AUTHORS
Joseph U. Becker, MD, Clinical Associate Professor, Department of Emergency Medicine, Stanford University, Palo Alto, CA
Lise Mumporeze, MD, MMED, Department of Emergency Medicine, King Faisal Hospital, Kigali, Rwanda
PEER REVIEWER
Daniel Migliaccio, MD, Clinical Assistant Professor, Ultrasound Fellowship Director, Department of Emergency Medicine, University of North Carolina, Chapel Hill
Executive Summary
- The vast majority of severe malaria results from infection with Plasmodium falciparum, although all species of malaria may cause significant clinical symptoms and, potentially, serious disease in children with preexisting disease or impaired immune function. P. falciparum is the most common form of malaria in Africa, accounting for more than 99% of cases and more than 50% of cases in Southeast Asia. Plasmodium vivax is the most common cause of malaria infection in the Americas, responsible for ~75% of cases. Plasmodium knowlesi recently has been shown to be responsible for a portion of human infection in Indonesia — and likely elsewhere.
- The majority of severe disease results from infection with P. falciparum, although P. vivax is a significant cause of severe anemia and mortality in Southeast Asia.
- Malaria infection occurs when an individual is bitten by an infected female Anopheles mosquito. The mosquito injects the malaria parasites in the form of sporozoites into human blood. Once in the bloodstream, sporozoites make their way to the liver, where they undergo asexual reproduction over the course of seven to 10 days. In the hepatic cells, the parasite forms a liver schizont, which matures and releases merozoites into the bloodstream. In cases of P. vivax and Plasmodium ovale infections, the parasite may form a dormant hypnozoite phase in the liver, which then may cause recurring infection potentially weeks to years after the initial infection. These merozoites then invade erythrocytes, where they may develop into a ring trophozoite stage, forming an erythrocytic schizont phase. This erythrocytic schizont phase then releases its own merozoites, eventually leading to lysis of red blood cells (RBCs), releasing more merozoites and leading to symptomatic disease and, potentially, severe hemolysis.
- The duration of the erythrocytic portion of the malarial life cycle determines the periodicity of fever, which occurs every 72 hours in Plasmodium malariae infection (quartan fever) and every 48 hours in P. falciparum, P. vivax, and P. ovale infection (tertian fever).
- The World Health Organization (WHO) defines severe malarial anemia as a hemoglobin concentration of less than 5 mg/dL (hematocrit less than 15%) with a parasitemia of greater than 10,000 parasites/μL.
- The WHO defines cerebral malaria as the presence of coma greater than one hour after a seizure, regardless of the use of antiseizure medications.
- Malaria must be considered in all travelers with fever who are returning from malaria-endemic areas, regardless of the use of prophylactic medications.
- Both thin and thick blood smears may be used to quantify the degree of parasitemia. Thin smears dry more rapidly than thick smears and, unlike thick smears, are fixed in methanol prior to Giemsa staining.
- While smears may be helpful in determining parasite burden, the sensitivity of rapid diagnostic tests generally is superior, with reported sensitivities in the 97% range, compared with 85% for malaria blood smear analysis.
- The choice of antimalarial therapy depends on multiple factors, including the severity of disease, the resistance patterns observed in the local region, and the causative species of malaria involved.
- Artemisinin combination therapies (ACTs) have been the treatment of choice for uncomplicated P. falciparum malaria for several years. ACTs now are recommended as the first-line treatment in P. knowlesi infections as well.
Introduction
From its prehistoric origin as a parasitic disease of the nonhuman primates of Africa through to the present day, the malaria parasite and its vector, the Anopheles genera of mosquitoes, have infested all the continents except Antarctica and have led to a massive toll of human illness and death.1 To date, the disease is endemic in 89 countries, mainly in Africa, Asia, and the Americas. The most affected region is Africa, with the vast majority of cases occurring in sub-Saharan Africa, which accounts for 94% of the global burden of disease.2 In 2019, children younger than 5 years of age accounted for 67% (274,000) of malaria deaths globally, making malaria a significant contributor to childhood mortality worldwide.2,3
Malaria is a mandatory consideration in fever in returned travellers, since the disease process can lead to significant mortality and rapid clinical decline. Understanding the pathophysiology, clinical presentation, and unique challenges of malaria in the pediatric population is critical to making the diagnosis, instituting appropriate life-saving therapy, and developing strategies for control and prevention.
Ecology
The female Anopheles mosquito is the vector for all species of malaria and is found across tropical and temperate climates spanning from North America to the South Pacific and Africa.4 (See Figure 1.) Many factors affect the suitability of a given species of Anopheles as a vector for malaria parasites, and the incidence of malaria infection in a given region is at least partially dependent on these factors. Species of Anopheles mosquitoes have varying degrees of immunity against infection by Plasmodium species, directly impacting the suitability of said mosquitoes as a vector for malaria transmission.4 Furthermore, the lifespan of an Anopheles mosquito is directly related to its ability to function as a vector for malaria. Mosquitoes that live shorter lives have a lower chance of being infected than mosquitoes that live longer. Therefore, shorter-living mosquitoes are less likely to transmit malaria infection. Additionally, the malaria parasite requires approximately seven to 10 days to complete the mosquito (extrinsic) portion of its development before being capable of infecting a new host. The smaller the population of Anopheles mosquitoes that live this long, the smaller the population capable of infecting a new human host, even if the mosquito is infected.5,6
Figure 1. Malaria-Free Countries and Malaria-Endemic Countries in Phases of Control*, Pre-Elimination, Elimination, and Prevention of Reintroduction, end 2008 |
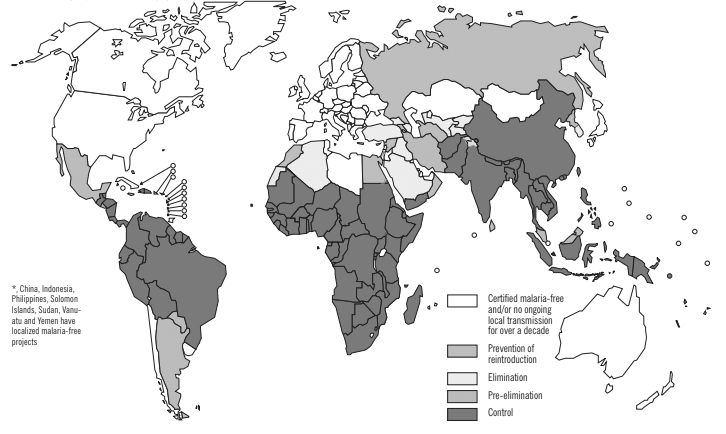 |
Reprinted with permission from the World Health Organization. World malaria report 2009. Published Nov. 29, 2009. https://bit.ly/377SfB7 |
The female Anopheles seeks out a blood meal principally to provide nutrition to eggs that she lays every two to three days.5 Feeding behaviors vary among different species and even among different population groupings of Anopheles of the same species. While the female Anopheles generally is thought of as feeding at dusk and dawn, some species feed throughout the night, making their interactions with sleeping humans more likely and rendering preventive interventions, such as bednets, critical.4 Some species of Anopheles prefer to bite and rest indoors while others remain largely outdoors. The activity and sleeping practices of human populations in a geographic area and the feeding preferences of colocated
Anopheles mosquitoes determine how efficiently a given Anopheles species will serve as a malarial vector. Furthermore, not all Anopheles species prefer humans (anthropophily) as a source for blood meals, with some species preferring cattle or other animals (zoophily). Anopheles species in Africa, Anopheles gambiae and Anopheles funestus, are particularly anthropophilic, which may contribute to the extent of malaria in the African region.5
The necessary requirements for malaria transmission to occur include the two host species (humans and Anopheles) as well as the parasite itself. With these three species present, the intensity of transmission is directly related to the entomologic inoculation rate (EIR), or the number of infectious female anopheline bites per person per year. The EIR for a given region might fluctuate based on season or human/vector behaviors, with higher EIRs realized in any situation wherein susceptible human or Anopheles populations increase, or the frequency of successful Anopheles biting increases.7
Environmental and ecological factors directly determine the degree to which Anopheles may reproduce and flourish. Female Anopheles mosquitoes lay their eggs in standing fresh water, and their offspring undergo several molts before emerging in the pupal stage.5 As a result, malaria epidemics may be seasonal in parts of the world with defined dry and wet seasons. As can be imagined, as the population of biting Anopheles increases, a previously small, stable human-mosquito malarial infection epidemic may grow. Increases in the population of infected mosquitoes may lead to a progression of human cases and the development of an epidemic.5,8
Life Cycle
Malaria comprises four species of Plasmodia, which are able to cause human infection. A fifth species, Plasmodium knowlesi, recently has been shown to be responsible for a portion of human infection in Indonesia — and likely elsewhere.9 The vast majority of severe malaria results from infection with Plasmodium falciparum, although all species of malaria may cause significant clinical symptoms and, potentially, serious disease in children with preexisting disease or impaired immune function.2,3 P. falciparum is the most common form of malaria in Africa, accounting for more than 99% of cases and more than 50% of cases in Southeast Asia. Plasmodium vivax is the most common cause of malaria infection in the Americas, responsible for ~75% of cases.2,3 While an in-depth discussion of the malarial life cycle is beyond the scope of this article, a brief description of the stages of reproduction is relevant to understanding the pathophysiology of disease and identifying parasitic forms on blood smears.
Malaria infection occurs when an individual is bitten by an infected female Anopheles mosquito. The mosquito injects the malaria parasites in the form of sporozoites into human blood. Once in the bloodstream, sporozoites make their way to the liver, where they undergo asexual reproduction over the course of seven to 10 days. This phase generally is asymptomatic, and patients largely are unaware that they are infected.
In the hepatic cells, the parasite forms a liver schizont, which matures and releases merozoites into the bloodstream. In the case of infection with P. vivax and Plasmodium ovale, the parasite may form a dormant hypnozoite phase in the liver, which then may cause recurring infection potentially weeks to years after the initial infection.10 These merozoites then invade erythrocytes, where they may develop into a ring trophozoite stage, forming an erythrocytic schizont phase. This erythrocytic schizont phase then releases its own merozoites, eventually leading to lysis of red blood cells (RBCs), releasing further merozoites and leading to symptomatic disease and, potentially, severe hemolysis.
Some parasites mature into a sexual phase known as the gametocytes (male and female). These gametocytes circulate in the bloodstream and then are ingested by another female Anopheles mosquito during a blood meal. The erythrocytic forms of the life cycle are visible via microscopy on peripheral blood smears, which were the basis of diagnosis for decades prior to the development of antigen-based tests. In the mosquito gut, male and female gametocytes fuse and eventually develop into oocysts. Oocysts eventually release sporozoites, which then invade the Anopheles salivary glands and, thus, become able to infect another human host on a subsequent Anopheles meal.10
See Figure 2 for an overview of the malaria life cycle.
Figure 2. Malaria Life Cycle |
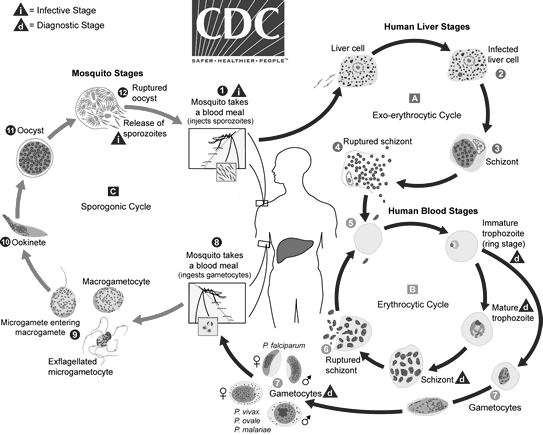 |
Source: Centers for Disease Control and Prevention. Malaria: Parasite biology. Updated Oct. 6, 2020. https://www.cdc.gov/dpdx/malaria/index.html |
Pathophysiology
Malaria can span the spectrum of severity from asymptomatic infection or minor febrile illness, which may occur in healthy adults with a history of prolonged (lifelong) prior immunologic exposure, to life-threatening disease encompassing multi-organ system failure. Severe disease is seen most often in the two highest risk groups: children younger than 5 years of age and pregnant women. Anyone with nutritional or immune deficiency also is at heightened risk of severe disease from malaria, with malnutrition and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) being important factors in malaria epidemiology in Africa.11,12 The majority of severe disease results from infection with P. falciparum, although P. vivax is a significant cause of severe anemia and mortality in Southeast Asia.2,3
As might be imagined, the severity of malaria infection is directly related to the extent to which the RBC population in a given host is infected. P. falciparum has evolved to be able to infect and reproduce unrestrained in RBCs of any age, producing large percentages of infected RBCs — at times as high as 20% to 30% of the total RBC population in a given individual. Other species of Plasmodium only infect young RBCs, which significantly limits the red cell population available for infection, leading to infected red cell percentages measured in the 1% to 2% range, and thus more limited parasitemia.13,14
The pathophysiology of malaria largely derives from the ability of the parasite to cause RBC lysis and destruction. Given the timing of parasite reproduction, the lysis of RBCs frequently occurs near simultaneously. The rupture of RBCs releases a variety of RBC debris along with parasitic waste products and antigens that stimulate the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-alpha), interferon gamma, and interleukin 1b. Two Plasmodium antigens, glycosylphosphatidylinositol (GPI) and hemozoin, are particularly potent in stimulating the host inflammatory response.15,16 This inflammatory cascade is thought to be responsible for the intense fevers, rigors, and inflammatory response characteristic of malaria infection. Indeed, the duration of the erythrocytic portion of the malarial life cycle determines the periodicity of fever, which occurs every 72 hours in Plasmodium malariae infection (quartan fever) and every 48 hours in P. falciparum, P. vivax, and P. ovale infection (tertian fever).15-17
RBCs infected by the malaria parasite express a variety of cell surface proteins and are identified and destroyed by phagocytic macrophages located in the spleen, leading to further RBC destruction and release of inflammatory cytokines.18 These cytokines, along with the byproducts of heme metabolism by the parasite, supress erythropoiesis in the bone marrow, further exacerbating the severity of anemia.19,20 The production of TNF-alpha and interferon gamma increases the expression of adhesion molecules by capillary endothelial cells and increases vascular permeability.
These effects are central in the progression of malaria pathophysiology leading to RBC adherence to the endothelial walls of capillaries. In turn, this leads to a procoagulatory state and the formation of RBC clots (known as red cell rosettes) and increased inflammation and vascular permeability, which causes the recruitment of leukocytes and their migration out of the capillaries and infiltration of tissues.21,22 This capillary obstruction, along with unchecked local inflammation, is thought to underpin the pathophysiology of cerebral malaria and acute respiratory distress syndrome that are common factors contributing to severe malaria.23 The significant burden of hemoglobin, and specifically the heme molecule itself, released into the circulation by red cell lysis, is responsible for the nephrotoxicity noted in severe malaria. Severe anemia, as well as severe volume depletion, also may contribute as prerenal causes of kidney dysfunction. The dark-colored urine seen in severe malarial hemolysis, called blackwater fever, results from myoglobinuria.24
Clinical Features
As mentioned, the severity of malaria depends on multiple factors, including host immune and nutritional state, comorbid diseases, malaria species, and degree of parasitemia. (See Table 1.) In patients with prolonged immunologic exposure to malaria, particularly in malaria-endemic regions of the world, infections may be mild, manifesting as mild febrile conditions, with only minimal hemolysis and a self-limited course.25 This disease course has been termed “uncomplicated malaria.”
Table 1. Characteristics of Severe Malaria | |
Clinical Manifestations |
Frequency |
Impaired consciousness |
High |
Respiratory distress (acidotic breathing) |
High |
Multiple convulsions |
High |
Prostration |
High |
Shock |
Low |
Pulmonary edema (radiological) |
Infrequent |
Abnormal bleeding |
Infrequent |
Jaundice |
Low |
Laboratory Indices |
|
Severe anemia |
High |
Hypoglycemia |
High |
Acidosis |
High |
Hyperlactatemia |
High |
Acute kidney injury |
Low |
Hyperparasitemia |
Average |
However, despite the unimpressive symptoms, malaria infection still may produce significant illness and morbidity in children in endemic areas. Even in asymptomatic children, anemia is a common laboratory finding in malaria-endemic areas, and concomitant parasitemia has been noted in as many as 50% of anemic children in some studies.26,27 While the cause of anemia in children often is multifactorial, with malnutrition/iron deficiency, concomitant HIV or hookworm infection, or hemoglobinopathies being important contributors, many studies have suggested a critical role for malaria infection in chronic childhood anemia. Interventions using periodic antimalarial treatment in children have been associated with improvements in hemoglobin concentration.28,29
Chronic malarial anemia in childhood may develop from chronic, persistent malaria infection (with or without symptoms) or repetitive, intermittent infections or from recrudescence of dormant malaria (P. vivax and P. ovale). No matter the cause, chronic anemia in childhood has significant consequences for immune system function, growth, and neurocognitive development.30,31 In neonates and infants, the symptoms of malaria may be general, such as fever, poor feeding, tachypnea, diarrhea, and jaundice and so might be mistaken for other causes of fever and sepsis.
In the first months of life, the signs and symptoms of malaria also may be muted or delayed, given the protection afforded by fetal hemoglobin and the extent of breastfeeding and maternal antimalarial antibody protection.30,32 In the immunologically naïve, such as children younger than 5 years of age and adults from non-endemic areas, infection with P. falciparum may progress rapidly, culminating in severe anemia and multi-organ system failure. Severe malaria generally presents as a manifestation of severe anemia, cerebral malaria, or a combination of the two.30
Severe Malarial Anemia
The World Health Organization (WHO) defines severe malarial anemia (SMA) as a hemoglobin concentration of less than 5 mg/dL (hematocrit less than 15%) with a parasitemia of greater than 10,000 parasites/μL. Children younger than 5 years of age with a hemoglobin concentration of less than 3 mg/dL are at the highest risk for death.2,3,33 Anemia associated with malaria is typically normocytic and normochromic, although concomitant iron deficiency anemia or other causes of anemia may complicate hematologic findings.
Severe malarial anemia may contribute to respiratory distress as infants and children struggle to increase minute ventilation in response to rapid and extreme decreases in blood oxygen-carrying capacity. Inadequate oxygen delivery to tissues also can contribute to severe metabolic acidosis, which is a common abnormality in children with severe malaria and can further increase respiratory demands.
As SMA progresses and the oxygen-carrying capacity of the blood is further reduced, children will demonstrate evidence of end-organ hypoperfusion and multi-organ system failure. Renal impairment may be significant in children with SMA, since hypovolemia, hemolysis, and microvascular obstruction cause acute tubular necrosis and potentially contribute further to the metabolic acidosis.34,35
Cerebral Malaria
Cerebral malaria (CM) may present as altered consciousness, delirium, or seizures. The WHO defines CM as the presence of coma greater than one hour after a seizure, regardless of the use of anti-seizure medications.31 The Blantyre Coma Scale has been used to quantify the degree of coma in CM.31 CM is most common among those with comorbid conditions, such as HIV/AIDS, malnutrition, or prior splenectomy. Because of the high frequency of parasitemia in even asymptomatic children in malaria-endemic areas, other explanations for seizures or central nervous system dysfunction, such as febrile seizures, meningitis, encephalitis, or hypoglycemia, must be considered. A characteristic retinopathy has been associated with CM and, if present, increases confidence in the diagnosis.31 Focal neurologic findings rarely are associated with CM and, if noted, should prompt consideration of other clinical explanations.
CM has a mortality rate ranging from 10% to 40%.31 Microvascular obstruction and inflammation, progressing to cerebral edema, increasing intracranial pressure, and ultimately leading to herniation is the suspected etiology in most fatal CM cases.31 A large body of literature has established that, among CM survivors, longstanding neurocognitive deficits (i.e., behavioral/cognitive delay, epilepsy) are more common compared to age-matched controls.31
Other Manifestations of Severe Malaria
Hypoglycemia is a common associated complication of severe malarial infection. It is thought to result from increased metabolic demands and utilization as well as impaired hepatic gluconeogenesis. Hypoglycemia occurs more commonly in younger children in whom fasting and delayed or inadequate nutritional intake may contribute. Hypoglycemia may be difficult to detect in children with concomitant CM and coma or delirium. Hypoglycemia is associated with worse clinical outcomes.31
Metabolic acidosis frequently is noted in children with severe malaria, and its causes often are multifactorial. Impaired tissue oxygen delivery resulting in increased anaerobic metabolism and lactic acidosis are significant contributors, although acute kidney injury also may lead to metabolic acidosis. The severity of metabolic acidosis correlates with poor outcomes.31
Fever in the Returned Traveler
Although malaria may produce impressive symptoms and laboratory abnormalities, some immunologically naïve patients may initially present with mild or nonspecific symptoms. Travelers who have been inconsistently using malarial prophylaxis may have partially treated infections and present with mild or intermittent symptoms. Furthermore, symptoms not traditionally associated with malaria, such as diarrhea and vomiting, may distract clinicians and frequently are misattributed to viral infection or gastrointestinal illness.35
As such, malaria must be considered in all travelers with fever who are returning from malaria-endemic areas regardless of the use of prophylactic medications. Additionally, P. vivax and P. ovale have the ability to lay dormant in hepatocytes in the hypnozoite phase and cause infection months after the patient returns home, potentially long after termination of antimalarial prophylaxis, meaning that a history of even remote travel to a malarial region may be relevant. In the case of infection with P. falciparum, delays in the administration of antimalarial medications may have fatal implications.
Diagnosis
Traditionally, the diagnosis of infection with any of the species of malaria has been accomplished via microscopic examination of peripheral blood smears. (See Figure 3.) In many parts of the world, this remains the standard of care. However, newer, reliable antigen-based rapid diagnostic tests (RDTs) have become available, allowing rapid diagnosis without the need for microscopes or trained microscopists. While rapid tests have streamlined the time to diagnosis, this comes at the expense of speciation and determination of parasite burden, both of which generally are obtainable via examination of peripheral blood smears.36
Figure 3. Preparing Thin and Thick Blood Smears |
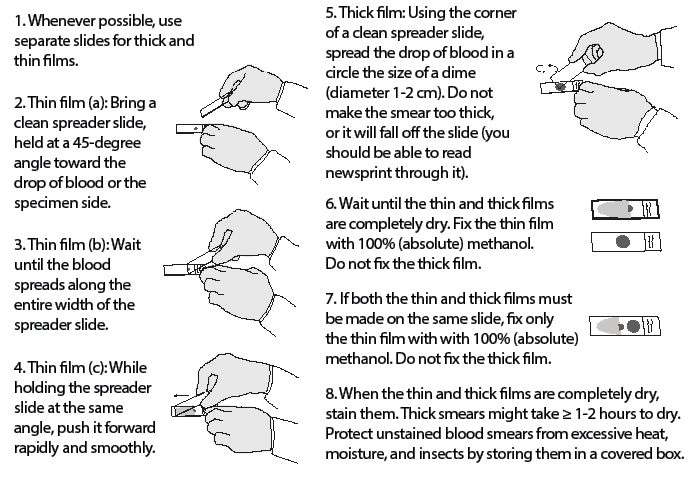 |
Adapted from Centers for Disease Control and Prevention. Laboratory diagnosis of malaria: Preparation of blood smears. https://www.cdc.gov/dpdx/resources/pdf/benchaids/malaria/malaria_procedures_benchaid.pdf |
The preparation of blood smears for malaria diagnosis usually involves the creation of both a “thick smear” and a “thin smear.” The thick smear is prepared by placing a single drop of venous blood in the middle of a microscope slide and then using the corner of another slide to smear the blood in a circular fashion. The slide then should be allowed to dry for at least half an hour. An appropriately prepared thick smear should allow the physician to barely read printed words through the blood smear.36 Thick smears allow for increased sensitivity in the detection and diagnosis of malaria, but they do not allow for speciation. The thin smear is prepared by placing a drop of blood in the middle of a microscope slide and then using the edge of another slide to smear the blood so that there is a gradient of thickness of the smear, allowing for a clearer picture of RBCs and parasite morphology. Both thin and thick smears may be used to quantify the degree of parasitemia. Thin smears dry more rapidly than thick smears and, unlike thick smears, are fixed in methanol prior to Giemsa staining.36
In the hands of an experienced microscopist, malaria percentages as low as 1% to 2% can be detected. If suspicion for malaria is high, multiple smears, prepared at different times (particularly when patients are actively symptomatic), may increase diagnostic sensitivity.37,38 While smears may be helpful in determining parasite burden, the sensitivity of RDTs generally is superior, with reported sensitivities in the 97% range compared with 85% for malaria blood smear analysis.39
RDTs for malaria have become an important tool in settings where microscopy is not reliable or obtainable. RDTs generally assay for one of several Plasmodium antigens: histidine rich protein (HRP2, P. falciparum only) or Plasmodium-specific lactate dehydrogenase and/or aldolase. These assays may provide the diagnosis of malaria in general or differentiate between P. falciparum and P. vivax only.
As such, the choice of which antigen tests to use in a particular area is dependent to some degree on the local saturation of non-falciparum malaria and the need to differentiate between species. Polymerase chain reaction tests for all Plasmodium species and for specific drug resistance mutations have been developed and are used mainly in research and epidemiology.38,40
Differential Diagnosis
The differential diagnosis for malaria infection is broad, given that the symptoms of early or uncomplicated malaria are quite nonspecific. The differential diagnosis for uncomplicated malaria includes influenza, dengue fever, chikungunya, leptospirosis, and other viral causes of fever and malaise. The differential diagnosis for severe malaria includes meningitis, disseminated intravascular coagulation, sepsis/bacteremia, hemolysis, hepatitis, viral hemorrhagic fever, and African trypanosomiasis.
A positive malaria smear in a patient with severe symptoms in a malaria-endemic region should prompt treatment with antimalarial medications. However, given the possibility of chronic or asymptomatic parasitemia in these areas, specific consideration should be paid to other diagnostic considerations before concluding that severe illness is attributable solely to malaria.
Malaria Treatment
The choice of antimalarial therapy depends on multiple factors, including the severity of disease, resistance patterns observed in the local region, and the causative species of malaria involved. (See Tables 2 and 3.) In P. vivax and P. ovale infections, the goal of treatment is eradicating not only blood of parasites, but also hypnozoite forms residing in hepatocytes. Appropriate antimalarial dosing is critical in children, since underdosing has been identified as causing clinical decline or recurrence and treatment failure.31,33
Table 2. Malaria Treatment | |
Plasmodium falciparum Acute Uncomplicated Malaria | |
Artemether-lumefantrine (20/120 mg) |
|
Artesunate-amodiaquine |
|
Artesunate-mefloquine |
|
Artesunate-sulfadoxine-pyrimethamine |
|
Dihydroartemisinin-piperaquine |
|
Atovaquone-proguanil (pediatric tab: 62.5/25 mg) (adult tab: 250/100 mg) |
|
Quinine plus doxycycline or tetracycline or clindamycin |
|
Mefloquine |
|
Chloroquine-Sensitive Regions | |
Chloroquine |
|
Hydroxycloroquine |
|
Table 3. Treatment for Severe Malaria | ||
Antimalarial |
Dose | |
Parenteral Therapy | ||
Artesunate (All patients with severe malaria, regardless of infecting species, should be treated with intravenous artesunate) |
2.4 mg/kg/dose intramuscular or intravenously initially, followed by 2.4 mg/kg/se at 12 and 24 hours; if patients cannot tolerate oral meds, then use artesunate each day for up to six additional days. | |
Artemether |
3.2 mg/kg intramuscular followed by 1.6 mg/kg intramuscularly each day for 3 days or until an ACT can be commenced | |
Reassessment: Four hours after the third dose if parasite density is < 1% and the patient is stable, an oral regimen may be completed. If the patient is clinically worsening or the parasite density is > 1%, continue IV artesunate until parasite density is < 1% and the patient is able to tolerate an oral regimen and is clinically improving. | ||
Oral Therapy | ||
Artemether-lumefantrine |
5-15 kg: Day 1: 1 tab and a second tab 8 hours later. Day 2 and Day 3: 1 tab twice per day | |
15-25 kg: D1: 2 tabs and a second 2 tabs 8 hours later. Day 2 and Day 3: 2 tabs twice per day | ||
25-35 kg: D1: 3 tabs and a second 3 tabs 8 hours later. Day 2 and Day 3: 3 tabs twice per day | ||
> 35 kg: D1: 4 tabs and a second 4 tabs 8 hours later. Day 2 and Day 3: 4 tabs twice per day | ||
Atovaquone-proguanil (pediatric tablet: 62.5/25 mg) (adult tablet: 250/100 mg) |
5-8 kg: 2 pediatric tabs once per day x 3 days | |
9-10 kg: 3 pediatric tabs once per day x 3 days | ||
11-20 kg: 1 adult tabs once per day x 3 days | ||
21-30 kg: 2 adult tabs once per day x 3 days | ||
31-40 kg: 3 adult tabs once per day x 3 days | ||
> 40 kg: 4 adult tabs once per day x 3 days | ||
Uncomplicated Malaria in Children
In February 2021, the WHO released updated guidelines about the treatment of malaria, incorporating evidence from recent studies and assigning level-of-evidence ratings to recommendations. The following treatment recommendations are based on that document.33
Artemisinin combination therapies (ACTs) have been the treatment of choice for uncomplicated P. falciparum malaria for several years. ACTs now are recommended as the first-line treatment in P. knowlesi infections as well.31,33 In some malaria-endemic regions, P. vivax remains sensitive to chloroquine, which may be used in those areas. However, in locations of high P. vivax resistance to chloroquine, treatment with ACTs is recommended. P. ovale and P. malariae are identified less commonly, so data regarding optimal treatment regimens are limited. However, the ACTs seem to have some effectiveness against these species and should be used initially, if available.33
Piperaquine and lumefantrine, along with artemesinin compounds, have prolonged half-lives (piperaquine: ~30 days, lumefantrine: ~4 days) and may play an important role in limiting recurrence of malaria in the immediate post-treatment period.31,33 Similarly, primaquine (used for P. vivax and P. ovale infection) is associated with reduced recurrence via elimination of the hypnozoite form. Primaquine also has been used in the treatment of P. falciparum malaria as a gametocidal therapy along with ivermectin (not recommended for children < 15 kg) to potentially reduce transmissibility during treatment more rapidly than purely schizonticidal therapy would.31,33 Treatment with primaquine typically requires antecedent testing for glucose-6-phosphate dehydrogenase deficiency (G6PD) to avoid hemolysis, although recent WHO guidelines recommend that no G6PD testing is required for single-dose primaquine.33
Visit https://bit.ly/3j7akEW to view a map of areas with drug-resistant malaria.
Severe Malaria in Children
The AQUAMAT (2015) study enrolled more than 5,000 children in nine African countries and compared parenteral quinine vs. artesunate-based treatment regimens for the treatment of severe malaria caused by P. falciparum. Those in the artesunate treatment arm had significantly lower mortality than those in the quinine-treated group. Therefore, WHO guidelines since have recommended the use of artesunate-based regimens for the treatment of severe P. falciparum malaria.33,41 Once a child has received at least 24 hours of parenteral artesunate and is able to tolerate oral therapy, treatment can be completed with three days of oral ACT.33
Children weighing < 20 kg should receive a higher dose of artesunate (3 mg/kg per dose) than larger children and adults (2.4 mg/kg per dose) to ensure equivalent exposure to the drug. If artesunate is not available, artemether is preferred over quinine for treating children and adults with severe malaria.33
Children with severe malaria often require intensive supportive care focused on seizure management, hypoglycemia avoidance, and fluid and blood product transfusion management. Prophylactic antiseizure medications are not recommended and have been associated with higher mortality.31
However, the judicious use of benzodiazepines to treat seizures and status epilepticus often is necessary. Blood product transfusion would seem to be an important intervention to counteract the deleterious effects of severe malarial anemia, but studies investigating larger volume transfusions (20 vs. 30 mL/kg body weight) and immediate, early transfusion have not shown a benefit, and WHO guidelines currently suggest transfusion be triggered by severity of condition or hemoglobin
< 4 mg/dL.31
Administration of bolus colloid or crystalloid solutions has been associated with increased mortality, and fluid resuscitation in severe malaria should be undertaken cautiously.42 Hypoglycemia is a common occurrence in severe malaria, and frequent evaluation of blood glucose levels is critical. Many patients have altered sensorium and seizure, which may mask serious hypoglycemia. Given the prevalence of chronic or recurrent malaria infection in children in areas of high endemicity, other differential diagnostic considerations should be entertained in critically ill children with positive malaria smears. Bacteremia has been associated with malaria smear positivity, so patients with severe disease should be considered for broad spectrum antibiotic coverage in conjunction with appropriate antimalaria therapy.31
Prevention
Malaria prevention strategies involve multipronged efforts to disrupt the vector-host life cycle. These include vector eradication efforts, insecticide-treated bednets, indoor residual spraying, insect repellents and insect-resistant clothing, antimalarial medications, and, more recently, the development and testing of a vaccine.
Vector Eradication
Insecticide spraying for Anopheles mosquitoes has long been used as a strategy for reducing malaria transmission. Widespread outdoor administration of dichlorodiphenyltrichloroethane (DDT) as an insecticide in agriculture and malaria control efforts was common until the 1970s, when growing evidence of the insecticide’s environmental and toxic effects limited its use. Indoor residual spraying of walls and ceilings with insecticides, such as DDT, pyrethroids, or malathion, has been shown to reduce malaria transmission by reducing the lifespan of Anopheles mosquitoes, preventing the completion of the portion of the malarial life cycle taking place in the mosquito, and reducing the number of blood meals taken.33,39,43
Vector resistance to all insecticides has been a recurring problem, and DDT has been re-examined for use in indoor residual spraying in high-malaria endemnicity areas where the public health benefit of limited indoor use is thought to outweigh the deleterious environmental effects. The WHO has stated that using DDT should be phased out over time in favor of other less toxic substitutes.33,39,43
Insecticide-Treated Bednets
Using untreated bednets has long been associated with reduced rates of malaria infection. However, insecticide-treated bednets have been shown to provide greater reductions in malaria transmission and mortality.30 The insecticide of choice for insecticide-treated bednets mostly has been pyrethroids, but resistance among mosquito populations has been documented, which is causing a shift in some regions to piperonyl butoxide-treated nets.44 Long-lasting insecticide-treated nets, which provide effective insecticide concentrations for at least three years, also have been developed. The WHO recommends that long-lasting insecticide-treated nets be used by all people living in areas of high malaria endemnicity.33
Larval Control
Female Anopheles mosquitoes lay eggs in standing bodies of fresh water, and the larvae that hatch from these eggs continue to live in the fresh water until they develop into pupae, then they leave the water as adult mosquitoes. The elimination of standing freshwater sources, such as filling depressions in the earth that collect water or draining swamps, has been shown to reduce malaria infection.45 Furthermore, the introduction of chemical larvicides or larvicidal treatments to bodies of fresh water in highly malarial areas likewise has demonstrated a reduction in malaria infection. Lastly, several species of larvivorous fish have been used to reduce larvae in ponds, wells, and lakes in India with a significant reduction in local cases of malaria.46
Pharmacologic Therapy in Malaria Prevention
Acute treatment for malaria infection can be critical to prevent severe complications, morbidity, and mortality and also can reduce transmission by limiting the duration of infections and the degree of parasitemia. However, most antimalarial treatments are not gametocidal and do not prevent the uptake of gametocytes by mosquitoes during a blood meal. As a result, standard pharmacologic therapy does not immediately prevent transmissibility. Treatment with low-dose primaquine at the commencement of antimalarial treatment in children older than age 6 months and nonpregnant adults has been shown to reduce transmission compared to standard antimalarial treatment alone. Previously, all individuals treated with primaquine were tested for G6PD deficiency, since it can cause life-threatening hemolysis, but more recent studies have questioned the necessity for widespread G6PD testing.31,33
Multiple studies have supported the use of mass drug administration (MDA) campaigns in the reduction of malaria incidence and transmission. Several different approaches to MDA have been evaluated, including identifying asymptomatic cases via rapid diagnostic testing or microscopy followed by targeted MDA to families or households residing within a specific spatial radius around the positive individual(s). MDA also has been used in a targeted fashion in regions of both low and high malarial prevalence. In Myanmar, the identification of malarial hot spots followed by targeted MDA with
dihydroartemisinin-piperaquine plussingle-dose primaquine once per month for three consecutive months resulted in an 80% reduction in the incidence of malaria in the target villages as well as surrounding villages. There was no observed increase in malaria resistance to the antimalarials used in the study.47 In Gambia, an area of seasonal malaria transmission, MDA with dihydroartemisinin-piperaquine prior to the beginning of the malarial season resulted in a reduction in infections.48
Mass administration of antimalarials also is recommended by the WHO for targeted populations. The WHO recommends intermittent preventive treatment (IPT) in infants or coadministration of slowly metabolized antimalarials (sulfadoxine-pyrimethamine) with measles and tetanus, diphtheria, and pertussis vaccination.33 The WHO also recommends prophylaxis with trimethoprim-sulfamethoxazole for neonates exposed to HIV at birth as a mechanism for preventing opportunistic infections, such as Pneumocystis jirovecii; this also has shown some effectiveness in preventing malaria infection.
Likewise, the WHO endorses IPT in children (IPTc) for children younger than 6 years of age in areas of seasonal malaria. IPTc has been shown to substantially reduce the incidence of malaria infection among children in multiple studies and across multiple regions. IPT in pregnant patients also is effective in reducing the incidence of malaria infection in this high-risk group.33 Moreover, ivermectin has been shown to reduce the lifespan of Anopheles mosquitoes who feed on the blood of ivermectin-treated individuals. Several studies have demonstrated decreased malarial incidence among treated individuals compared to control groups.49
Prophylactic dosing of antimalarial medications for travelers headed to malaria-endemic regions is vital to preventing disease in these potentially immunologically naïve individuals. Multiple regimens are available depending on regional resistance patterns, speciology, and patient medical history. A discussion of malaria prophylaxis for travelers is beyond the scope of this review, and readers are referred to the Centers for Disease Control and Prevention (www.cdc.gov/malaria) for recommendations on appropriate prophylaxis.
Malaria Vaccine
Work on an effective malaria vaccine has been ongoing for decades, with recent progress allowing some optimism for the first time. The RTS,S/AS01 P. falciparum vaccine is the only vaccine candidate to date to demonstrate effectiveness in reducing malaria caseload and severity of disease as well as the need for hospitalization and blood transfusion in a broad range of transmission settings. While there is currently no WHO policy on the widespread administration of the RTS,S/AS01 vaccine, the Strategic Advisory Group of Experts and the Immunization and Malaria Policy Advisory Committee jointly have supported pilot implementation of the vaccine in further regions across sub-Saharan Africa.31,50
Conclusion
Malaria has been the focus of intense public health investment and attention. In recent years, there has been evidence of significant progress toward reducing the global burden of malarial disease and death. Preventive strategies, including insecticide treated nets, long-lasting insecticide treated nets, and indoor residual spraying likely have contributed to a global reduction in malaria cases. Antimalarials, obviously a cornerstone of malaria therapy, have become instruments of prevention, with MDA, SMA, and IPT gaining evidentiary support in the prevention of infection. The development of resistance both to therapeutics as well as anti-mosquito insecticides remains a challenge and treatment guidelines will require continued updating to take into account regional resistance emergence. Underdosing has been identified as a significant problem in treating malaria in children, and attention to updated, weight-based dosing according to WHO recommendations is critical.
Malaria will undoubtedly continue to be responsible for a significant burden of illness among the world’s children and travelers to malarial areas, and severe malaria will continue to be a challenging clinical condition for pediatricians to both diagnose and treat. However, adherence to evidence based guidelines for prevention and treatment, such as those delineated by the WHO, will optimize outcomes and reduce malaria case burdens.
References
- Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev 2002;15:564-594.
- World Health Organization. Malaria. Published April 1, 2021. https://www.who.int/news-room/fact-sheets/detail/malaria
- World Health Organization. World malaria report 2019. Published Dec. 4, 2019. https://www.who.int/publications/i/item/9789241565721
- Belachew EB. Immune response and evasion mechanisms of Plasmodium falciparum parasites. J Immunol Res 2018;2018:6529681. https://doi.org/10.1155/2018/6529681
- Centers for Disease Control and Prevention. About malaria: Biology: Mosquitoes. Updated July 16, 2020. https://www.cdc.gov/malaria/about/biology/#tabs-1-5
- Sherrard-Smith E, Skarp J, Beale AD, et al. Mosquito feeding behavior and how it influences residual malaria transmission across Africa. Proc Natl Acad Sci USA 2019;116:15086-15095.
- Smith T, Maire N, Dietz K, et al. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg 2006;75(2 Suppl):11-18.
- Nguyen M, Howes RE, Lucas TC, et al. Mapping malaria seasonality in Madagascar using health facility data. BMC Med 2020;18:26.
- Figtree M, Lee R, Bain L, et al. Plasmodium knowlesi in human, Indonesian Borneo. Emerg Infect Dis 2010;16:672-674.
- Centers for Disease Control and Prevention. About malaria: Biology: Lifecycle. Updated July 16, 2020. https://www.cdc.gov/malaria/about/biology/index.html
- World Health Organization. Malaria in HIV/AIDS patients. Updated April 27, 2017. 2021. https://web.archive.org/web/20180924214552/http://www.who.int/malaria/areas/high_risk_groups/hiv_aids_patients/en/
- Das D, Grais RF, Okiro EA, et al. Complex interactions between malaria and malnutrition: A systematic literature review. BMC Med 2018;16. https://doi.org/10.1186/s12916-018-1177-5
- Tamez PA, Liu H, Fernandez-Pol S, et al. Stage-specific susceptibility of human erythroblasts to Plasmodium falciparum malaria infection. Blood 2009;114:3652-3655.
- Malleret B, Li A, Zhang R, et al. Plasmodium vivax: Restricted tropism and rapid remodeling of CD71-positive reticulocytes. Blood 2015;125:1314-1324.
- Schwarzer E, Turrini F, Ulliers D, et al. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med 1992;176:1033-1041.
- Krishnegowda G, Hajjar AM, Zhu J, et al. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: Cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 2005;280:8606-8616.
- Ferreira A, Balla J, Jeney V, et al. A central role for free heme in the pathogenesis of severe malaria: The missing link? J Mol Med (Berl) 2008;86:1097-1111.
- del Portillo HA, Ferrer M, Brugat T, et al. The role of the spleen in malaria. Cellular Microbiol 2011;14:343-355.
- Casals-Pascual C, Kai O, Cheung JOP, et al. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 2006;108:2569-2577.
- Lamikanra AA, Merryweather-Clarke AT, Tipping AJ, Roberts DJ. Distinct mechanisms of inadequate erythropoiesis induced by tumor necrosis factor alpha or malarial pigment. PLoS One 2015;10:e0119836.
- Newbold C, Craig A, Kyes S, et al. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int J Parasitol 1999;29:927-937.
- Kaul DK, Roth EF Jr, Nagel RL, et al. Rosetting of Plasmodium falciparum-infected RBCs with uninfected RBCs enhances microvascular obstruction under flow conditions. Blood 1991;78:812-819.
- Ponsford MJ, Medana IM, Prapansilp P, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 2012;205:663-671.
- Bodi JM, Nsibu CN, Aloni MN, et al. Black water fever associated with acute renal failure among Congolese children in Kinshasa. Saudi J Kidney Dis Transpl 2014;25:1352-1358.
- Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev 2009;22:13-26.
- World Health Organization. World malaria report 2020. https://www.who.int/docs/default-source/malaria/world-malaria-reports/9789240015791-double-page-view.pdf?sfvrsn=2c24349d_5
- Menon MP, Yoon SS; Uganda Malaria Indicator Survey Technical Working Group. Prevalence and factors associated with anemia among children under 5 years of age--Uganda, 2009. Am J Trop Med Hyg 2015;93:521-526.
- Menendez C, Kahigwa E, Hirt R, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants.Lancet 1997;350:844-850.
- Schellenberg D, Menendez C, Kahigwa E, et al. Intermittent treatment for malaria and anaemia control at time of routine vaccinations in Tanzanian infants: A randomised, placebo-controlled trial. Lancet 2001;357:1471-1477.
- Ashley EA, Poespoprodjo JR. Treatment and prevention of malaria in children. Lancet Child Adolesc Health 2020;4:775-789.
- No authors listed. Severe malaria. TropMed Int Health 2014;19(Suppl 1):7-131.
- Pasvol G, Weatherall DJ, Wilson RJ, et al. Fetal haemoglobin and malaria. Lancet 1976;1:1269-1272.
- World Health Organization. World health organization guidelines for malaria. Published Feb. 16, 2021. https://www.who.int/publications/i/item/guidelines-for-malaria
- Milner DA Jr. Malaria pathogenesis. Cold Spring Harb Perspect Med 2018;8:a025569.
- Wassmer SC, Taylor TE, Rathod PK, et al. Investigating the pathogenesis of severe malaria: A multidisciplinary and cross-geographical approach. Am J Trop Med Hyg 2015;93(3 Suppl):42-56.
- Centers for Disease Control and Prevention. Blood specimens: Specimen processing. Updated Oct. 1, 2020. https://www.cdc.gov/dpdx/diagnosticprocedures/blood/specimenproc.html
- Visser T, Daily J, Hotte N, et al. Rapid diagnostic tests for malaria. Bull World Health Organ 2015;93:862-866.
- Wilson ML. Malaria rapid diagnostic tests. Clin Infect Dis 2012;54:1637-1641.
- Stauffer WM, Cartwright CP, Olson DA, et al. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in U.S. clinical practice. Clin Infect Dis 2009;49:908-915.
- Boggild AK, Page AV, Keystone JS, et al. Delay in diagnosis: Malaria in a returning traveller. CMAJ 2009;180:1129-1131.
- Dondorp AM, Fanello CI, Hendriksen ICE, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): An open label randomised trial. Lancet 2010;376:1647-1657.
- Maitland K, Babiker A, Kiguli S, et al. The FEAST trial of fluid bolus in African children with severe infection. Lancet 2012;379:613; author reply 613-614.
- Tangena J-AA, Hendriks CM, Devine M, et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: An adjusted retrospective analysis. Malar J 2020;19:150.
- Steinhardt LC, Yeka A, Nasr S, et al. The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda. Am J Trop Med Hyg 2013;88:855-861.
- Pryce J, Richardson M, Lengeler C. Insecticide-treated nets for preventing malaria. Cochrane Database Syst Rev 2018;11:CD000363.
- Centers for Disease Control and Prevention. Larval control and other vector controI interventions. Updated July 16, 2020. https://www.cdc.gov/malaria/malaria_worldwide/reduction/vector_control.html
- Walshe DP, Garner P, Adeel AA, et al. Larvivorous fish for preventing malaria transmission. Cochrane Database Syst Rev 2017;12:CD008090.
- Landier J, Parker DM, Thu AM, et al. Effect of generalised access to early diagnosis and treatment and targeted mass drug administration on Plasmodium falciparum malaria in Eastern Myanmar: An observational study of a regional hospital. Lancet 2018;391:1916-1926.
- Mwesigwa J, Achan J, Affara M, et al. Mass drug administration with dihydroartemisinin-piperaquine and malaria transmission dynamics in the Gambia: A prospective cohort study. Clin Infect Dis 2019;69:278-286.
- World Health Organization. Malaria vaccine implementation programme: One year on. Updated April 22, 2020. https://web.archive.org/web/20210106004558/https://www.who.int/immunization/diseases/malaria/malaria_vaccine_implementation_programme/en/
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
