Vomiting and Diarrhea in Immunocompromised Patients
October 1, 2020
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
AUTHORS
Ashley E. Pickering, MD, MPH, Resident, Emergency Medicine, University of Maryland, Baltimore
Mercedes Torres, MD, Clinical Assistant Professor, Department of Emergency Medicine, University of Maryland School of Medicine, Baltimore
PEER REVIEWER
Catherine A. Marco, MD, FACEP, Professor of Emergency Medicine and Surgery, Wright State University, Dayton, OH
EXECUTIVE SUMMARY
- Vomiting and diarrhea are frequent reasons for immunocompromised patients to seek emergency department care and can be harbingers of life-threatening disease.
- Immunosuppression increases the risk for serious infection and decreases inflammatory response, leading to atypical, delayed presentations.
- Consider a broad differential diagnosis for the evaluation of even mild gastrointestinal symptoms, with increased diagnostic testing in the special populations highlighted in this review.
- Many immunosuppressed patients presenting with vomiting and diarrhea will require hospitalization or prompt follow-up with their primary treatment team upon discharge.
Introduction
Patients who are immunosuppressed range from those with mild immunocompromise, such as elderly or diabetic patients, to those with immunodeficiency, including patients living with acquired immunodeficiency syndrome (AIDS), malignancy, or organ transplants. They may exhibit subtle or atypical presentations of gastrointestinal (GI) infection, as well as complications of their underlying disease processes or treatments. Emergency physicians (EPs) should maintain a high level of suspicion for life-threatening pathology and evaluate these patients using broad differentials.
Infectious Causes of Vomiting and Diarrhea in Immunosuppressed Patients
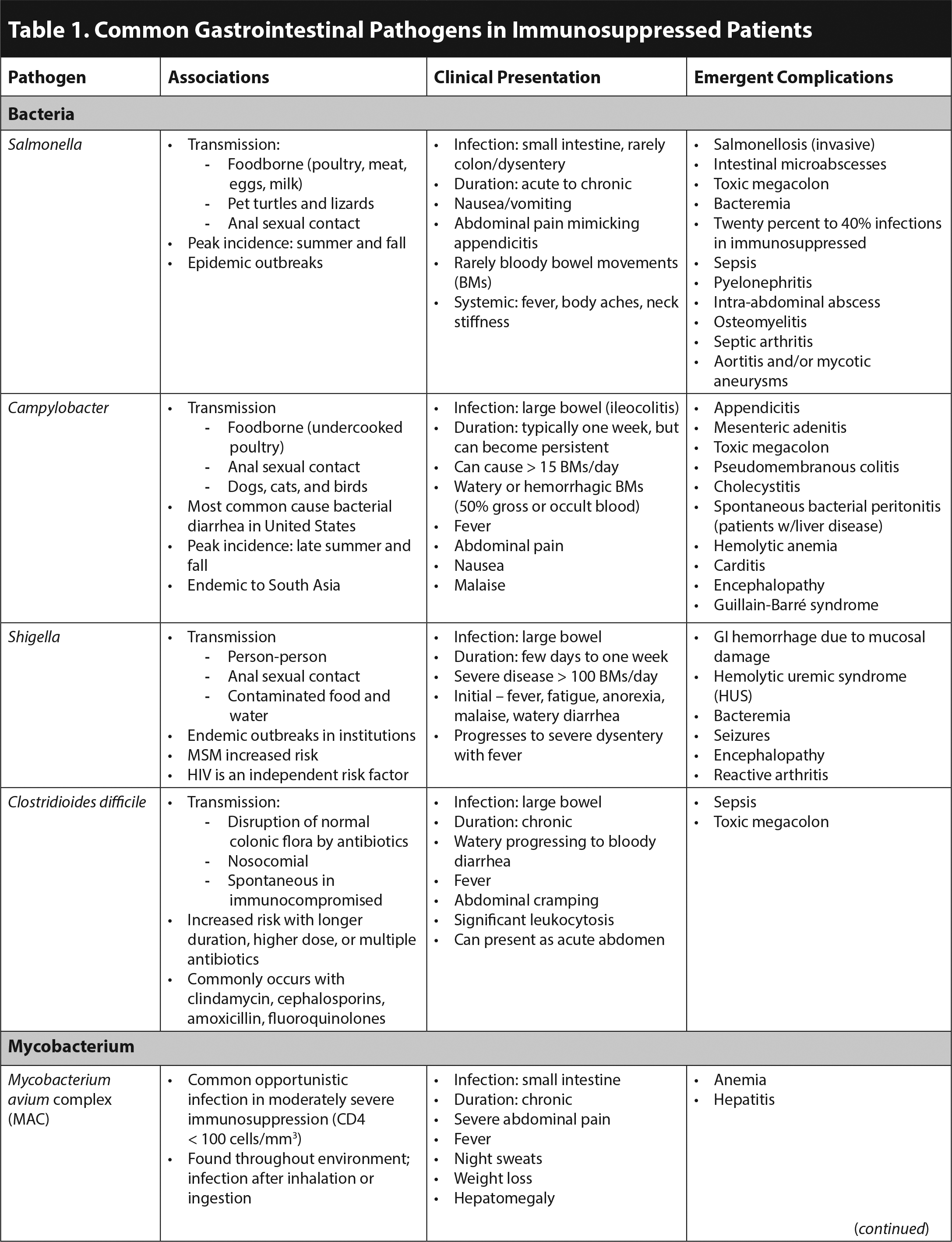
In the general population, viral gastroenteritis causes 50% to 70% of acute infectious vomiting and diarrhea. The most common viral etiologies are norovirus, rotavirus, and astrovirus.1 Early diagnostic closure due to the assumption that GI symptoms are caused by viral gastroenteritis is dangerous in all patients, since vomiting and diarrhea can be harbingers of surgical emergencies or severe systemic illness.2 The evaluation of immunocompromised patients should be broad, considering bacterial, mycobacterial, parasitic, and severe viral infections. Table 1 outlines common GI infections in immunosuppressed patients. Although some pathogens are opportunistic infections mainly occurring in the severely immunosuppressed, others are common infections in the general population that may present with more severe symptoms or complications.1,3,4 In solid organ transplant patients, Clostridioides difficile, norovirus, and cytomegalovirus (CMV) are the most common causes of infectious diarrhea.5 C. difficile also is the most common cause of infectious diarrhea in patients with human immunodeficiency virus (HIV).2
Acute diarrhea is defined as three or more loose stools per day for less than two weeks, while chronic diarrhea is present for four or more weeks. Severe acute diarrhea is defined as greater than six unformed stools per day.5 Infections of the small intestine produce copious watery stools, with associated gas, cramping, bloating, and nutritional deficits. Dysentery affects the large bowel, producing frequent, small, often mucoid or bloody, painful stools, with associated tenesmus and lower abdominal cramping.1,2 (See Table 1. Click to enlarge the tables.)
Furthermore, certain symptoms accompanying diarrhea can be suggestive of specific pathogens. Fever and/or bloody diarrhea typically are associated with CMV, invasive bacterial pathogens (Campylobacter, Salmonella, Shigella), and Entamoeba histolytica. Abdominal pain occurs in patients with the aforementioned invasive bacterial pathogens and C. difficile.4 When present together, fever, abdominal pain, and bloody diarrhea point to an invasive bacterial pathogen.1 Non-bloody, watery diarrhea generally is more reassuring and associated with non-CMV viral GI infections.1,5
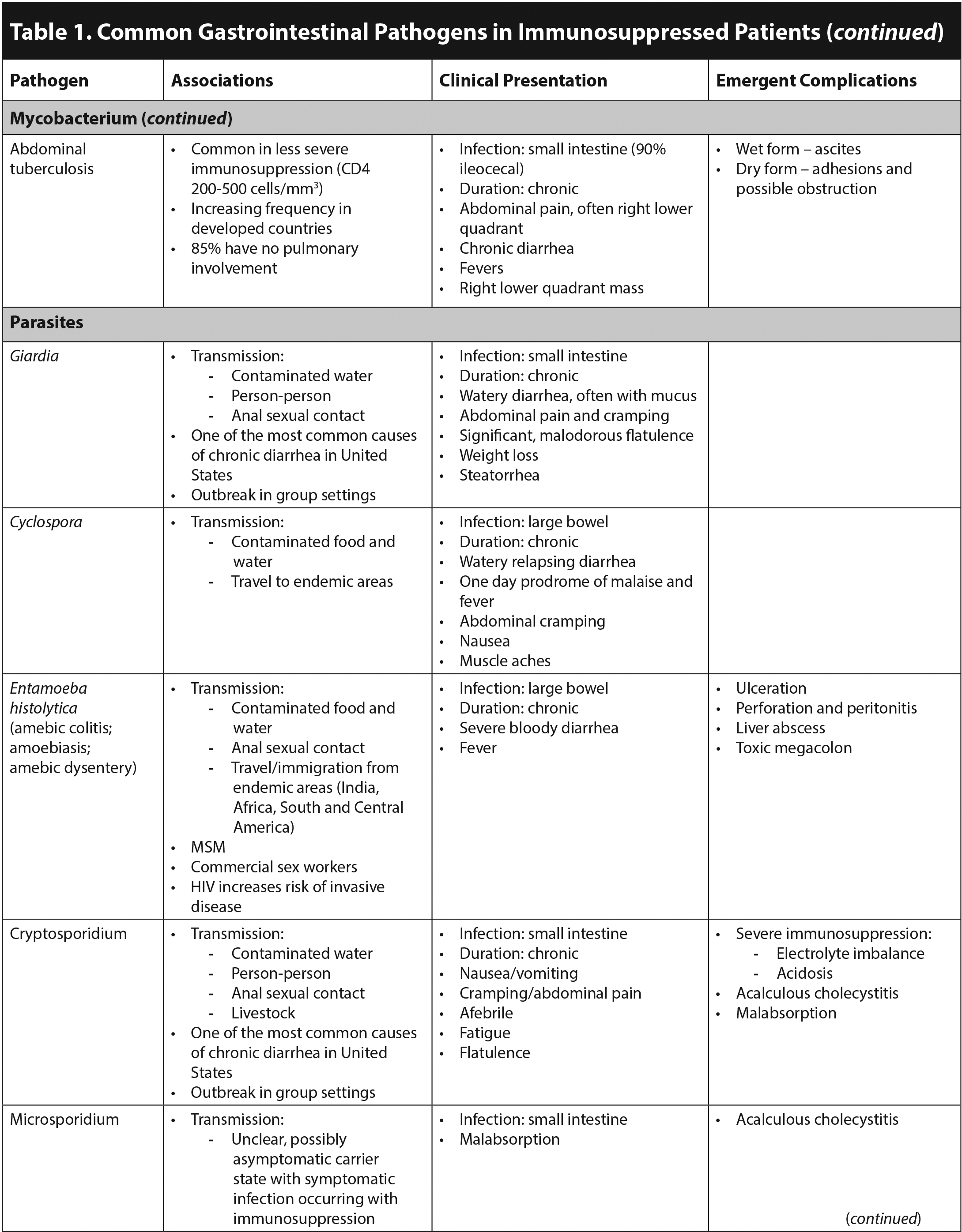
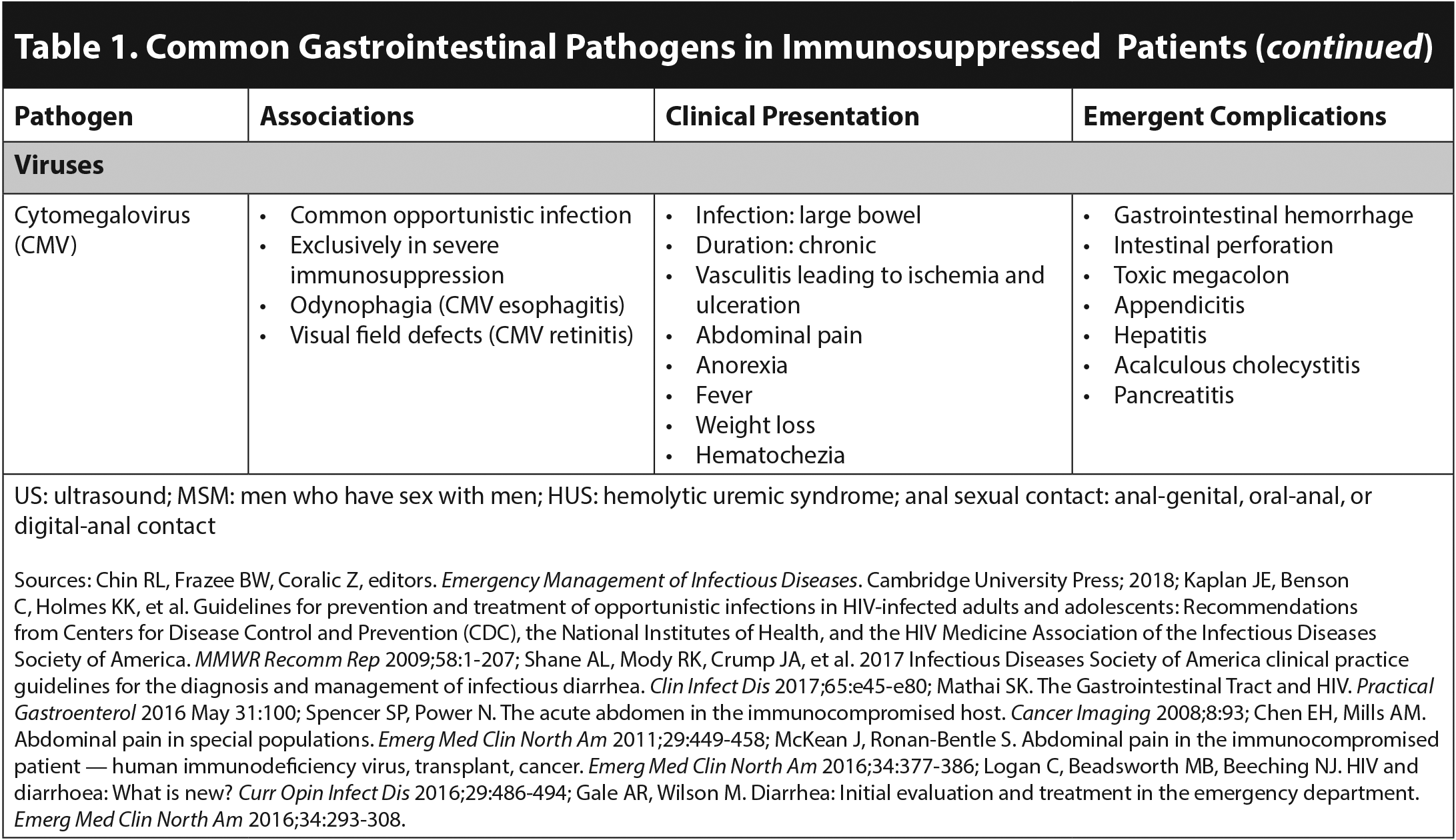
Immunocompromised patients are at risk for experiencing emergent surgical complications of acute GI infections. CMV is a well-known cause of appendicitis in this population.2 Acalculous cholecystitis commonly presents with abdominal pain, vomiting, and diarrhea, occurring concurrently with Listeria, CMV, Cryptosporidium, and microsporidia infections.6 Toxic megacolon that does not respond rapidly to medical therapy also is a surgical emergency because of the risk of perforation.2 This is commonly associated with C. difficile infection; however, it also can be caused by Salmonella, Campylobacter, E. histolytica, and CMV.1,2 Invasive disease without evidence of toxic megacolon, due to Salmonella or E. histolytica, can cause ulceration resulting in perforation and peritonitis. CMV is unique in causing a vasculitis leading to ischemia and possible perforation.1 Furthermore, adhesions due to abdominal tuberculosis can cause obstruction with or without perforation.3
HIV
HIV and GI Infections
In people living with HIV (PLWH), 50% to 60% of diarrhea has an identified infectious source.2 The CD4 count, or even the absolute lymphocyte count, can help with the diagnosis. (See Table 2.)
Table 2. Association Between Common Gastrointestinal Pathogens in HIV and CD4 Count |
||
|
CD4 Count |
CD4 Count |
CD4 Count |
|
|
|
|
*Common pathogens in human immunodeficiency virus Sources: Chin RL, Frazee BW, Coralic Z, editors. Emergency Management of Infectious Diseases. Cambridge University Press; 2018; Cello JP, Day LW. Idiopathic AIDS enteropathy and treatment of gastrointestinal opportunistic pathogens. Gastroenterology 2009;136:1952-1965. |
||
In relatively immunocompetent PLWH (CD4 count greater than 200 cells/mm3), viral gastroenteritis and medication side effects are the most common etiologies of GI symptoms. In this population, acute vomiting and diarrhea often are self-limited. Conversely, in cases of severe immunosuppression (CD4 counts less than 200 cells/mm3), diarrhea is an independent predictor of death.1
Acute HIV Infection
Acute retroviral syndrome (ARS) is a symptomatic manifestation of acute HIV, occurring approximately one to four weeks after infection in 40% to 90% of exposed individuals.7,8 Typically, ARS is an influenza-like illness, presenting with vomiting and/or diarrhea, fever, malaise, lymphadenopathy, and rash.7-11 During acute infection, HIV affects the GI lymphoreticular system, resulting in a 30% to 70% frequency of nausea/vomiting and/or diarrhea.7,9,12,13 In a recent study of HIV screening in the emergency department (ED), 67% of patients diagnosed with acute HIV endorsed GI symptoms, with 54% experiencing nausea/vomiting and 27% experiencing diarrhea. Twenty-two percent of these patients presented with chief complaints suggestive of GI disease.10
Generally, the nonspecific symptoms of ARS make diagnosis challenging.7 Although ARS is self-limited and resolves within one to three weeks, there are significant benefits to diagnosing this and initiating treatment for acute HIV. For the individual, early antiretroviral therapy (ART) decreases the severity of ARS symptoms, limits viral reservoirs, suppresses chronic inflammation, and slows disease progression. Overall, early ART halts the decline in CD4 count and slows the progression to AIDS. The public health benefits also are significant, decreasing the risk of HIV transmission during this especially infectious early stage of disease.9-11 Up to 50% of new HIV infections are due to transmission during acute HIV infection. Studies have shown that up to 1% of at-risk individuals presenting to the ED with a viral syndrome test positive.11
Noninfectious Diarrhea
Noninfectious diarrhea is extremely common in PLWH even if the virus is well-controlled. Estimates of prevalence range from 39% to 60%.6,7 It can lead to nutritional deficiency, weight loss, poor quality of life, and decreased adherence to ART regimens.7 The two primary causes are antiretroviral medication side effects and HIV enteropathy.6 Pancreatic insufficiency and HIV-associated malignancy are less frequent but also are implicated as causes of diarrhea in PLWH.12,13
Antiretroviral Drugs. Diarrhea is one of the most common side effects of all classes of ART. EPs should assess for a temporal association between medication initiation and the onset of diarrhea.1 In one study, 63% of PLWH on ART reported diarrhea.14 This diarrhea, with or without associated vomiting, can be severe enough to cause dehydration or even hemodynamic instability.15 It is reported to be the adverse effect most likely to cause patients to switch or discontinue their ART.12 Protease inhibitors (PIs) are more likely than other classes of ART to cause diarrhea, as well as nausea, gastric discomfort, and bloating.13,16 Ritonavir is the most common culprit, causing diarrhea in 15% to 68% of patients.1 Enfuvirtide, a fusion inhibitor, also causes diarrhea in approximately one-third of patients. Currently, many PLWH are treated with multidrug combination medications, with overall rates of diarrhea at approximately 10%.13 Diarrhea as a side effect of ART often is self-limited, improving after four to six weeks of treatment.6,17 Prescribing antibiotics in conjunction with ART increases the risk of diarrhea because of the increased risk of C. difficile and proliferation of Gram-negative enteric bacteria, which cause chronic diarrhea and malabsorption.1,14
AIDS Enteropathy. AIDS enteropathy is due to direct infection of the GI mucosa and gut associated lymphoid tissue (GALT). AIDS enteropathy is characterized by chronic diarrhea without an identifiable infectious pathogen.16 Following
initial infection, continued HIV infection of the GI tract damages the intestinal mucosa, impairing absorption, decreasing the intestinal epithelial barrier function, and leading to translation of microbes to the systemic circulation. The associated immune system activation perpetuates chronic inflammation and directly damages the autonomic nerves that control GI motility, contributing to diarrhea.14,18,19 The effects of HIV infection on the GI tract are most prominent in those with untreated HIV, particularly those with a CD4 count less than 100 cells/mm3 or late infection.1 However, up to 30% of PLWH on effective ART develop enteropathy, especially as they age.16,18
Pancreatitis and Pancreatic Insufficiency. Fat malabsorption related to pancreatic insufficiency often is overlooked as a cause of chronic diarrhea, bloating, and weight loss in HIV patients. Studies have found that pancreatic insufficiency affects nearly 50% of PLWH.12,16 This may be related to chronic pancreatitis resulting from infection, specifically CMV and Mycobacterium avium complex (MAC). A recent study also linked pancreatic insufficiency to coinfection with hepatitis C virus (HCV) in PLWH, suggesting that this may be due to direct infection of pancreatic tissue with HCV, or interferon-alpha treatment.20 Tumor invasion by malignancies, discussed later, also can lead to pancreatic tissue destruction and insufficiency.19 Didanosine traditionally has been thought to cause pancreatitis; recent studies have not supported this evidence.20 Additionally, PIs and nucleoside reverse transcriptase inhibitors (NRTIs) can cause significant hypertriglyceridemia leading to pancreatitis.16,17,19 Pentamidine and trimethoprim/sulfamethoxazole, used for Pneumocystis jiroveci pneumonia (PJP) prophylaxis and treatment, also are known to cause pancreatitis.19,16 Pancreatitis caused by any of these etiologies should be considered in the differential diagnosis for a PLWH presenting with vomiting and epigastric pain.
HIV-Associated Malignancies. Approximately one-third of PLWH will die from cancer.14,21 Kaposi’s sarcoma (KS) and non-Hodgkin’s lymphoma, AIDS-defining cancers affecting the GI tract, are most common in untreated HIV. However, even in the age of ART, these malignancies account for 15% of deaths in PLWH in the United States. KS most commonly occurs in patients with CD4 counts less than 350 cells/μL3.22,23 Patients with GI KS also have cutaneous lesions, which are well demarcated, violaceous, and papular or nodular. Although GI symptoms occur in a minority of patients, they include nausea, vomiting, diarrhea, and abdominal pain. Primary non-Hodgkin’s lymphoma of the small bowel is uncommon; however, 50% of patients diagnosed are HIV-infected, typically with CD4 counts less than 200 cells/μL3. It usually involves the terminal ileum and can cause diarrhea, obstruction, and ascites.22 Age and duration of time living with HIV are the most significant risk factors for non-HIV-related malignancy.21
Noninfectious Diarrhea Treatment. Noninfectious diarrhea in PLWH is a common and bothersome symptom. Nonpharmacologic treatments include dietary modification, focusing on low-fat, low insoluble fiber regimens that are caffeine-free and high in complex carbohydrates (for example, toast, rice, noodles, oats).1,2,8 PLWH may benefit from a lactose-free diet since lactose intolerance is frequent.8 Bulking agents, such as psyllium and oat bran, are useful. Although evidence is scarce to support using bismuth subsalicylate in PLWH, it is highly effective for traveler’s diarrhea and does not interact with ART. Loperamide has the best evidence of effectiveness; however, it has significant interactions with ART. Crofelemer, a botanical agent that blocks chloride secretion, is specifically used for chronic diarrhea in PLWH on ART. Octreotide injections also have been found to decrease dehydration and malnutrition in severe chronic diarrhea, although they are not generally prescribed in the ED. Probiotics are ineffective in addressing noninfectious diarrhea in PLWH.
Metabolic Side Effects of ART
Older NRTIs are known to cause lactic acidosis through their inhibition of oxidative phosphorylation, therefore increasing anaerobic metabolism. This is less common with newer ART.6,15 The integrase inhibitor dolutegravir causes lactic acidosis when given concurrently with metformin through decreased metformin excretion.24 Symptoms of lactic acidosis can be vague, while the consequences of a missed diagnosis are severe. Nausea, vomiting, and abdominal pain are prominent, accompanied by fatigue and dyspnea. When severe, lactic acidosis can lead to altered mental status, seizures, arrhythmias, and heart failure.24 Another metabolic side effect of ART, insulin resistance is precipitated by PIs and can be acute, presenting as diabetic ketoacidosis or hyperglycemic hyperosmolar state, with symptoms of nausea, vomiting, and abdominal pain.16
Organ Transplant
Post-transplant patients present a dynamic challenge for EPs. The infections and complications that may cause GI symptoms vary by time since transplantation and degree of immunosuppression. In one study, infection (36%) and GI/genitourinary (GU) pathology (20%) were the most common ED diagnoses in solid organ transplant (SOT) patients, regardless of the time elapsed since surgery.25 Post-transplant ED patients presenting with GI symptoms should be evaluated for infection, surgical complications, drug toxicity, and organ rejection.26 Even mild GI symptoms warrant thorough evaluation in this population, especially in patients with intra-abdominal transplants.15
Infectious Disease in Post-Transplant Patients
Infections in post-transplant patients can be due to surgical complications in the case of SOT, chemotherapy in hematopoietic stem cell transplant (HCT), and immunosuppression in both groups. The risk of infection is greatest in the first year following transplant.6 The time since the transplant as well as the type of transplant can help to narrow the differential. (See Table 3.) The majority of clinically important infections in SOT patients occur within the first 180 days post-transplant. They can lead to sepsis and high rates of mortality, as well as contribute to acute and chronic organ rejection. Estimates of severe sepsis in SOT range from 20% to 60%, and associated mortality ranges from 5% to 40%.26 HCT patients are even more susceptible to severe infection. In one study, 93% of HCT patients developed infection within 30 days following transplant and more than half of this group had bacteremia.27
Table 3. Etiologies of Gastrointestinal Symptoms Based on Degree of Immunosuppression and Post-Transplant Phase in SOT Patients |
|
Early phase (< 1 month): Low-dose immunosuppression; postsurgical complications and healthcare-associated infections
Intermediate phase (1-6 months): High-dose immunosuppression; opportunistic infections
Last phase (> 6 months): Maintenance immunosuppression (level varies); community-acquired infections and chronic rejection
|
|
Information from: Zhong D, Liang SY. Approach to transplant infectious diseases in the emergency department. Emerg Med Clin North Am 2018;36:811-822; Chen EH, Mills AM. Abdominal pain in special populations. Emerg Med Clin North Am 2011;29:449-458; McKean J, Ronan-Bentle S. Abdominal pain in the immunocompromised patient — human immunodeficiency virus, transplant, cancer. Emerg Med Clin North Am 2016;34:377-386; Long B, Koyfman A. The emergency medicine approach to transplant complications. Am J Emerg Med 2016;34:2200-2208. |
In the post-transplant population, a lack of infectious symptoms should not dissuade an EP from pursuing an infectious workup, since immunomodulation can lead to mild or atypical presentations.6,26 EPs should look for sepsis in the SOT population, even in the absence of fever, leukocytosis, and the classic systemic inflammatory response criteria.26 Immunosuppressed patients who are seropositive for CMV, hepatitis C, and Epstein-Barr virus (EBV) are more susceptible to infection.6
The timeline of immunosuppression and infection risk following HCT is largely similar to that of SOT. However, the initial post-transplant phase is more variable based on the individual patient’s time to immune reconstitution. Immune constitution typically occurs several months to more than a year after HCT. Diarrhea is very common due to mucosal injury following pre-transplant chemotherapy and radiation; however, EPs also should consider neutropenic enterocolitis, which is discussed further later.28 Following engraftment, the post-HCT trajectory is similar to that of SOT. In the early post-engraftment phase, typically 30-100 days post-transplant, immunosuppression is maintained at high levels, and opportunistic infections are common. In the late post-engraftment phase, immunosuppression is weaned to maintenance levels.29
In addition to the typical considerations of nosocomial, opportunistic, and community-acquired infections, there is the risk of donor-derived infections. In these cases, post-transplant patients present with infections for which they do not have common risk factors. Concerns for transmission are highest in “latent’”organisms, such as viruses (HIV, CMV, Epstein-Barr virus, and hepatitis B and C), parasites (Toxoplasma gondii, Trypanosoma cruzi), bacteria (mycobacteria, specifically tuberculosis and syphilis), and fungi (Aspergillus, endemic mycoses).30 These same organisms can be reactivated in the setting of immunosuppression in the post-transplant patient.
CMV is the most common viral infection following both SOT and HCT.29,31 It affects 15% to 55% of SOT recipients and is commonly donor-derived.31 Patients who were seronegative for CMV prior to transplantation and receive an organ from a seropositive donor are at the greatest risk of severe symptomatic CMV infection.32 Many CMV infections are asymptomatic; however, GI symptoms are the most common manifestation.5 CMV infection leading to GI bleeding or perforation should be considered in SOT presenting with unexplained hypotension or anemia.31 CMV infection can lead to transplanted organ rejection and increased risk of developing other opportunistic infections.5 Most patients are on CMV prophylaxis with ganciclovir or valganciclovir for at least the first six months post-transplant.33 However, delayed-onset CMV can occur, commonly within three to six months of discontinuation of prophylaxis.32
The prevalence of diarrhea is estimated to be 20% to 50% post-transplant, with some reports as high as 80% in post-HCT. Diarrhea can be severe and life-threatening, resulting in medication toxicity, electrolyte derangements, transplanted organ rejection, and severe dehydration.5,34 EPs should consider infection and organ rejection in all post-transplant patients presenting with diarrhea.31 Norovirus, typically benign and self-limited in the general population, can cause significant disease in post-transplant patients, including acute renal failure, bacteremia, peritonitis, and death.5,31 In one study, 55% of SOT patients presenting with norovirus required hospitalization and 27% required ICU-level care.31 Another study noted that 94% of post-transplant patients developed chronic diarrhea from norovirus.5 C. difficile is the most common bacterial infection in the post-transplant population, with an incidence of approximately 2% to 30%, often with severe disease.5,29 Microsporidium also should be considered as a cause of diarrhea in these patients.33
GU problems can present as GI symptoms post-transplant. Patients should be evaluated for urinary tract infections, and those with renal transplants should be evaluated for anostomotic leaks.29,31
A stepwise approach to the diagnosis of infectious diarrhea in SOT patients is recommended. Initial diagnostics include C. difficile PCR, CMV blood PCR/NAT, and stool bacterial culture or PCR for a broad range of enteric pathogens. If no diagnosis is found and diarrhea continues, stool testing for viral PRC, ova, parasites, Giardia, and Cryptosporidium is recommended. Consultation with the patient’s transplant team at the time of the visit or as a follow-up is indicated.5
Transplant Rejection
Rejection is common, occurring within six weeks post-transplant in 64% of liver transplants. Rejection should be considered when evaluating post-transplant patients with fever and malaise. Renal transplant rejection can cause abdominal symptoms, the most prominent being pain at the graft site. Patients may have nausea, vomiting, and electrolyte abnormalities associated with a rapid decline in renal function. ED diagnostics focus on renal ultrasound (US), which may demonstrate increased graft size, a loss of definition of the corticomedullary junctions, and increased vascular resistance. Liver transplant failure can cause prominent GI symptoms along with hepatosplenomegaly and ascites. Similarly, US is the most useful ED diagnostic test, typically demonstrating a focal necrotic lesion or vascular abnormality. If diagnosed, urgently consult with the transplant team.35
Surgical Emergencies in the Post-Transplant Patient
Many SOTs involve abdominal surgeries, leaving post-transplant patients vulnerable to typical postsurgical complications. Transplanted organs lack innervation, making abdominal pain a less reliable symptom of a surgical emergency in this population. In the early post-surgical period (less than one month), surgical site infections, anastomotic leaks, or strictures are typical causes of GI symptoms.6,15 Common causes of GI symptoms, such as appendicitis and diverticulitis, occur as well and may present atypically.
Following liver transplantation, acute peritonitis or abdominal abscess can present with GI symptoms. These complications are most common in the early post-transplant period.26
In pancreatic transplant patients, pancreatitis due to reperfusion injury is common immediately postoperatively, but pancreatitis also presents as a more delayed complication. Acute graft pancreatitis is most prominent in the first three months following transplant. Typically, this is due to vascular thrombosis of the graft (60% to 70%), but it also can result from inflammation or infection.6 Pancreatitis also is common in renal and liver transplant patients. Causes include immunosuppression, hypercalcemia, hyperlipidemia, and viral infection.6,33 Pancreatitis can present with abdominal pain and vomiting, or in a delayed fashion as systemic illness due to the inflammatory response.6 Mortality is high, up to 64% in one study of liver transplant patients.33
There are high rates of biliary tract pathology in the post-transplant populations and increased rates of mortality following cholecystectomy. In one study of SOT patients, 37% had evidence of biliary disease.33 Biliary leak and stricture are the most common biliary complications in liver transplant. They cause a significant increase in mortality, cholestatic liver disease, and acute rejection.36 Cholangitis also occurs due to biliary stricture and can be complicated by liver abscess and/or sepsis.31 Cholestasis is a side effect of cyclosporine immunosuppression.
Graft-Versus-Host Disease
Graft-versus-host disease (GVHD) is the most common life-threatening complication of HCT and the second leading cause of death in these patients.37,38 It occurs in 65% of HCT patients in one study, with 14.8% manifesting GI and 7.7% liver disease.39 Onset typically is two to six weeks after transplant.6 In one study of post-HCT patients, 30% of diarrhea was attributed to GVHD.34 Clinical presentation includes a maculopapular rash, watery or bloody diarrhea with or without abdominal pain, fever, and pancytopenia.5,37 Damage to small bile ducts can lead to jaundice and cholestasis causing nausea, vomiting, and anorexia.37 GVHD also leads to recurrent infections.15 Cyclosporine and methotrexate are immunosuppressants typically used post-transplant for GVHD prophylaxis.37,38 When GVHD occurs, glucocorticoids are the first-line treatment.
Post-Transplant Lymphoproliferative Disorder
Post-transplant lymphoproliferative disorder (PTLD) is diverse, ranging from benign polyclonal B-cell proliferation to malignant diffuse B-cell lymphoma. The GI tract is the most common extranodal site affected. Approximately 70% of PTLD is associated with EBV infection in the setting of post-transplant immunosuppression. The risk of PTLD is greatest with primary EBV infection post-transplant.29,40 The presentation can be diverse, ranging from a mononucleosis-like syndrome to GI obstruction, bleeding, or perforation, but it often includes hepatic and pancreatic dysfunction.5,6,15 Onset usually is more than a year post-transplant but can occur years later. Reduction of immunosuppression may lead to remission; however, chemotherapy also may be required.40
Immunosuppressive Medication Side Effects
As noted earlier, diarrhea is common and can be associated with infection, organ rejection, or surgical complications. One study found that 73% of diarrhea in post-transplant patients was medication-related, with the majority of these cases due to immunosuppression.41 All immunosuppressive medications are known to cause diarrhea within therapeutic dose ranges. Tacrolimus and mycophenolate mofetil (MMF) are both highly associated with vomiting and diarrhea. Tacrolimus can cause diarrhea severe enough to necessitate parenteral nutrition; however, this typically is self-limited. MMF-related diarrhea, vomiting, and abdominal pain typically occur in the first six months of therapy.34,35 Consider referral of patients to their transplant team in cases of severe, lifestyle-limiting vomiting or diarrhea.23,24,33
Metabolic syndrome is common in post-transplant patients because of adverse effects of immunosuppression. Insulin resistance and diabetes occur in patients receiving glucocorticoids, cyclosporine (19%), tacrolimus (13% to 28%), or sirolimus (25%).23,24 Consider diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar syndrome (HHS) in patients on these medications presenting with vomiting and abdominal pain. Hyperlipidemia, caused most often by sirolimus and cyclosporine, also may precipitate pancreatitis.42,43 Furthermore, azathioprine can cause both hepatotoxicity and pancreatitis directly.42
Immunosuppression with MMF or steroids in combination with nonsteroidal anti-inflammatory drugs (NSAIDs) increases the risk for colonic perforation. Perforation in the early post-transplant period is associated with aggressive immunosuppression, often in the setting of CMV infection or diverticulosis.33
Cancer
GI symptoms are the most common reason for oncology patients to present to the ED, regardless of their specific malignancy.6 They may experience symptoms directly related to their malignancy or as a complication of treatment. One ICU-based study of critically ill cancer patients presenting with acute GI symptoms found that a majority suffered from infection: 33% with neutropenic enterocolitis and another 31% with infectious colitis/peritonitis. In contrast, 9% of patients in the study were critically ill with perforation or obstruction of the bowel due to infiltration by malignancy, and an additional 7% due to mucosal toxicity of their chemotherapy regimens.44
Infection Causing GI Symptoms in Cancer Patients
Patients on chemotherapy and with hematologic malignancies, myelodysplastic syndromes, or solid organ tumors with bone marrow infiltration may have neutropenia and severe immunosuppression. The risk of infection increases as the absolute neutrophil count (ANC) declines; less than 1,000-1,500 cells/μL represents mild neutropenia, while less than 100 cells/μL indicates profound neutropenia.45 Patients presenting to the ED who received chemotherapy within six weeks are assumed to be neutropenic until cell counts are back.46
In one study, more than half of those who were critically ill had Gram-negative sepsis (Escherichia coli, Pseudomonas). Fifteen percent were found to have invasive fungal infections, mainly Candida. CMV was the most common viral infection identified. Twenty-five percent were positive for C. difficile on stool studies.44 These data support the assertion that all neutropenic patients with new-onset diarrhea (three or more unformed stools per day) should undergo stool cultures, including for C. difficile.45 Patients with malignancy are at a higher risk of C. difficile infection and associated severe disease, regardless of their history of recent antibiotic use.46,47
Neutropenic Enterocolitis
Neutropenic enterocolitis (NE), also referred to as typhlitis and necrotizing enterocolitis, is a concern in any neutropenic patient with abdominal pain and fever. This includes those with malignancy, AIDS, aplastic anemia, cyclic neutropenia, and those on immunosuppression post-transplant.15,48 NE is particularly prevalent with acute leukemia and lymphomas. Treatment with high-dose cytarabine plus anthracyclines also is a more frequent cause. NE has been reported following radiotherapy.49 Damage to the intestinal mucosa by chemotherapy or neutropenia allows translocation of microorganisms into the bowel tissue as well as the bloodstream.45,49 Following invasion of the bowel wall, endotoxin production leads to inflammation, ulceration, transmural necrosis, perforation, and hemorrhage.45,46 Although Gram-negative Enterobacteriaceae are the most frequent cause, other bacteria, C. difficile, and viral and fungal infections have been implicated.49
Symptoms include fever, nausea, vomiting, diarrhea (which often is bloody), and pain, which often is localized to the right lower quadrant.44,49 Abdominal distension and frank peritoneal signs are associated with perforation.49 One study reported that 93% of patients with NE presented with fever.44
Computed tomography (CT) imaging is critical to the diagnosis and management of NE. CT helps identify complications (pneumatosis, bowel perforation) early and avoid unnecessary urgent surgical treatment.44 Diagnostic criteria for NE center on CT or US demonstrating bowel wall thickening. Typically, NE initially will affect the cecum, progressing to the ascending colon and ileum. Bowel wall thickening greater than 4 mm on CT, in association with fever and abdominal pain, is diagnostic.49 CT often demonstrates mesenteric stranding, bowel dilation, mucosal contrast enhancement, and pneumatosis.46 While CT is definitive, bedside US may be considered for the initial evaluation of critically ill or hemodynamically unstable patients.45 Additionally, blood cultures, stool cultures, and C. difficile toxin testing is recommended.49
Initial NE management includes fluid resuscitation. Empiric antibiotic coverage should be broad, covering not only Gram-negative and Gram-positive bacteria, but also Pseudomonas, C. difficile, and Candida. Piperacillin-tazobactam or imipenem-cilastatin both are options. In one study of critically ill patients, 80% were successfully managed conservatively;44 however, surgery is indicated if bowel ischemia or perforation is present.15 Historically, NE has carried a very high mortality rate.48
Malignancy Directly Causing GI Symptoms
Intra-abdominal cancers increase the risk for bowel obstruction, similar to diffuse peritoneal carcinomatosis due to breast cancer, ovarian cancer, or melanoma. Gastrointestinal primary tumors or metastatic lesions of the bowel wall can be the lead point for intussusception, as well as causing perforation due to transmural erosion.15
Paraneoplastic diarrhea is rare. Non-B islet cell pancreatic tumors may secrete vasoactive intestinal peptide leading to profound watery diarrhea, accompanied by hypokalemia and hypochlorhydria. Carcinoid tumors can produce flushing and diarrhea due to serotonin syndrome. Multiple other hormone-secreting malignancies can produce diarrhea, including glucagonomas, gastrinomas, hepatocellular carcinoma, somatostatinomas, and pheochromocytomas. Small cell lung cancer also can produce diarrhea via the production of antibodies causing intestinal autonomic neuropathy.49
Increased intracranial pressure (ICP) due to primary or metastatic malignancy of the brain is worth considering when assessing cancer patients presenting with vomiting alone. Nausea and vomiting often are the first signs of elevated ICP, with seizures and altered mental status occurring later. CT with contrast is the imaging modality of choice. High-dose corticosteroids are administered urgently to reduce ICP prior to definitive treatment.50
Malignancy Treatment-Related GI Symptoms
Abdominal and pelvic radiation involving the intestinal tract can cause severe mucosal damage, vasculitis, and narrowing of the intestinal lumen.15,49 Radiation enteritis can cause acute ulceration of the bowel wall, leading to perforation or GI bleeding and chronic strictures or fistulas.15 The peak severity of symptoms typically is seven to 14 days following the first radiotherapy treatment.49
Chemotherapy-induced nausea and vomiting (CINV) can be severe enough to cause volume depletion and electrolyte abnormalities. Carmustine, cisplatin, cyclophosphamide, dacarbazine, dactinomycin, mechlorethamine, streptozotocin, and temozolomide have reported rates of CINV greater than 90%.51 EPs should treat CINV with typical antiemetic agents; however, involvement of the patient’s oncology team is important to guide additional therapy.52
Although diarrhea is common in patients with malignancy, infectious etiologies are identified in only a small proportion of cases, suggesting cancer treatment toxicity. A number of traditional chemotherapeutic agents are known to cause diarrhea with notable frequency, including 5-fluorouracil, irinotecan, capecitabine, and anthracyclines. Bloody diarrhea can be seen with 5-fluorouracil due to cytotoxic damage to the intestinal mucosa. Chemotherapy agents also cause lactose intolerance in a significant number of patients, which may present with diarrhea.49
Newer immunotherapy treatments are associated with both a high incidence and severity of diarrhea.49 Immunotherapy targets malignancy by increasing the immune system’s response to tumor cells. Adverse effects of immunotherapy are due to immune system over-activation rather than the immunosuppression seen with traditional chemotherapy and are termed immune-related adverse events.53 Ipilimumab, approved for the treatment of unresectable metastatic melanoma, is the agent most likely to have GI immune-related adverse events.51,54 Diarrhea is common, affecting up to 30% of patients on anti-CTLA therapy, and can be severe. Colitis also can develop, presenting with abdominal pain and progressing to bleeding, perforation, and/or intractable diarrhea in some cases.53 However, the programmed cell death (PD1) inhibitors, including nivolumab and pembrolizumab, are approved for use in more diverse malignancies and have low rates of significant GI immune-related adverse events.51,55 ED diagnostics of diarrhea in patients on immunotherapy should include a CT scan and infectious workup.53 EPs should consult with the patient’s oncology team for any medication modifications.
Diagnostics, Management, and Disposition
In mildly immunosuppressed patients, viral gastroenteritis, enteric bacterial illness (C. difficile, Salmonella, and Shigella), and protozoal diseases (Cryptosporidium, Giardia, Entamoeba) are the most common etiologies of GI symptoms.1 In this population, acute vomiting and diarrhea often are self-limited. Lack of associated abdominal pain, fever, and other symptoms are suggestive of viral gastroenteritis or other benign self-limiting etiologies. However, a lower threshold for ordering diagnostic testing is recommended, including ordering stool for culture, C. difficile toxin, ova, parasites, and Giardia.56
In contrast with immunocompetent patients, empiric antibiotic treatment is worth considering in this population. In the well-appearing immunocompromised patient, treatment for Gram-negative enteric pathogens with ciprofloxacin 500 mg twice daily or levofloxacin 500 mg daily is recommended. If there is suspicion for parasitic infection, metronidazole 250 mg to 500 mg three times daily for a week or tinidazole 2 g can be prescribed empirically. Concerns with increased rates of hemolytic uremic syndrome precipitated by antibiotic treatment of bloody diarrhea in afebrile patients are outweighed by the risk of the GI infection progressing to invasive or systemic disease in this population. However, if the risk of a shiga toxin-producing organism (Shigella or E. coli 0157:H7) is high, providers should obtain shiga toxin testing results prior to starting antibiotics if possible.5 EPs may consider conservative management with oral rehydration and discharge, provided that the patient is not febrile, severely dehydrated, or systemically ill.1 Patients who are discharged should receive strict, time-specific return precautions, which include return for fever, bloody diarrhea, intolerance of oral intake, or any signs of dehydration.2
In severely immunocompromised patients (CD4 counts < 200 cells/mm3, high-dose immunosuppression, or neutropenia), GI symptoms and opportunistic infections become increasingly common. There also is an increased frequency of noninfectious diarrhea in this population. Therefore, a more comprehensive diagnostic approach is required.56 Pertinent options for diagnostic evaluation are presented in Table 4. In patients who are severely immunocompromised or ill-appearing, broad-spectrum empiric antibiotic treatment should cover enteric Gram-negative rods and anaerobes until a specific pathogen has been identified.6 Emergency management of dehydration, hemodynamic compromise, electrolyte, and/or acid base abnormalities should be undertaken if present. Admission is prudent for severe immunosuppression, systemic involvement, concern for or identified bacteremia, Salmonella infection with risk of bacteremia, severe dehydration, significant electrolyte imbalances, or acidosis.1
Table 4. Initial Laboratory Investigations for Gastrointestinal Symptoms in the Immunosuppressed |
|
|
Sources: Chin RL, Coralic Z, Frazee BW, editors. Emergency Management of Infectious Diseases. Cambridge University Press; Aug. 9, 2018; Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 2017;65:e45-e80; Angarone M, Snydman DR, AST ID Community of Practice. Diagnosis and management of diarrhea in solid‐organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13550. |
Although stool studies are notoriously low yield in immunocompetent populations, they demonstrate high utility in the immunocompromised, identifying an infectious etiology in approximately 25% of cases. PCR testing has significantly increased the yield of stool studies, and are three times as likely to identify a pathogen (72% vs. 26%). Further, because of the increased risk of severe or invasive disease, identification of a specific pathogen is paramount in this population.5 Although the exact infectious pathogen causing GI symptoms often is unknown in the ED setting, specific diagnoses and treatments are presented in Table 5. (See the supplement available online at https://bit.ly/3hyYj8A and the Rapid Access Card included with this issue.)
Although not universal, most severely immunocompromised patients will require CT scan or magnetic resonance imaging (MRI) to diagnose surgical complications of infection. Cross-sectional imaging also is useful to diagnose emergent surgical conditions unrelated to immunocompromise or specific underlying disease processes that may present atypically in this population.
Mild to moderate dehydration typically responds to oral rehydration. Balanced electrolyte and glucose solutions should be encouraged, and commercial oral rehydration solutions (ORS) are preferred to address both dehydration and metabolic acidosis.2 Antiemetic treatment can assist with oral fluid tolerance and decrease the need for intravenous fluids (IVFs).56 Abnormal vital signs, fever, orthostatic hypotension, decreased urine output, and altered mental status are concerning signs of severe dehydration.1,2 More severe dehydration, inability to tolerate oral intake, or NPO status necessitates IVF hydration. Lactated Ringers is the optimal fluid because it contains both glucose and potassium.1 Once the patient is rehydrated, it is important to ensure that oral or IV maintenance fluids cover ongoing losses.56 For patients being discharged, 2 L to 4 L of ORS a day is recommended until the diarrhea resolves.5
Dietary modification and bulking agents may be helpful. Antimotility agents, specifically loperamide, should be avoided in the immunosuppressed patient if there is any concern for infection because they can increase tissue invasion due to delayed pathogen clearance.1 For patients with chronic diarrhea, or select afebrile patients with acute non-bloody diarrhea, loperamide or diphenoxylate/atropine may be a reasonable option. Bismuth subsalicylate is effective in reducing the number of diarrhea stools and expediting symptom resolution in traveler’s diarrhea with minimal side effects; however, there is no specific evidence of its effectiveness or safety in immunosuppressed patients.2
Conclusion
There are numerous etiologies for nausea, vomiting, and diarrhea in immunocompromised populations, such as those with HIV/AIDS, organ transplants, and cancer. Although benign disease processes may prevail in the general population, immunosuppressed patients are at increased risk for more severe, life-
threatening problems.
(The list of references is available online: https://bit.ly/2ZLz5NY)
Patients who are immunosuppressed may exhibit subtle or atypical presentations of gastrointestinal infection, as well as complications of their underlying disease processes or treatments. Emergency physicians should maintain a high level of suspicion for life-threatening pathology and evaluate these patients using broad differentials.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.