Introduction
Supraventricular tachycardia (SVT) is a common medical condition. Diagnosis and treatment often occur simultaneously. Rapid diagnosis can be achieved by a stepwise, logical approach that can be easily learned, remembered, and then deployed at the bedside. To a great degree, long-term treatment options depend on the history of symptoms and the patient’s desire, rather than on the specific type of SVT.
SVT affects every specialty in medicine, including primary care, obstetrics, and surgery, and is a common problem not only in general cardiology, but also in adult congenital heart disease. Accurate diagnosis of SVT guides acute treatment. Although many doctors are good at pattern recognition on electrocardiograms (ECGs), having a methodical approach to think through SVT is extremely helpful. This article will lay out a stepwise method to diagnose and thus treat SVT.
Definition and Clinical Presentation of SVT
Generally, SVT is defined as a rhythm with a heart rate greater than 100 beats per minute (bpm) and a QRS width less than 120 msec. However, in certain instances, SVT can present with a QRS width greater than 120 msec if there is underlying bundle branch block or the SVT is aberrantly conducted. In contrast, by definition, wide complex tachycardia (WCT) has a heart rate greater than 100 bpm but a QRS duration of greater than 120 msec, with the most concerning diagnosis being ventricular tachycardia.
The incidence of SVT is about 2.25/1,000 persons. Women are twice as likely as men to have SVT. Atrioventricular reentry tachycardia (AVRT) is a type of SVT most common in young patients, whereas in patients older than age 65 years, atrial tachycardia (AT) is the type of SVT most commonly found.1
The clinical presentation is varied. Patients may present from completely asymptomatic to feeling palpitations to post-syncopal.2 (See Figure 1.) Syncope as the initial presentation of SVT is rare, representing only 4% of cases. Palpitations and chest pain are more common (22% and 5%, respectively). SVT often can be misdiagnosed as panic attacks.3 Sudden cardiac death is extremely rare as an initial presentation and accounts for only 0.2% of cases. An unusual but common clinical complaint is polyuria. Most likely, this occurs as the result of atrial stretch and increased B-type natriuretic peptide production stimulating diuresis.4
Figure 1. Supraventricular Tachycardia (SVT) as a Cause of Syncope |
 |
Telemetry strip showing acute drop in blood pressure and pulsus alternans in a systolic heart failure patient who went into SVT. The bottom line is the arterial line tracing. |
SVT can occur spontaneously or can be triggered by several mechanisms. Increased adrenergic stimulation from dehydration, sepsis or other acute illness, cocaine (less than 1% incidence), and even energy drinks all can induce SVT.5,6 Another common mechanism is increased intracardiac pressures and atrial stretch from conditions such as pulmonary embolism, myocardial infarction, congestive heart failure, or valvular stenosis or regurgitation. SVT also can occur in healthy patients without significant structural heart disease. SVT is becoming an increasing problem in adult congenital patients not only as the result of primary abnormalities of the atria themselves, but also because of increased atrial pressures over time, leading to atrial dilation and, finally, to SVT. Overall, the prevalence in adult congenital heart disease is around 10%, but it increases with age. In one study, the prevalence of SVT in the adult congenital disease population was as high as 38% by age 50 years.7,8 In a similar manner, pregnancy is associated with SVT because of increased volume status and cardiac chamber stretch. The incidence has been reported to be around 8%, but SVT in this condition has not been well studied.9
The physical examination of a patient in SVT also is varied. Other than an elevated heart rate, the rest of the examination often is completely normal. Blood pressure can be normal, low, or elevated depending on the trigger and the patient’s response to the tachycardia. The patient may or may not have any signs of congestive heart failure (gallops, rales, elevated jugular venous pressure). One interesting finding is denoted as “frog sign,” which may occur during atrioventricular nodal reentry tachycardia (AVNRT), where the atria fire against a closed tricuspid valve causing regular cannon A waves in the jugular vein.10 This gives the appearance of a billowing neck, hence the name “frog sign.” Regular cannon A waves also can occur in ventricular tachycardia, but the ECG would demonstrate a wide QRS complex.
Sinus tachycardia: Strictly taking prevalence into account, this is the most common SVT. Multiple triggers can lead to sinus tachycardia, from simple stress, hypovolemia (dehydration, blood loss, sepsis), fever, stimulants (caffeine, cocaine, methamphetamine), or the stress of any severe illness. Inappropriate sinus tachycardia is defined by a resting heart rate greater than 100 bpm or an average heart rate greater than 90 bpm in a 24-hour period with no underlying cause.11
Sinus node reentrant tachycardia: This is a rare condition (less than 5% of all SVT) in which the reentry involves the sinus node itself.12 Unlike sinus tachycardia, sinus node reentrant tachycardia starts and ends suddenly.
AVNRT: The most common pathologic regular SVT is AVNRT.13 AVNRT accounts for up to 60% of the regular sustained SVTs. Seventy percent of patients are female.14 Up to 80% of humans possess dual atrioventricular (AV) nodal physiology, meaning they have both a slow and a fast pathway.15 These pathways not only conduct from atria to ventricles, but also retrograde from the ventricles to the atria, thus forming a potential reentrant circuit. Common AVNRT often is initiated by a well-timed premature atrial contraction (PAC), resulting in conduction block in the fast pathway, and antegrade conduction using the slow pathway, followed by retrograde activation up the fast pathway and initiation of tachycardia. On the 12-lead ECG, this sometimes can be seen with a PAC, followed by a prolonged PR interval (antegrade conduction down the slow pathway), followed by initiation of a short RP tachycardia.
AVRT: AVRT is a reentrant tachycardia that uses the AV node and also requires a bypass tract. Usually, orthodromic AVRT is a narrow complex tachycardia because the impulse travels anterogradely down the AV node and then retrogradely up the accessory pathway.
However, in antidromic AVRT, the impulse travels anterogradely down the bypass tract, creating a wide complex QRS before traveling retrogradely up the AV node. About 25% of SVTs involve the use of a bypass tract.16
Junctional tachycardia: This occurs commonly in children or in postoperative patients with high adrenergic tones. However, it also can occur as a result of a drug overdose, such as digoxin.17,18 Junctional tachycardia resulting from a drug overdose typically is self-limited and is treated by stopping the offending agent.
Paroxysmal atrial tachycardia (PAT): PAT represents 15% of all SVT. A previously common arrhythmia was PAT with block that typically was associated with digoxin toxicity.19,20 Atrial tachycardia is more common in older patients with structurally abnormal atria, but like all SVTs, it can occur in any age group.
Multifocal atrial tachycardia (MAT): By definition, MAT is diagnosed when the heart rate is above 100 bpm, the overall rate is irregular, and there are at least three well-defined P wave morphologies present. The two related diagnoses are AFib and wandering atrial pacemaker (WAP). Often, it is hard to discern MAT from AFib, especially if the AFib has coarse F waves. In MAT, there should be at least three different, but repetitive, P waves seen throughout the ECG tracing. WAP by definition has a heart rate of less than 100 bpm, so it is much easier to discriminate from MAT.
Although both MAT and WAP primarily occur in elderly patients, their mechanisms are different. Typically, WAP is seen with a high vagal tone, allowing different atrial foci to usurp control of the atrium.21 MAT results from increased stimulus and stress, causing increased firings from multiple atrial foci, causing a more AFib-like condition.22 The underlying mechanisms and differences in these two rhythms are important to remember, since when MAT is rate controlled (heart rate below 100 bpm), that does not mean the patient now has WAP.
Atrial flutter will be discussed only briefly, since often it does present as a regular SVT. Since the typical atrial flutter atrial rate is 300 bpm, the ventricular response often is 150 bpm because of the 2:1 AV nodal block. Atrial flutter should be suspected in any narrow complex tachycardia at a heart rate around 150 bpm.
Diagnosis of SVT
The diagnosis of SVT can be accomplished by using a stepwise approach. The use of several filters can greatly reduce the number of differential diagnoses in each group. (See Figure 1 and Table 1.)
The first major filter is whether the rhythm is irregular or regular. Irregular SVTs include AFib, atrial flutter with variable block, and MAT. AFib is the most common sustained abnormal SVT. For more information on AFib or flutter, see the November 2019 and December 2019 issues of Primary Care Reports.
Table 1. Differential Diagnosis of SVT |
Irregular SVT |
|
Regular SVT |
|
SVT: supraventricular tachycardia; AFib: atrial fibrillation; AVNRT: atrioventricular nodal reentry tachycardia; MAT: multifocal atrial tachycardia; AVRT: atrioventricular reentry tachycardia; PAT: paroxysmal atrial tachycardia |
The regular SVTs include sinus tachycardia (the most common SVT), AVNRT, AVRT, PAT, and junctional tachycardia.
Regular SVTs can be classified further, or subdivided, into those with a short RP tachycardia or a long RP tachycardia. Short RP tachycardias are limited to the common type of AVNRT, AVRT, or junctional tachycardia. Long RP tachycardias include uncommon type AVNRT. Atrial tachycardia traditionally is a long RP tachycardia, although the RP interval can vary occasionally. As the term suggests, long RP tachycardias have a longer RP length than the next PR length. (See Figure 2.) Determining whether the RP interval is long or short necessitates finding the P waves. In short RP tachycardias, P waves can be obscured (“buried”) by the QRS complex itself or often are seen right after the QRS complex. Since they are retrograde, typically they are inverted and appear as “pseudo S waves.” (See Figures 3 and 4.) Short RP tachycardias are almost diagnostic for the common type of AVNRT, since junctional tachycardia is so rare. Short RP tachycardia also can be diagnostic for AVRT, but the RP interval often is slightly longer than in AVNRT because the atria are activated in series as opposed to activated in parallel in AVNRT.
Figure 2. Flow Diagram for the Diagnosis of SVT |
 |
SVT: supraventricular tachycardia; AV: atrioventricular; MAT: mutifocal atrial tachycardia; AVNRT: atrioventricular nodal reentry tachycardia; AVRT: atrioventricular reentry tachycardia |
Figure 3. Atrioventricular Nodal Reentry Tachycardia (AVNRT) |
Common Type |
 |
Down the slow pathway, up the fast pathway, leading to a short R to P interval. Hence, a short RP tachycardia. |
Uncommon Type |
 |
Down the fast pathway, up the slow pathway, leading to a long |
Most people have dual atrio-ventricular nodal physiology, two pathways that can conduct in both directions. In common type AVNRT, the impulse goes down the slow pathway and back up the fast pathway. In uncommon type AVNRT, the impulse goes down the fast pathway and back up the slow pathway. |
Figure 4. Atrioventricular Nodal Reentry Tachycardia |
Common Type |
 |
Uncommon Type |
 |
Examples of common and uncommon type AVNRT. The top ECG shows a short RP tachycardia consistent with the common type of AVNRT. The arrows denote retrograde P waves that are close to the preceding R wave (short RP interval). The bottom ECG shows uncommon AVNRT. The arrows denote the retrograde P waves farther away from the R wave (long RP interval). |
AVNRT: atrioventricular nodal reentry tachycardia; ECG: electrocardiogram |
Although the critical exercise in diagnosing SVT is finding P waves, this is not always possible. Fortunately, there are ways to treat and diagnose SVT simultaneously.
Acute Treatment
Vagal maneuvers have long been used not only to treat but to help diagnose SVT. Carotid massage, cold immersion, or Valsalva maneuver can increase vagal tone, slow conduction within a reentry circuit, and, thus, terminate the SVT. These interventions are only successful 19% of the time.23 If the change in vagal tone is not enough to terminate, it often is strong enough to reduce AV conduction. This allows for a slow down of the ventricular response, allowing for the appearance of P waves, which can be used to diagnose the specific SVT. A new way to improve the effectiveness of these maneuvers is to add a passive leg lift. In the REVERT study, lifting the legs of the patient increased the conversion rate from 17% to 43%.24
Likewise, any pharmacologic intervention that acts on the AV node can have a similar therapeutic effect by terminating the reentry SVT or helping to diagnose it by unmasking P waves.25 (See Figure 5.) It is important to note that if SVT terminates with vagal maneuvers or AV nodal blocking agents, it suggests that the SVT involves the AV node (AVNRT or AVRT) as opposed to atrial tachycardia, flutter, or fibrillation. While some atrial tachycardias can terminate with adenosine, more commonly the atrial tachycardia will continue while unmasking P waves during AV block. If an SVT continues and AV block occurs during treatment with adenosine, AVRT and AVNRT typically are excluded. The most common medication used today is intravenous (IV) adenosine. Adenosine infusion gave additional diagnostic information in 25% of the cases in one study.26 This is done by the rapid push of 6 mg or 12 mg of IV adenosine to block the AV node and terminate reentry SVTs. The conversion rate for adenosine is 57% for the 6 mg dose vs. 93% for the 12 mg dose. Heart block is a common, but short-lived, side effect of IV adenosine.27 Other typical side effects include transient chest pain, dyspnea, flushing, and headache. For comparison, the standard slowly infused dose for pharmacologic stress testing is 140 mcg/kg per minute given over six minutes.28 Adenosine infusion for stress testing mostly has been replaced by regadenoson bolus dosing because of ease of use. The key benefit of adenosine is its short half-life (seconds), allowing for transient AV block to resolve quickly with transient symptoms.
Figure 5. Unmasking P Waves |
Panel A: Atrial Flutter with 2:1 Atrioventricular Block |
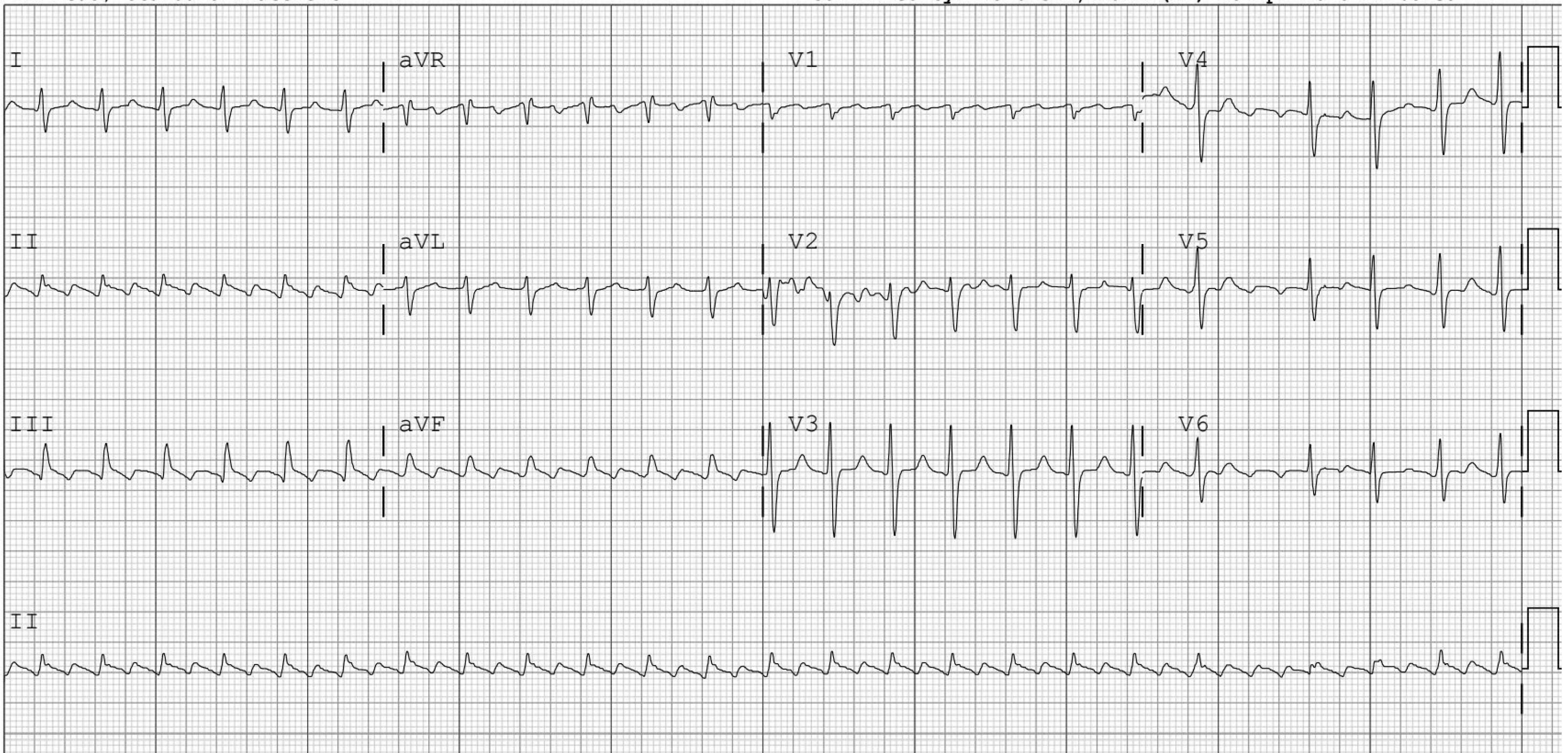 |
Panel B: Atrial Flutter with Variable Atrioventricular Block |
 |
Panel C: Atrial Tachycardia |
 |
Panel D: Valsalva-Inducing Intermittent 2:1 Atrioventricular Block |
 |
Panel A shows a rapid supraventricular tachycardia. After rate control agents are administered, atrial flutter waves clearly can be seen in Panel B. Panel C shows a long RP tachycardia. Panel D shows intermittent 2:1 atrioventricular block with the Valsalva maneuver, confirming this is atrial tachycardia. |
IV calcium channel blockers, such as diltiazem and verapamil, have become a mainstay of the acute treatment of SVT. Diltiazem, especially, has become very popular in the acute and emergency room (ER) setting and is highly effective. Conversion rates of up to 98% have been reported.29 Convenience probably is a factor, since diltiazem comes in preloaded syringes that can be given rapidly. Typical bolus doses of 5 mg up to 20 mg can be given. A diltiazem drip also is readily available for use. It is less of a negative inotrope than verapamil and is well tolerated, similarly to metoprolol.30 Calcium channel blockers, particularly diltiazem, in general should be given with caution in patients with systolic heart failure but can be given acutely.
IV esmolol is a B1 and B2 receptor blocker that also can be used to treat SVT acutely. The drawbacks include a more complicated dosing protocol (mcg/kg/min) than IV diltiazem (mg/hour), and it is significantly less effective. Like adenosine, the major advantage of esmolol is the rapid metabolism (minutes). The infusion can be used for diagnostic/treatment purposes and then can be reversed rapidly by stopping the infusion if side effects occur. This is a benefit over IV diltiazem or other IV beta-blockers. In a small study of 32 patients, IV diltiazem converted all patients, but esmolol converted only 25%.31 Typically, cost is a factor, since esmolol commonly is more expensive than diltiazem.
Other IV beta-blockers, such as metoprolol or labetalol, also can be used to treat SVT acutely. Commonly, metoprolol is given, and most medical personnel are very familiar with its use. It can be used in an intermittent dosing strategy if it does not convert the SVT after initial administration.
IV labetalol also can be used but has a weaker effect on AV nodal blocking and a greater effect on blood pressure because of its alpha blocking properties. This makes it less ideal in the patient who will require repeated doses.
IV digoxin is not an ideal choice for acute SVT. Its inotropic effects come from inhibiting the cellular sodium/potassium pump, leading to an increase in intracellular calcium and greater contractile force. As far as its effect on the AV node, it works indirectly by increasing vagal tone, which may already be low in acutely ill patients, and may be difficult to raise.32
Oral calcium blockers, beta-blockers, and digoxin also can be used in SVT. Typically, oral agents take too long to be absorbed and become bioavailable, making them less attractive as acute agents. A new medication that can be delivered via nasal spray has been shown to be very effective in terminating SVT in Phase I and Phase II clinical trials. Etripamil, a self-administered, intranasal calcium channel blocker, converted SVT in 65% to 95% of the patients, depending on the study dose given in a Phase II clinical trial.33 The median time to convert to sinus rhythm was three minutes. There was only a significant blood pressure drop in the highest dose tested. It remains to be seen if this will continue to show promise in further clinical trials.(See Table 2.)
Table 2. Acute Treatment of SVT |
|||
Treatment |
Typical Dose |
Advantages |
Disadvantages |
Vagal maneuvers with or without leg lift |
|
|
|
IV adenosine |
6 mg or 12 mg |
|
|
IV diltiazem |
5 mg to 20 mg |
|
|
IV metoprolol |
5 mg IV push |
|
|
IV digoxin |
0.125 mg to 1.0 mg IV push |
|
|
IV esmolol |
50 to 300 micrograms/kg/min |
|
|
Etripamil nasal spray |
70 mg, 105 mg, or 140 mg |
|
|
Synchronized cardioversion |
50 J synchronized countershock |
|
|
SVT: supraventricular tachycardia; IV: intravenous |
|||
Chronic Treatment
Although oral agents are not the preferred treatment for acute SVT, they are highly useful in the chronic treatment and prevention of recurrent SVT. Calcium channel blockers, such as diltiazem and verapamil, are well tolerated and can serve both to prevent SVT and control blood pressure. First-generation dihydropyridines, such as nifedipine, and second-generation dihydropyridines, such as amlodipine or felodipine, are not useful since they do not have any significant effect on the AV node. The side effects of calcium channel blockers include reflux, constipation, and lower extremity edema.34
Beta-blockers can be used not only for the prevention of SVT but also can serve other functions, such as blood pressure control and reverse remodeling the left ventricle, and can reduce morbidity and mortality in congestive heart failure. Side effects include fatigue, weight gain, and wheezing.35,36 Metoprolol, a pure B1-receptor blocker, is a more effective rate control agent, while carvedilol and labetalol are better blood pressure control agents because of their additional alpha receptor-blocking properties. Atenolol, although similar to metoprolol, is used less since it has a higher proportion of renal excretion, making it less safe in patients with chronic renal disease.
Since William Withering’s initial description of it in his 1785 book An Account of the Foxglove and Some of its Medical Uses, digoxin has had a controversial record. As an inotrope, it was shown to not increase mortality similarly to other cardiac stimulants in the DIG trial, but in large-scale, propensity score-matched studies, it has been associated with worse outcomes in the long-term treatment of AFib.37,38 This study by Lopes et al was not randomized, and much of the risk centered on patients who had a serum digoxin level of greater than 1.2 ng/mL. It is possible that patients with AFib that is harder to control may have required additional treatment with digoxin, which would explain why outcomes were worse and not necessarily caused by the drug itself. Patients not on digoxin in the Lopes et al study had a higher ejection fraction, more permanent AFib, lower brain natriuretic peptide levels, and lower heart rates.39 Until more is known, it makes sense to reserve digoxin as a second-line agent, not only in the treatment of AFib, but also for the long-term treatment of SVT.
Specific to inappropriate sinus tachycardia, ivabradine has had success in lowering heart rate. Ivabradine blocks the If, or “funny” channel, which is responsible for the slow outward sodium current leak and, thus, the automaticity of the cardiac pacemaker cells. Similar to what was seen in heart failure trials with ivabradine, the heart rate was reduced from 98 bpm to 84 bpm in patients with inappropriate sinus tachycardia.40 Ivabradine was tested against metoprolol and had similar resting heart rate reduction, but was more effective in regard to symptom relief and control of heart rate with activity.41 Since ivabradine acts specifically on pacemaker cells, it is ineffective for SVTs other than inappropriate sinus tachycardia.
Probably the most important decision is not which pharmacologic agent to use long term, but if one is needed at all. The doctor and the patient need to engage in shared decision making to decide on the best course of action — or inaction.42,43 Both must weigh the risks and benefits between the frequency and severity of SVT vs. any possible treatment. If the SVT is rare or mild, and self-limited, it is reasonable to observe the patient rather than start a daily medical program with its associated cost, effort, and potential side effects.
If episodes are rare but cause severe symptoms, then an as-needed approach could be used. This “when necessary,” or “PRN,” approach has been detailed extensively for AFib and is known by the term “pill in the pocket.”44 Similar protocols can be used for SVT, including PRN use of short-acting calcium channel blockers (for faster onset), short-acting beta-blockers, and even PRN anti-arrhythmic medications such as class Ic agents, such as flecainide or propafenone. The blood pressure and pro-arrhythmic effects of any of these treatments need to be evaluated and discussed with the patient.
Since the first surgical ablation of Wolff-Parkinson-White (WPW) syndrome in 1968, curative procedures have worked their way rapidly up the therapeutic ladder.45
Catheter ablation should not be looked at as a last resort. For the younger patient, or the patient with frequent or severe symptoms, ablation may be the best option, may eliminate years of medications and ER visits, and may drastically improve the quality of life. Unlike for AFib, catheter ablation of atrial flutter, AVNRT, AVRT, PAT, and WPW are highly successful. Typically, initial procedural success rates are higher than 90%, with only a 5% to 10% long-term recurrence rate. The procedures also have become very safe, with very low complication rates. The most common complications with these types of ablation procedures are minor bleeding complications.
Other, more serious complications include pericardial effusions necessitating pericardiocentesis and AV block necessitating permanent pacemaker placement. Both of these complications occur in less than 1% of the cases.46
A Special Case: WPW
WPW syndrome was first described in 1930 by the three aforementioned doctors.47 WPW is defined as the existence of ventricular preexcitation coupled with clinical SVT. The existence of the bypass tract (or tracts) leads to early depolarization of ventricular myocardium prior to or fused with ventricular activation from the His-Purkinje system, which is the genesis of the delta wave. The bypass tract, also known as the “bundle of Kent,” can conduct in an anterograde and/or retrograde fashion, much like what was described earlier for AV node. However, bypass tracts conduct retrogradely more commonly than anterogradely. Twenty-five percent of the bypass tracts can conduct only in a retrograde, or orthodromic, manner, while 10% can conduct in only anterograde, or antidromic, fashion.16,48 The most common presenting SVT with WPW is orthodromic AVRT, which represents about 30% of all cases of SVT, making this the second most common regular SVT by prevalence after AVNRT.49 WPW also is commonly associated with both AFib and atrial flutter.50
WPW commonly is asymptomatic, and sudden cardiac death is rarely the presenting symptom of AFib with rapid antegrade conduction down a bypass tract.51,52 Risk assessment can be done with pharmacologic intervention or an electrophysiology (EP) study to determine the refractoriness of the bypass tract.
More commonly, exercise treadmill testing is performed to see if the delta wave can become abolished at a low heart rate, which would make the patient lower risk. The ability of an exercise stress test to predict rapid bypass tract conduction in a follow-up EP study was excellent. The specificity and positive predictive value of a low-risk stress test both were 100% when compared to invasive EP testing.53
Treatment depends on the presence or absence of preexcitation on the ECG. The bypass tract, if recruited in a retrograde fashion (orthodromic conduction), will not show preexcitation or an aberrantly widened QRS complex. If AVNRT or AVRT is suspected, AV nodal blocking agents should terminate the tachycardia.
On the other hand, when the bypass tract is recruited in an antegrade manner (antidromic conduction) the ECG typically will show preexcitation and a widened and bizarre QRS complex. (See Figure 6.) During atrial fibrillation, AV nodal agents (as detailed earlier) depress conduction across the AV node, but do not affect the bypass tract, which can result in rapid antegrade conduction and rapid ventricular rates that can degenerate to ventricular fibrillation.54 If there is SVT with preexcitation, then treatments that block the AV node, such as verapamil, could lead to 1:1 conduction to the ventricle and to ventricular fibrillation, necessitating emergency defibrillation.55,56 This also has been reported with amiodarone.57,58 In patients with WPW and SVT demonstrating delta waves, procainamide can be used, but it may be safer and easier to manage this type of patient by performing a controlled, elective cardioversion rather than an emergent defibrillation.
Figure 6. Wolff-Parkinson-White Syndrome |
 |
 |
The top electrocardiogram (ECG) shows an irregular supraventricular tachycardia with inconsistent bizarre QRS complexes. This is an example of atrial fibrillation and preexcitation (the Wolff-Parkinson-White syndrome). The bottom ECG is the same patient after elective, synchronized cardioversion back to sinus rhythm. Delta waves (preexcitation) can be seen on this ECG. |
Summary
SVT is a common, but straightforward, diagnosis to evaluate and treat. Diagnosis often can be made in a thoughtful, stepwise approach that is easy to obtain. Short- and long-term treatment options can be tailored to the presenting symptoms and the patient’s ability to tolerate the varying options available.
REFERENCES
- Orejarena LA, Vidaillet H Jr, DeStefano F, et al. Paroxysmal upraventricular tachycardia in the general population. J Am Coll Cardiol 1998;31:150-157.
- Cain N, Irving C, Weber S, et al. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. Am J Cardiol 2013;112:961-965.
- Lessmeier TJ, Gamperling D, Johnson-Liddon V, et al. Unrecognized paroxysmal supraventricular tachycardia: Potential for misdiagnosis as panic disorder. Arch Intern Med 1997;157:537-543.
- Abe H, Nagatomo T, Kobayashi H, et al. Neurohumoral and hemodynamic mechanisms of diuresis during atrioventricular nodal reentrant tachycardia. Pacing Clin Electrophysiol 1997;20:2783-2788.
- Singh V, Rodriguez AP, Thakkar B, et al. Hospital admissions for chest pain associated with cocaine use in the United States. Am J Med 2017;130:688-698.
- Grasser EK, Miles-Chan JL, Charrière N, et al. Energy drinks and their impact on the cardiovascular system: Potential mechanisms. Adv Nutr 2016;7:950-960.
- Trojnarska O, Grajek S, Kramer L, Gwizdała A. Risk factors of supraventricular arrhythmia in adults with congenital heart disease. Cardiol J 2009;16:218-226.
- Bouchardy J, Therrien J, Pilote L, et al. Atrial arrhythmias in adults with congenital heart disease. Circulation 2009;120:1679-1686.
- Chang SH, Kuo CF, Chou IJ, et al. Outcomes associated with paroxysmal supraventricular tachycardia during pregnancy. Circulation 2017;135:616-618.
- Gursoy S, Steurer G, Brugada J, et al. Brief report: The hemodynamic mechanism of pounding in the neck in atrioventricular nodal reentrant tachycardia. N Engl J Med 1992;327:772-774.
- Marcus B, Gillette PC, Garson A Jr. Intrinsic heart rate in children and young adults: An index of sinus node function isolated from autonomic control. Am Heart J 1990;119:911-916.
- Sanders WE Jr, Sorrentino RA, Greenfield RA, et al. Catheter ablation of sinoatrial node reentrant tachycardia. J Am Coll Cardiol 1994;23:926-934.
- Akhtar M, Jazayeri MR, Sra J, et al. Atrioventricular nodal reentry: Clinical, electrophysiological, and therapeutic considerations. Circulation 1993;88:282-295.
- Porter MJ, Morton JB, Denman R, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm 2004;1:393-396.
- Markowitz SM, Lerman BB. A contemporary view of atrioventricular nodal physiology. J Interv Card Electrophysiol 2018;52:271-279.
- Oren JW IV, Beckman KJ, McClelland JH, et al. A functional approach to the preexcitation syndromes. Cardiol Clin 1993;11:121-149.
- Rosen KM. Junctional tachycardia: Mechanisms, diagnosis, differential diagnosis, and management. Circulation 1973;47:654-664.
- Kerr CR, Mason MA. Incidence and clinical significance of accelerated junctional rhythm following open heart surgery. Am Heart J 1985;110:966-969.
- Porter MJ, Morton JB, Denman R, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm 2004;1:393-396.
- Smith TW, Antman EM, Friedman PL, et al. Digitalis glycosides: Mechanisms and manifestations of toxicity. Prog Cardiovasc Dis 1984;27:21-56.
- Bagliani G, Leonelli F, Padeletti L. P wave and the substrates of arrhythmias originating in the atria. Card Electrophysiol Clin 2017;9:365-382.
- Markowitz SM, Lerman BB. A contemporary view of atrioventricular nodal physiology. J Interv Card Electrophysiol 2018;52:271-279.
- Markowitz SM, Lerman BB. A contemporary view of atrioventricular nodal physiology. J Interv Card Electrophysiol 2018;52:271-279.
- Appelboam A, Reuben A, Mann C, et al. Postural modification to the standard Valsalva manoeuvre for emergency treatment of supraventricular tachycardias (REVERT): A randomised controlled trial. Lancet 2015;386:1747-1753.
- Rankin AC, Oldroyd KG, Chong E, et al. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J 1989;62:195-203.
- DiMarco JP, Miles W, Akhtar M, et al. Adenosine for paroxysmal supraventricular tachycardia: Dose ranging and comparison with verapamil. Assessment in placebo-controlled, multicenter trials. The Adenosine for PSVT Study Group. Ann Intern Med 1990;113:104-110.
- Rankin AC, Oldroyd KG, Chong E, et al. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J 1989;62:195-203.
- Brink HL, Dickerson JA, Stephens JA, Pickworth KK. Comparison of the safety of adenosine and regadenoson in patients undergoing outpatient cardiac stress testing. Pharmacotherapy 2015;35:1117-1123.
- Lim SH, Anantharaman V, Teo WS, Chan YH. Slow infusion of calcium channel blockers compared with intravenous adenosine in the emergency treatment of supraventricular tachycardia. Resuscitation 2009;80:523-528.
- Hirschy R, Ackerbauer KA, Peksa GD, et al. Metoprolol vs. diltiazem in the acute management of atrial fibrillation in patients with heart failure with reduced ejection fraction. Am J Emerg Med 2019;37:80-84.
- Gupta A, Naik A, Vora A, Lokhandwala Y. Comparison of efficacy of intravenous diltiazem and esmolol in terminating supraventricular tachycardia. J Assoc Physicians India 1999;47:969-972.
- Albert CL, Kamdar F, Hanna M. Contemporary controversies in digoxin use in systolic heart failure. Curr Heart Fail Rep 2016;13:197-206.
- Stambler BS, Dorian P, Sager PT, et al. Etripamil nasal spray for rapid conversion of supraventricular tachycardia to sinus rhythm. J Am Coll Cardiol 2018;72:489-497.
- Frishman WH. Calcium channel blockers: Differences between subclasses. Am J Cardiovasc Drugs 2007;7(Suppl 1):17-23.
- Mann SJ. Redefining beta-blocker use in hypertension: Selecting the right beta-blocker and the right patient. J Am Soc Hypertens 2017;11:54-65.
- Jaiswal A, Nguyen VQ, Carry BJ, le Jemtel TH. Pharmacologic and endovascular reversal of left ventricular remodeling. J Card Fail 2016;22:829-839.
- The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-533.
- Lopes RD, Rordorf R, De Ferrari GM, et al. Digoxin and mortality in patients with atrial fibrillation. J Am Coll Cardiol 2018;71:1063-1074.
- Aloia E. There is a place for digoxin: We think so! J Am Coll Cardiol 2018;72:123-124.
- Cappato R, Castelvecchio S, Ricci C, et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: A prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J Am Coll Cardiol 2012;60:1323-1329.
- Ptaszynski P, Kaczmarek K, Ruta J, et al. Metoprolol succinate vs. ivabradine in the treatment of inappropriate sinus tachycardia in patients unresponsive to previous pharmacological therapy. Europace 2013;15:116-121.
- Krist AH, Tong ST, Aycock RA, Longo DR. Engaging patients in decision-making and behavior change to promote prevention. Stud Health Technol Inform 2017;240:284-302.
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: What it is and what it isn’t. BMJ 1996;312:71-72.
- Alboni P, Botto GL, Baldi N, et al. Out-patient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N Engl J Med 2004;351:2384-2391.
- Cobb FR, Blumenschein SD, Sealy WC, et al. Successful surgical interruption of the bundle of Kent in a patient with Wolff-Parkinson-White syndrome. Circulation 1968;38:1018-1029.
- Spector P, Reynolds MR, Calkins H, et al. Meta-analysis of ablation of atrial flutter and supraventricular tachycardia. Am J Cardiol 2009;104:671-677.
- Wolff L, Parkinson J, White PD. Bundle-branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. Am Heart J 1930;5:685-704.
- Atie J, Brugada P, Brugada J, et al. Clinical and electrophysiologic characteristics of patients with antidromic circus movement tachycardia in the Wolff-Parkinson-White syndrome. Am J Cardiol 1990;66:1082-1091.
- Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretations. 2nd ed. Lea & Febiger; 1993.
- Campbell RW, Smith RA, Gallagher JJ, et al. Atrial fibrillation in the preexcitation syndrome. Am J Cardiol 1977;40:514-520.
- Klein GJ, Bashore TM, Sellers TD, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. N Engl J Med 1979;301:1080-1085.
- Montoya PT, Brugada P, Smeets J, et al. Ventricular fibrillation in the Wolff-Parkinson-White syndrome. Eur Heart J 1991;12:144-150.
- Wackel P, Irving C, Webber S, et al. Risk stratification in Wolff-Parkinson-White syndrome: The correlation between noninvasive and invasive testing in pediatric patients. Pacing Clin Electrophysiol 2012;35:1451-1457.
- Hamer A, Peter T, Platt M, Mandel WJ. Effects of verapamil on supraventricular tachycardia in patients with overt and concealed Wolff-Parkinson-White syndrome. Am Heart J 1981;101:600-612.
- Garratt C, Antoniou A, Ward D, Camm AJ. Misuse of verapamil in pre-excited atrial fibrillation. Lancet 1989;1:367-369.
- Shiraishi H, Ishibashi K, Urao N, et al. Two cases of polymorphic ventricular tachycardia induced by the administration of verapamil against paroxysmal supraventricular tachycardia. Intern Med 2002;41:445-448.
- Sheinman BD, Evans T. Acceleration of ventricular rate by fibrillation associated with the Wolff-Parkinson-White syndrome. Br Med J (Clin Res Ed) 1982;285:999-1000.
- Boriani G, Biffi M, Frabetti L, et al. Ventricular fibrillation after intravenous amiodarone in Wolff-Parkinson-White syndrome with atrial fibrillation. Am Heart J 1996;131:1214-1216.
Want to earn free Primary Care CME courses? With FreeCME you can earn accredited CME courses for free online. Get started today!
Supraventricular tachycardia (SVT) is a common medical condition. Diagnosis and treatment often occur simultaneously. To a great degree, long-term treatment options depend on the history of symptoms and the patient’s desire, rather than on the specific type of SVT.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
