Caring for Young Febrile Infants
April 1, 2021
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
AUTHORS
Jacob Wannemacher, MD, Chief Resident, Combined Emergency Medicine and Pediatrics Residency, University of Arizona College
of Medicine, Tucson
Aaron Leetch, MD, FACEP, Associate Professor, Director, Combined Emergency Medicine and Pediatrics Residency, University of Arizona College of Medicine, Tucson
PEER REVIEWER
John Cheng, MD, Pediatric Emergency Medicine Physician, Pediatric Emergency Medicine Associates, LLC, Children’s Healthcare of Atlanta, Wellstar Health System, Atlanta
Executive Summary
• Fever in the neonate has been defined widely in both research and clinical practice as a rectal or core temperature ≥ 38.0°C. Febrile neonates are defined as infants ≤ 28 days old.
• Most studies of infants younger than 60 days old in the United States report an incidence of serious bacterial infection (SBI) of around 10% in febrile young infants, with the highest prevalence in infants ≤ 28 days of age.
• Epidemiologic research has shown that the most common cause of urinary tract infection (UTI) is Escherichia coli. E. coli also is the most common cause of bacteremia, followed by group B Streptococcus (GBS). The most common cause of meningitis is GBS, followed by E. coli. Bacteremia co-occurs in about 50% of cases of meningitis.
• Biomarkers for SBI perform particularly poorly in infants ≤ 28 days of age, which contributes to the poor sensitivity of clinical decision rules in neonates. As of now, most clinicians would agree that all febrile infants ≤ 28 days of age need a full sepsis (and possibly herpes simplex virus [HSV]) workup, as well as empiric antimicrobials and admission to the hospital, given the lack of definitive low-risk criteria to reliably rule out SBI and HSV in infants.
• If well-appearing infants meet low-risk criteria, they potentially can be discharged with close follow-up and strict return precautions. These studies to define low-risk criteria were performed in infants who were generally healthy and did not have additional baseline risk for SBI compared to a healthy infant. Infants who are born prematurely (< 37 weeks), and infants with unexplained hyperbilirubinemia, chronic or complex medical conditions, prior antibiotic use, and prolonged initial hospitalization after birth generally were excluded from these studies. The presence of any of these conditions puts the infant at higher risk for SBI, and a full septic workup, including lumbar puncture (LP), should be strongly considered.
• In general, premature infants should be considered to be at high risk for SBI until at least 44 weeks post-conceptional age, and a full septic workup should be strongly considered for them as well. Unfortunately, typical risk stratification rules used in the emergency department cannot be accurately applied to premature infants, given the lack of adequate studies.
• A recent systematic review looked at infants younger than 3 months of age with UTIs and concluded that existing evidence shows that most cases of UTI with concurrent meningitis are in infants younger than 1 month of age or in ill-appearing infants.
• Infants ≤ 28 days who are positive for viral infection still require a full septic workup and antimicrobial treatment. In contrast, co-occurrence of bacteremia or meningitis in infants older than 28 days of age with documented viral infections is rare, with the incidence of bacteremia less than 1% and meningitis around 0.2%. So, infants of this age who test positive for a viral source of infection still should have urine obtained for testing, but they may be able to forgo blood and cerebrospinal fluid testing if they are well-appearing and urinalysis is negative.
Every clinician has struggled with managing a febrile infant. We know the majority will have a benign viral illness, but we fear the serious bacterial infection that may have devastating consequences in this vulnerable population. This evidence-based article reviews the current literature and approach to infants less than 60 days of age.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Definition of the Problem
How to clinically approach a well-appearing, febrile young infant has been a dilemma in emergency medicine for years. It is no easy task to balance the need to identify and treat all life-threatening infections in this vulnerable demographic with the desire to avoid unnecessary invasive testing, antibiotic exposure, and hospitalization. Although stagnant for many years, high-quality research in this area has recently proceeded at a rapid pace.
Febrile young infants and neonates are demographics in which the majority of fevers are caused by self-limited viral infections. However, a significant proportion of fevers are caused by serious bacterial infections (SBIs), which include meningitis, bacteremia, pneumonia, and urinary tract infection (UTI). Additionally, young infants often do not exhibit many signs and symptoms of dangerous infection other than temperature instability early in the disease course, making differentiation of serious infection from self-limited viral illness more difficult. Although there is a general consensus in some aspects of the diagnosis and management of febrile young infants, there is no single universally accepted guideline. This review focuses on management of young, well-appearing febrile infants, primarily in the context of the United States, Canada, and Western Europe, and it may not be applicable in regions with different epidemiological characteristics.
Fever in the neonate has been defined widely in both research and clinical practice as a rectal or core temperature ≥ 38.0°C. Febrile neonates are defined as infants ≤ 28 days old. The febrile young infant has historically been characterized as infants 29-90 days of age. However, over the last 20 years, many studies, hospital protocols, and reviews have focused on infants 29-60 days of age and have lumped infants > 60 days old into algorithms for 2- to 24-month-olds with fever. This review will focus on the workup and management of febrile infants ≤ 60 days of age, since this group has been identified as particularly high-risk for life-threatening etiologies.
The focus of most febrile infant algorithms is on well-appearing infants with fever without a source (FWS), since these infants often present more of a clinical dilemma than infants with a clear source of infection. The general definition of FWS is a fever with no clear localizing signs of infection, such as cellulitis, osteomyelitis, omphalitis, respiratory signs or symptoms, or significant diarrhea. In studies of FWS, the primary categorizations of infection are SBI (UTI, bacteremia, and meningitis) and invasive bacterial infections (bacteremia or meningitis), with an acknowledgement that herpes simplex virus (HSV) infection also must be considered a serious life-threatening infection. Although UTIs are still common in febrile infants older than 60 days of age, invasive bacterial infections (IBIs) are less common in that age group than in younger infants.1
Nuances of Fever Measurement
Many cases of SBI and IBI present because of fever at home, but the patients are afebrile on arrival to the emergency department (ED). A common reason that a septic workup is not performed when recommended in the ED is that providers do not believe there was a true fever, or they attribute the elevated temperature to environmental factors.2 However, a measured fever at home is significantly correlated with SBI and IBI and, from a diagnostic and management standpoint, should be believed and approached the same way as an infant with fever measured in the ED.3,4 Likewise, infants with fevers measured at home via an axillary or temporal route, although not measurements of core temperature, still should be treated as truly febrile infants. Axillary and temporal measurements often underestimate the prevalence and height of fever compared to rectal measurements.5
Children sometimes present for tactile fevers at home without a temperature measurement. Many studies of fever in neonates do not include tactile fever at home as an inclusion criterion, and tactile fever at home is not as reliable an indicator of possible SBI as a measured fever. A recent meta-analysis of the reliability of tactile fever in children showed a sensitivity of 87.5% and specificity of 54.6%. There was a positive likelihood ratio of 1.93 for true fever when there was tactile fever at home. Most included studies were outside of the neonatal period, but overall, this study suggests that a tactile fever at home should be taken seriously, although it is not fully diagnostic of a true fever.6 Given the high prevalence of SBI in this population, physicians should consider observation of the infant in the ED to watch for the development of a fever or for performing a septic workup, especially in a neonate.
Excessive bundling also can be a possible cause of an elevated temperature in neonates.7 Generally, term newborn infants should have one additional layer of clothing on compared to an appropriately dressed adult to help maintain a normal temperature. Infants with more layers than they need may have some element of environmental warmth contributing to their presentation. However, given the high prevalence of SBI in infants in the first month of life, it would be prudent not to assume that bundling is the cause of fever in these infants and to seriously consider completing a full septic workup. In infants 29-60 days of age who truly have excessive bundling, a more selective approach may be taken, including observation in the ED or hospital for occurrence of fever when appropriately clothed. Alternatively, initial laboratory testing could be performed to ensure that the infant meets low-risk criteria and is safe to be discharged.
Epidemiology, Etiology, and Pathophysiology
The epidemiology of infections in young infants has changed significantly since the advent of widespread group B streptococcal (GBS) screening during pregnancy and intrapartum antibiotic treatment of GBS colonization, as well as the use of Haemophilus influenzae type B (Hib) and pneumococcal vaccinations that have provided significant herd immunity.
Early-onset sepsis generally is defined as occurring within seven days of birth in term infants and within 72 hours of birth in preterm infants. It usually is caused by vertically transmitted infections. Infection after this timeframe is defined as late-onset sepsis and can be from either vertical or horizontal transmission of pathogens, including community-acquired pathogens. The incidence of early-onset sepsis was around three to four per 1,000 live births prior to the initiation of intrapartum antibiotics for GBS prophylaxis. In the current era, incidence is estimated at 0.5 per 1,000 live births in term infants and one per 1,000 live births in late preterm infants.8 Note that GBS colonization occasionally can occur between the time of maternal screening and delivery, so not all cases are caught by screening. Adequate antibiotic treatment of maternal GBS colonization during delivery decreases (but does not eliminate) the incidence of early-onset GBS sepsis. However, intrapartum GBS prophylaxis does not significantly decrease the incidence of late-onset sepsis from GBS.
Most studies of infants younger than 60 days old in the United States report an incidence of SBI of around 10% in febrile young infants, with the highest prevalence in infants ≤ 28 days of age.9,10 Studies conducted in Europe in infants with FWS show an even higher rate of SBI, mostly because of higher rates of UTI.11,12 Overall, the majority of SBI cases in this age group are from UTIs. More recent studies have shown that the incidence of IBI in febrile infants younger than 60 days old is around 2%, with the highest rates in infants younger than 29 days old. The incidence of bacteremia is around 3% in the first month of life and around 1% to 1.6% in the second month of life. The incidence of meningitis is around 1% in the first month of life and 0.2% to 0.4% in the second month of life.13-15
Epidemiologic research has shown that the most common cause of UTI is Escherichia coli.16 E. coli also is the most common cause of bacteremia, followed by GBS.15,16 The most common cause of meningitis is GBS, followed by E. coli. Bacteremia co-occurs in about 50% of cases of meningitis.17
There are multiple other important bacterial pathogens in this age group. Staphylococcus aureus is an emerging cause of IBI as well and can be present without an obvious clinical source.18 The prevalence has been reported at 8% to 12% of IBIs.15,16,18 The proportion of methicillin-resistant Staphylococcus aureus (MRSA) isolates in this age group varies by region, but it has been reported to be more than 10% in some areas.16,19 Pneumococcal vaccination against S. pneumoniae infection has decreased invasive pneumococcal disease nearly tenfold since 1988 in children younger than 5 years of age.20 In the modern era, Streptococcus pneumoniae and Neisseria meningitidis are rare causes of bacteremia and meningitis in this age group.13,15,17 However, in communities with lower vaccination coverage, Hib and pneumococcal infection may have higher prevalence. Enterococcus is another minority cause of SBIs and IBIs and is notable for its resistance to cephalosporins. However, it typically is sensitive to ampicillin.18
Additional risk to neonates comes from Listeria monocytogenes infection, although it is very rare in the United States, with an estimated prevalence of < 0.1% of febrile neonates.21 Maternal infections mostly are from contaminated foods and can be transferred to the neonate. Most infections occur in the first week of life, although it can occur from ages 1 to 4 weeks as well. Infection after the first month of life is extremely rare. If infected, neonates have a high rate of meningitis, with a case fatality rate ranging from 14% to 56%.22
The incidence of HSV infections is relatively low, with around 0.2% to 0.3% of neonates presenting with fever.23 However, it cannot be overlooked; the consequences of neonatal HSV can be devastating. The risk of HSV transmission is highest during a primary maternal HSV infection near the time of delivery in a vaginally delivered infant. Maternal genital lesions/blisters, dysuria, or unexplained fever near the time of delivery can be the signs of maternal genital HSV. However, the lack of these maternal symptoms does not rule out neonatal HSV because HSV infection can be subclinical in the mother but still infect the neonate.
The vast majority of neonatal HSV infections occur in infants whose mothers report no history of genital HSV.24 HSV infection risk is significantly decreased in infants delivered by cesarean delivery compared to vaginal delivery. For infants with HSV infection, about 45% of cases are skin/eye/mouth disease (about 20% of these cases do not have skin vesicle involvement), about 25% of cases are disseminated HSV (around two-thirds of which have central nervous system [CNS] involvement), and about 30% of cases are CNS involvement only. Initial signs of neonatal HSV infection most often occur by 4 weeks of age, although they sometimes can be delayed until around 6 weeks of life.25
Common viral causes of febrile illness in this age cohort include parechovirus, adenovirus, and human herpesvirus 6.26 One of the most common viral pathogens is enterovirus, particularly in the summer and early fall months. Influenza also can cause severe febrile illness in young infants.
A significant reason for the increased risk of infection in young infants is their relative immunodeficiency in both the innate and adaptive immune systems. Neonates get most of their antibody protection from maternal antibodies, since they have poor antibody production themselves. They also have low opsonization capacity. There is poor chemotaxis of neutrophils to sites of infection and decreased effectiveness of neutrophils in bacterial killing. Additionally, neonates have less effective T-lymphocytes and deficiencies in complement activation.27,28
Mortality and serious adverse events are rare in well-appearing febrile term infants without meningitis who receive treatment. Prematurity, ill appearance, and the presence of meningitis are indicators of a higher likelihood of morbidity or mortality.29
Clinical Features
In addition to a typical SAMPLE (signs and symptoms, allergies, medications, pertinent medical history, last ins and outs, events) history, ask about infant and maternal risk factors for infection. Gestational age at birth is important, since premature infants are at an increased risk of SBI. Birth history should include route of delivery, need for neonatal intensive care unit (NICU) stay or prolonged hospitalization, and whether the infant received antibiotics after birth. Maternal risk factors include premature rupture of membranes and presence of fever during delivery.30,31 Pertinent maternal screening tests include screening results for GBS, chlamydia, gonorrhea, HIV, and syphilis. If the mother was GBS-positive, ask about antibiotic treatment during delivery. The history should include how the temperature was taken at home. Ask about excessive fussiness or decreased energy level and inquire whether stooling, urination, and feeding have been normal. Respiratory symptoms, rash, gastrointestinal (GI) symptoms or other focal symptoms also should be elicited. Note that many infant symptoms of infection are nonspecific. For example, GI symptoms, such as vomiting and diarrhea, also may be seen in UTI, meningitis, or otitis media and cannot be considered to be clearly diagnostic of viral or bacterial gastroenteritis.
Physical examination of the febrile neonate starts with close attention to vital signs and rapid evaluation of the Pediatric Assessment Triangle, which includes an assessment of general appearance, work of breathing, and circulation to the skin.32 If the infant has abnormal vital signs, concerning Pediatric Assessment Triangle findings, or poor feeding, then he or she should not be managed according to the well-appearing febrile infant algorithms. Tachycardia should be taken seriously as a sign of illness if it is present when the infant is calm. One large retrospective study noted that in 13% of infants with IBI, tachycardia was the only concerning abnormality on physical exam.33 It also should be noted that neonatal sepsis does not always present with fever. Hypothermia is a common presentation of neonatal sepsis, as is ill appearance without fever.34
Attention should be paid to any focal signs of infection. Assess for fullness or depression of the fontanel. However, fullness may be absent initially in cases of meningitis, and signs of meningeal irritation, such as nuchal rigidity, usually are absent in this age group. Assess the eyes for any evidence of conjunctivitis, keratitis, or corneal clouding, which could suggest HSV infection. Purulent discharge from the eyes in a young neonate may suggest gonococcal or chlamydial conjunctivitis or HSV. All skin, including the scalp, must be exposed and examined to assess for rash, petechiae, or signs of cellulitis or abscess. In particular, vesicular lesions with an erythematous base are suggestive of HSV. Be sure to check the oral mucosa for lesions as well. Other than exanthems and enanthems, jaundice can occur in sepsis and HSV infection. All bones should be palpated and joints ranged to assess for focal tenderness or swelling that might suggest osteomyelitis or septic arthritis. Decreased infant movement of a particular limb may be suggestive of pathology in that limb.
Even a normal exam in a well-appearing febrile young infant does not exclude SBI. Multiple studies have shown that well-appearing young infants still may harbor SBI and that clinical suspicion and observational scales alone do not adequately rule out SBI in young infants. In a recent Pediatric Emergency Care Applied Research Network (PECARN) study, in 21% of young infants with SBI, clinicians initially thought they had a < 1% chance of having an SBI.9 Additionally, more than 50% of infants with meningitis in this study had normal Yale Observation Scale scores and ≤ 5% unstructured clinical suspicion for SBI.9,35
Diagnostic Studies
Given that the incidence of SBI in young infants is high and the physical exam is not reliable in excluding dangerous infection, these infants require a diagnostic workup. (See Table 1.) Much of the workup, including point-of-care (POC) glucose, comprehensive metabolic panel (CMP), urinalysis and urine culture, white blood cell (WBC) count with differential, and blood culture, should be performed in all cases. Other aspects, such as C-reactive protein (CRP) and procalcitonin (PCT), can be useful if they are available for risk-stratification and helping to determine the need for lumbar puncture (LP). HSV studies should be done in higher risk patients. Stool studies to assess for bacterial or viral GI pathogens, respiratory viral polymerase chain reaction (PCR) panels, and imaging studies can be useful in patients with focal symptoms.
Table 1. Full Septic Workup Components |
|
Urinalysis and urine culture |
|
Blood culture |
|
Complete metabolic panel (including liver function tests) |
|
Complete blood count with differential |
|
C-reactive protein and procalcitonin, if available |
|
Cerebrospinal fluid culture, cell count, glucose, and protein |
|
Chest X-ray, if there are lower respiratory symptoms |
|
Stool culture and stool polymerase chain reaction if mucus or blood in stool |
|
Point-of-care glucose |
|
Adapted from Palladino L, Woll C, Aronson PL. Evaluation and management of the febrile young infant in the emergency department. Pediatr Emerg Med Pract 2019;16:1-24. |
Serum Tests
All of these infants will require a blood culture to assess for bacteremia. POC glucose and CMP can help in ruling out other contributing factors to clinical presentation, such as hypoglycemia and electrolyte abnormality. Elevated liver function tests (LFTs) can occur in cases of sepsis, HSV infection or other viral infection, or can be indicative of biliary pathology or inborn errors of metabolism.
Other serum tests are used primarily to risk-stratify infants who are at higher vs. lower risk for SBI and IBI. Significant effort has been invested in researching whether some infants are at a low enough risk for SBI to safely avoid antibiotic administration and additional invasive testing, such as LP. Historical risk-stratification criteria include Philadelphia, Rochester, and Boston criteria.36-38 Newer criteria include the Step-by-Step and PECARN criteria.9,11
One of the major differences between older and newer low-risk criteria is the types of serum studies used. All criteria include a urinalysis, but older criteria tend to include WBC and band counts, while newer criteria use absolute neutrophil count (ANC) and PCT. The Step-by-Step criteria use CRP as well. Not all hospitals currently have laboratory capacity to test for band count or PCT, so test availability may contribute to the decision about which low-risk criteria to use. No single serum test has been found to be adequately predictive of SBI in this age group. Band count has good specificity, but poor sensitivity for invasive bacterial infection.39 A normal WBC count (considered to be 5,000 to 15,000 cells/μL by Philadelphia and Rochester criteria) and “low-risk” cutoffs for ANC also have poor sensitivity for SBI (although a cutoff for elevated ANC of 4,100 cells/mcL is much more sensitive than 10,000 cells/mcL).40 PCT and CRP perform relatively well but still are not sufficiently sensitive on their own to rule out SBI.41,42 Most low-risk criteria consider a combination of multiple laboratory tests and clinical appearance to predict risk of SBI. (See Table 2.)
Table 2. Risk Stratification Criteria for Young Febrile Infants Not Immediately Requiring Lumbar Puncture |
||||
|
Prediction Criteria |
Studied |
Appearance, Population, Low-Risk Labs |
Management |
Management |
|
Modified Philadelphia |
29-56 days |
|
Discharge home if the patient lives within 30 minutes of the hospital; 24-hour follow-up and no empiric antibiotics |
Perform lumbar puncture, hospitalize, and give empiric antibiotics |
|
Rochester |
0-60 days |
|
Discharge home with a 24-hour follow-up and no empiric antibiotics |
Perform lumbar puncture, hospitalize, and give empiric antibiotics |
|
Step-by-Step |
21-90 days |
|
Discharge home with a 24-hour follow-up and no empiric antibiotics |
Perform lumbar puncture, hospitalize, and give empiric antibiotics |
|
PECARN |
0-60 daysa |
|
Not fully defined, but generally discharge home with a 24-hour follow-up and no empiric antibiotics |
Perform lumbar puncture, hospitalize, and give empiric antibiotics |
|
WBC: white blood cell; UA: urinalysis; HPF: high-power field; PCT: procalcitonin; CRP: C-reactive protein; ANC: absolute neutrophil count; PECARN: Pediatric Emergency Care Applied Research Network a Most clinicians would not apply PECARN criteria for patients ≤ 28 days of age. b Consider cutoffs of ANC ≤ 4,000 cells/mcL and PCT of ≤ 0.5 ng/mL for PECARN criteria. Adapted from Palladino L, Woll C, Aronson PL. Evaluation and management of the febrile young infant in the emergency department. Pediatr Emerg Med Pract 2019;16:1-24. |
||||
Urinalysis
Sterile samples obtained by straight catheterization or suprapubic aspiration are preferred to bagged urine specimens in this age group, given the risk of contamination with bagged specimens. All urine samples also should be sent for urine culture as well as urinalysis. Catheterized urinalysis is highly accurate for diagnosis of UTI in this age group, with both sensitivity and specificity greater than 90%.43 It is slightly less sensitive if a UTI is defined as a colony count of > 10,000 cfu/mL rather than > 50,000 cfu/mL, but it is currently an area of debate whether bacteriuria without pyuria constitutes a true UTI in this age group.44 The criteria for an abnormal urinalysis are positive leukocyte esterase, nitrites, or > 5 WBC/hpf. Urinalysis with microscopy is preferred to dipstick in this age group, but some centers have used dipstick as a relatively accurate surrogate.11 Even a trace leukocyte esterase should be considered diagnostic for pyuria in this age group, since infants void frequently and leukocyte esterase is the most sensitive aspect of the urinalysis.43
Cerebrospinal Fluid Studies
Interpretation of cerebrospinal fluid (CSF) parameters depends on the age of the infant. A very large recent study compared normal values for CSF WBC count, protein, and glucose for infants aged ≤ 28 days to those in infants aged 29-60 days.45 According to the study, normal values for CSF in infants aged ≤ 28 days are 45:
- WBC: ≤ 15 cells/mm3
- Protein: ≤ 127 mg/dL
- Glucose: ≥ 35 mg/dL (lower bound: 25 mg/dL).
Normal values for CSF in infants aged 29-60 days are45:
- WBC: ≤ 9 cells/mm3
- Protein: ≤ 99 mg/dL
- Glucose: ≥ 37 mg/dL (lower bound: 27 mg/dL).
Any abnormality in CSF should be taken seriously as a sign of possible meningitis, even isolated elevated protein or low glucose. Low glucose and high protein in CSF have been associated with complications of meningitis, such as hydrocephalus or infarction.46 Low CSF glucose can be determined by a low absolute number or a low CSF-to-serum glucose ratio. A normal ratio is around 0.6, and if the ratio is significantly less, it becomes concerning for meningitis.47 For improved accuracy of the ratio, a fingerstick glucose should be obtained just prior to preparing for LP, and the CSF-to-serum ratio of glucose should be calculated from that POC glucose result. Viral PCR testing of CSF for HSV, enterovirus, or parechovirus can be done if risk factors are present. Performing enterovirus PCR testing on CSF, if obtained during the summer and early fall, has been shown to decrease the length of stay for some infants; however, a positive result does not completely exclude SBI.48 Some hospitals may have the availability of meningoencephalitis CSF PCR panels for broader testing for specific viral, bacterial, and fungal pathogens. These panels have high sensitivity and specificity for particular pathogens and may be helpful in cases of questionable meningitis, or they may give an earlier turnaround time in testing for these pathogens.49 They can be done in addition to a CSF culture, but they should not replace one. One ED approach would be to collect extra CSF in the fourth CSF tube for the inpatient team to send for additional PCR testing if indicated by local institutional protocols.
Classically taught CSF parameter cutoffs for bacterial vs. viral meningitis may not hold true in young infants. Bacterial meningitis does not always present with a neutrophil predominance in the CSF in young infants. In this age group, CSF pleocytosis with a mononuclear predominance can be caused by life-threatening infections, such as HSV meningitis or bacterial meningitis. Physicians should not assume it is caused by a self-limited viral meningitis.50 In addition to this, a significant portion of meningitis cases in this age range do not show CSF pleocytosis on initial presentation; as such, normal initial CSF studies make bacterial meningitis less likely but do not completely rule it out.17,51
Unsuccessful LP and traumatic lumbar punctures are both, unfortunately, common. Use of local anesthetic has been shown to increase LP first-pass success, as has removal of the stylet after the skin and subcutaneous tissue have been entered but before the subarachnoid space is entered. Traumatic LPs are far more common if more than one attempt is required.52 Generally, a traumatic tap is defined as > 10,000 RBCs/mm3 of CSF. Studies suggest caution with using corrected WBC and protein values for traumatic LPs. The typical correction factor for protein is 1:1,000 RBCs/mm3; however, correcting for protein may miss meningitis cases, since studies have shown infants, mainly those younger than 1 month of age, with both bacterial and HSV meningitis having normal corrected protein but abnormal uncorrected protein.53
Correction for WBCs was evaluated similarly in a large recent retrospective study.54 A correction factor of 1 WBC:1,000 RBCs/mm3 only misclassified one case of meningitis in infants older than 28 days, but misclassified cases in infants 28 days old or younger with a much higher frequency. However, using the correction factor did allow a much lower rate of diagnosing pleocytosis in infants older than 28 days. Overall, the correction factors should not be used in infants younger than 29 days of age and should only be used very cautiously in 29- to 60-day-old infants.
There is no established standard of care regarding which infants to test and empirically treat for HSV infection. Practice varies widely from testing and empirically treating all infants younger than 21 (or 28) days in whom empiric antibiotics are given, to testing and treating infants only who present with high-risk clinical signs and symptoms or risk factors.24 To do a full workup for HSV, one should test in all of the possible locations for infection. HSV PCR of the CSF and blood should be performed. Swabs should be taken of the conjunctiva, nasopharynx, buccal mucosa, rectum, and vesicular skin lesions, if present (vesicles should be unroofed for specimen collection). These swabs should be sent for viral culture at a minimum; some institutions may send these swabs for PCR as well. Note that HSV PCR of CSF is not 100% sensitive for herpes encephalitis and may be falsely negative, particularly early in the disease course.55
Imaging
Infants with significant lung pathology tend to display signs and symptoms of lower respiratory tract infection, such as tachypnea, hypoxia, retractions, cough, or focal abnormalities on lung exam. A chest X-ray has poor diagnostic utility in infants who do not have any of these signs or symptoms, and obtaining one is not recommended unless evidence of lower respiratory tract infection exists on history and physical exam.56,57
In contrast to the common practice in older children of obtaining a computed tomography (CT) scan prior to performing an LP, in well-appearing infants, the risk of dangerously elevated intracranial pressure is very low, and CT is not needed prior to LP in these cases. Open fontanelles help make herniation less likely, although they do not completely eliminate the possibility of herniation. CT of the head is not a fully reliable indicator of herniation risk in infants with meningitis.58 In very ill-appearing infants or febrile young infants with focal neurologic deficits or seizures, CT may be indicated prior to LP. However, antimicrobials should not be delayed for CT and should be given immediately in these cases. It is better to obtain a pretreated LP than to delay antimicrobials in a critically ill infant.
Future Studies
Potential future diagnostic techniques include ribonucleic acid (RNA) biosignature analysis and the biomarker presepsin. Host RNA expression patterns vary in response to different infections. A PECARN study of RNA biosignatures showed 87% sensitivity and 89% specificity for SBI in febrile infants ≤ 60 days of age.59 This is a promising area of research and needs further study and validation before it can be applied broadly. Presepsin is a truncated form of soluble CD14 that is released from the surface of immune cells after stimulation by a pathogen. Studies in neonates have been promising, but further study is needed on this biomarker to determine clinical utility.60
Differential Diagnosis
Infections are by far the most common cause of fever in neonates and young infants. Well-appearing febrile young infants are very unlikely to have a noninfectious pathologic cause of their presentation. In ill-appearing febrile young infants, other causes of ill appearance must be considered during resuscitation and management. (See Table 3 and Figure 1.)
Table 3. Non-Infectious Differential Diagnosis for Critically Ill Neonates with Fever* |
|
Intestinal catastrophe: necrotizing enterocolitis, intestinal malrotation with volvulus |
|
Congenital hyperthyroidism |
|
Abusive head trauma |
|
Congenital heart disease |
|
Inborn errors of metabolism |
|
Congenital adrenal insufficiency |
|
Metabolic abnormality |
|
* Note that most of these conditions often occur without fever as well. |
Figure 1. Algorithm to Treat Infants Aged 29-60 Days Presenting with Fever Without a Source |
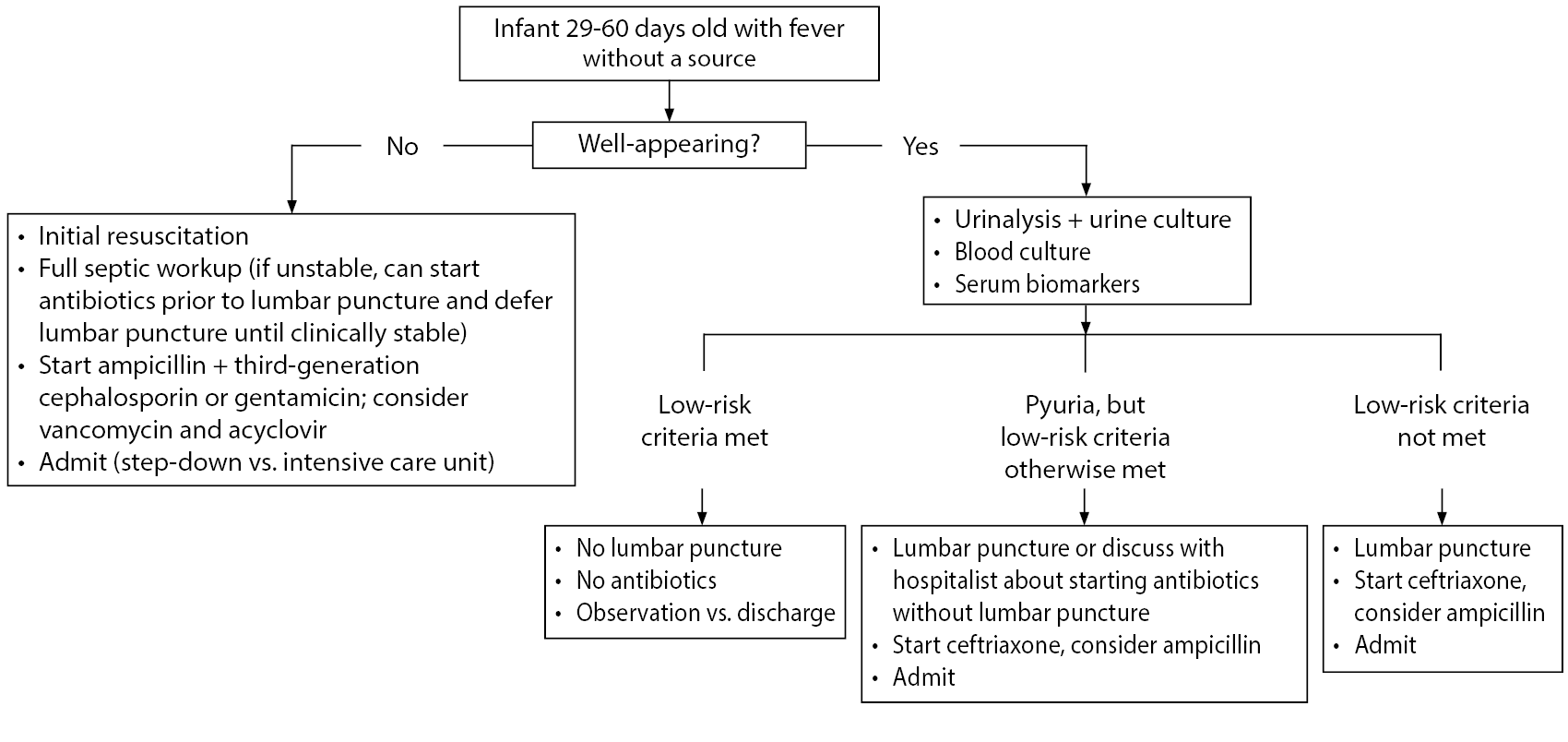 |
Management
Antimicrobial Choice
Antibiotic choice must cover the most common causes of infection, including GBS, E. coli, MSSA, S. pneumoniae, and less common gram-negative and gram-positive pathogens. In addition to antibiotic coverage, empiric coverage for HSV with acyclovir 20 mg/kg/dose every eight hours should be strongly considered in infants younger than 42 days of age, especially those younger than 28 days.25
In infants ≤ 28 days of age, ampicillin is required for coverage of Listeria in addition to covering for resistance of some important pathogens to third-
generation cephalosporins (e.g., Enterococcus). Ampicillin also is the preferred treatment for GBS. Some potential pathogens, such as E. coli, have a significant resistance to ampicillin, which is why two antibiotics are required for initial coverage.18,61 In the first month of life, the preferred second antibiotic is cefotaxime or gentamicin. Cefotaxime penetrates CSF better than gentamicin, so it may be preferred if meningitis is suspected based on ill appearance or CSF abnormalities.18 Recent cefotaxime shortages have prompted the substitution of cefotaxime with ceftazidime, cefepime, or other antibiotics at some institutions.62 The addition of vancomycin can be considered as well if there is suspicion for MRSA sepsis in areas with high prevalence of MRSA. Note that vancomycin has increased nephrotoxicity when given along with aminoglycosides, and vancomycin dosing is somewhat controversial in young infants because of decreased clearance and increased volume of distribution, leading to a longer half-life.63 As such, it is recommended to discuss with a pharmacist if the infant is in the neonatal period or if they will be boarding in the ED for a long time and will need subsequent dosing.
In the second month of life, ceftriaxone can be substituted for cefotaxime. Ceftriaxone has been shown to competitively displace bilirubin from albumin in vivo, potentially increasing the risk of bilirubin encephalopathy and kernicterus in young infants.64 Because of this, it should not be given to infants younger than 1 month of age or to slightly older infants with high bilirubin. For well-appearing infants 29-60 days of age who are being admitted, ceftriaxone monotherapy may be adequate, but consider also giving ampicillin, since it is the best-studied agent for treatment of GBS, which still has a high prevalence in the second month of life (although GBS resistance to ceftriaxone is rare).61 If the infant is not well appearing, then ampicillin should be added, and consider the addition of vancomycin for coverage of resistant S. pneumoniae and MRSA.
Special Considerations for Antimicrobials
Depending on the locality, there can be varying resistance of E. coli to gentamicin or third-generation cephalosporins. However, generally, E. coli is > 90% susceptible to either antibiotic in infants without prematurity or chronic health conditions.16 Note that premature infants who have spent time in the NICU or infants with complicated chronic conditions or indwelling medical devices may have higher rates of atypical infections with organisms, such as MRSA, Enterobacter species, or Serratia species, which may be resistant to usual antibiotic coverage.46 These infants would fall outside the typical well-appearing febrile infant algorithms.
Note that it also is generally preferred to obtain all cultures prior to administration of antimicrobials. However, critically ill infants may not be clinically stable enough to obtain an LP immediately. If this is the case, antibiotics should be started once blood and urine cultures are quickly obtained. An LP can be deferred until the infant is more clinically stable.
Risk Stratification of Well-Appearing Infants
Biomarkers for SBI perform particularly poorly in infants ≤ 28 days of age, which contributes to the poor sensitivity of clinical decision rules in neonates.66 As of now, most clinicians would agree that all febrile infants ≤ 28 days of age need a full sepsis (and possibly HSV) workup, as well as empiric antimicrobials and admission to the hospital, given the lack of definitive low-risk criteria to reliably rule out SBI and HSV in infants.10
However, as described earlier, in infants ages 29-60 days, a more nuanced and individualized approach may be warranted. Validated algorithms that address approaching these infants without necessarily requiring an LP, hospitalization, and broad-spectrum antibiotics include the Step-by-Step approach and the PECARN prediction rule, as well as the older Rochester criteria and Modified Philadelphia criteria. (See Table 2.) In these algorithms, if well-appearing infants meet low-risk criteria, they potentially can be discharged with close follow-up and strict return precautions. These studies to define low-risk criteria were performed in infants who were generally healthy and did not have additional baseline risk for SBI compared to a healthy infant. Infants who are born prematurely (< 37 weeks), and infants with unexplained hyperbilirubinemia, chronic or complex medical conditions, prior antibiotic use, and prolonged initial hospitalization after birth generally were excluded from these studies. The presence of any of these conditions puts the infant at higher risk for SBI, and a full septic workup, including LP, should be strongly considered. In general, premature infants should be considered to be at high risk for SBI until at least 44 weeks post-conceptional age, and a full septic workup should be strongly considered for them as well.67 Unfortunately, typical risk stratification rules used in the ED cannot be accurately applied to premature infants, given the lack of adequate studies. Another additional low-risk criterion that has been discussed in the literature is the “Lab Score.”68 This was originally derived in children 7 days to 3 years of age and uses low-risk laboratory results to suggest the risk of SBI. However, the “Lab Score” performs poorly for detection of IBI in young febrile infants and should not be used in this age group.69
A recent PECARN study looked at the risk of SBI in well-appearing infants 0-60 days of age using ANC, PCT, and urinalysis results. This study excluded infants with soft tissue infections, but not with other focal signs of infections. It was not performed in a cohort consisting of infants with FWS. The rule was derived from a set of 908 infants with an SBI rate of 9%. Low-risk infants were defined as having a negative urinalysis, ANC of 4,090/µL or lower, and serum PCT of 1.71 ng/mL or lower. The negative predictive value (NPV) was very similar when using easier-to-remember values of ANC of 4,000/µL and PCT of 0.5 ng/mL (although the specificity was lower when using these adjusted cutoffs). In the validation set of 913 infants, 497 (54.4%) were categorized as low risk by the decision rule, which missed two UTIs. One case of Enterobacter cloacae bacteremia was missed by the rule in the derivation study, and no cases of meningitis were missed. The sensitivity of the rule was 97.7%, the specificity was 60%, and the NPV was 99.6%. The lower end of the 95% confidence interval of the NPV in the validation set was 98.4%. The PECARN study did not comment on excluding HSV infection reliably, and at this point, the application of these criteria to infants younger than 29 days of age would be controversial.9
The PECARN criteria also were recently externally validated in infants with FWS who were not immediately admitted to the ICU. This was a single-center study and was a secondary application of the PECARN criteria to a prior prospective cohort. This study showed slightly poorer accuracy than the initial validation study. The sensitivity for SBI was 89.8% with an NPV of 95.5%. When limited to infants aged 29-60 days, it had similar sensitivity for SBI. For IBI, the sensitivity was 86.8%, and the NPV was 99.1%. Notable aspects of this validation study were overall higher rates of SBI and IBI, which may be because it only included infants with FWS, as well as because of a generally shorter duration of fever in this cohort compared to PECARN. In this study, five cases of IBI were missed by the PECARN criteria, including two cases of meningitis. However, if the PECARN criteria were applied only to infants in this study who were older than 28 days of age and the lower PCT cutoff of 0.5 ng/mL was used, it would have missed only one case of IBI (Staphylococcus bacteremia). Also notable was the result that three of the five missed cases of IBI had a fever for less than three hours prior to arrival in the ED.12 Overall, this study urges some caution in applying the PECARN criteria to infants with FWS, especially those younger than 28 days of age or who present with a short duration of fever. Further external validation studies ideally will be done in the future by other centers to give a fuller picture of the accuracy of the PECARN criteria.
The Step-by-Step criteria were derived and validated in large multicenter trials in western Europe. They apply to infants 22-90 days of age with FWS, so they excluded infants with obvious sources of infection and significant respiratory symptoms. Low-risk criteria include no pyuria, PCT < 0.5 ng/mL, CRP ≤ 20 mg/L, and ANC ≤ 10,000/mm3. Sensitivity and specificity for detecting IBI were 92% and 46%, respectively. The NPV was 99.3%. No cases of bacterial meningitis were missed in the validation study. Four of the seven cases of bacteremia missed in the validation study were in infants 22-28 days of age, and application of the Step-by-Step criteria to that age group is controversial. Additionally, six of the seven missed cases of IBI had a fever for less than two hours at the time of evaluation, or the fever was first detected in the ED.11 This may reflect the fact that biomarkers like PCT and CRP take time to rise, making them less sensitive very early in the infectious process. This suggests a potential role of short-term observation in the ED or an observation unit in young infants with very short duration of fever to watch for clinical worsening.
The initial studies of the Rochester and Philadelphia criteria were done before GBS prophylaxis was common and before the deployment of modern pneumococcal vaccinations. They have not been studied extensively for accuracy in the modern era, although a few studies have been performed. Recent evaluations of the Rochester criteria have shown sensitivity for detecting IBI of 81% to 94% in infants aged 29-60 days.11,33,70 Sensitivity of the Philadelphia criteria for IBI was 83% in this age group in one modern study.70 In hospitals that lack quick turnaround time for CRP and PCT studies, but do include band counts on CBC differentials, employing the Rochester or Philadelphia criteria for well-appearing febrile infants could be an option, although they appear likely to be inferior to the Step-by-Step criteria and PECARN criteria in the modern era. Note that hyperpyrexia (temperature > 40°C) has been shown to be a risk factor for SBI in infants younger than 3 months of age and could be considered a trigger for a full septic workup in infants ages 1-3 months in whom a more limited workup might have been considered otherwise.71 The application of low-risk criteria is not as clear in patients with hyperpyrexia.
Do Febrile Infants with UTIs Need LPs?
Older infants who have evidence of UTIs on urinalysis, but who are otherwise well-appearing and have low-risk biomarkers, may not need LPs to exclude concomitant meningitis. A recent systematic review looked at infants younger than 3 months of age with UTIs and concluded that existing evidence shows that most cases of UTI with concurrent meningitis are in infants younger than 1 month of age or in ill-appearing infants.72
Additional studies have shown that having a UTI does not increase the risk of meningitis compared to the background risk in infants older than 28 days of age.73,74 Applying several of the Step-by-Step low-risk criteria (PCT < 0.5 ng/mL, CRP < 20 mg/L, age > 21 days) to infants with UTI to assess the risk of IBI did not miss any known cases of meningitis in infants older than 28 days in two studies.75,76 There is a higher risk of concomitant bacterial meningitis in infants
≤ 28 days of age with a UTI; these infants should have an LP performed.51
Another factor in this discussion is that sterile CSF pleocytosis occurs in around 20% of young infants with UTIs. If this is present, it tends to lead to longer antibiotic courses for these infants, even though infants with UTIs and sterile pleocytosis who are otherwise clinically well with an uncomplicated course have been shown to have similar clinical outcomes to infants with UTIs without pleocytosis.77 Current evidence appears to suggest that a more selective approach to LP may be taken with infants 29-60 days of age with UTIs, with the application of the Step-by-Step criteria to these infants having the most direct evidence. Infants with abnormal urinalysis but low-risk biomarkers can potentially be admitted to the hospital for intravenous antibiotics pending blood and urine cultures without obtaining an LP. However, a discussion with the admitting pediatrician should occur regarding whether they agree with starting antibiotics without obtaining an LP in these cases, since institutional preference may vary.
Focal Infections
Other more obvious focal infections, such as pneumonia, omphalitis, septic arthritis, cellulitis, osteomyelitis, or bacterial gastroenteritis, should be aggressively investigated and treated in this age group. The fact that the infant is presenting with fever is potentially suggestive that the focal infection has an invasive aspect to it, as a retrospective study of S. aureus infections suggests that fever is more common in invasive infections than in focal cellulitis, pustulosis, or abscess.78 The particular organisms causing focal infections in young infants tend to be more virulent in this relatively immunosuppressed group and often have the ability to cause bacteremia and meningitis in young infants, so a full septic workup, in addition to broad spectrum antibiotics, often is indicated. Additional focal cultures should be obtained, if feasible.
Infants with Bronchiolitis or Other Viral Infections
Infants with clinical bronchiolitis or who are respiratory syncytial virus (RSV)-positive in this age group do have a risk of concomitant SBI, but it is lower than in infants with negative viral panel results. In provider surveys, positive viral swab results have been shown to influence provider decision making as to whether to start antibiotics for young febrile infants.79 However, the rates of UTI are greater than 2% in infants 29-60 days of age with documented viral infection, and the rates of co-occurring bacteremia and meningitis are still significant in infants younger than 29 days old. Therefore, infants ≤ 28 days who are positive for viral infection still require a full septic workup and antimicrobial treatment. In contrast, co-occurrence of bacteremia or meningitis in infants older than 28 days of age with documented viral infections is rare, with the incidence of bacteremia less than 1% and meningitis around 0.2%.80,81 So, infants of this age who test positive for a viral source of infection still should have urine obtained for testing, but they may be able to forgo blood and CSF testing if they are well-appearing and urinalysis is negative.
Note, though, that a positive rhinovirus result does not correlate as well with a reduced risk of SBI as other viral infections.82 Rhinovirus can have prolonged shedding and frequently is detected in asymptomatic patients. Therefore, an infant with only a positive rhinovirus result on viral testing should be managed as if the fever is unlikely to be caused by rhinovirus infection, and an alternative cause of fever should be sought. Swabbing for influenza can be helpful, since treatment with oseltamivir often is indicated in infants older than 2 weeks of age if they test positive for influenza.83 Infants younger than 8 weeks of age with bronchiolitis are at a higher risk of apnea and poor outcomes and may require admission regardless of the particular virus isolated. Any viral cause of bronchiolitis, including rhinovirus, can result in apneic events.84 At this age, they also should have a chest X-ray performed if they have significant respiratory symptoms to evaluate for any focal bacterial pneumonia.
When to Test and Treat for HSV
Infants who are at a high risk for HSV infection should be tested for HSV and treated with empiric acyclovir while test results are pending. Features that are more common in infants with HSV infection are elevated transaminases, consumptive coagulopathy, pneumonitis, seizure, ill appearance, mouth lesions, vesicular rash, and eye findings. Patients younger than 29 days of age with these presentations should be tested for HSV and started on acyclovir. Patients in the fifth or sixth week of life with one of these presentations should be strongly considered for HSV testing and empiric treatment with acyclovir, especially if they are ill-appearing. Any patient with signs of skin/eye/mouth disease on exam in the first two months of life should have full HSV testing performed and should be treated. Isolated cutaneous disease frequently progresses to involve other sites within one week if not treated. Delays in HSV treatment are associated with increased mortality.85
HSV certainly can present with fever only, and infants can initially be relatively well-appearing. Most nonspecific presentations of HSV occur in the first month of life.24,86,87 However, given the low overall prevalence of HSV infection in febrile infants, in well-appearing infants without known risk factors and no other evidence of HSV infection other than fever, there is no clear standard of care for testing and empiric treatment. In these cases, the cost and potential complications from acyclovir administration and more prolonged hospitalization need to be weighed against the low but potentially devastating risk of missing an early HSV infection. Acyclovir can cause neutropenia and nephrotoxicity. However, in infants who need treatment for HSV infection, it is uncommon for these side effects to be severe enough to necessitate discontinuing the medication.24
Disposition
All febrile infants younger than 29 days of age should have a full septic workup obtained, receive broad spectrum antibiotics (± acyclovir), and be admitted to the hospital. Well-appearing infants 29-60 days of age with FWS should have a urinalysis, urine culture, blood culture, and serum risk stratification studies performed. Low-risk criteria then can be applied based on the testing available at the institution. If the infant does not qualify as low-risk for IBI, then an LP should be performed and the infant should be admitted to the hospital for observation, with initiation of antibiotics as well. Infants in whom both urinalysis and serum studies are low risk can be discharged after reevaluation in the ED if they meet the following criteria: well-appearing, adequate hydration, good caregiver supervision, reliable telephone access, close proximity to the hospital, and ability to follow up with their pediatrician within 12-24 hours. Ideally, the pediatrician should be contacted prior to discharge to confirm the availability of follow-up. Any worsening of clinical condition should prompt caregivers to return to the ED with the infant. If an LP is not obtained prior to discharge, antibiotics generally should not be given to avoid affecting CSF culture yield if an LP is needed later. If a patient is low risk for SBI but does not have a safe discharge situation, the patient can be admitted for observation without an LP or antibiotics pending culture results and clinical stability. If an LP was obtained in a low-risk infant and initial CSF studies are normal, the infant could be administered a dose of ceftriaxone prior to discharge and can follow up with their pediatrician within 24 hours. In infants with a short-duration fever, a longer ED observation period or admission to the hospital for observation should be considered. The role of repeat testing for inflammatory markers, either as inpatient or outpatient, is not clear.
Considering COVID-19
The rise of SARS-CoV-2 infection has presented a new potential cause of infection in febrile young infants. COVID-19 infection in young infants may present with or without fever; and there is not enough evidence currently to suggest that febrile infants with positive COVID-19 testing are at decreased risk for SBI.88 Infected neonates seem to have a lower prevalence of fever but a higher incidence of needing respiratory support than older children.89 As of now, febrile infants should be tested for COVID-19 if they have had possible exposure from their mother or from the community. However, a positive COVID-19 test should not decrease testing for SBIs or HSV in young febrile infants. Multisystem inflammatory syndrome in children (MIS-C) is another new potential cause of fever in children. However, current research suggests the vast majority of MIS-C cases occur in older children; when they have occurred in infants, they generally have been in infants older than 2 months of age.90 This is an area of rapidly evolving knowledge.
Conclusion
Management of febrile infants younger than 29 days of age has not changed much despite recent research, given the high-risk status of these infants. Whether further research into RNA biosignatures or other biomarkers eventually will change this remains to be seen. Acceptable management strategies for febrile infants 29-60 days of age have evolved in recent years, and there is opportunity for more individualization of care based on risk factors, appearance, and biomarkers. Management of these infants likely will continue to change in the near future as research continues at a rapid pace, hopefully promoting better outcomes for vulnerable infants and their families.
References
A complete list of references can be found online at http://bit.ly/3kWok4Y.
Every clinician has struggled with managing a febrile infant. We know the majority will have a benign viral illness, but we fear the serious bacterial infection that may have devastating consequences in this vulnerable population. This evidence-based article reviews the current literature and approach to infants less than 60 days of age.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
