
For lacerations on the forehead and anterior portion of the scalp, the supraorbital and supratrochlear blocks can be invaluable in providing regional anesthesia. Although those nerves are subdivisions of the trigeminal nerve via the ophthalmic and subsequent frontal nerve divisions of that cranial nerve and innervate different portions of the forehead, there is significant overlap, and blocking one generally will block the other. The supraorbital nerve provides sensation to the lateral portion of the forehead on the ipsilateral side, while the supratrochlear nerve provides sensation to the ipsilateral medial portion of the forehead.20 To perform the block, find the supraorbital foramen by mentally tracing a straight line up from the pupil and palpating the superior margin of the eyebrow. Once the small notch is felt along the upper portion of the orbital ridge, the foramen can be approached with the needle either laterally or medially while infiltrating until the midline is reached.5 Be careful not inject into the foramen itself, since nerve necrosis may occur from the pressure resultant from the volume of the anesthetic agent.20
The infraorbital nerve, a branch of the trigeminal nerve via its maxillary division, can be approached both intraorally and extraorally.20 Blocking this nerve will anesthetize the ipsilateral upper lip, the medial portion of the ipsilateral cheek, and the lower portion of the eyelid.21 The infraorbital foramen tracks in line with the supraorbital foramen and midline of the pupil, mentioned earlier, so this also can be used as a landmark. For the intraoral approach, the maxillary canine can serve as an additional landmark after the lip has been retracted, and the needle can be inserted into the oral mucosa superior to this. As with any injection, aspirate the plunger of the syringe to check for blood return, then slowly inject the anesthetic.5 While the intraoral approach is overall less painful for the patient, there will be circumstances, such as patient intolerance of the intraoral approach, in which the extraoral approach will need to be used. In this case, palpate the infraorbital foramen using the aforementioned landmarks, then insert the needle superior to this and inject the anesthetic as previously described.20
The mental nerve provides sensation to the ipsilateral chin and lower lip.20 As with the infraorbital nerve, a mental nerve block has both intraoral and extraoral approaches. Similarly to the supraorbital and infraorbital foramina, the mental foramen lies in the line traced through the middle of the midline pupil. For the intraoral approach, locate the second mandibular bicuspid, then advance the needle inferiorly through the oral mucosa. As with every other block, ensure that there is retraction of the plunger to check for blood return prior to injecting the anesthetic. Similar to the infraorbital block, there may be circumstances in which the intraoral approach is not feasible, and the extraoral approach will be needed. Advance the needle after the skin has been penetrated superior to the foramen until the tip hits the mandible, then inject the anesthetic agent.5
Finally, an auricular block can be used to anesthetize the ear. There are multiple nerves that provide sensation to the ear, including the greater auricular, lesser occipital, and auriculotemporal nerves, with the meatus having sensory innervation from both the auriculotemporal and vagus nerves.5 Because of this separate innervation, separate blocks would be needed to anesthetize the external auditory canal/meatus and the other external structures of the ear. The ear is notoriously difficult to anesthetize because of the multiple innervations with different origins.20 For the external ear block, which will be most relevant to physicians performing the block in the ED, the injection site should be located at the mandibular angle. The needle should be advanced superiorly from this point both anteriorly and posteriorly to the ear; the anterior orientation will anesthetize the auriculotemporal nerve, while the posterior orientation will anesthetize both the greater auricular and lesser occipital nerves.20
Areas of the scalp not innervated by the lesser occipital nerve can be anesthetized by blocking the supraorbital, supratrochlear, auriculotemporal, lesser occipital, and great auricular nerves.20 To anesthetize the posterior portion of the scalp and provide comprehensive anesthesia to the entire scalp, the greater occipital nerve may be blocked as well. Given that the greater occipital nerve courses in close proximity to the posterior occipital artery, the determination of landmarks and assiduous avoidance of intra-arterial injection of anesthetic is vital to the success and safety of the procedure. Both landmark identification and ultrasound can be used to facilitate this. The mastoid process and the external occipital protuberance should be palpated, then one-third of the distance of the imaginary line between these landmarks should be traced to palpate the occipital artery. After the location of the occipital artery has been determined, the anesthetic should be injected on either side of the pulse, taking care to aspirate for blood return prior to injecting. Ultrasound with color-flow also can be used to determine the artery’s location to avoid vessel violation by the needle.20
Anxiolysis and Sedation
Now that an overview of local and regional anesthesia has been provided, the discussion will turn to sedation. Before moving on to deeper forms of sedation, it is worth noting at this point that anxiolysis often is used in combination with topical anesthetics for pediatric laceration repair and can be very useful as an adjuvant to local anesthetics or nerve blocks. Benzodiazepines are the most common, but nitrous oxide is another option, if available.22 Intranasal midazolam (trade name Versed) with an anxiolytic dose of 0.3 to 0.5 mg/kg is an excellent choice, since the intranasal route of administration avoids the use of needles, and midazolam is a short-acting benzodiazepine that begins to take effect around 10 minutes after administration.22 The duration of action is approximately 20-25 minutes.23
Nitrous oxide, by comparison, begins to take effect only a few minutes after administration, and the effect wears off quickly after it is withdrawn. Additionally, nitrous oxide has an analgesic effect, whereas benzodiazepines provide only anxiolysis. Nitrous oxide is delivered by face mask or nasal hood combined with oxygen in a concentration range of nitrous oxide from 30% to 70%. Ranges from 30% to 50% are considered anxiolytic, whereas ranges from 51% to 70% are considered moderate sedation.22 Nitrous oxide also has a rapid onset and offset of action.24
Because of the magnitude of the topic, conscious sedation will be addressed more briefly here, and the reader is encouraged to consult one of the many other resources that are dedicated to pediatric sedation in the ED. (See Pediatric Emergency Medicine Reports, June 2021.) Sedation exists along a spectrum, and tools such as the Ramsay Scale for Standardized Levels of Sedation can be used to describe the clinical findings at different depths of sedation, with scores on the Ramsay Scale ranging from 1 to 6. A patient with a score of 1 would be anxious and/or restless and essentially be unanesthetized, while a patient with a score of 6 would have no response to auditory stimuli or tapping of the glabella and would represent general anesthesia.25 As general principles, there are Level B recommendations to not defer sedation based on last oral intake and to use capnography for ventilation and apnea monitoring.26 Both ketamine and propofol have Level A recommendations for use in procedural sedation in the ED.26
Postsedation, patients should return to their presedation Aldrete scores.27 While further details are available in other resources, the relevant parameters include activity, respiration, blood pressure, level of consciousness, and oxygen saturation.28
Preparation
Proper wound preparation is essential to the overall success of the laceration repair. Following examination of the wound for foreign bodies, evaluation of the neurovascular status of the surrounding tissue, and the provision of anesthesia and/or sedation, the wound should be prepared for the repair. It is important to make special note of hair removal. First and foremost, the eyebrows should never be shaved for laceration repair because they may grow back irregularly and, thus, lead to a suboptimal cosmetic outcome.11 Additionally, the eyebrow borders may serve as useful markers for linear approximation and thereby a better overall scar appearance. Scalp hair is not often removed prior to laceration repair, but if the physician elects to do so, a depilatory cream should be used in place of a razor, since the latter has been demonstrated to result in increased postrepair infection rates, likely via microdisruptions to the epidermis by the blade.29
The wound should be irrigated using either tap water or saline, since infection rates with either are equivalent.30 Additionally, tap water is far more cost effective than sterile saline.31 High-pressure irrigation with a syringe (35 cc syringe with a 19-gauge catheter in the study) has been shown to reduce wound bacterial count and subsequent infection rates.32 More than 5 psi were required to be considered a high-pressure stream, and the aforementioned setup generated approximately 7.5 psi.32 Although there is a reduced chance of infection, irrigation has not been shown to have a positive impact on wound healing.33
Several options of antiseptic agents are available for wound preparation, but their availability is likely to vary by hospital and healthcare system. These agents are used in addition to saline irrigation.34 In increasing order of efficacy for prolonged contact antiseptic action, options include povidone-iodine, chlorhexidine, polyhexanide, and octenidine, with the latter two having equivalent efficacy.35 The order of this list does change according to objective, however, such as quick-action antiseptic prior to more invasive procedures.
Povidone-iodine uses povidone as a carrier for the antiseptic iodine, slowly releasing the molecule into tissue. Chlorhexidine is a biguanide antiseptic that works immediately as a bactericidal through cell membrane disruption.36 It has been shown to have less of an effect on fibroblast activity in lower amounts and, thus, less of a detrimental effect with respect to wound healing when compared to some other antiseptics.37 Polyhexanide, another biguanide, has strong data to support its use in wound infection prevention and has been shown to reduce time-to-closure for superficial wounds and overall wound healing improvement with greater efficacy than any other antiseptic examined in the study.38,39 A recent review of antiseptics recommends polyhexanide for acute, contaminated wounds and povidone-iodine for bite, stab, or puncture wounds with lesser degree of contamination.40 The same study also recommended a combined octenidine/phenoxyethanol solution for similar wound types.
It also is worth addressing using hydrogen peroxide for wound management. Although it is popularly used as an over-the-counter antiseptic, the overall utility of hydrogen peroxide in the ED is limited, since it has been shown to lift new epithelium off of the dermis due to the oxygen bubbles and to have a relatively weak antiseptic effect.41 Additionally, hydrogen peroxide (as well as povidone-iodine) diminishes fibroblast migration and proliferation, thereby slowing wound healing.37
Suture Material/Needle Size and Alternatives to Sutures
After cleaning, it is time for the repair itself. Materials available for wound approximation on facial and scalp lacerations include sutures, staples, and tissue adhesives. Each of these will be considered in turn, as well as common indications for the use of each. There are crossover indications for use, so physician preference and facility availability also will play a role in selection. In addition to the materials discussed in the following section, it is worth mentioning that either sterile or nonsterile gloves may be used, since no clinically significant difference in infection rate has been demonstrated between the two glove types for laceration repair.42 However, the physician may wish to select sterile gloves for comfort, given their more precise fit for various hand sizes.
If sutures are to be used, a needle driver, tissue forceps, and scissors will be required in addition to the suture itself. Many EDs have kits that provide all of these components together, with the exception of the suturing material. Traditionally, needle sizes of 5-0 or 6-0 have been used for lacerations on the face, particularly with pediatric patients, and the practice generally is to use the smallest practical needle size for the laceration repair. Principal divisions within suture material nomenclature include absorbable vs. nonabsorbable and braided vs. non-braided. A meta-analysis did not demonstrate statistically significant differences in cosmesis, infection rate, dehiscence, or scar hypertrophy between absorbable and nonabsorbable suture materials.43,44 However, the studies in this meta-analysis relied on more subjective evaluation of the scar. Digital imaging analysis has been used more recently to provide a more objective evaluation, and absorbable sutures have been shown to elicit less tissue reaction as skin erythema.45 No significant difference was found between braided and monofilament sutures in terms of infection, dehiscence, or cosmesis.46
Another option for wound approximation is to use tissue adhesives, the most commonly available of which is octyl cyanoacrylate (Dermabond). Studies have shown that tissue adhesive can be used in certain instances as an alternative to sutures, although there is a higher rate of wound dehiscence with adhesives.47,48 Tissue adhesives’ advantages include not requiring procedural sedation, less pain for the pediatric patient, time efficiency for the physician, and parent satisfaction.49 The adhesives also have been shown to reduce overall length of stay in the ED.50 Octyl cyanoacrylate also provides a barrier to microbes as well as an excellent, moist wound healing environment.51 However, it is important to understand which lacerations are amenable to approximation with tissue adhesives and which are not. Criteria for tissue adhesive use include low tension, well-approximated, and hemostatic lacerations that are located in areas where runoff can be contained.5 Interestingly, lacerations repaired with tissue tape (such as Steri-Strips) in one study had a similar cosmetic outcome to octyl cyanoacrylate, so the tape could be considered as an alternative if cost is a concern or if tissue adhesive is unavailable.52
Staples are an excellent option for the approximation of scalp lacerations, since they are quicker to place, less expensive, and less tissue-reactive.15 There also is a decreased risk of infection with staples vs. sutures.53 Although the cosmetic outcome can be less optimal than with other methods (and, thus, should not be used for lacerations on the face), studies have shown that the outcomes are cosmetically acceptable with staples when compared with sutures.54 Additionally, a recent study demonstrated that parents could be trained to remove the staples themselves at home, obviating the need for a follow-up visit and increasing convenience for the patient, parents, and physician.55
The hair apposition technique is an alternative to staples and sutures for scalp lacerations that are not irregular in shape, not actively bleeding after pressure has been held, or in patients with very short hair. It has been demonstrated to be more cost-effective than either of the other methods.56 It also has a lower infection rate for scalp lacerations than sutures do and, in contrast to sutures and staples, does not require return for removal.57 Table 1 (available online at https://bit.ly/3tnvT9f) shows the different wound closure techniques/materials and their characteristics.
Approximation Technique
Successful suture placement requires matching the layer of tissue on one side to the same layer of tissue on the contralateral side of the wound and eversion of the edges of the wound. Depending on the tissue level of suturing, epidermis must be matched with contralateral epidermis, dermis with dermis, and so on. Eversion is necessary because of the contraction due to myofibroblasts that occurs with scar formation over time. If the initial scar is everted, it will flatten with contraction instead of pitting.5 To ensure eversion, the needle should be placed perpendicular to the wound margin and should both enter and exit the skin as close to the margin as possible, with the needle tip exactly perpendicular to the surface of the skin. The least amount of tension, just enough to approximate the wound margins, should be placed on the initial throw of the knot to prevent tissue damage.58 Halving should be used when selecting where and in what sequence to place sutures: the first, or central, suture should “cut” the laceration in half by being placed in the middle of the laceration. Sutures then should be placed on either side of the central suture to “cut” that portion of the laceration in half, and so on, until the wound is approximated.58
The type of stitch used depends largely on the location of the laceration, wound characteristics, physician preference, and patient factors. Although there are many different types of stitches, not all stitch types are appropriate for the face. The simple interrupted suture is the most common type and has the advantage of adjusting tension by stitch. Since the stitches are interrupted, it is useful for irregularly shaped lacerations. Half-buried horizontal mattress stitches are useful for flap closures, such as with Y- or V-shaped or stellate lacerations, because they achieve approximation while avoiding flap-margin tension.58 The continuous subcuticular suture (either pull-out or permanent) is excellent for low-tension lacerations on the face, since there are no suture marks on the surface of the skin. (See Figure 2.)
Figure 2. Suture Diagrams |
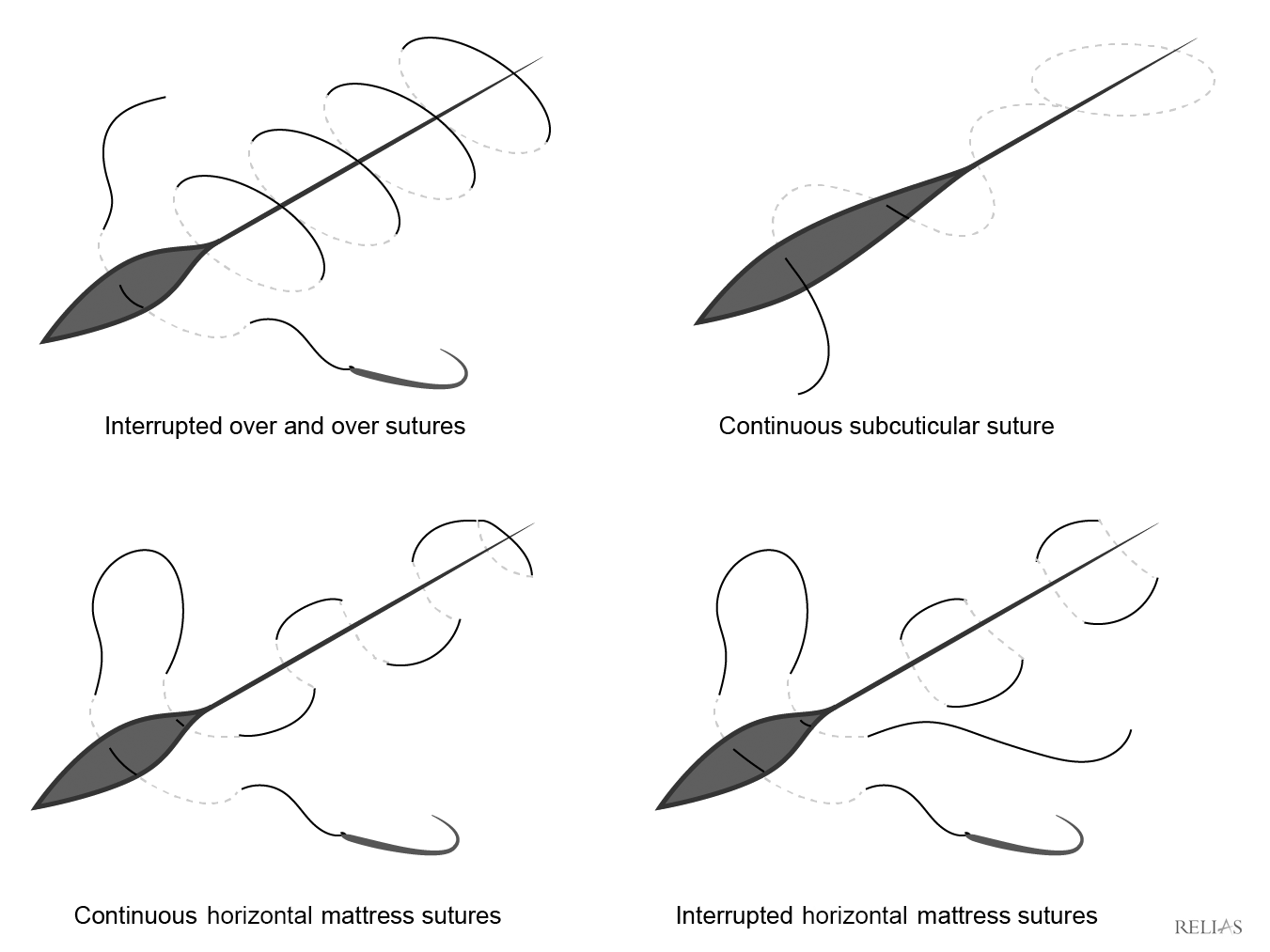 |
Tissue adhesive application is fairly straightforward. The wound edges should be approximated with the fingers. Then, an initial layer of the adhesive should be applied, repeating three to four more times while leaving 30-60 seconds between the application of each layer to allow the previous layer to dry. Runoff can be an issue with adhesive, particularly around the eyes, so the patient should be placed in Trendelenberg or reverse Trendelenberg position as required to avoid runoff. A petroleum jelly “wall” also can be formed around the wound to prevent runoff. After applying adhesive, it will take approximately two to three minutes for the full tensile strength to be achieved.59
Staples (metal or dissolvable) commonly are used to approximate linear scalp lacerations. Ideally, the physician will have an assistant for staple placement who will evert the wound margins with a pair of tissue forceps, but a single operator also is possible. The staple device then should be placed at a 90-degree angle with the skin so that the laceration itself runs through the center of the staple delivery mechanism. It is not necessary to indent the skin. The trigger then should be squeezed to place the staple, and the process should be repeated for the length of the wound. The staples should remain in place for the same amount of time as for sutures before the patient and parents return for follow-up to have them removed — or to have them removed at home, if appropriate training is available.5
The modified hair apposition technique has been shown to be a reasonable alternative to staples or sutures for scalp laceration approximation.57 The original hair apposition technique required tying knots, while the modified technique relies on twisting. Essentially, the technique consists of using two pairs of Kelly clamps to grasp a small bundle of hair on each side of the laceration, then twisting the clamps around each other before placing a drop of tissue adhesive on the twist to hold it in place. This process then is repeated along the length of the wound until it is approximated. The glue should dissolve over the course of the next couple of weeks, and the patient only needs to follow up as needed.
Areas and Circumstances of Particular Concern
Anatomical areas and circumstances of particular concern include lacerations of the lip, mouth, tongue, eyes, ears, nose, and galea aponeurotica. It is important to understand which types of lacerations in these areas should fall under the purview of a specialist and which can be managed appropriately by the emergency physician.
Lacerations of the lip that pass through the vermilion border are of extreme cosmetic importance, since misapproximation of as little as 1 mm will leave a deformity that can be noticed by a casual observer.60 If there is a large avulsion, or if the laceration is complex with multiple violations of the vermilion border, a specialist should be consulted for the repair.
The aforementioned infraorbital and mental nerve blocks for the ipsilateral upper and lower lips, respectively, can be extremely useful in older children, since they will provide analgesia without distorting the wound margins or lip anatomy and thus will permit more accurate wound margin approximation. Younger children likely will require conscious sedation for this repair type. In contrast to the halving principle discussed earlier, the first suture that is placed in lacerations that cross the vermilion border is to approximate the border perfectly with a nonabsorbable suture type.60 The orbicularis oris muscle should be approximated with absorbable sutures, if applicable. From that point, skin defects can be repaired with non-absorbable suture, and mucosal defects can be repaired with absorbable suture.61
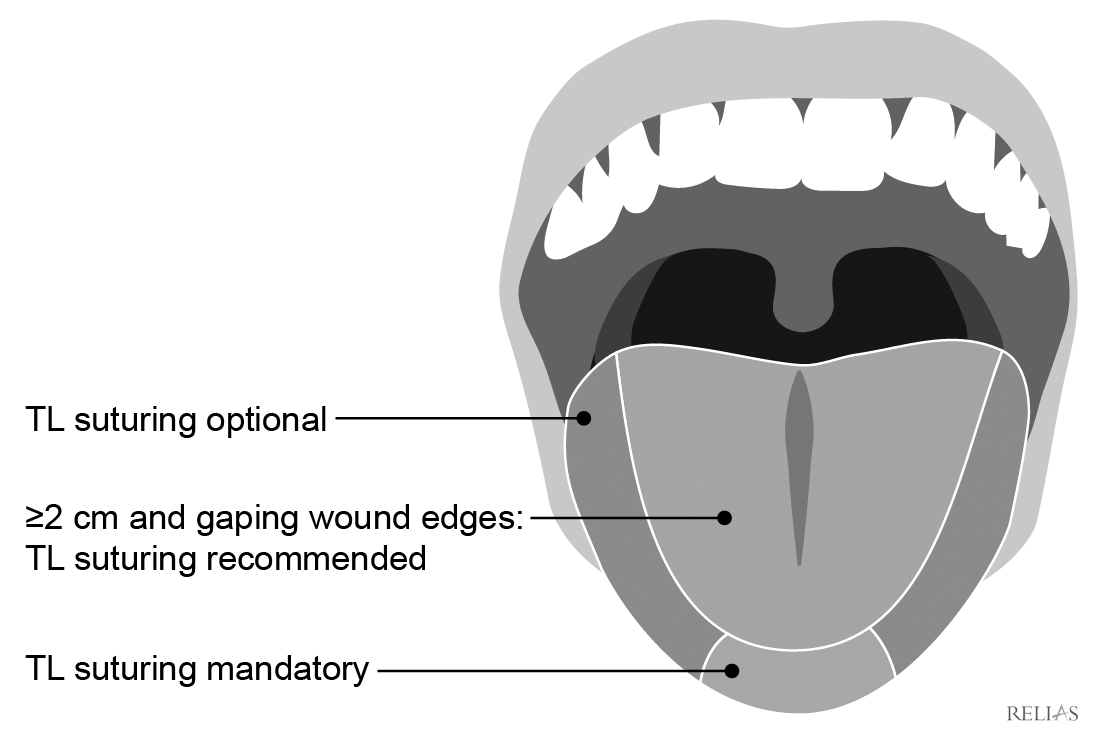
Intraoral lacerations need to be repaired only if they are large enough to catch food particles.61 If the laceration needs to be repaired, a nonabsorbable suture type should be used. For lacerations of the tongue, specific recommendations for repair can be somewhat controversial. For pediatric patients, a good general rule is that lacerations less than 2 cm long and not involving the tip of the tongue do not require repair, but the Zurich Tongue Scheme can provide more specific guidance as to which types will require repair.62 (See Figure 3.)
Figure 3. Zurich Tongue Scheme |
Lacerations surrounding the eye that require consultation include violation of the tear duct apparatus, the medial palpebral ligament, the levator palpebrae, or the tarsal plate.5 Obviously, a globe rupture also would require immediate ophthalmology consultation in addition to operative management.63 Extramarginal lid lacerations can be repaired by the emergency physician with nonabsorbable sutures or with polyglactin-910 with equivalent cosmetic outcomes.64 However, repair of these lacerations can be particularly challenging, considering the thinness of the dermis. Care should be taken not to place sutures too shallowly, thus resulting in a percutaneous approximation that will not provide the strength for a cosmetically favorable outcome.65 As with all repairs, skin eversion is vital for optimal healing.1 With lacerations that cross the eyebrow, the borders of the eyebrow should be aligned to avoid suboptimal cosmetic outcomes, and sutures should be removed in three to five days.5
Ear injuries with auricular avulsion should be deferred to consultant management, along with lacerations with exposed cartilage that the emergency physician does not feel confident in repairing.61 Techniques such as wedge excision and subsequent repair may be used by the emergency physician, but approaches to them may vary considerably by institution, and it would probably be most prudent for the provider to consult with the appropriate service prior to attempting more advanced techniques. In addition to the usual goals of achieving a cosmetically pleasing outcome while avoiding infection, laceration management on the ear has the added concern of preventing the formation of a hematoma that would prevent the diffusion of nutrients from the perichondrium to the avascular cartilage, thereby leading to chondritis, necrosis, and subsequent “cauliflower ear.”66
In terms of wound preparation, the area should not be irrigated with as much force as other lacerations, since using higher psi runs the risk of separating the cartilage from the perichondrium.60 A piece of cotton may be placed at the meatus during irrigation so as to not cause unnecessary discomfort to the patient. If the laceration is simple, use nonabsorbable sutures to place simple interrupted stitches to distribute tension. Begin on the anterior portion of the ear because of its greater cosmetic importance.67 Do not suture the cartilage itself. It is vital that all exposed cartilage is covered by the repair to prevent chondritis. In the event that the patient presents with a perichondral hematoma within 72 hours of onset, it should be drained and a pressure dressing should be placed.5 Approaches to this are widely covered in other resources.
Lacerations of the nose have concerns similar to the ears in that cartilage may be involved, and management of nose cartilage is similar — exposed cartilage should be covered, approximation should be precise, debridement should be minimal, and consultants should be involved if the injury is complex or if the emergency physician is uncomfortable performing the repair. During the initial assessment of the patient with a nasal injury, it is important for the emergency physician to examine the patient for evidence of a septal hematoma, which, if left untreated, could lead to permanent deformity.
The abundant blood supply to the scalp is provided by the occipital, superficial temporal, posterior auricular, supraorbital, and supratrochlear arteries.61 The potential space that forms the subaponeurotic space beneath the galea aponeurotica and above the pericranium is at risk for hematoma or infection if the galea aponeurotica is violated.68,69 It is important to understand that such infections have the potential to create brain abscesses or osteomyelitis, so one must be diligent in the initial assessment of scalp lacerations.5 With larger lacerations to the galea, impairment of facial expression may result if the defect is not repaired, given that the galea serves as the frontalis muscle’s insertion point.5 When repairing lacerations of the forehead, it is important to determine whether the frontalis muscle is involved, since involvement will require the placement of deep sutures to reapproximate the muscle fibers to preserve function of the muscles of facial expression.61 When the forehead skin itself is approximated, the horizontal lines of the forehead should be aligned for the repair to ensure a more cosmetically favorable outcome.61
Postrepair Care and Antibiotics
As is common practice, all pediatric patients should be instructed to follow up with their primary care physician as needed for wound checks and/or suture removal, and return instructions always should be provided. Patients should be advised to keep the wound in a moist environment with Xeroform, topical antibiotic ointment, or other similar agents, since moist environments have been shown to reduce healing time by approximately three days.41 If nonabsorbable sutures or staples have been selected for the repair, the general rule is that these should be removed after three to five days if on the face and after seven to 10 days if on the scalp.46 Parents also should be advised to avoid sun exposure and to use sunscreen as much as possible on the area to optimize the cosmetic outcome.70
In general, antibiotic prophylaxis is not necessary and has not been shown to be beneficial.15 Reasonable instances to provide oral antibiotics include involvement of cartilage, a severely contaminated wound, a bite, an immunocompromised patient, and facial wounds more than a day old, although emergency physician discretion will play a large role in the decision for or against antibiotic prophylaxis.5
Conclusion
Facial lacerations are a common reason for the evaluation of pediatric patients in the ED. It is essential for the emergency physician to have a firm grasp of the management of these lacerations, including an understanding of vaccination status, injury history, physical examination, analgesia, the various options for wound approximation, potential complications, areas of special interest, and post-repair care. A thorough familiarity with these principles will ensure that the emergency physician will be able to formulate and execute an appropriate plan of care that encompasses excellent patient care, parent satisfaction, and an optimal cosmetic outcome.
REFERENCES
- Murray BL. Soft-tissue injury and wound repair. In: Tenenbein M, Macias CG, Sharieff GQ, et al, eds. Strange and Schafermeyer’s Pediatric Emergency Medicine. 5th ed. McGraw-Hill;2019:217-230.
- Baker MD, Selbst SM, Lanuti M. Lacerations in urban children. A prospective 12-January study. Am J Dis Child 1990;144:87-92.
- Choi J, Lorenz HP, Spain DA. Review of facial trauma management. J Trauma Acute Care Surg 2020;88:e124-e130.
- Imahara SD, Hopper RA, Wang J, et al. Patterns and outcomes of pediatric facial fractures in the United States: A survey of the National Trauma Data Bank. J Am Coll Surg 2008;207:710-716.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Saunders; 2012.
- Centers for Disease Control and Prevention. Diphtheria, tetanus, and pertussis vaccine recommendations. Updated Jan. 22, 2020. https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/recommendations.html
- Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the Advisory Committee on Immunization Practices — United States, 2019. MMWR Morb Mortal Wkly Rep 2020;69:77-83.
- Vazquez M-P, Kadlub N, Soupre V, et al. [Facial trauma and injury in children.] Ann Chir Plast Esthet 2016;61:543-559.
- Rall JM, Cox JM, Songer AG, et al. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage.” Trauma Acute Care Surg 2013;75:S150-S156.
- Peng HT. Hemostatic agents for prehospital hemorrhage control: A narrative review. Mil Med Res 2020;7:13.
- Sabatino F, Moskovitz JB. Facial wound management. Emerg Med Clin North Am 2013;21:529-538.
- Zehtabchi S, Tan A, Yadav K, et al. The impact of wound age on the infection rate of simple lacerations repaired in the emergency department. Injury 2012;43:1793-1798.
- Kumar V, Abbas A, Aster J. Robbins and Cotran Pathologic Basis of Disease. 9th ed. Elsevier; 2015.
- Hollander JE, Blasko B, Singer AJ, et al. Poor correlation of short- and long-term cosmetic appearance of repaired lacerations. Acad Emerg Med 1995;2:983-987.
- Mankowitz SL. Laceration management. J Emerg Med 2017;53:369-382.
- Kraissl CJ. The selection of appropriate lines for elective surgical incisions. Plast Reconstr Surg (1946) 1951;8:1-28.
- Singer AJ, Quinn JV, Thode Jr HC, et al. Determinants of poor outcome after laceration and surgical incision repair. Plast Reconstr Surg 2002;110:429-435, discussion 436-437.
- Ali S, Poonai N. Pain management and procedural sedation for infants and children. In: Tintinalli JE, Ma JO, Yealy DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill; 2020:723-730.
- Supino M, Yousef D. Local anesthesia. In: Reichman EF, ed. Reichman’s Emergency Medicine Procedures. 3rd ed. McGraw-Hill;2018:1249-1256.
- Reichman EF, Meer J. Regional nerve blocks (regional anesthesia). In: Reichman EF, ed. Reichman’s Emergency Medicine Procedures. 3rd ed. McGraw-Hill;2018:1271-1311.
- Gibbs MA, Wu T. Local and regional anesthesia. In: Tintinalli JE, Ma JO, Yealy DM, eds. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill; 2020:236-247.
- Wathen JE, Wiersma AJ. Procedural sedation and analgesia. In: Tenenbein M, Macias CG, Sharieff GQ, et al. Strange and Schafermeyer’s Pediatric Emergency Medicine. 5th ed. McGraw-Hill; 2019:55-64.
- Chiaretti A, Barone G, Rigante D, et al. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child 2011;96:160-163.
- Becker DE, Rosenberg M. Nitrous oxide and the inhalation anesthetics. Anesth Prog 2008;55:124-130, quiz 131-132.
- Ramsay MA, Savege TM, Simpson BR, Goodwin R, et al. Controlled sedation with alphaxalone-alphadolone. Br Med J 1974;2;656-659.
- Godwin SA, Burton JH, Gerardo CJ, et al. Clinical policy: Procedural sedation and analgesia in the emergency department. Ann Emerg Med 2014;63:247-258.e18.
- Daud YN, Carlson DW. Pediatric sedation. Pediatr Clin North Am 2014;61:703-717.
- Butterworth JF, Wasnick JD, Mackey DC, eds. Ambulatory and non-operating room anesthesia. Morgan & Mikhail's Clinical Anesthesiology. 6th ed. McGraw-Hill;2018:943-958.
- Adisa AO, Lawal OO, Adejuyigbe O. Evaluation of two methods of preoperative hair removal and their relationship to postoperative wound infection. J Infect Dev Ctries 2011;5:717-722.
- Moscati RM, Mayrose J, Reardon RF, et al. A multicenter comparison of tap water versus sterile saline for wound irrigation. Acad Emerg Med 2007;14:404-409.
- Sepehripour S, Dheansa BS. Wound irrigation and the lack of evidence-based practice. J Plast Reconstr Aesthet Surg 2018;71:940-941.
- Stevenson TR, Thacker JG, Rodeheaver GT, et al. Cleansing the traumatic wound by high pressure syringe irrigation. JACEP 1976;5:17-21.
- Wilkins RG, Unverdorben M. Wound cleaning and wound healing: A concise review. Adv Skin Wound Care 2013;26:160-163.
- Singer AJ, Hollander JE. Wound preparation. In: Tintinalli JE, Ma JO, Yealy DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill;2020:269-271.
- Koburger T, Hübner N-O, Braun M, et al. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 2010;65:1712-1719.
- Atiyeh BS, Dibo SA, Hayek SN. Wound cleansing, topical antiseptics, and wound healing. Int Wound J 2009;6:420-430.
- Thomas GW, Rael LT, Bar-Or R, et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma 2009;66:82-90, discussion 90-91.
- Kramer A, Roth B, Müller G, et al. Influence of the antiseptic agents polyhexanide and octenidine on FL cells and on healing of experimental superficial aseptic wounds in piglets. A double-blind, randomised, stratified, controlled, parallel-group study. Skin Pharmacol Physiol 2004;17:141-146.
- Roth B, Neuenschwander R, Brill F, et al. Effect of antiseptic irrigation on infection rates of traumatic soft tissue wounds: A longitudinal cohort study. J Wound Care 2017;26:79-87.
- Kramer A, Dissemond J, Kim S, et al. Consensus on wound antisepsis: Update 2018. Skin Pharmacol Physiol 2018;31:28-58.
- Gruber RP, Vistnes L, Pardoe R. The effect of commonly used antiseptics on wound healing. Plast Reconstr Surg 1975;55:472-476.
- Perelman VS, Francis GJ, Rutledge T, et al. Sterile versus nonsterile gloves for repair of uncomplicated lacerations in the emergency department: A randomized controlled trial. Ann Emerg Med 2004;43:362-370.
- Al-Abdullah T, Plint AC, Fergusson D. Absorbable versus nonabsorbable sutures in the management of traumatic lacerations and surgical wounds: A meta-analysis. Pediatr Emerg Care 2007;23:339-344.
- Karounis H, Gouin S, Eisman H. A randomized, controlled trial comparing long-term cosmetic outcomes of traumatic pediatric lacerations repaired with absorbable plain gut versus nonabsorbable nylon sutures. Acad Emerg Med 2004;11:703-735.
- Parara SM, Manios A, de Bree E, et al. Significant differences in skin irritation by common suture materials assessed by a comparative computerized objective method. Plast Reconstr Surg 2011;127;1191-1198.
- Forsch RT, Little SH, Williams C. Laceration repair: A practical approach. Am Fam Physician 2017;95:628-636.
- Farion KJ, Osmond MH, Hartling L, et al. Tissue adhesives for traumatic lacerations: A systematic review of randomized controlled trials. Acad Emerg Med 2003;10:110-118.
- Beam JW. Tissue adhesives for simple traumatic lacerations. J Athl Train 2008;43:222-224.
- Bruns TB, Simon HK, McLario DJ, et al. Laceration repair using a tissue adhesive in a children's emergency department. Pediatrics 1996;98:673-675.
- Otterness K, Thode Jr HC, Singer AJ. Methods of laceration closure in the ED: A national perspective. Am J Emerg Med 2020;38:1058-1061.
- Singer AJ, Thode Jr HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg 2004;187:238-248.
- Zempsky WT, David Parrotti, Grem C, Nichols J. Randomized controlled comparison of cosmetic outcomes of simple facial lacerations closed with Steri Strip Skin Closures or Dermabond tissue adhesive. Pediatr Emerg Care 2004;20:519-524.
- Ricci NA, Rizzolo D. Laceration repair: Avoid infection, optimize healing, minimize scarring. JAAPA 2011;24:28-33.
- Khan ANGA, Dayan PS, Miller S, et al. Cosmetic outcome of scalp wound closure with staples in the pediatric emergency department: A prospective, randomized trial. Pediatr Emerg Care 2002;18,171-173.
- Quinn BJ, Mancinelli A, Rooney-Otero K, et al. Scalp staples placed in a pediatric emergency department: Feasibility and benefits of home removal. Pediatr Emerg Care 2020;doi:10.1097/PEC.0000000000002213. [Online ahead of print].
- Ong MEH, Coyle D, Lim SH, Stiell I. Cost-effectiveness of hair apposition technique compared with standard suturing in scalp lacerations. Ann Emerg Med 2005;46:237-242.
- Karaduman S, Yürüktümen A, Güryay SM, et al. Modified hair apposition technique as the primary closure method for scalp lacerations. Am J Emerg Med 2009;27:1050-1055.
- Reichman EF. Basic wound closure techniques. In: Reichman EF, ed. Reichman’s Emergency Medicine Procedures. 3rd ed. McGraw-Hill;2018:994-1017.
- Forsch RT. Essentials of skin laceration repair. Am Fam Physician 2008;78;945-951.
- Brown DJ, Jaffe JE, Henson JK. Advanced laceration management. Emerg Med Clin North Am 2007;25:83-99.
- Coates WC. Face and scalp lacerations. In: Tintinalli JE, Ma JO, Yealy DM, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill;2020:269-271:285-291.
- Seiler M, Massaro SL, Staubli G, Schiestl C. Tongue lacerations in children: To suture or not. Swiss Med Wkly 2018;148:w14683.
- Ko AC, Satterfield KR, Korn BS, Kikkawa DO. Eyelid and periorbital soft tissue trauma. Facial Plast Surg Clin North Am 2017;25:605-616.
- Talbot AWR, Meadows AER, Tyers AG, Shah-Desai S. Use of 7/0 Vicryl (coated polyglactin 910) and 7/0 Vicryl-rapide (irradiated polyglactin 910) in skin closure in ophthalmic plastic surgery. Orbit 2002;21:1-8.
- Kantor J. The eyelids. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. McGraw-Hill;2017:332-333.
- Belleza WG, Kalman S. Otolaryngologic emergencies in the outpatient setting. Med Clin North Am 2006;90:329-353.
- Kennedy TM, Russo CJ. Management of specific soft tissue injuries. In: Reichman EF, ed. Reichman’s Emergency Medicine Procedures. 3rd ed. McGraw-Hill;2018:1034-1038.
- Barry J, Fridley J, Sayama C, Lam S. Infected subgaleal hematoma following blunt head trauma in a child: Case report and review of the literature. Pediatr Neurosurg 2015;50:223-228.
- Antón J, Pineda V, Martin C, et al. Posttraumatic subgaleal hematoma: A case report and review of the literature. Pediatr Emerg Care 1999;15:347-349.
- Meaume S, Pullouer-Prost A, Richert B, et al. Management of scars: Updated practical guidelines and use of silicones. Eur J Dermatol 2014;24:435-443.
Pediatric facial lacerations are common, and every emergency medicine physician needs to be familiar with the approach to pediatric facial and scalp lacerations, child-friendly methods for repair, and different options for analgesia.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.