Throat Infections Part II: Deadly, Must-not-Miss, Pediatric Throat Infections
August 1, 2021
AUTHOR
Daniel Migliaccio, MD, Clinical Assistant Professor, Ultrasound Fellowship Director, Department of Emergency Medicine, Universityof North Carolina, Chapel Hill
PEER REVIEWER
Catherine Marco, MD, Wright State University, Dayton, OH
Executive Summary
- Peritonsillar abscesses are the most common deep-seated oropharyngeal infection seen by emergency providers. Peritonsillar cellulitis, or phlegmon, involves an inflammatory reaction in the area between the pharyngeal muscle and palatine tonsillar pillars. When a discrete pocket of pus forms in this area, it is known as a peritonsillar abscess.
- Imaging is not necessary to diagnose peritonsillar abscess, and the diagnosis may be made clinically. In patients with undifferentiated sore throat with concern for other deep-seated neck space infections, the diagnosis may be facilitated with advanced imaging, such as ultrasound or computed tomography (CT) with intravenous (IV) contrast.
- Retropharyngeal infections represent a subtype of deep neck space infection that arises between the alar fascia anteriorly and prevertebral fascia posteriorly and extends from the base of the skull to the posterior mediastinum. The retropharyngeal space communicates with the lateral pharyngeal space and is a potential path for pharyngeal infections to be transmitted into the mediastinum. Abscess formation in this area typically occurs following an upper respiratory tract infection, pharyngitis, or recent trauma to the pharynx, such as dental procedures or recent intubation. Retropharyngeal abscess is most common in young children between the ages of 2 and 4 years.
- In children with retropharyngeal infections, a lateral neck radiograph typically will demonstrate a widened retropharyngeal or prevertebral space. The film should be taken in the lateral plane during patient inspiration with the neck in extension. Failure to follow proper technique can lead to false-positive studies. In children younger than 4 years of age, the retropharyngeal space at the level of C2 should be less than half the width of the vertebral body at that level. An abnormally wide space is considered when the diameter of the space is greater than the size of the vertebral body at C2, or a gradient of 7 mm at C2 or 14 mm at C6.
- Infectious thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, is a rare complication following an oropharyngeal infection, most commonly bacterial pharyngitis. The infection spreads contiguously to the nearby internal jugular vein, leading to thrombosis of the vein. Subsequently, septic emboli are formed and can lead to various clinical presentations.
- Emergency department diagnosis of Lemierre’s syndrome typically is facilitated with radiological imaging. Blood and/or throat cultures subsequently may reveal F. necrophorum while a patient is hospitalized. Imaging modalities include CT scanning of the neck and chest with IV contrast. Ultrasound of the neck and internal jugular may be considered as well, although that is less sensitive than CT and does not help to differentiate potential complications, such as septic pulmonary emboli. The benefits of ultrasound include the rapidity in which it may be performed at the bedside and the ability to perform the study on unstable patients at the bedside. Ultrasound of the internal jugular vein may demonstrate thrombus within the vein.
- Ludwig’s angina refers to a life-threatening infection of the bilateral submandibular, submental, and/or sublingual space. Infection typically originates from an odontogenic source (most commonly second/third mandibular molar), but it may spread from a pharyngitis, parotiditis, or peritonsillar abscess.
- Patients presenting with Ludwig’s angina typically present with systemic symptoms of fevers, chills, and malaise, along with neck pain, odynophagia, neck stiffness, drooling, changes in voice, and trismus. Patients usually present with rapid/acute spreading infection to the floor of the mouth with a potential “woody” or solid texture on palpation of the floor of the mouth while it is elevated. The patient’s neck may appear erythematous and tender, with a symmetric brawny swelling and induration of the submandibular region.
- Acute laryngotracheitis (croup) is a common cause of acute hoarseness and/or stridor in children younger than 6 years of age. It is most common in children from 6 months of age to 3 years of age. The most common etiology is viral, with parainfluenza virus type I being the most likely culprit.
- Patients with moderate to severe croup should be provided nebulized racemic epinephrine at a dose of 0.05 mL/kg per dose (diluted in a total of 3 mL) over 15 minutes in addition to corticosteroids such as dexamethasone.
Introduction
While less frequently encountered than pathologies described in part 1, pediatric patients presenting with sore throat may come into the emergency department with an acute life threatening illness. The emergency provider must be aware of these pathologies and look for diagnostic clues to rapidly diagnose and treat deadly neck space infections. As will be discussed, any ill-appearing pediatric patient with sore throat and/or with a change in voice or stridor should prompt a rapid and thorough evaluation to ensure expedited management. This can potentially avoid an airway catastrophe.
Peritonsillar Abscess
Background
Peritonsillar abscesses are the most common deep-seated oropharyngeal infection seen by emergency providers. Peritonsillar cellulitis, or phlegmon, involves an inflammatory reaction in the area between the pharyngeal muscle and palatine tonsillar pillars.1 The most common location for a peritonsillar abscess is in the superior pole of the tonsil. Typically, the infection forms from a direct spread from a prior tonsillitis and/or pharyngitis. Potential complications of untreated peritonsillar abscesses include airway obstruction, sepsis, mediastinitis, carotid artery pseudoaneurysm formation, and/or internal jugular vein thrombophlebitis, also known as Lemierre’s syndrome. Patients with peritonsillar abscess are at risk for future episodes of abscess formation.
Presentation
Patients with peritonsillar abscess usually will present with a subacute sore throat along with systemic symptoms, such as fever, chills, and malaise. Classically, patients will have voice changes, described as having a “hot potato voice.” Many times, patients will have ipsilateral otalgia associated with the peritonsillar abscess secondary to inflammation, which spreads along the masseter muscle. An emergent assessment of the airway needs to be made with patients presenting with concerning signs for peritonsillar abscess. Patients with stridor, upper airway obstruction, drooling, and posturing should receive expedited evaluation and management.
Diagnosis
Diagnosis of peritonsillar abscess typically is clinical and involves an examination of the posterior oropharynx, demonstrating a deviated uvula with significant unilateral peritonsillar edema. (See Figure 1.) Symmetric bilateral swelling of the peritonsillar areas can be seen in tonsillitis and typically is not seen in peritonsillar abscess. Significant cellulitis can mimic abscess formation secondary to the edema. However, uvular deviation typically is absent in cellulitis. Trismus is common in patients with peritonsillar abscess and is related to the inflammation and spasm of the internal pterygoid muscle. Examination may be challenging in patients with significant trismus. While imaging is not necessary to diagnose peritonsillar abscess, the diagnosis in patients with undifferentiated sore throat with concern for other deep-seated neck space infections may be facilitated with advanced imaging, such as ultrasound or computed tomography (CT) with intravenous (IV) contrast.2 A bedside ultrasound may be performed via the traditional intraoral technique and/or submandibular technique with a sensitivity of greater than 90%. (See Figure 2.) The classic finding with peritonsillar abscess on ultrasound is a discrete fluid collection with heterogeneous constituent with an irregular border.3 Ultrasound also may help with procedural guidance and landmark evaluation of the distance to the center of the abscess as well as the location of the surrounding vasculature.
Figure 1. Peritonsillar Abscess |
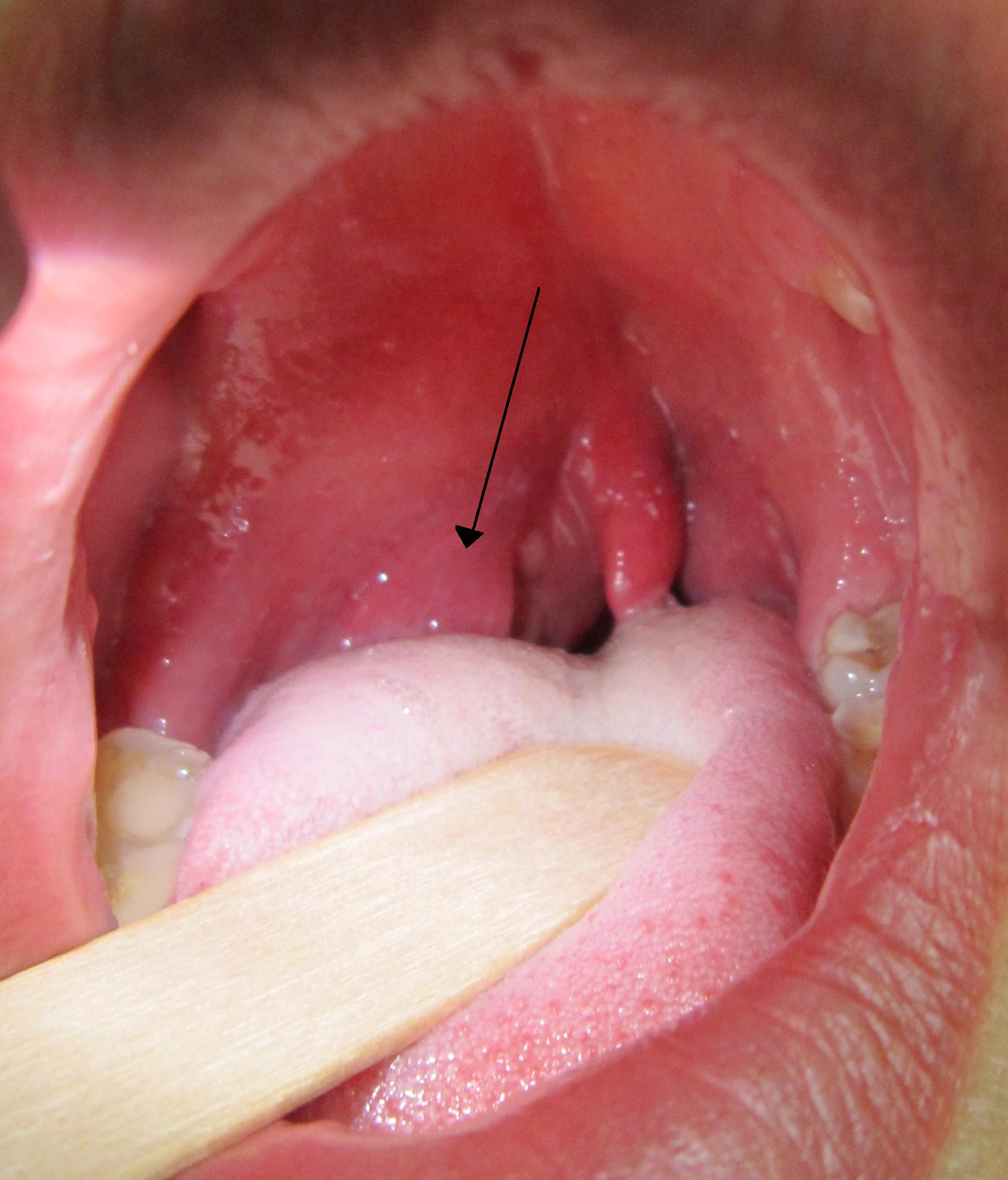 |
|
Courtesy of James Heilman, MD. PeritonsilarAbsess.jpg. https://commons.wikimedia.org/wiki/File:PeritonsilarAbsess.jpg |
Figure 2. Point-of-Care Ultrasound with an Endocavitary Probe to Evaluate the Posterior Oropharynx |
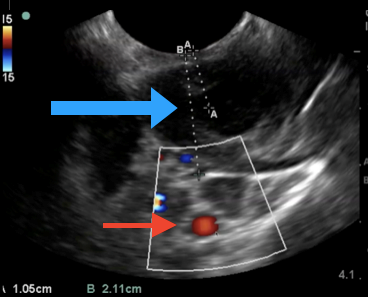 |
|
A peritonsillar abscess (blue arrow), carotid artery (red arrow), and relative distances to each. Courtesy of Daniel Migliaccio, MD. |
On CT imaging with IV contrast, peritonsillar abscesses appear as a hypodense fluid collection with surrounding rim enhancement. Patients that are toxic-appearing with concern for airway obstruction may need to be evaluated and/or examined in the operating room with otolaryngology specialists. There is no indication for adjunctive laboratory testing to diagnose peritonsillar abscess. However, laboratory testing done on pediatric patients with peritonsillar abscess may demonstrate an elevated white blood cell count with neutrophil predominance. Systemically ill patients admitted for operative management or IV antibiotics should have sepsis labs drawn, although blood cultures in patients with peritonsillar abscesses rarely are positive.
Management
Management of peritonsillar abscess includes drainage, antibiotic therapy, and analgesia. Children who are well-appearing and able to maintain oral hydration may be candidates for discharge with close outpatient follow-up after drainage is performed. An experienced emergency provider may perform needle aspiration and/or incision and drainage in the emergency department. If the emergency department provider is not experienced/well versed in the procedure, consultation may be required with an otolaryngologist. Ultimately, the patient requires close outpatient follow-up with an otolaryngologist for repeat examination, to evaluate the need for repeat incision and drainage, and/or to discuss possible tonsillectomy.4
Ultrasound can help guide procedural placement of the needle in a needle aspiration attempt. Incision and drainage as well as needle aspirations have similar success rates (above 90%) in the resolution of abscess, while there is a slight trend toward an increased need for a repeat procedure in patients who received needle aspiration. Complications of needle aspiration include carotid artery puncture, hemorrhage, bleeding into the airway, and aspiration. The carotid artery sits posterolateral to the peritonsillar area. Appropriate technique, landmark identification, and the use of ultrasound may decrease the incidence of these complications.
For instance, ultrasound can delineate the distance to the middle of the abscess and the distance to the surrounding vasculature. This can allow the provider to safely cap a needle to that distance to avoid unnecessary needle length penetrating the peritonsillar space. It is important to note that the oral mucosa is well perfused, which leads to bleeding. Using lidocaine with epinephrine prior to needle aspiration helps decrease the rate of capillary bleeding with needle puncture. Using suction also can help decrease the risk of aspiration of blood and pus products.
Furthermore, after the incision and drainage and/or needle aspiration, the patient may gargle hydrogen peroxide to help with hemostasis as well. If bleeding persists, some topical tranexamic acid also may be used to assist with obtaining hemostasis. The mucosal bleeding typically is self-limited and will resolve within minutes. Monitoring patients after the procedure is important to ensure hemostasis, control of any pain, and that the patient is able to maintain oral hydration and tolerate oral antibiotics. Patients presenting with concern for acute airway compromise should have an immediate evaluation by a surgical specialist or ear, nose, and throat physician, preferably in a controlled environment or operating room setting.5
After source control with needle aspiration or incision and drainage, inpatient hospitalization may be required if patients are toxic-appearing, there is concern for possible sepsis, and/or they have a concomitant immunocompromised state. Similarly, patients who have difficulty with oral hydration, symptom control, or who have poor follow-up/resources precluding outpatient antibiotic therapy also may need to be hospitalized. Appropriate antibiotic therapy should include coverage for group A Streptococcus, Staphylococcus aureus, and anaerobic bacteria.
If cultures are obtained after drainage, empiric antibiotics should be provided while awaiting the results. Patients requiring admission and parenteral antibiotics can be started on ampicillin-sulbactam IV 50 mg/kg per dose every six hours or 3 g every six hours in young adults. Alternatively, clindamycin may be used at a dose of 30 mg/kg per dose every eight hours or 600 mg every six to eight hours in adults. Importantly, ampicillin-
sulbactam does not provide coverage against methicillin-resistant Staphylococcus aureus (MRSA). If there is a suspicion for this, patients should receive either clindamycin or vancomycin. For those who meet criteria for outpatient treatment, appropriate oral antibiotic selections include amoxicillin–clavulanate 45 mg/kg with a maximum of 875 mg every 12 hours or clindamycin 10 mg/kg per dose at a max single dose of 600 mg every eight hours.6 Concomitant administration of glucocorticoids, such as intravenous or oral dexamethasone, frequently is provided, but evidence regarding its benefit is unclear.3
Retropharyngeal Abscess
Background
Retropharyngeal infections represent a subtype of deep neck space infection that arises between the alar fascia anteriorly and prevertebral fascia posteriorly and extends from the base of the skull to the posterior mediastinum.7 The retropharyngeal space communicates with the lateral pharyngeal space and is a potential path for pharyngeal infections to be transmitted into the mediastinum.8 Abscess formation in this area typically occurs following an upper respiratory tract infection, pharyngitis, or recent trauma to the pharynx such as dental procedures or recent intubation. Retropharyngeal abscess is most common in young children between the ages of 2 and 4 years.9 Most commonly, retropharyngeal abscess is secondary to Streptococcus pyogenes, group A Streptococcus, S. aureus (including a rising incidence of MRSA), and anaerobes, including Fusobacterium.10-12 Complications associated with retropharyngeal abscess include laryngospasm and associated airway compromise, sepsis, and mediastinitis.
Presentation
Pediatric patients with retropharyngeal abscess typically will present with significant odynophagia with neck pain and may present with severe airway obstruction. The child may be toxic in appearance, may be in the sniffing position with increased respiratory effort and retractions as well as stridor and a change in voice. The classic muffled voice is that of a duck also known as “cri du canard.”13 Children typically will be unwilling to move their neck secondary to pain and inflammation in the neck muscles. Trismus also may be seen in these patients. The clinical picture may be consistent with that of epiglottitis; however, retropharyngeal abscess typically is more subacute in its onset.
Diagnosis
In patients in whom the provider has concerns for airway compromise, including those who present with drooling, stridor, and/or in the tripoding position should have immediate diagnostic as well as definitive airway management and treatment in the operating room by an otolaryngologist. Providers should consider retropharyngeal abscess as a possible diagnosis in patients presenting with sore throat and neck pain with significant swelling of the posterior pharyngeal wall. Tender anterior cervical lymph nodes frequently are encountered with these patients as well. Hyoid tenderness to palpation also may be appreciated. Laboratory evaluation typically is nonspecific and demonstrates a leukocytosis with a neutrophil predominance.14 Blood cultures also may be obtained if there is concern for concomitant sepsis, but those cultures typically are negative.
If a patient is stable for radiographic evaluation, imaging can include plain film lateral neck radiographs and/or CT with IV contrast of the neck. However, plain films may be an initial screening study if suspicion is high that the patient should have a CT with IV contrast performed.15 Care must be taken to closely monitor the airway during imaging, since lying supine may exacerbate airway compromise in patients with a retropharyngeal abscess. If there is concern for potential airway compromise, consulting a pediatric airway specialist, such as an anesthesiologist and/or otolaryngologist, should be considered for the diagnostic evaluation.
Lateral neck radiographs typically demonstrate a widened retropharyngeal or prevertebral space. (See Figure 3.) The film should be taken in the lateral plane during patient inspiration with the neck in extension. Failure to follow proper technique can lead to false-positive studies. In children younger than 4 years of age, the retropharyngeal space at the level of C2 should be less than half the width of the vertebral body at that level. An abnormally wide space is considered when the diameter of the space is greater than the size of the vertebral body at C2, or a gradient of 7 mm at C2 or 14 mm at C6.16 (See Figure 4.) If there is concern for concomitant mediastinitis, an anterior-posterior and lateral chest X-ray should be obtained as well. CT imaging is the diagnostic modality of choice to identify deep-seated neck space abscess. (See Figure 5.) It can differentiate an abscess from cellulitis and can facilitate surgical planning with the otolaryngology team. Typically, the abscess will appear as a discrete fluid collection with surrounding rim enhancement.
Figure 3. Retropharyngeal Abscess |
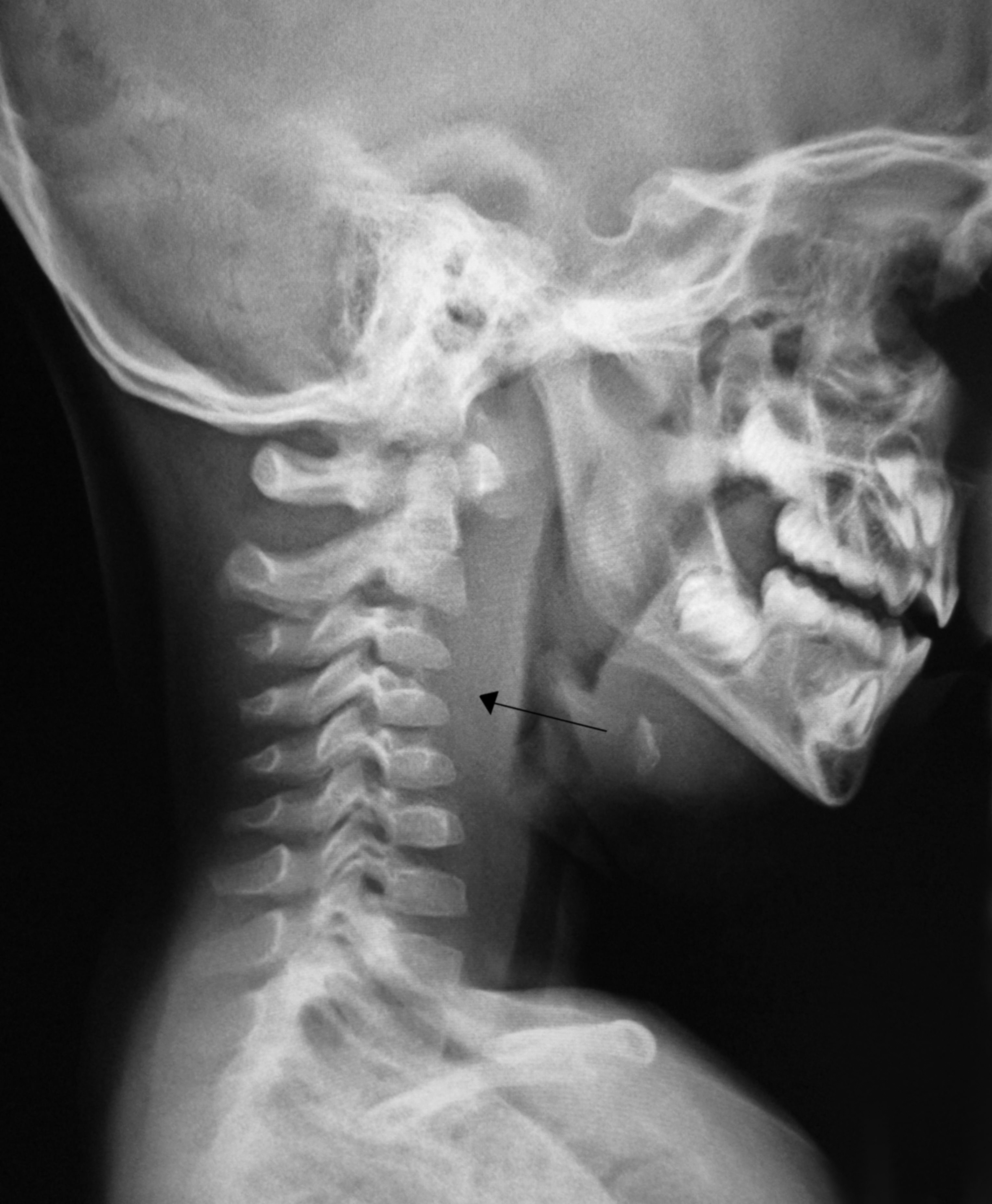 |
|
Lateral X-ray demonstrating retropharyngeal abscess (black arrow). Note the increased prevertebral space (7 mm at C2). Courtesy of James Heilman, MD. Retroabscess10.JPG. https://commons.wikimedia.org/wiki/File:Retroabscess10.JPG |
Figure 4. Normal Lateral Neck Film |
 |
|
Courtesy of Nevit Dilman. Medical X-ray imaging RNT07 nevit. https://commons.wikimedia.org/wiki/File:Medical_X-Ray_imaging_RNT07_nevit.jpg |
Figure 5. Computed Tomography Scan of a Retropharyngeal Abscess |
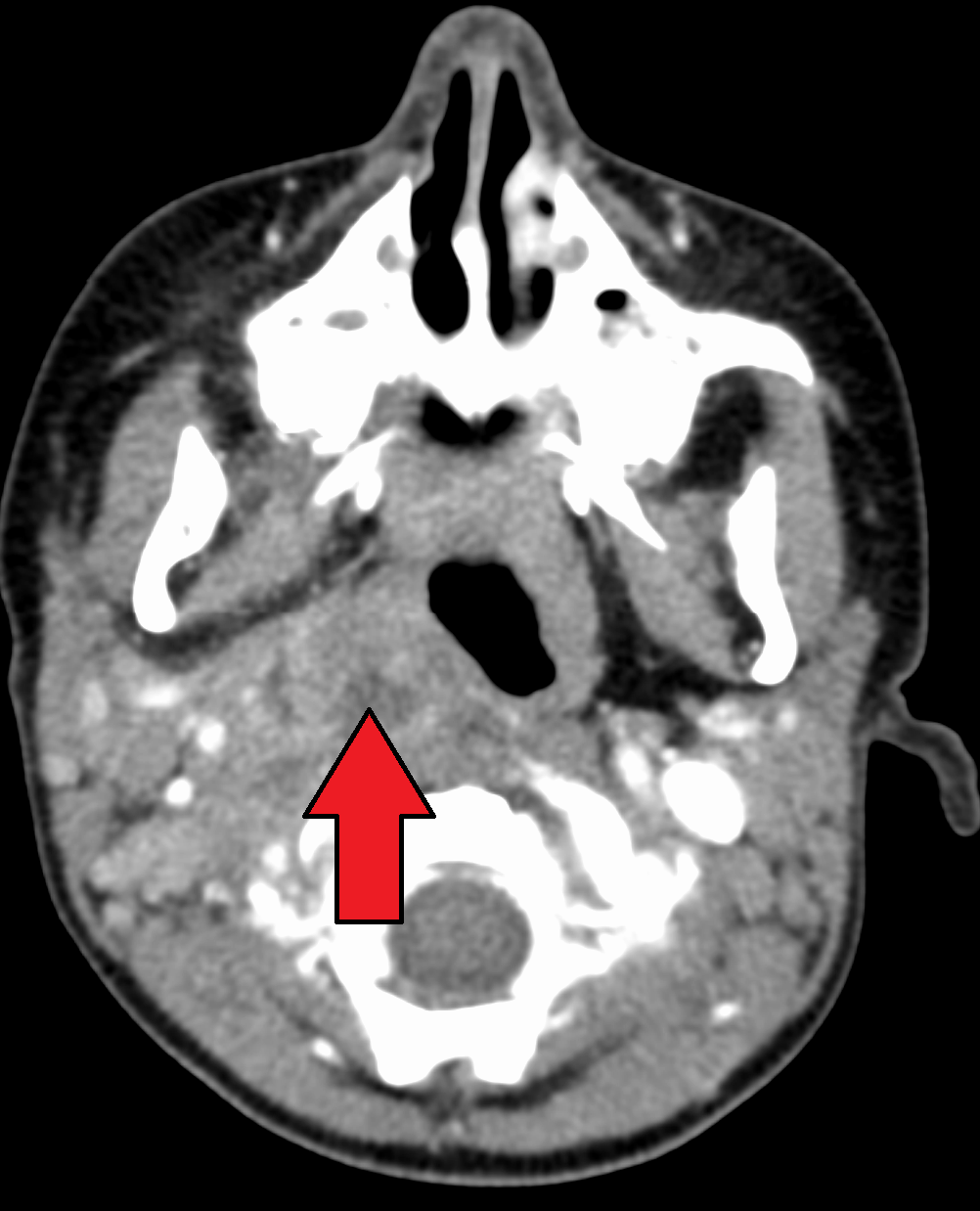 |
|
Computed tomography scan demonstrating retropharyngeal abscess (red arrow). Courtesy of James Heilman, MD. LargeRetroAbsMarkTra. https://commons.wikimedia.org/wiki/File:LargeRetroAbsMarkTra.png |
Management
If the child presents with concern for airway compromise with drooling, stridor, and/or in the tripoding position, care must be taken not to aggressively evaluate the patient at the bedside. This can potentially worsen airway compromise. Similarly, avoiding venipuncture, which potentially can lead to patient agitation and increase airway obstruction, until resources are mobilized in the operating room potentially can avoid an airway disaster. The provider must organize care rapidly to have the patient evaluated in the operating room by an anesthesiologist and otolaryngologist or obtain advanced imaging if the differential is unclear (in patients who are still maintaining their airway appropriately). Patients with airway compromise should have airway protection in the operating room and should not be sent to obtain imaging, including CT and/or plain film radiography without immediate availability of an airway team..
In patients who are stable without acute airway concern and are found to have retropharyngeal abscess on imaging, emergent otolaryngology consultation is required. Additionally, patients with retropharyngeal abscess should be given empiric parenteral antibiotics and admitted to the hospital. Potential empiric regimens include ampicillin-sulbactam intravenously or clindamycin every eight hours intravenously.17
If there is concern for MRSA, clindamycin or vancomycin at a dose of should be provided.11 Adjunctive analgesia and IV hydration also should be provided. Patients without airway compromise can be candidates for a trial of IV antibiotic therapy without initial surgical intervention after consultation with otolaryngology. If there is a failure of response in the antibiotic therapy, or if there is a deterioration in the respiratory status or airway of the patient, surgical intervention is recommended.
Lemierre’s Syndrome
Background
Infectious thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, is a rare complication following an oropharyngeal infection, most commonly bacterial pharyngitis. The infection spreads contiguously to the nearby internal jugular vein, leading to thrombosis of the vein.18 Subsequently, septic emboli are formed and can lead to various clinical presentations.
Patients with Lemierre’s syndrome typically are quite ill and present with signs of sepsis and bacteremia. The limited available data demonstrate the highest incidence in adolescents and young adults aged 14 to 24 years.19 The most common organism that results in Lemierre’s syndrome is the anaerobic bacteria Fusobacterium necrophorum.20
Less commonly implicated bacteria include MRSA, streptococci, and other oropharyngeal flora, such as Eikenella corrodens and Bacteroides.21 The most common infection predisposing a young adult to Lemierre’s syndrome is a bacterial pharyngitis. However, other oropharyngeal/neck space infections also can cause the disease.
Presentation
The presentation of Lemierre’s syndrome typically is subacute and follows a preceding pharyngitis. Patients may present with fever, significant sore throat, trismus, and neck tenderness to palpation.22 They typically are ill-appearing and toxic, with signs of sepsis.23 Patients may present with various signs consistent with septic emboli, including septic pulmonary emboli, polyarthritis, pyomyositis, endocarditis, and/or renal abscess.24,25 The most common complication is pulmonary, and patients may present in a picture akin to a traditional pulmonary embolism with tachypnea, tachycardia, hypoxia, hemoptysis, and an increased work of breathing. Central nervous system infections are less frequent and may include meningitis, epidural abscess, and/or brain abscess.26
Diagnosis
The diagnosis of Lemierre’s syndrome should be considered in patients presenting with pharyngitis as well a as concern for patients with systemic symptoms, such as septic pulmonary emboli and bacterial seeding of joints. Emergency department diagnosis typically is facilitated with radiological imaging. Blood and/or throat cultures subsequently may reveal F. necrophorum while a patient is hospitalized. Imaging modalities include CT scanning of the neck and chest with IV contrast.27
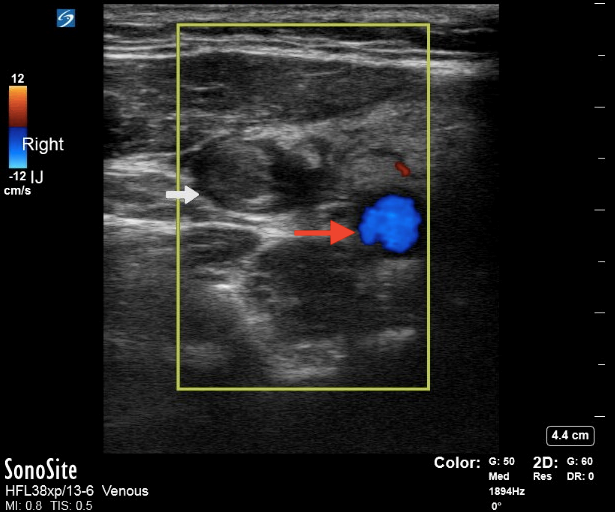
Ultrasound of the neck and internal jugular may be considered as well, although that is less sensitive than CT and does not help to differentiate potential complications, such as septic pulmonary emboli. The benefits of ultrasound include the rapidity in which it may be performed at the bedside and the ability to perform the study on unstable patients at bedside. Ultrasound of the internal jugular vein may demonstrate thrombus within the vein. (See Figure 6.) Additionally, bedside point-of-care ultrasound also enables the ability to perform a bedside cardiac evaluation for potential right heart strain in the setting of septic pulmonary emboli and potentially allows for the diagnosis of right-sided endocarditis, which can mimic Lemierre’s syndrome.
Figure 6. Ultrasound of a Thrombus in the Internal Jugular |
 |
|
Point-of-care ultrasound with Doppler demonstrating a thrombus in the internal jugular vein (white arrow) and carotid artery (red arrow). Courtesy of Daniel Migliaccio, MD. |
Management
A patient with Lemierre’s syndrome presenting with signs of sepsis and/or septic shock should have aggressive resuscitation with IV fluids and broad-spectrum IV antibiotics. All patients with suspected Lemierre’s syndrome should be admitted for IV empiric antibiotic therapy targeting the most common culprit organisms, including F. necrophorum and other commonly implicated oropharyngeal flora. It is important to note that F. necrophorum may have associated beta-lactamase production, and the provider should include an antibiotic that is resistant to beta-lactamase.28 Empirical options for antibiotics include piperacillin-tazobactam or ceftriaxone plus metronidazole.29 Vancomycin should be added in patients who are presenting in septic shock and/or if there is a clinical concern for MRSA infection.
Anticoagulation in the treatment of thrombus associated with septic emboli is not routinely used, since there are no good data available for its efficacy. In the setting of prolonged, continued thrombosis despite antibacterial therapy, the provider may consider the use of anticoagulants.30 In those circumstances, it is prudent to involve specialty hematology consultation regarding anticoagulation in these patients.
Ludwig’s Angina
Background
Ludwig’s angina refers to a life-threatening infection of the bilateral submandibular, submental, and/or sublingual space. Infection typically originates from an odontogenic source (most commonly second/third mandibular molar), but it may spread from a pharyngitis, parotiditis, or peritonsillar abscess.31 The infection spreads contiguously from the floor of the mouth to the submandibular region, leading to an aggressive cellulitis. Infections typically are polymicrobial in nature and include anaerobic bacteria. There may be an associated abscess formation as well, although that is rare.
Morbidity and mortality occur secondary to progression of the infection toward the patient’s airway, leading to laryngospasm and asphyxia.32 The most important complication for emergency providers to be aware of is airway compromise, and providers should be attuned to signs of impending airway collapse, such as change in voice, drooling, stridor, and/or respiratory distress. As with retropharyngeal abscess, mediastinitis also is a possible complication, although it is much less frequent in occurrence.33
Presentation
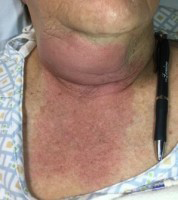
Patients presenting with Ludwig’s angina typically present with systemic symptoms of fevers, chills, and malaise, along with neck pain, odynophagia, neck stiffness, drooling, changes in voice, and trismus. Patients usually present with rapid/acute spreading infection to the floor of mouth with a potential “woody” or solid texture on palpation of the floor of the mouth while it is elevated.34 The patient’s neck may appear erythematous and tender, with a symmetric brawny swelling and induration of submandibular region. (See Figure 7.) Submandibular crepitance occasionally may be appreciated on exam.35
Figure 7. Ludwig's Angina |
 |
|
Ludwig's angina, submandibular erythema, warmth, and fluctuance Courtesy of Daniel Migliaccio, MD. |
Diagnosis
The provider evaluating a patient with submandibular erythema and swelling should immediately consider the diagnosis of Ludwig’s angina. CT imaging of the neck with IV contrast is the modality of choice to evaluate the nature of the deep space neck infection.36 CT imaging typically demonstrates soft tissue swelling, edema, and haziness suggestive of cellulitic change and can indicate to the provider the proximity of the swelling to the airway. It also may demonstrate concomitant abscess formation, which may facilitate surgical planning.
Management
In patients with concern for potential airway compromise, advanced imaging should be delayed until discussion with specialty services, including an otolaryngologist and/or anesthesiologist, for possible advanced methods of airway protection.37 Consultation in these circumstances should be emergent. For providers practicing in locations without the subspecialty services, advanced airway maneuvers, such as fiberoptic intubation, may be required. The suggested route of intubation in these patients is via nasal fiberoptic intubation.38 Equipment for emergent cricothyrotomy should be available at the bedside. However, it is important to note that the significant submandibular swelling likely will make the palpation of landmarks such as the cricothyroid membrane extremely difficult, complicating the procedure even further.
Concomitantly, empiric broad-spectrum antibiotics for oropharyngeal flora and anaerobic bacteria should be provided. Example antibiotic regimens include clindamycin or ampicillin-sulbactam. In immunocompromised patients, MRSA or gram-negative aerobes also may contribute to the infection.39 In these patients, providers should consider using broad-spectrum antibiotics, such as cefepime plus metronidazole plus vancomycin (if the patient has risk factors for MRSA).40 Risk factors for MRSA include recent hospitalization, living in long-term care facilities or prison, a history of IV drug use, or prior abscesses requiring incision and drainage. Patients who have evidence of abscess development on CT imaging should have an otolaryngology evaluation for source control with either needle aspiration or incision and drainage in the operating room.
In patients with a suspected odontogenic source of the submandibular cellulitis, there should be concomitant consultation with an oral maxillofacial surgery team to extract the tooth.
Epiglottitis
Background
Epiglottitis is a life-threatening infection and inflammation of the epiglottis and surrounding tissues. The swelling typically is prominent in the supraglottic region of the patient’s airway, which can dramatically decrease the functioning caliber of the airway, leading to stridor and potential respiratory compromise. The most dreaded complication includes obstruction of the airway that can lead to asphyxia and cardiopulmonary collapse.41
Most commonly, epiglottitis occurs secondary to an infectious etiology.42 Prior to widespread adoption of immunization in the United States, Haemophilus influenzae type b (Hib) was the bacteria most commonly implicated in infectious epiglottitis.43 While epiglottitis still can be caused by Hib, other etiologies are nontypeable H. influenzae A, group A Streptococcus, and S. aureus, including MRSA.44 Viral and fungal infections also can lead to epiglottitis, although that is much less common. Other less common noninfectious etiologies of epiglottitis include direct injury, such as clinical trauma, caustic injury, or thermal injury to the epiglottis. Since implementation of the Hib vaccine, the median age of children with epiglottitis has increased to approximately 6 to 12 years of age.45,46 Similarly, the incidence of epiglottitis among pediatric patients younger than 20 years of age has decreased from approximately five cases per 100,000 children to 0.5 cases per 100,000 children.47 The most common risk factors for the development of epiglottitis include a lack of immunization against Hib, concomitant comorbidities, such as diabetes, and/or an immunocompromised state.48
Presentation
Classically, younger pediatric patients with epiglottitis present with rather rapid onset of respiratory distress. Patients may have a muffled voice (also described as a “hot potato” voice), drooling, or leaning forward with the neck hyperextended in a sniffing/tripod posture.49 This position optimizes the remaining diameter of the obstructed airway. Similarly, these patients typically are quite reluctant to lie supine, given the increase in airway obstruction from the swollen edematous epiglottis.
However, older children and young adults may not have any of the classic findings but may present with a severe pharyngitis clinical picture with associated odynophagia. Patients also may have tenderness with movement of the thyroid cartilage secondary to the inflammation of the epiglottis. In comparison to children presenting with croup and stridor, patients with epiglottitis typically do not have a concomitant cough or a hoarse voice.50
Diagnosis
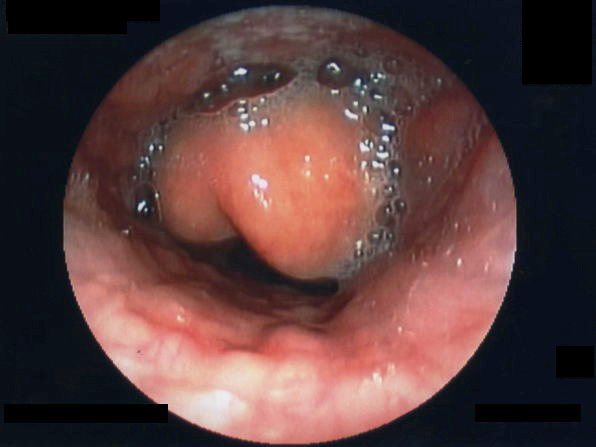
Diagnosis should be considered in high-risk children (unimmunized, for example) presenting with signs or symptoms classic for epiglottitis, such as tripod positioning, drooling, change in voice, and respiratory distress.51 Direct visualization of a swollen edematous epiglottis is considered to be the gold standard for the diagnosis of epiglottitis. (See Figure 8.)
Figure 8. Laryngoscopy of a Swollen Epiglottis |
 |
|
Courtesy of Fujisawa Takashi. Epiglottitis endoscopy. https://commons.wikimedia.org/wiki/File:Epiglottitis_endoscopy.jpg |
In patients presenting with respiratory distress and with a high concern for epiglottitis, manipulation and visualization of the epiglottis should be avoided given the potential for agitation and anxiety, since this can lead to further airway obstruction. Manipulating the area surrounding the epiglottis also can lead to laryngospasm and complete airway collapse. In these patients, the provider must swiftly and expeditiously coordinate evaluation by airway specialists (otolaryngology and anesthesiology) in a controlled setting, such as the operating room. The airway should be secured prior to any further diagnostic evaluation (such as advanced imaging).
In children for whom epiglottitis is a differential diagnostic consideration but who are not acutely ill with immediately impending respiratory compromise, evaluation of the oropharynx should be performed. However, it is important to note that the exam may be normal in patients with epiglottitis. Consultation with an otolaryngologist to perform a nasal fiberoptic evaluation of the epiglottis in patients with whom the provider has a high suspicion for epiglottitis is a reasonable diagnostic approach. Emergency providers also can consider flexible fiberoptic evaluation of the epiglottis if they have significant experience and comfort with this procedure. However, inadvertently touching the epiglottis with the fiberoptic device can cause a reflex laryngospasm and complete airway collapse.52
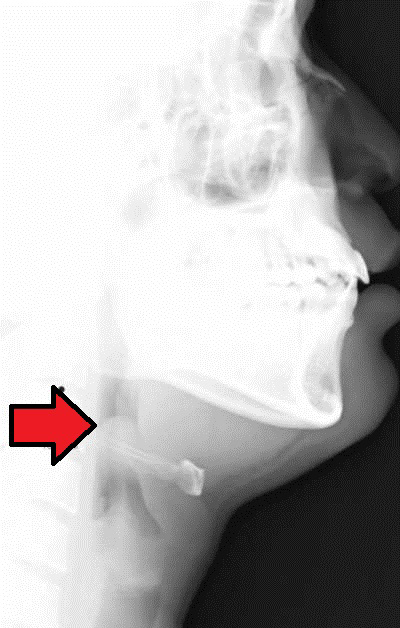
Adjunctive imaging in patients without concern for immediate airway collapse can include lateral neck plain film radiography.53 The patient should not leave the care of a team skilled in advanced airway management. Classically, lateral neck radiograph demonstrates an enlarged epiglottis, which appears with a thumb-like appearance, known as “thumb sign.”54,55 (See Figure 9.) It is important to note that radiographs are not necessary to make the diagnosis of epiglottitis.56 Laboratory evaluation includes nonspecific findings, such as an elevation in the white blood cell count with a neutrophil predominance and blood cultures positive for a possible bacterial etiology.57
Figure 9. Lateral Neck X-Ray of an Enlarged Epiglottis |
 |
|
Lateral neck X-ray demonstrating enlarged epiglottis (red arrow), also known as “thumb sign” concerning for epiglottitis. Courtesy of Med Chaos. Epiglottitis. https://commons.wikimedia.org/wiki/File:Epiglottitis.jpg |
Management
The most important consideration in the management of patients with suspected epiglottitis is airway protection. In patients presenting with concern for impending airway collapse, immediate control of the airway precludes further diagnostic evaluation. Many times, upon intubation, the diagnosis is made with direct visualization of the epiglottis. Providers should obtain specialty consultation services (if emergently available), such as otolaryngology and/or anesthesiology, to evaluate and protect the airway in a controlled environment. Emergent attempts at airway control must be made in patients who have signs of complete airway obstruction, such as respiratory collapse, hypoxia, or cardiopulmonary collapse. In these circumstances, if the emergency provider is concerned for epiglottitis, the patient can be given a trial of oxygenation with bag-valve mask (BVM) ventilation while mobilizing resources for evaluation of the airway in a controlled operating room environment.58
If oxygenation is not maintained with BVM and/or subspecialty services are not available, an emergent attempt at tracheal intubation should be performed by the emergency physician. Preferably, video laryngoscopy should be the first attempted method for endotracheal intubation. Fiberoptic intubation techniques, such as nasal fiberoptic laryngoscopy, can be attempted as well, but the edematous, inflamed epiglottis can be extremely challenging to surpass with the flexible camera. Blind nasotracheal intubation is contraindicated in patients with epiglottitis, given the visual distortion in the upper airway.59 Because of the narrow diameter of the airway, using an endotracheal tube that is one to two sizes smaller than would be predicted by the patient’s age may be necessary. Preparation for surgical airway should be concomitant with initial endotracheal intubation attempts. Options for surgical airway include needle cricothyrotomy in children younger than 10 years of age.60 In older patients, surgical cricothyroidotomy is preferred.61
Pediatric patients who are protecting their airway should be allowed to sit in a position of comfort while further diagnostic evaluation is being performed. Diagnostic evaluation may include radiography and/or specialty evaluation of the epiglottis via fiberoptics as aforementioned. Unnecessary anxiety- and agitation-provoking tests, such as multiple attempts at IV access, or vigorous attempts to visualize the oropharynx with a tongue depressor, should be avoided, since they can lead to airway compromise.
Ultimately, patients diagnosed with epiglottitis should have an assessment of the need for airway securement with consultation of an otolaryngologist. Generally, children younger than 6 years of age with epiglottitis should have emergent/controlled airway securement, since they are at increased risk for rapid progression of the epiglottitis to airway obstruction. Older children and young adults with epiglottitis but without respiratory distress can be considered candidates for observation if they are nontoxic-appearing, have had a diagnostic evaluation of the airway that demonstrates that the narrowing of the airway is no greater than 50%, and there is a low suspicion for anticipated airway difficulty in the case of progression of the epiglottitis.62
Lastly, empiric antibiotic therapy should be provided to the most common culprit organisms while awaiting the results of blood cultures. Possible antibiotic regimens include ceftriaxone and vancomycin for possible MRSA. Concomitant use of glucocorticoids has not been demonstrated to be of benefit in decreasing the hospital length of stay, the need for intubation, and/or days in the intensive care unit. Patients with epiglottitis should be monitored closely in an intensive care setting until resolution of epiglottic swelling is noted (typically via direct visualization).
Laryngitis/Laryngotracheitis
Background
Pediatric patients who present with a hoarse voice and sore throat may have acute laryngitis, which is an inflammation of the laryngeal mucosa from infection, irritation, or overuse. The most common etiology in children is related to viral upper respiratory infections. It is rarely secondary to bacterial infection, but in immunocompromised patients, this should be considered. Acute vocal cord strain, such as excessive screaming and yelling (temper tantrums), can cause mucosal vocal cord swelling leading to dysphonia. Acute laryngotracheitis (croup) in children is a common cause of acute hoarseness and/or stridor in children younger than 6 years of age. It is most common in children from 6 months of age to 3 years of age.63 The most common etiology is viral, with parainfluenza virus type I being the most likely culprit.64
Presentation
Patients typically will present with a sore throat, dry cough, and hoarseness/dysphonia. The dysphonia can be classified as a hoarse voice. Patients may present with fever, coryza myalgias, and potentially dysphagia. Children with croup typically will present with viral symptoms, such as nasal congestion and fever, and may demonstrate inspiratory stridor, a barking-like cough, and a hoarse voice. Depending on the degree of airway edema compromise, the patient may have increased work of breathing and associated retractions.65
Diagnosis
Diagnosis of laryngitis and/or laryngotracheitis is clinical. Important differential diagnostic considerations in patients presenting with laryngotracheitis include epiglottitis as well as bacterial tracheitis. Throat pain, dysphagia, drooling, and hoarseness of voice are atypical for patients with epiglottitis. A severity assessment of the croup should be obtained and is based on the patient’s work of breathing as well as evidence of stridor or respiratory failure.
Management
Acute laryngitis typically is self-limited and resolves within one to two weeks.66 Supportive care includes rest, oral hydration, use of humidifiers, and avoiding yelling. These all may alleviate some of the discomfort associated with laryngitis. Analgesics, such as acetaminophen, and/or nonsteroidal anti-inflammatory drugs, such as ibuprofen, also are useful in treating the accompanying sore throat with the laryngitis. Management of acute laryngotracheitis in children involves corticosteroids and use of the nebulized epinephrine when indicated. Patients with mild croup should be given a single dose of dexamethasone 0.6 mg/kg with a maximum dose of 16 mg. Corticosteroid use has demonstrated a decreased duration of symptoms in patients with croup.67,68 Patients with moderate to severe croup should be provided nebulized racemic epinephrine at a dose of 0.05 mL/kg per dose (diluted in a total of 3 mL) over 15 minutes in addition to the corticosteroids such as dexamethasone.69,70 Patients who receive nebulized epinephrine should be monitored in the emergency department for at least four to six hours.71,72 If, after a period of observation, the patient has no stridor at rest, is clinically well-hydrated, has normal oral intake, and is well-appearing with reliable follow-up, the patient may be a candidate for discharge.73
Tracheitis
Background
Bacterial tracheitis is a potentially life-threatening infection of the trachea with or without associated involvement of the upper bronchial airways and/or pneumonia (acute bacterial laryngotracheobronchitis).74 It is most common in children younger than 6 years of age. Bacterial tracheitis typically occurs after a recent upper respiratory infection, which damages the mucosa of the larynx, allowing for laryngeal pathogens (most commonly S. aureus followed by Streptococcus pneumoniae) to invade.74,75 A predisposing viral infection with parainfluenza, the most commonly implicated virus in croup, is a common mechanism for the patient to develop bacterial tracheitis.76
Presentation
Children will present with subacute respiratory tract infection symptoms antecedent to the development of signs of bacterial tracheitis. The signs of bacterial tracheitis tend to be severe and include airway obstruction, stridor, and respiratory distress.77 Children may present with fatigue, marked retractions, and/or listlessness. Hoarseness and/or changes in voice are common in patients who have bacterial tracheitis.52 As opposed to epiglottitis, drooling typically is uncommon.78
Diagnosis
A provider must have a high degree of suspicion for bacterial tracheitis based on the clinical presentation of a patient presenting with acute airway obstruction in the setting of a subacute recent upper respiratory illness.79 Patients who are toxic in appearance with a poor response to nebulized epinephrine as well as glucocorticoids should prompt the provider to consider bacterial tracheitis.76 Definitive diagnosis is achieved via direct visualization of the airway with laryngoscopy with possible bronchoscopy.80
In patients who have impending airway compromise and/or are of concern for respiratory failure, diagnostic evaluation should be delayed until there has been definitive management of the patient’s airway.81 Optimally, if the provider has adequate resources and enough time prior to airway collapse, it is safest to have the airway managed in a controlled operative environment with subspecialty services, such as otolaryngology and anesthesiology.
Further evaluation of the airway may be performed after airway securement with bronchoscopy in the operating room.82 Bronchoscopy findings include significant subglottic narrowing, purulence, and adherent pseudomembranous lesions along the bronchial airways.83 Respiratory specimens should be obtained for Gram stain and culture. In the evaluation of patients without impending airway compromise, further diagnostic evaluation with radiographic imaging may be obtained. Plain film radiography of the neck (lateral and/or anterior posterior) classically demonstrates a subglottic tracheal narrowing, known as the steeple sign.84 However, this finding, is nonspecific and can be seen in children with viral croup who are nontoxic.85 Patients may also have irregularly thickened mucosa of the upper trachea distal to the conus elasticus without recognizable membrane or recognizable membranes in the proximal trachea.86
Similarly, laboratory tests are nonspecific in patients with bacterial tracheitis and may include a mild elevation in inflammatory markers, such as erythrocyte sedimentation rate and/or C-reactive protein. Blood cultures also should be obtained, although they typically are negative in patients with tracheitis.
Management
The mainstay of management of patients with bacterial tracheitis includes aggressive airway management and protection, if clinically indicated.78 Supportive care, such as a trial of inhaled nebulized epinephrine, may be attempted concomitantly while evaluating the patient. Similarly, patients who are toxic with concerns for bacterial tracheitis and possible sepsis should be given empiric IV antibiotics as well as resuscitative fluids. Patients with suspected bacterial tracheitis in the emergency department should be admitted to an intensive care setting after consultation with specialty services such as otolaryngology, anesthesiology, and potentially pulmonology to evaluate the patient’s airway and possibly perform a bronchoscopy. In addition, aggressive supportive care, including antipyretics, IV fluids, supplemental oxygen, and analgesia, should be given to patients. However, care should be given when providing analgesia, since the potential sedating effects can lead to respiratory depression and further respiratory compromise.
Patients with suspected tracheitis who do not need emergent airway protection should have aggressive respiratory care, including pulmonary hygiene with suctioning, hypertonic saline, and nebulized epinephrine. These patients also should be provided glucocorticoids and admitted for continued monitoring in the intensive care unit. Using these adjunctive therapies typically is trialed, given the significant overlap with laryngotracheitis, although data regarding the utility of these medications are limited.82 Empirical antibiotic therapy should include coverage against the most commonly implicated organisms, such as S. aureus (including MRSA) and group A Streptococcus. Typical regimens may include vancomycin plus ceftriaxone.81
Conclusion
Pediatric patients presenting with sore throats frequently are encountered in emergency departments worldwide. While most cases are secondary to benign or self-limiting etiologies, emergency providers must be methodological in the evaluation of each patient presenting with sore throat. Having a broad differential diagnosis and thoroughly evaluating each patient presenting with the chief presenting symptom of sore throat can ensure that a provider decreases the chances of missing a potential life-threatening pathology.
Importantly, providers must avoid diagnostic biases, such as premature closure (when the provider fails to obtain additional information once they believe they have a diagnostic inclusion) or framing (developing a diagnostic conclusion and subsequently assembling elements that support this conclusion). Instead, obtaining a thorough history and physical exam is crucial in being able to differentiate various etiologies and presentations of sore throat in the emergency department.
References
To view the references for this article, visit https://bit.ly/3qCGifz.
Although less frequent than the conditions discussed in part I, recognition of the critical, life-threatening throat infections is essential. Ill-appearing pediatric patients with a change in voice or stridor should prompt a rapid and thorough evaluation to ensure expedited management. This article reviews the critical, must-not-miss etiologies of a sore throat.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
